Abstract
Early vascularized soft tissue closure has long been recognized to be essential in achieving eventual infection free union. The question of whether muscle or fasciocutaneous tissue is superior in terms of promoting fracture healing remains unresolved. Here we review the experimental and clinical evidence for the different tissue types and advocate that the biological role of flaps should be included as a key consideration during flap selection.
Introduction
Open tibial fractures are severe injuries, largely affecting young men of working age, and take on average 43 weeks to unite, with 13% developing non-union in the best centres[1]. There is, therefore, an urgent need to enhance the process of bone repair in these patients. There have been numerous innovations in the techniques used for fracture stabilization as well as biological therapy, such as bone morphogenetic proteins (BMPs)[2]. Improvement in the care pathway, through a multidisciplinary and integrated orthoplastic approach, has also led to significant improvements in patient outcomes[3-6]. These refinements have reduced the mean union time to 26 weeks[5].
Considerations when planning soft tissue coverage include the size and location of the defect as well as donor site morbidity. An area which has not featured prominently in determining flap choice thus far is the potential biological role the flap may play in the fracture repair process. However, there is a growing body of experimental evidence that demonstrates that the biological characteristics of the tissues in a flap can significantly influence fracture healing, thereby potentially reducing union time and the rate of delayed or non-union.
Experimental Evidence
Wound healing properties of soft tissue flaps
The role of soft tissue reconstruction in open fractures is not limited to wound coverage to prevent wound desiccation and infection. Soft tissues also contribute to fracture repair by serving as a local source of stem or osteoprogenitor cells, growth factors and vascular supply[7-10].
Vascular Supply
A key role of soft tissue flaps in lower limb trauma is to serve as source of vascular supply to bone ends that have been stripped of periosteum and undergone disruption of the endosteum[11]. There is evidence that muscle contributes greater vascularity to a defect than fasciocutaneous tissue[12-16]. A study using a canine model to compare the blood flow at the musculocutaneous and fasciocutaneous flap/wound interfaces with no underlying fracture showed that whilst there was an initial increase in muscle blood flow in the first 24 hours, the deep surface of the fasciocutaneous flap underwent a slower and steadier increase in blood flow over the experimental period of 6 days to exceed that of muscle by this time point[17], yet there was greater evidence of healing in the muscle group [18]. Using a murine tibial fracture model, Harry et al. found that at all time points the vascular density was greater in fasciocutaneous tissue in apposition with a periosteally stripped fracture than muscle, and in spite of this, fracture repair was more rapid in the muscle group[19, 20]. These observations suggest that while vascularity is essential for wound healing, including bone repair, other biological factors become limiting, once an adequate blood supply threshold has been met.
Cellular contribution
Fracture repair requires the recruitment of osteoprogenitor cells. Mesenchymal stem cells (MSCs) are, by definition, multipotent and can therefore serve as a source of osteoprogenitor cells. MSCs may originate from a variety of tissues including the bone marrow, periosteum, dermis, adipose tissue and muscle, as well as blood vessels and the circulation. In closed fractures, the main sources of osteoprogenitor cells are thought to be the bone marrow and periosteum[21-25]. However, high energy open fractures of long bones are characterized by loss of the periosteum and bone marrow, especially following insertion of an intramedullary rod. Under these circumstances the main osteoprogenitor cells must originate from the local soft tissues or the circulation[10, 26].
It is well established that muscle provides a suitable environment for osteogenesis, although damaged muscle is less effective[27]. In 1965, Urist[28] found that new bone formed readily when decalcified bone was implanted into muscle and deduced that the inductor cells were derived from the host bed. Furthermore, purified BMPs injected into muscle are capable of inducing ectopic bone formation[29, 30]. Using a mouse model, Zacks et al.[31] found that muscle (but not liver tissue) demonstrated a significant osteogenic effect. Extraskeletal ossification observed in patients with fibrodysplasia ossificans progressiva[32] and heterotopic ossification following either orthopaedic surgery or blast injuries tend to occur in muscle[33, 34].
Both fasciocutaneous tissue and muscle are rich reservoirs of MSCs[9, 10]. However, the characteristics, including the osteogenic potential, of MSCs vary depending on their tissue origin. For example, human stromal cells derived from muscle exhibit a significantly greater potential for osteogenesis than those from fasiocutaneous tissue, including both skin and adipose, and are equivalent to those from bone marrow[35]. Using a critical sized rat femoral diaphyseal defect model, muscle was found to be more effective in promoting bone repair than adipose tissue[36]. Muscle-derived stem cells can be recruited from muscle and stimulated to undergo osteogenic differentiation by proinflammatory cytokines, especially TNF-α, released at the site of injury[35].
Cytokine/ growth factor environment
Muscle also provides a bone anabolic environment through the expression of members of the transforming growth factor-β (TGF-β) superfamily of growth and differentiation factors, including the BMPs. The reciprocal relationship between muscle and bone mass is well described, particularly the strong association between sarcopenia (age-related loss of muscle mass) and osteopenia. Muscle and bone are believed to be mutually regulating via physical forces and cytokine control. Indeed, recent evidence indicates that muscle serves as an endocrine organ that releases trophic factors, known as myokines, which have been identified as key regulators of the muscle and bone mass. Further observations suggest that intact muscle supports bone repair via the release of bone anabolics, including IGF-1, IL-6, BDNF and FGF-2[37-39] while severely injured muscle, such as following military trauma, impairs this process through the release catabolic myokines, including myostatin (GDF-8)[40-42]. Therefore, the net effect on bone is dependent on the balance of these factors.
Anti-microbial property
Soft tissue flaps are believed to possess an anti-bacterial property that is independent of vascularity. Chang and Mathes used a canine model to compare the anti-microbial properties of different tissues during wound healing[43]. Chambers inoculated with bacteria were inserted beneath random pattern flaps raised on the flanks with no underlying fracture. Muscle was found to be superior in eliminating bacteria from the wound bed. In a separate study, they compared bacterial growth within the wound fluid at interface of musculocutaneous and fasciocutaneous flaps and found that despite a higher blood flow and tissue oxygen tension in the fasciocutaneous group, muscle exhibited a greater ability to reduce the bacterial count[17, 18]. Moreover, histological examination revealed greater evidence of wound repair, including increased collagen deposition, at the muscle interface[18].
Comparison in animal models of bone repair
Recent evidence suggests that the presence of muscle is an important contributor to bone healing[9, 10, 44]. For example, the size of fracture callus is greater adjacent to muscle[45] and muscle coverage accelerates fracture repair in murine models[19, 46].
Schemitsch et al.[11, 47-50] compared cutaneous and muscle tissues in a series of studies using a canine open tibial fracture model. A devascularized segment of tibia was covered with either transposed tibialis muscle and the skin incision closed (muscle flap group) or skin closed directly following excision of the underlying fascia (skin group), and fracture healing was assessed. There was a significant increase in the bone blood flow and rate of union in the muscle flap group compared to the skin group[11, 48]. Muscle flaps were also found to significantly increase cortical porosity, enveloping callus and intracortical new bone formation[49]. Notably, there was no direct correlation between the soft tissue blood flow and the indices of bone repair, and resting muscle blood flow was found to be higher in the control limb using the microsphere technique[47]. Subsequent investigation of flap perfusion showed no difference in extraosseous soft tissue perfusion at the fracture site between the different groups[50]. However, this model does not emulate the clinical scenario as fascia beneath the anterior skin was excised in both groups, and only one-third of the circumference of the osteotomised tibial segment was in contact with the soft tissue flap, with the posterior segment in direct apposition with intact periosteum and musculature in both groups.
Our group developed a murine tibial fracture model to emulate the high-energy injuries encountered in clinical practice. One third of the circumference of the fracture was permitted direct contact with either muscle of fasciocutaneous tissue by excluding the remainder with polytetrafluoroethylene[19]. At 28 days following fracture, there was greater healing in the experimental muscle coverage group compared to skin and fascia alone with almost 50% more mineralized bone content and a three-fold stronger union in the muscle group compared to fasciocutaneous group despite a higher vascular density in the fasciocutaneous tissue compared to the muscle at all time points[19, 20].
In a series of studies, Utvag et al.[27, 46, 51, 52] examined the effect of separating muscle from the fracture site in the long bones of the lower limb in rodents. Interposition of an impermeable membrane between periosteum and muscle resulted in impaired healing in a rat femoral model[51]. However, a delay of 2 weeks in insertion of the impermeable membrane did not have any detrimental effect, indicating that early direct contact of muscle with the fracture site enhances fracture healing[52]. Excision of the anterolateral compartment muscles in a rat tibial fracture model also resulted in delayed healing. This effect was abolished when the muscle defect was corrected by transposition of the gluteal muscle[46]. Furthermore, isolating a tibial fracture in a rat model with nitrocellulose membranes with pore sizes ranging from 3 to 50kDa still resulted in impaired healing, confirming that direct contact of muscle with the fracture site, likely the cellular component, is an important factor in the healing of diaphyseal fractures[53].
Clinical Evidence
Most of the relevant clinical evidence comprises descriptive retrospective observational case series (Table 1) and all studies are categorized as Level 4 evidence according to the Oxford Centre for Evidence-based Medicine. Few of these specifically compared muscle with fasciocutaneous flaps and those that did were severely limited by the lack of power and case heterogeneity, including a wide variety of patients with clinical indications ranging from open fractures to burns or contour deficits. There were insufficient details in the publications to allow us to separate the flaps used to cover open fractures. Furthermore, the outcome measures differed considerably between studies, for example, not all studies reported time to fracture union, rates of deep infection or even flap survival. Therefore, the currently the published literature precludes amalgamation of data from different studies and hence any meaningful meta-analysis or systematic review that can provide guidance for the use of different flap options in the management of open fractures of the lower limb.
Table 1.
Evidence base for soft tissue coverage of open fractures of the lower limb
| Authors | Study design | Study groups | Flaps | Outcomes | Comments |
|---|---|---|---|---|---|
| Yazar et al 2006 [72] | Retrospective observational study |
174 patients, 177 free flaps distal 1/3rd tibia and ankle fractures (segmental bone defects and vascularized bone grafts excluded) Mean age: 35.4 years Follow-up time: 2 years |
Group I: 98 free muscle flaps Group II: 79 free fasciocutaneous flaps Flap selection not randomised Muscle flaps used for large wounds FC flaps for shallow defects Mean number of previous debridement procedures 3-4 |
Complete flap survival: Group I: 92.9%, Group II: 91.1% Post-op infection: Group I: 11.2%, Group II: 12.7% Primary fracture union: Group I: 84.5%, Group II: 81% Time to union not available Unaided walking at 2 years” Group I: 95.8%, 98.7% |
Outcomes for free muscle v FC flaps in severe lower limb trauma are equivalent. |
| Hong et al 2005 [67] | Retrospective observational study |
28 patients with chronic osteomyelitis of lower limb Mean age: 42.8 years Mean follow-up: 18.2 months |
All ALT flaps (6 included vastuslateralis muscle) | All flaps survived All achieved acceptable gait function No recurrence or persistence of osteomyelitis 2 flaps had partial wound dehiscence which healed spontaneously (both diabetic) |
Excellent success rate with free ALT flap. No comparison between FC alone and chimeric flaps.. |
| Van Landuyt et al 2005 [68] |
Retrospective observational study |
25 patients, 28 flaps; Mixed indications including trauma, burns, amputation Mean age: 37.6 years Mean follow-up time: not available |
All DIEP flaps 5 flaps for open lower limb fractures |
Complications in 60% including wound dehiscence & partial necrosis | Heterogeneous cohort, low numbers. Apparent high complication rate with free DIEP flaps for open leg fractures. |
| Hallock 2004 [66] | Retrospective observational study |
19 patients, 20 flaps 14/19 trauma-related Mean age: not available Mean follow-up: not available |
FC perforator flaps: Pedicled (5/20) or free (15/20), 7 chimeric flaps including gracilis muscle Flap selection not randomised 45% for foot and ankle defects, all free flaps |
6/20 pedicled flaps: 1 partial necrosis, 1 cellulitis and 1 heel pressure sore 15/20 free flaps successful but 2 had venous thrombosis (salvaged); 1 partial necrosis 2 anastomotic thrombosis (information of flap fate unavailable) |
Small series, high complication rate in local compared to free flaps. No comparison between FC only and chimeric flaps. |
| Baumeister et al 2003 [71] | Retrospective multi-center observational study |
67 patients, 70 flaps Mixed indications: 13 acute defects (11 trauma, 2 tumor resection); 36 longstanding post- traumatic defects or unstable scars, 15 chronic ulcers, 6 pressure sores Mean age: 54.1 years Mean follow-up time: not available |
All pedicledsural artery FC flaps |
|
Pedicledsural artery FC flaps associated with high complication rates especially in older age groups and those with comorbidities. |
| Pollak et al 2000 [60] | Prospective multicentre observational study |
601 patients enrolled: 190 patients, 195 limbs requiring flap coverage Mean age: 36 years Follow-up time: 6 months |
Rotational flap: 88 limbs Free flap: 107 limbs Rotation flap group comprised 26 FC and 62 muscle flaps - but no comparison made between groups Free flaps: 95.3% muscle, remainder ’other’ |
Overall 27% complication rate, 87% of which required further operative treatment; Flap loss rate equivalent in rotational (8.0%) and free flap groups (8.4%). Among patients with most severe osseous injury, 44% rotational flap group v 23% free flap group had wound complications. Rotational flap group was 4.3 fold more likely to have wound complication requiring operative intervention compared to free flap group |
Free flaps - lower complication rate than local flaps (muscle or FC) when used to cover severe open fractures. |
| Gopal et al 2000 [55] | Retrospective observational study |
80 patients, 84 fractures All GustiloIIIb or IIIc open tibial fractures Mean age: 37 years Mean follow-up: min 1 year, until bony union |
All muscle flaps: 9 pedicled, 75 free 63/84 within 72 hours, 21/84 delayed Flap selection not randomised |
Limb salvage 95% Primary bone union 66% Mean fracture union time 41 weeks Flap failure (requiring further surgery) 3.5% Deep fracture site infection 9.5% but at final review, all patients infection free and ambulant |
Combined early orthoplastic approach comprising debridement, fixation and early soft tissue cover with muscle flaps are associated with optimal outcome and reduced complications. |
| Gopal et al 2004 [56] (follow up of cohort published in 2000 [55]) |
Retrospective observational study |
33 patients, 34 Gustilo type IIIb and IIIc fractures Mean age: 48 years Mean follow-up time 46 months |
Orthoplastic approach:
|
Mean fracture union time 41 weeks 34% required further surgery to achieve union 2 patients developed deep infection that resolved Immediate flap cover associated with improved union and infection rates 100% patient satisfaction 41% returned to work SF-36 scores (mental and physical health) comparable with previous studies |
Orthoplastic, protocol-driven approach with early muscle flap coverage associated with satisfactory outcome in severe lower limb trauma. No comparison between free or local muscle flaps. |
| Hallock 2000 [65] | Retrospective observational study |
155 patients, 160 limbs, 184 flaps Indication: soft tissue closure for lower limb trauma but not all patients had open fractures 10 limbs Gustilo type IIIc fractures Mean age: not available Mean follow-up: 2 years |
Local muscle: 60 Local FC 50 (5 distally-based) flaps Free muscle: 61 Free fascial: 13 Flap selection not randomised |
91% of limbs salvaged Overall, 33% all flaps had complications, more likely in more severe injuries Complications (i.e. requiring additional surgery, complete flap failure, inability to achieve intended purpose, or significant morbodity to injured limb): Free flaps 39%, local muscle 27%, local fascia 30% 6/10 Gustilo type IIIc had complications :
Early soft tissue closure reduced risk of complications: Acute 19%, subacute 46%, chronic 45% Local FC flaps less reliable than local muscle flaps during acute period: 23% v 11% complication rate 5 distal-based local FC flaps used: 1 total necrosis due to venous congestion. |
Early closure is associated with lower complication rate. Free flaps are used for large and severe wounds. Local flaps are an alternative for limited defects, but higher complication rates with local fascial flaps compared to local muscle flaps. |
| Erdmann et al 1997 [64] |
Retrospective observational study |
61 patients, 66 flaps Acute trauma fractures: 80.3% Mean age: 50.5 years Mean follow-up time 13 months |
All distally-based islanded FC flaps: Distal 1/3rd leg: 71.2% 48/61 (78.7%) had associated fractures; 25 were Gustilo type IIIb fractures Flap procedures within 72h: 78.8% |
Mean time to fracture union 5.9 months (2-18 months) 6/66 (9.8%) patients developed non-union requiring bone grafting Loss of flap requiring further surgery in 5/66 (7.6%) All 5 patients sustained Gustilo type IIIb fractures and were heavy smokers. Tip necrosis in 6/66 (9.1%) |
Distally based islanded FC flaps for covering severe open fractures are associated with relatively high flap failure rates (16.7%). |
| Hallock 1993 [63] | Retrospective observational study |
269 flaps Mixed indications and sites 117 below knee Age: from <20 to >70 years Follow-up time: not available |
Flap selection not randomised |
Overall donor site complication rate 14% (same for both groups) Most frequent donor site problem in FC flaps was SSG ’take’ 73% v 16% in muscle group Major complication rate (i.e. requiring second operation to donor site e.g. further flap or SSG): 3% both groups 50% of major complications occurred below the knee: muscle: 5/42 (11.9%), FC: 9/72 (12.5%) Flap complications: muscle: major 4/42 (9.5%), minor 4/42 FC: major 10/72 (13.9%), minor 7/72 (9.7%) (Minor complications included seromas, minor wound dehiscence, epidermolysis, wound infections) |
Higher rate of major complications in FC (13.9%) compared to muscle flaps (9.5%). |
| Georgiadis et al 1993 [59] |
Retrospective observational study |
55 patients Gustilo type IIIb or IIIc fracturesnecessitating free tissue transfer or amputation Mean age: 32.5 years Mean follow-up: 40 months |
Group I: free tissue cover (latissmusdorsi, rectus abdominis, tensor fascia lata) 27 patients •Mean time to soft tissue cover: 21 days Group II: early amputation 18 patients
|
Group I: 81.5% (22/27) successfully salvaged and 96% after 2nd attempt Overall complication rate: 89%
Group II:
|
No comparison on outcome relating to flap types. Early amputation for unsalvageable limbs leads to good outcomes |
| Small and Mollan 1992 [61] | Retrospective observational study |
165 patients, 168 open tibial fractures Mean age: 24 years Follow-up time: not available |
174 flaps in 133 fractures:
SSG only in 22 fractures primary amputationin 13 fractures Delay to soft tissue cover: 4 days to 2.8 months Flap selection not randomised |
Flap necrosis (partial and complete):
18 of 22 failed flaps underwent further flap 15/22 (68.2%) SSGs: long term problems including unstable skin with ulceration, chronic bone infection and non-union |
Local FC flaps are unreliable for early coverage in severel lower limb trauma; local muscle flaps have a lower complication rate but free muscle flaps are superior. SSG alone is inadequate for coverage of open fractures. |
| Fischer et al 1991 [58] | Retrospective observational study |
43 patients Gustilo type IIIb fractures Mean age: 32 years Mean follow-up time: 104 weeks |
All muscle flaps (local 12 and free 12):
Flap selection not randomised |
Flap survival:
Rates of deep infection; chronic osteomyelitis:
Bone grafting after soft tissue healing was associated with lower rate of deep infection (31% v 73%), chronic osteomyelitis (0% v 26.7%) and shorter time to fracture union (54 v 63 weeks) |
Early muscle coverage is associated with lower infection rates. |
| Hallock 1991 [62] | Retrospective observational study |
100 flaps Mixed indications and sites: 67 lower extremity 54% all cases - trauma Mean age: not available Mean follow-up time: not available |
All local FC flaps | 97% success - i.e. ultimate wound healing and limb preservation Overall complication rate 26%. 15% all cases required further surgical procedures Higher complication rate for lower extremity and trauma (27.8% total or partial necrosis rate) 63.6% in older patients with peripheral vascular insufficiency Timing: early coverage - 5.6% complication rate subacute 38.5% chronic 36% 37.5% of distal-based flaps had complications |
Local FC flaps for open tibial fractures had high failure rate. Early coverage is associated improved outcomes. |
| Ponten 1981 [69] | Retrospective observational study |
22 patients, 23 flaps Mixed indications including unhealed, recent/old fractures, pseudoarthrosis, osteomyelitis, carcinoma Mean age: 42 years Follow-up time: not available |
All local FC flaps | 3/23 fair (i.e. flap tip loss) 3/23 failures:
|
Not possible to segregate the flaps in acute open fractures only. Although local FC flaps are simpler and quicker, they are associated with a high flap loss (partial or complete) of 26.1% in this series of mixed indications. |
| Byrd et al 1981 [57] | Prospective observational study |
18 patients, 20 Gustilo type IIIb fractures Mean age: 30.4 years Follow-up times: 1-34 months |
Orthoplastic approach: •early debridement •local muscle or myocutaneous flaps or free myocutaneous flaps within 5 days of injury •external fixation |
All fractures united (mean 4 months) Soft tissues stable in all patients Overall complication rate 5% (1 delayed union, 1 necrotising fasciitis following shot gun wound) |
Orthoplastic, protocol-driven approach with early debridement and soft tissue cover leads to favourable outcomes and low complication rates. No comparison of different flap types. |
FC = fasciocutaneous
SSG = split skin graft
Muscle flaps
It has been observed that open fractures of bones not surrounded by muscle, such as the tibia, unite slowly[54] and that healing of open bone defects is accelerated when a muscle flap is used to cover the wound. Furthermore, intact muscle appears to be more effective at promoting bone repair than injured muscle[55]. In a retrospective review of 84 consecutive patients with severe open tibial fractures, which included 79 grade IIIB and five Gustilo grade IIIC fractures, Gopal et al. presented their ‘fix and flap’ approach comprising early effective debridement, skeletal stabilization and subsequent obliteration of the dead space with a well-vascularized muscle flap[55]. Their longer-term outcome of 34 severe open tibial fractures, including 30 graded as Gustilo grade IIIB, showed a mean union time of 41 weeks, and rates of limb salvage and amputation compared favourably with other series[56].
Other authors have also commented that muscle provides superior coverage of open tibial fractures[55, 57-60]. Georgiadis et al.[59] highlighted the ability of muscle flaps to reduce both healing time and deep infection while Small and Mollan[61] retrospectively reviewed 168 open tibial fractures treated over a 15-year period and found a lower necrosis rate in local muscle flaps (13.3%) and free tissue transfer (most were muscle only latissimus dorsi and rectus abdominis flaps; 10%) compared to fasciocutaneous flaps (21.2%).
Fasciocutaneous flaps
Fasciocutaneous flaps are popular and have been used successfully in large clinical series to reconstruct open tibial defects[62-68]. Local fasciocutaneous flaps are reliable for lower limb reconstruction, as demonstrated by Ponten[69] in his study of 23 cases. They offered significant advantages, including simplicity, availability and versatility, replacing ‘like with like’ without sacrificing muscle function[62, 63, 65, 70]. However, in a series of 100 consecutive local fasciocutaneous flaps, which included 67 to the lower extremity, Hallock[62] reported that 15% required further surgical intervention, with the majority in lower limb wounds and attributed to peripheral vascular insufficiency. Although the majority of patients requiring vascularized tissue had been subject to trauma, it was not clear that all patients had fractures. The coverage of contaminated wounds was highlighted, with short-term healing achieved, suggesting that local fasciocutaneous flaps could be used to cover previously infected fractures[18].
The major advantage of local fasciocutaneous flaps is their relative simplicity of procedure. However, in patients with high-energy injuries, they may be susceptible to tip necrosis. Erdmann et al.[64] published their experience of pedicled fasciocutaneous flaps in lower limb trauma. Over a five-year period, they used distally-based, islanded fasciocutaneous flaps to reconstruct open tibial fractures to cover the distal one-third of the leg, ankle, heel or foot in 61 patients, with 25 fractures graded as Gustilo IIIB. The overall complication rate was 7.6%, which included five patients with Gustilo IIIB fractures suffering complete flap loss and four patients developing chronic osteomyelitis that led to non-union. Thus, the complication rate for coverage of Gustio IIIB fractures with distally-based islanded fasciocutaneous flaps reached 20%. The mean time to fracture healing was 5.9 months. In a prospective multicentre study involving high energy lower limb trauma, rotational flaps, including fasciocutaneous tissue and muscle, were compared to free muscle flaps in 195 limbs in 190 patients[60]. In patients with the most severe grade of osseous injury, wound complications including infection, necrosis or flap loss, were significantly higher in the rotational flap group (44% compared to 23%), and furthermore, these were 4.3 times more likely to require operative intervention.
Fasciocutaneous flaps have been found to be useful in chronic osteomyelitis of the lower limb by Hong et al.[67]. Over a three-year period, they treated 28 consecutive patients with surgical debridement and reconstruction using free anterolateral thigh perforator flaps, although six of these fasciocutaneous flaps were combined with a segment of vastus lateralis muscle. The well-contoured soft tissue flaps allowed effective resurfacing at the level of the ankle, permitting normal footwear, and unlike the muscle flaps, the elasticity of the skin flaps permitted easy re-exploration for secondary bone grafting procedures, with tension-free closure. Although lacking long-term follow-up, they felt that with adequate debridement and obliteration of dead space, the anterolateral thigh perforator flap was a time-efficient, functional, aesthetic and safe procedure that provided successful coverage for chronic infection.
More recently, the sural artery flap has gained popularity. However, in a multicentre review of 70 flaps, Baumeister et al.[71], found that up to 36% developed necrosis, and this was most likely to occur in patients with comorbidities, including diabetes mellitus, venous insufficiency and peripheral arterial disease. This is the sub group that is erroneously considered by some surgeons to be unsuitable for free flaps.
Studies comparing fasciocutaneous and muscle flaps
In a retrospective review over an 18-year period, Hallock assessed the role of muscle and fascia flaps in lower extremity trauma[65]. Details of flap coverage in 160 limbs in 155 patients, of which 60 were local muscle, 50 local fascial and 74 free muscle and fascial flaps, were reported. Flap selection was not randomly assigned, but based on clinical need. Complications were related to the severity of the injury, with 39% associated with free flap transfer, whereas local muscle and local fascia flaps had similar morbidities of 27% and 30%, respectively.
Donor site morbidity is often a factor in flap selection. In a retrospective review, the same author compared the relative donor site morbidity of muscle and fascial flaps[63]. In total, 147 local muscle/musculocutaneous and 122 fascia/fasciocutaneous flaps were used to reconstruct all regions of the body. These included a total of 45 muscle and 72 fasciocutaneous flaps for the lower limb, although it was not clear whether all these patients had exposed fractures. Overall, donor site complications were equivalent at 14% for each group while major complications, including nerve injury, failed graft, necrosis or ulceration, were infrequent in both. Most difficulties, however, were encountered below the knee with fasciocutaneous flap donor sites, where no local muscle option was available, and the skin grafted donor sites were described as cosmetically unappealing.
Finally, a retrospective review of patients with open tibial fractures treated with either free muscle or facsciocutaneous flaps showed that similar numbers went on to achieve bony union and were able to walk unaided at two years[72]. The authors found that muscle conformed better to complex defects but fasciocutaneous flaps better tolerated secondary surgical procedures.
Clinical Implications
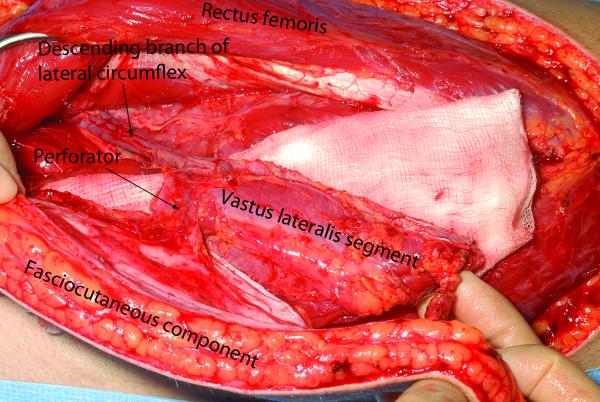
Meticulous wound debridement removes any non-viable soft tissue including muscle that may serve as a nidus of infection and a source of catabolic myokines to inhibit bone repair. From the available data and our own experience, we suggest that fasciocutaneous flaps may be superior to muscle for coverage of rapidly uniting metaphyseal fractures, particularly around the ankle, thereby avoiding skin grafts, which might be susceptible to minor trauma. However, muscle in direct apposition with diaphyseal fractures would aid healing. While muscle flaps covered with skin grafts are aesthetically unappealing and can be difficult to elevate for secondary procedures such as bone grafting, an alternative which retains the biological benefits of muscle apposition is to use chimeric flaps, such as a free anterolateral thigh flap that includes a segment of vastus lateralis[73] (Fig. 1). The plasticity of muscle also helps to obliterate the dead space, thereby reducing potential complications associated with hematoma formation[74]. In summary, thorough wound debridement and early flap coverage of open fractures achieves infection-free union and the biological contribution of the constituent tissues should be taken into consideration during flap selection.
Figure 1.
Chimeric free ALT flap, with a segment of vastus lateralis
Acknowledgments
Financial Disclosures and Products Page Project No F-09-23N was supported by the AO Foundation. JKC is in receipt of a Wellcome Trust Research Training Fellowship and a Royal College of Surgeons of England Research Fellowship. The Kennedy Institute of Rheumatology receives a core grant from ARUK (Registered Charity No. 207711).
Footnotes
Author participation JN, JKC conceived the idea of the manuscript.
JKC, JN, GW and LH co-wrote and edited the manuscript.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bosse MJ, et al. An analysis of outcomes of reconstruction or amputation after leg-threatening injuries. N Engl J Med. 2002;347(24):1924–31. doi: 10.1056/NEJMoa012604. [DOI] [PubMed] [Google Scholar]
- 2.Lissenberg-Thunnissen SN, et al. Use and efficacy of bone morphogenetic proteins in fracture healing. Int Orthop. 2011;35(9):1271–80. doi: 10.1007/s00264-011-1301-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mackenzie EJ, et al. The impact of trauma-center care on functional outcomes following major lower-limb trauma. J Bone Joint Surg Am. 2008;90(1):101–9. doi: 10.2106/JBJS.F.01225. [DOI] [PubMed] [Google Scholar]
- 4.Nanchahal J, et al. Standards for the management of open fractures of the lower limb. 1st ed British Association of Plastic, Reconstructive and Aesthetic Surgeons; 2009. [Google Scholar]
- 5.Naique SB, Pearse M, J N. Management of severe open tibial fractures: the need for combined orthopaedic and plastic surgical treatment in specialist centres. J Bone Joint Surg Br. 2006;88(3):351–7. doi: 10.1302/0301-620X.88B3.17120. [DOI] [PubMed] [Google Scholar]
- 6.Godina M. Early microsurgical reconstruction of complex trauma of the extremities. Plast Reconstr Surg. 1986;78(3):285–92. doi: 10.1097/00006534-198609000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Gerstenfeld LC, Einhorn TA. Developmental aspects of fracture healing and the use of pharmacological agents to alter healing. J Musculoskelet Neuronal Interact. 2003;3(4):297–303. discussion 320-1. [PubMed] [Google Scholar]
- 8.Gates CB, et al. Regenerative medicine for the musculoskeletal system based on muscle-derived stem cells. J Am Acad Orthop Surg. 2008;16(2):68–76. doi: 10.5435/00124635-200802000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Liu R, Schindeler A, Little DG. The potential role of muscle in bone repair. J Musculoskelet Neuronal Interact. 2010;10(1):71–6. [PubMed] [Google Scholar]
- 10.Schindeler A, Liu R, Little DG. The contribution of different cell lineages to bone repair: exploring a role for muscle stem cells. Differentiation. 2009;77(1):12–8. doi: 10.1016/j.diff.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 11.Richards RR, Schemitsch EH. Effect of muscle flap coverage on bone blood flow following devascularization of a segment of tibia: an experimental investigation in the dog. J Orthop Res. 1989;7(4):550–8. doi: 10.1002/jor.1100070413. [DOI] [PubMed] [Google Scholar]
- 12.Gothman L. Local arterial changes associated with diastasis in experimental fractures of the rabbit’s tibia treated with intramedullary nailing. A microangiographic study. Acta Chir Scand. 1962;123:104–10. [PubMed] [Google Scholar]
- 13.Trueta J, Buhr AJ. The Vascular Contribution to Osteogenesis. V. The Vasculature Supplying the Epiphysial Cartilage in Rachitic Rats. J Bone Joint Surg Br. 1963;45:572–81. [PubMed] [Google Scholar]
- 14.Rhinelander FW. The normal microcirculation of diaphyseal cortex and its response to fracture. J Bone Joint Surg Am. 1968;50(4):784–800. doi: 10.2106/00004623-196850040-00016. [DOI] [PubMed] [Google Scholar]
- 15.Holden CE. The role of blood supply to soft tissue in the healing of diaphyseal fractures. An experimental study. J Bone Joint Surg Am. 1972;54(5):993–1000. [PubMed] [Google Scholar]
- 16.Whiteside LA, et al. The acute effects of periosteal stripping and medullary reaming on regional bone blood flow. Clin Orthop Relat Res. 1978;(131):266–72. [PubMed] [Google Scholar]
- 17.Gosain A, et al. A study of the relationship between blood flow and bacterial inoculation in musculocutaneous and fasciocutaneous flaps. Plast Reconstr Surg. 1990;86(6):1152–62. discussion 1163. [PubMed] [Google Scholar]
- 18.Calderon W, Chang N, Mathes SJ. Comparison of the effect of bacterial inoculation in musculocutaneous and fasciocutaneous flaps. Plast Reconstr Surg. 1986;77(5):785–94. doi: 10.1097/00006534-198605000-00016. [DOI] [PubMed] [Google Scholar]
- 19.Harry LE, et al. Comparison of the healing of open tibial fractures covered with either muscle or fasciocutaneous tissue in a murine model. J Orthop Res. 2008;26(9):1238–44. doi: 10.1002/jor.20649. [DOI] [PubMed] [Google Scholar]
- 20.Harry LE, et al. Comparison of the vascularity of fasciocutaneous tissue and muscle for coverage of open tibial fractures. Plast Reconstr Surg. 2009;124(4):1211–9. doi: 10.1097/PRS.0b013e3181b5a308. [DOI] [PubMed] [Google Scholar]
- 21.Hutmacher DW, Sittinger M. Periosteal cells in bone tissue engineering. Tissue Eng. 2003;9(Suppl 1):S45–64. doi: 10.1089/10763270360696978. [DOI] [PubMed] [Google Scholar]
- 22.Gerstenfeld LC, et al. Fracture healing as a post-natal developmental process: molecular, spatial, and temporal aspects of its regulation. J Cell Biochem. 2003;88(5):873–84. doi: 10.1002/jcb.10435. [DOI] [PubMed] [Google Scholar]
- 23.Jaiswal N, et al. Osteogenic differentiation of purified, culture-expanded human mesenchymal stem cells in vitro. J Cell Biochem. 1997;64(2):295–312. [PubMed] [Google Scholar]
- 24.Bianco P, et al. Bone marrow stromal stem cells: nature, biology, and potential applications. Stem Cells. 2001;19(3):180–92. doi: 10.1634/stemcells.19-3-180. [DOI] [PubMed] [Google Scholar]
- 25.Sekiya I, et al. In vitro cartilage formation by human adult stem cells from bone marrow stroma defines the sequence of cellular and molecular events during chondrogenesis. Proc Natl Acad Sci U S A. 2002;99(7):4397–402. doi: 10.1073/pnas.052716199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shirley D, M.D., Jordan G, McQuaid S, Li G. Systemic recruitment of osteoblastic cells in fracture healing. J Orthop Res. 2005;23(5):9. doi: 10.1016/j.orthres.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 27.Utvag SE, et al. Influence of extensive muscle injury on fracture healing in rat tibia. J Orthop Trauma. 2003;17(6):430–5. doi: 10.1097/00005131-200307000-00007. [DOI] [PubMed] [Google Scholar]
- 28.Urist MR. Bone: formation by autoinduction. Science. 1965;150(3698):893–9. doi: 10.1126/science.150.3698.893. [DOI] [PubMed] [Google Scholar]
- 29.Iwata H, et al. Demineralized bone matrix and native bone morphogenetic protein in orthopaedic surgery. Clin Orthop Relat Res. 2002;395:99–109. doi: 10.1097/00003086-200202000-00010. [DOI] [PubMed] [Google Scholar]
- 30.Jingushi S, et al. Intramuscular bone induction by human recombinant bone morphogenetic protein-2 with beta-tricalcium phosphate as a carrier: in vivo bone banking for muscle-pedicle autograft. J Orthop Sci. 2002;7(4):490–4. doi: 10.1007/s007760200085. [DOI] [PubMed] [Google Scholar]
- 31.Zacks SI, Sheff MF. Periosteal and metaplastic bone formation in mouse minced muscle regeneration. Lab Invest. 1982;46(4):405–12. [PubMed] [Google Scholar]
- 32.Shore EM, et al. A recurrent mutation in the BMP type I receptor ACVR1 causes inherited and sporadic fibrodysplasia ossificans progressiva. Nat Genet. 2006;38(5):525–7. doi: 10.1038/ng1783. [DOI] [PubMed] [Google Scholar]
- 33.Forsberg JA, P.J, Wagner S. Heterotopic Ossification in high energy wartime extremity injuries: prevalence and risk factors. J Bone Joint Surg Am. 2009;91(5):8. doi: 10.2106/JBJS.H.00792. [DOI] [PubMed] [Google Scholar]
- 34.Iorio R, H.W. Heterotopic ossification after hip and knee arthroplasty: risk factors, prevention and treatment. J Am Acad Orthop Surg. 2002;10(6):8. doi: 10.5435/00124635-200211000-00005. [DOI] [PubMed] [Google Scholar]
- 35.Glass GE, et al. TNF-alpha promotes fracture repair by augmenting the recruitment and differentiation of muscle-derived stromal cells. Proc Natl Acad Sci U S A. 2011;108(4):1585–90. doi: 10.1073/pnas.1018501108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Evans CH, et al. Use of genetically modified muscle and fat grafts to repair defects in bone and cartilage. Eur Cell Mater. 2009;18:96–111. doi: 10.22203/ecm.v018a09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vogt PM, et al. Significant angiogenic potential is present in the microenvironment of muscle flaps in humans. J Reconstr Microsurg. 2005;21(8):517–23. doi: 10.1055/s-2005-922429. [DOI] [PubMed] [Google Scholar]
- 38.Pedersen BK. Muscles and their myokines. J Exp Biol. 2011;214(Pt 2):337–46. doi: 10.1242/jeb.048074. [DOI] [PubMed] [Google Scholar]
- 39.Cairns DM, et al. The role of muscle cells in regulating cartilage matrix production. J Orthop Res. 2010;28(4):529–36. doi: 10.1002/jor.21014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hamrick MW. A role for myokines in muscle-bone interactions. Exerc Sport Sci Rev. 2011;39(1):43–7. doi: 10.1097/JES.0b013e318201f601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hamrick MW, et al. Recombinant myostatin (GDF-8) propeptide enhances the repair and regeneration of both muscle and bone in a model of deep penetrant musculoskeletal injury. J Trauma. 2010;69(3):579–83. doi: 10.1097/TA.0b013e3181c451f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McPherron AC, Lawler AM, Lee SJ. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature. 1997;387(6628):83–90. doi: 10.1038/387083a0. [DOI] [PubMed] [Google Scholar]
- 43.Chang N, Mathes SJ. Comparison of the effect of bacterial inoculation in musculocutaneous and random-pattern flaps. Plast Reconstr Surg. 1982;70(1):1–10. doi: 10.1097/00006534-198207000-00001. [DOI] [PubMed] [Google Scholar]
- 44.Liu R, et al. Myogenic progenitors contribute to open but not closed fracture repair. BMC Musculoskelet Disord. 2011;12(1):288. doi: 10.1186/1471-2474-12-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stein H, et al. The muscle bed--a crucial factor for fracture healing: a physiological concept. Orthopedics. 2002;25(12):1379–83. doi: 10.3928/0147-7447-20021201-16. [DOI] [PubMed] [Google Scholar]
- 46.Utvag SE, et al. Poor muscle coverage delays fracture healing in rats. Acta Orthop Scand. 2002;73(4):471–4. doi: 10.1080/00016470216315. [DOI] [PubMed] [Google Scholar]
- 47.Anderson GI, et al. Soft-tissue blood flow after segmental osteotomy of the canine tibia. Ann Plast Surg. 1991;27(1):49–55. doi: 10.1097/00000637-199107000-00008. [DOI] [PubMed] [Google Scholar]
- 48.Richards RR, et al. A comparison of the effects of skin coverage and muscle flap coverage on the early strength of union at the site of osteotomy after devascularization of a segment of canine tibia. J Bone Joint Surg Am. 1991;73(9):1323–30. [PubMed] [Google Scholar]
- 49.Richards RR, et al. The influence of muscle flap coverage on the repair of devascularized tibial cortex: an experimental investigation in the dog. Plast Reconstr Surg. 1987;79(6):946–58. doi: 10.1097/00006534-198706000-00016. [DOI] [PubMed] [Google Scholar]
- 50.Schemitsch EH, et al. The relative importance of intramedullary, intracortical, and extraosseous soft-tissue blood flow to the repair of devascularized canine tibial cortex. Ann Plast Surg. 1997;38(6):623–31. doi: 10.1097/00000637-199706000-00010. [DOI] [PubMed] [Google Scholar]
- 51.Utvag SE, Grundnes O, Reikeras O. Effects of lesion between bone, periosteum and muscle on fracture healing in rats. Acta Orthop Scand. 1998;69(2):177–80. doi: 10.3109/17453679809117623. [DOI] [PubMed] [Google Scholar]
- 52.Utvag SE, Grundnes O, Reikeras O. Early muscle-periosteal lesion inhibits fracture healing in rats. Acta Orthop Scand. 1999;70(1):62–6. doi: 10.3109/17453679909000960. [DOI] [PubMed] [Google Scholar]
- 53.Kaufman H, et al. The biological basis of the bone-muscle inter-relationship in the algorithm of fracture healing. Orthopedics. 2008;31(8):751. [PubMed] [Google Scholar]
- 54.Landry PS, et al. Effect of soft-tissue trauma on the early periosteal response of bone to injury. J Trauma. 2000;48(3):479–83. doi: 10.1097/00005373-200003000-00018. [DOI] [PubMed] [Google Scholar]
- 55.Gopal S, et al. Fix and flap: the radical orthopaedic and plastic treatment of severe open fractures of the tibia. J Bone Joint Surg Br. 2000;82(7):959–66. doi: 10.1302/0301-620x.82b7.10482. [DOI] [PubMed] [Google Scholar]
- 56.Gopal S, et al. The functional outcome of severe, open tibial fractures managed with early fixation and flap coverage. J Bone Joint Surg Br. 2004;86(6):861–7. doi: 10.1302/0301-620x.86b6.13400. [DOI] [PubMed] [Google Scholar]
- 57.Byrd HS, Cierny G, 3rd, Tebbetts JB. The management of open tibial fractures with associated soft-tissue loss: external pin fixation with early flap coverage. Plast Reconstr Surg. 1981;68(1):73–82. doi: 10.1097/00006534-198107000-00015. [DOI] [PubMed] [Google Scholar]
- 58.Fischer MD, Gustilo RB, Varecka TF. The timing of flap coverage, bone-grafting, and intramedullary nailing in patients who have a fracture of the tibial shaft with extensive soft-tissue injury. J Bone Joint Surg Am. 1991;73(9):1316–22. [PubMed] [Google Scholar]
- 59.Georgiadis GM, et al. Open tibial fractures with severe soft-tissue loss. Limb salvage compared with below-the-knee amputation. J Bone Joint Surg Am. 1993;75(10):1431–41. doi: 10.2106/00004623-199310000-00003. [DOI] [PubMed] [Google Scholar]
- 60.Pollak AN, McCarthy ML, Burgess AR. Short-term wound complications after application of flaps for coverage of traumatic soft-tissue defects about the tibia. The Lower Extremity Assessment Project (LEAP) Study Group. J Bone Joint Surg Am. 2000;82-A(12):1681–91. [PubMed] [Google Scholar]
- 61.Small JO, Mollan RA. Management of the soft tissues in open tibial fractures. Br J Plast Surg. 1992;45(8):571–7. doi: 10.1016/0007-1226(92)90022-p. [DOI] [PubMed] [Google Scholar]
- 62.Hallock GG. Complications of 100 consecutive local fasciocutaneous flaps. Plast Reconstr Surg. 1991;88(2):264–8. doi: 10.1097/00006534-199108000-00015. [DOI] [PubMed] [Google Scholar]
- 63.Hallock GG. Relative donor-site morbidity of muscle and fascial flaps. Plast Reconstr Surg. 1993;92(1):70–6. doi: 10.1097/00006534-199307000-00010. [DOI] [PubMed] [Google Scholar]
- 64.Erdmann MW, Court-Brown CM, Quaba AA. A five year review of islanded distally based fasciocutaneous flaps on the lower limb. Br J Plast Surg. 1997;50(6):421–7. doi: 10.1016/s0007-1226(97)90329-5. [DOI] [PubMed] [Google Scholar]
- 65.Hallock GG. Utility of both muscle and fascia flaps in severe lower extremity trauma. J Trauma. 2000;48(5):913–7. doi: 10.1097/00005373-200005000-00016. [DOI] [PubMed] [Google Scholar]
- 66.Hallock GG. Lower extremity muscle perforator flaps for lower extremity reconstruction. Plast Reconstr Surg. 2004;114(5):1123–30. doi: 10.1097/01.prs.0000135847.49178.f2. [DOI] [PubMed] [Google Scholar]
- 67.Hong JP, et al. The use of anterolateral thigh perforator flaps in chronic osteomyelitis of the lower extremity. Plast Reconstr Surg. 2005;115(1):142–7. [PubMed] [Google Scholar]
- 68.Van Landuyt K, et al. The versatile DIEP flap: its use in lower extremity reconstruction. Br J Plast Surg. 2005;58(1):2–13. doi: 10.1016/j.bjps.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 69.Ponten B. The fasciocutaneous flap: its use in soft tissue defects of the lower leg. Br J Plast Surg. 1981;34(2):215–20. doi: 10.1016/s0007-1226(81)80097-5. [DOI] [PubMed] [Google Scholar]
- 70.Khan U, Pickford M. Use of an islanded fasciocutaneous flap in the lower limb following distraction callotasis. Br J Plast Surg. 2000;53(8):705–6. doi: 10.1054/bjps.2000.3425. [DOI] [PubMed] [Google Scholar]
- 71.Baumeister SP, et al. A realistic complication analysis of 70 sural artery flaps in a multimorbid patient group. Plast Reconstr Surg. 2003;112(1):129–40. doi: 10.1097/01.PRS.0000066167.68966.66. discussion 141-2. [DOI] [PubMed] [Google Scholar]
- 72.Yazar S, et al. Outcome comparison between free muscle and free fasciocutaneous flaps for reconstruction of distal third and ankle traumatic open tibial fractures. Plast Reconstr Surg. 2006;117(7):2468–75. doi: 10.1097/01.prs.0000224304.56885.c2. discussion 2476-7. [DOI] [PubMed] [Google Scholar]
- 73.Wong CH, Ong YS, Wei FC. The anterolateral thigh - Vastus lateralis conjoint flap for complex defects of the lower limb. J Plast Reconstr Aesthet Surg. 2012;65(2):235–9. doi: 10.1016/j.bjps.2011.08.043. [DOI] [PubMed] [Google Scholar]
- 74.Glass GE, Nanchahal J. The methodology of negative pressure wound therapy: Separating fact from fiction. J Plast Reconstr Aesthet Surg. 2012 doi: 10.1016/j.bjps.2011.12.012. Epub ahead of print. [DOI] [PubMed] [Google Scholar]