Abstract
Memory CD4+ T cells combat viral infection and contribute to protective immune responses through multiple mechanisms, but how these pathways interact is unclear. We found that several pathways involving memory CD4+ T cells act together to effectively clear influenza A virus (IAV) in otherwise unprimed mice. Memory CD4+ T cell protection was enhanced through synergy with naive B cells or CD8+ T cells and maximized when both were present. However, memory CD4+ T cells protected against lower viral doses independently of other lymphocytes through production of IFN-γ. Moreover, memory CD4+ T cells selected for epitope-specific viral escape mutants via a perforin-dependent pathway. By deconstructing protective immunity mediated by memory CD4+ T cells, we demonstrated that this population simultaneously acts through multiple pathways to provide a high level of protection that ensures eradication of rapidly mutating pathogens such as IAV. This redundancy indicates the need for reductionist approaches for delineating the individual mechanisms of protection mediated by memory CD4+ T cells responding to pathogens.
Introduction
Naive CD4+ T cells do not contribute to the clearance of influenza A virus (IAV) (1, 2), but memory CD4+ T cells are required for heterosubtypic immunity in IAV-primed animals (3–7) and have been correlated with protection in humans (8). How memory CD4+ T cells contribute to protection against viral pathogens is not well understood, but their attributes suggest that different subsets play multiple roles, including inducing inflammation, helping CD8+ T and B cells, and directly combating virus (9). In IAV-primed animals, it is challenging to analyze individual protective mechanisms mediated by memory CD4+ T cells and to distinguish them from those provided by memory CD8+ T cells, memory B cells, and Ab as well as elements of an altered lung environment. Moreover, we predict that distinct functions likely synergize with each other, further complicating analysis (10).
To unravel some of this complexity, we designed models to evaluate memory CD4+ T cell functions in unprimed hosts, lacking other IAV-primed lymphocytes, and showed a previously unappreciated ability of memory CD4+ T cells to act in the first 2–3 days after infection to induce innate immunity and reduce early viral titers (11). Using a similar model, another study concluded that memory CD4+ T cell protection is dependent on IFN-γ and that the protective capacity of these cells remains robust in lymphocyte-deficient hosts (12). However, this study was largely restricted to monitoring the first week of infection, and the importance of IFN-γ in protection against IAV remains controversial, with diverse reports citing either no role (2, 13–17) or a critical contribution (17–19).
Here, we systematically investigate protection against IAV mediated by memory CD4+ T cells in the absence of IAV-specific memory B and CD8+ T cells and preexisting IAV-specific Ab and analyze the contributions of major protective mechanisms likely to be mediated by CD4+ T cells, including helper activities, perforin-dependent cytotoxic function, and IFN-γ production. We found that memory CD4+ T cells transferred to unprimed hosts protected against high doses of IAV. In the absence of B or CD8+ T cells, protection was markedly and similarly reduced. By reconstituting lymphocyte-deficient mice with defined cellular populations, we showed that memory CD4+ T cells can mediate distinct modes of protection that do not require IFN-γ production and in which they synergize with either a neutralizing Ab response that does not require follicular help or with CD8+ T cell effectors acting during a brief window during the phase of viral clearance.
Furthermore, by eliminating both CD8+ T and B cells, we revealed unique protective mechanisms that are mediated directly by memory CD4+ T cells. Under these circumstances, memory CD4+ T cells can select for epitope-specific mutant viruses through a perforin-dependent mechanism that also contributes to viral control. Strikingly, in contrast with protection mediated through synergy with B or CD8+ T cells, protection mediated by memory CD4+ T cells in the absence of other lymphocytes was critically dependent on IFN-γ.
By deconstructing protective immunity mediated by memory CD4+ T cells, our results demonstrate they are capable of combating IAV through multiple pathways. The presence of these multiple mechanisms, which are redundant at low-challenge doses, helps to explain previous contradictory results and reveals the necessity of reductionist approaches for examining the full potential of memory CD4+ T cell protection against IAV. Furthermore, the fact that memory CD4+ T cells provide multiple layers of protection through synergizing mechanisms that develop concurrently supports the value of vaccines that induce multipotential CD4+ T cell memory.
Results
Memory CD4+ T cells protect against IAV infection.
We transferred memory CD4+ T cells (HNT TcR Tg) recognizing the PR8 virus (20) to WT mice that were then infected with PR8 to assess their protective capacity. Memory cells were obtained by reisolating donor cells from mice that had received naive HNT cells and a sublethal dose of PR8 at least 30 days prior (IAV-primed) (11) or by resting in vitro–generated TH1-, TH2-, or TH17-polarized, or unpolarized (TH0) effectors for at least 3 days in the absence of antigen and cytokines (21).
We first transferred 5 × 106 memory cells and challenged with 10,000 egg infective dose (EID50) (2 LD50) of PR8. Assuming a 10% “take” (22), this number (5 × 105) is in line with estimates of total IAV memory CD4+ T cells generated after priming based on our unpublished observations analyzing IAV-primed BALB/c mice as well as studies analyzing DR-1 transgenic mice (23), where the magnitude of the total memory CD4+ T cell response enumerable by ELISPOT assay employing multiple HA peptides alone was about 1 × 105 cells. Assuming that HA accounts for 20%–50% of the IAV response (23, 24), the total memory CD4+ T cell pool is about 2 to 5 × 105 cells, likely an underestimate, as not all cells are expected to produce the cytokines assayed. Analysis of memory CD4+ T cells generated by Sendai virus, another acute respiratory infection, leads to similar estimates of at least 1.5 × 105 cells based on IFN-γ ELISPOT assays utilizing an immunodominant peptide (25).
To further substantiate this, we transferred 1 × 106 naive HNT cells to unprimed mice, a minimum number required to consistently track donors into the memory phase (26), as a reporter population to analyze CD4+ T cell memory generated following IAV challenge. As the transferred cells came to dominate the IAV-specific response, enumerating the donor cells at the memory phase provided an estimate of the size of the total IAV memory CD4+ T cell pool. In these experiments, we found the number of donors in the memory phase to be about 3 × 105 cells (Supplemental Figure 1; supplemental material available online with this article; doi: 10.1172/JCI63689DS1), in line with the estimates above.
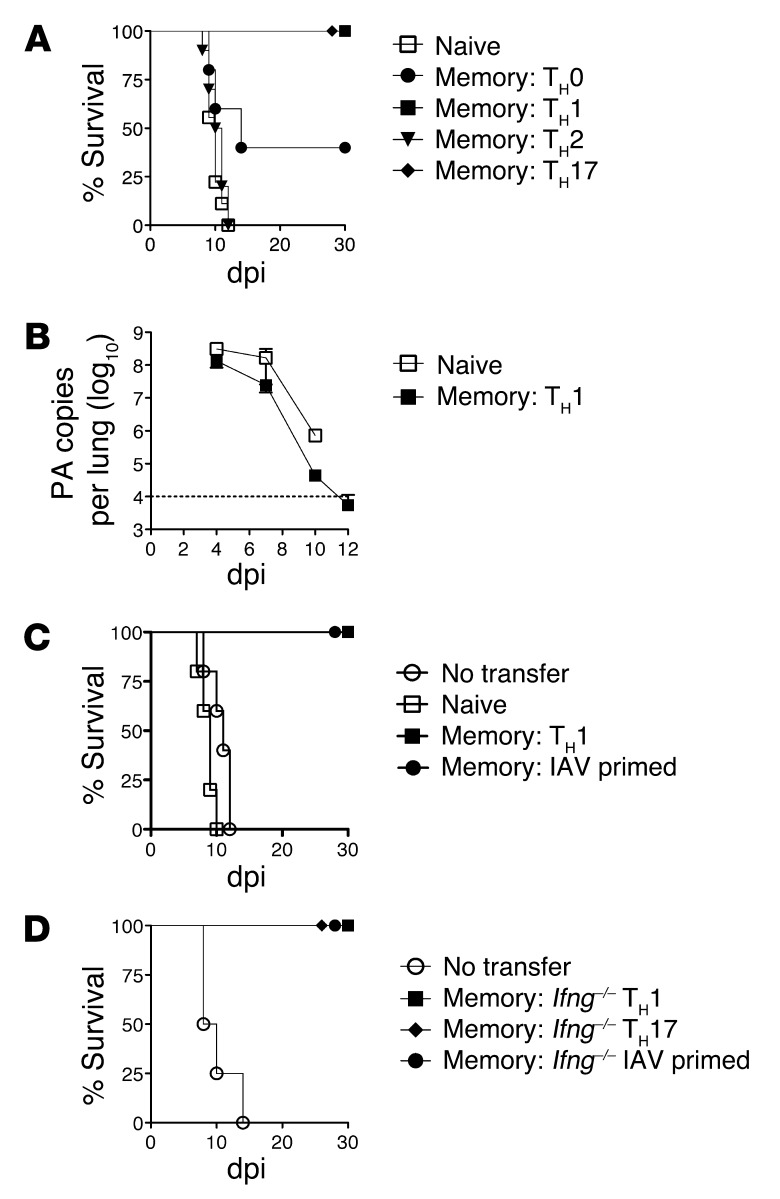
Both TH1 and TH17 memory cells fully protected mice from 2 LD50 PR8, but TH2-polarized cells did not (Figure 1A). Interestingly, TH0 memory cells provided partial protection (Figure 1A). Enhanced viral clearance in mice receiving TH1 memory cells was evident by 7 days post infection (dpi) and was complete by around 12 dpi (Figure 1B). In vivo IAV-primed memory cells also protected unprimed mice (Figure 1C), with kinetics of viral clearance and weight recovery similar to those observed with TH1 memory transfer (K.K. McKinstry et al., unpublished observations). In contrast, naive HNT cells provided no protection (Figure 1, A and C). The impact of protective memory cells was proportional to the number transferred: 1 × 106 cells marginally protected against 10,000 EID50, while 5 × 106 cells rescued mice from doses up to 40,000 EID50 PR8 (Supplemental Figure 2, A and B). No protection was observed against 2 LD50 of A/Philippines, which was not recognized by the HNT TcR (Supplemental Figure 2C).
Figure 1. Memory CD4+ T cell protection against lethal IAV infection.
5 × 106 naive or in vitro–generated memory HNT cells, polarized as stated, were transferred to unprimed WT hosts that were then infected with 10,000 EID50 PR8. (A) Survival for n = 10/group and (B) viral titers for n = 4/group (representative of 3 similar experiments). (C) 5 × 106 in vivo, IAV-primed memory HNT cells were transferred to WT host mice, then infected as in A (representative of 2 independent experiments with n = 5/group). (D) 5 × 106 Ifng–/– TH1 or TH17-polarized or in vivo–primed memory HNT cells were transferred to unprimed WT hosts, then infected with 10,000 EID50 PR8. Survival for n = 10/group (representative of 3 separate experiments).
We detected no role for memory CD4+ T cell–derived IFN-γ in protection, as in vivo IAV-primed, or TH1 or TH17 memory cells generated from Infg–/– HNT cells all rescued mice against 10,000 EID50 PR8 (Figure 1D). These results establish that memory CD4+ T cells effectively combat high doses of IAV in unprimed mice and that their protective capacity does not depend on IFN-γ. For further experiments, we focused on TH1 memory populations, as our studies strongly support that this subset most closely resembles memory CD4+ T cells generated by IAV challenge and accurately reflect their functions (11, 27).
Effect of removing host B or T cells.
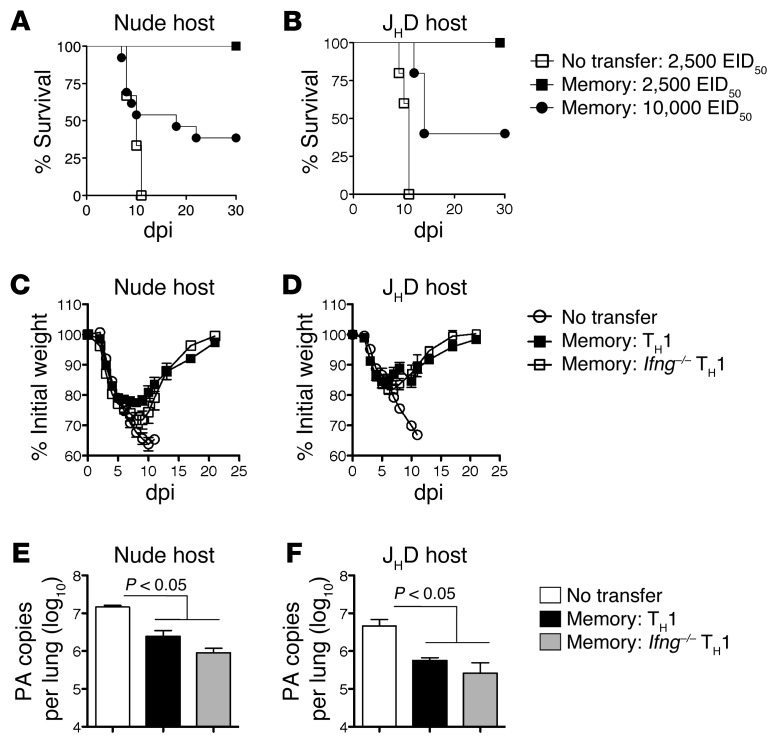
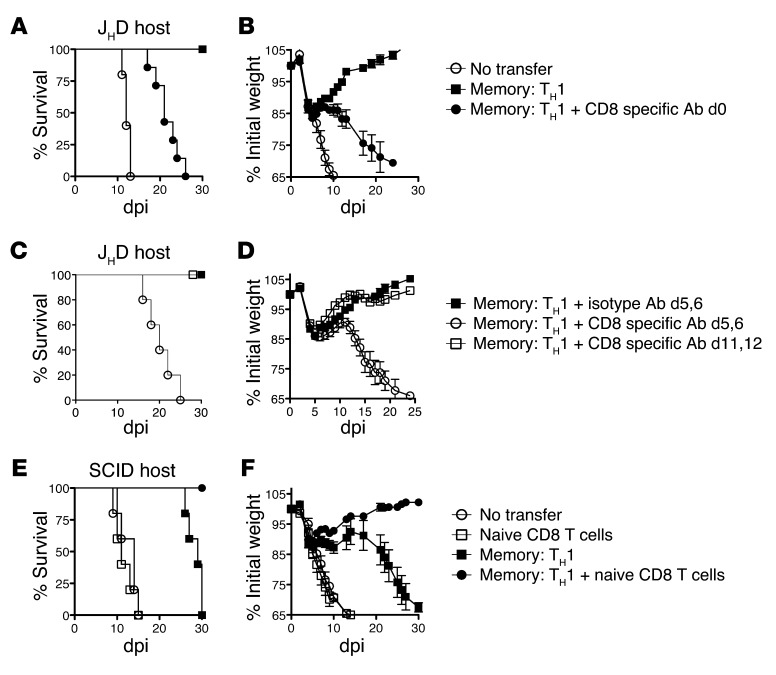
Memory CD4+ T cells could potentially protect through direct antiviral activities, through synergy with B cells or with other T cells, or through a combination of these mechanisms. To evaluate the role of host lymphocytes in memory CD4+ T cell–mediated protection, we utilized B cell–deficient (JHD) and T cell–deficient (nude) mice. These immunodeficient mice succumb to a lower dose of PR8 (2,500 EID50 = 0.5 LD50 for WT mice) at about 10 dpi (Figure 2, A and B), the same kinetics at which WT mice succumb to the higher 10,000 EID50 dose (Figure 1). Protection against 2,500 EID50 PR8 was complete in both JHD and nude mice receiving 5 × 106 memory HNT cells (Figure 2, A and B), as was viral clearance in both strains by 14 dpi (Figure 3C and Figure 4D). However, when challenged with 10,000 EID50, only a fraction of nude or JHD hosts survived. Nude and JHD hosts were protected equally by WT or Ifng–/– memory cells, as judged by monitoring weight loss and recovery and viral clearance (Figure 2, C–F). As WT hosts were completely protected against 10,000 EID50 PR8 (Figure 1A), this suggests that optimal memory CD4+ T cell–mediated protection against high doses of IAV requires synergy with both naive T and B cells.
Figure 2. Memory CD4+ T cell protection in the absence of host T or B cells.
5 × 106 TH1-polarized memory HNT cells were transferred to either (A) nude or (B) JHD hosts, then infected with stated doses of PR8 and survival monitored. Results representative of 3 separate experiments with each host (n = 10/group for nude and n = 5 for JHD hosts). WT or Ifng–/– TH1-polarized memory HNT cells were transferred to (C) nude and (D) JHD hosts and weight loss monitored after 2500 EID50 PR8 infection (n = 10/group for nude and n = 10 for JHD hosts) and (E) and (F) viral titers determined at 7 dpi (n = 5/group).
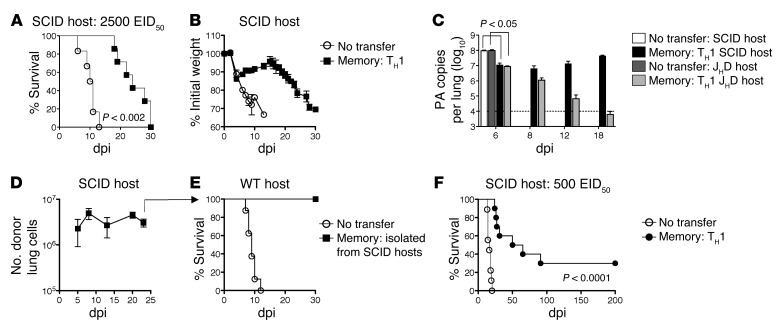
Figure 3. Memory CD4+ T cell protection in the absence of both host B cells and T cells.
5 × 106 TH1-polarized memory HNT cells were transferred to SCID hosts, then infected with 2,500 EID50 PR8. Survival (A) and weight loss (B) for n = 5–10/group. (C) Memory HNT cells were transferred to SCID or JHD mice and viral titer determined on indicated dpi, n = 5–10/group. (D) Recovery of donor cells in SCID hosts and (E) ability of 3 × 106 d22 reisolated cells from infected SCID hosts to protect WT hosts infected with 10,000 EID50 PR8. (F) Memory HNT cells were transferred to SCID hosts, then infected with 500 EID50 PR8 and morbidity monitored. n = 10/group. Data represent at least 2 independent experiments.
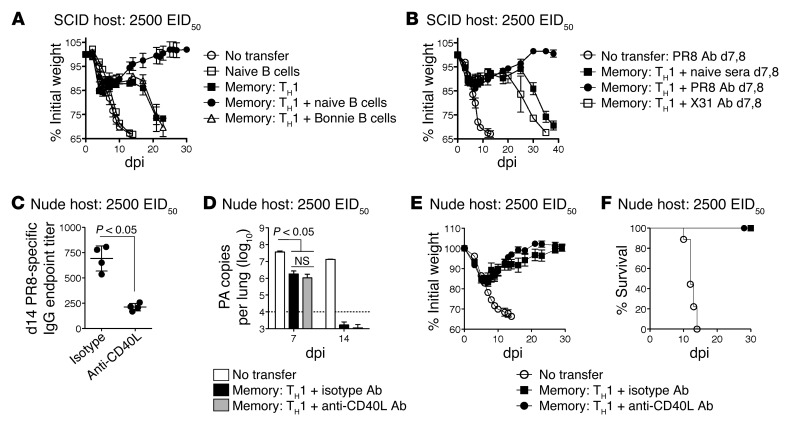
Figure 4. Memory CD4+ T cells synergize with unhelped B cell Ab responses.
Memory HNT cells (5 × 106) were transferred to SCID hosts, then infected with 2,500 EID50 PR8 (A) in conjunction with WT naive B cells or mIg Tg Bonnie B cells or (B) followed by administration of naive, PR8-, or X31-immune serum at 7 and 8 dpi and weight loss monitored for n = 5/group. Memory HNT cells were transferred to nude hosts, then infected with 2,500 EID50 PR8 and treated daily with 500 μg of isotype or anti-CD40L ab. (C) Mean PR8-specific total IgG (horizontal bar) endpoint titers at 14 dpi of n = 4/group (circles) and (D) viral titers at 14 dpi, n = 4/group as well as (E) weight loss and (F) survival were determined (n = 5/group). Data representative of at least 2 independent experiments.
Memory CD4+ T cell protection in the absence of T and B cells.
We next assessed protection in the absence of both T and B cells using SCID hosts challenged with the lower 2,500 EID50 dose of PR8. SCID mice receiving memory CD4+ T cells initially recovered but lost weight again during the second or third week of infection, and all mice eventually succumbed, although memory CD4+ T cells did increase the mean survival time of infected SCID mice (Figure 3, A and B). Memory CD4+ T cells reduced virus by more than 1 log during the first week of infection, but titers rebounded thereafter and remained high until experiments were terminated, in contrast with the progressive viral clearance observed in JHD mice receiving memory CD4+ T cells and challenged with the same dose of PR8 (Figure 3C).
The failure of memory CD4+ T cells to fully protect SCID mice against 2,500 EID50 PR8 could be due to the gradual loss of or the altered function of donor cells in the SCID host environment. Compromised protection was not due to the loss of donor cells, as high numbers were detected at 21 dpi (Figure 3D). To determine whether an altered functional program or exhaustion of donors was responsible for viral resurgence, we isolated donor cells from SCID hosts at 22 dpi and transferred 3 × 106 to unprimed WT mice, followed directly by infection with 10,000 EID50 PR8. Donor cells isolated from SCID hosts provided complete protection (Figure 3E), suggesting that memory CD4+ T cells responding in SCID mice are neither functionally defective nor exhausted. Indeed, memory CD4+ T cells protected SCID mice challenged with a lower 500 EID50 dose of PR8 (0.1 LD50 for WT mice), which was lethal to SCID mice not receiving memory CD4+ T cells. About 30% of mice survived for more than 200 dpi (Figure 3F) and evidenced complete viral clearance (K.K. McKinstry et al., unpublished observations). These results stress that as virus dose increases, more mechanisms are required to completely clear virus and achieve long-lasting protection.
Memory CD4+ T cells’ synergy with early B cell Ab response.
We next transferred naive WT B cells alone or together with memory HNT cells to SCID hosts to determine whether the addition of B cells would restore protection of SCID mice to that observed in nude hosts. B cell transfer alone provided no protection, but cotransfer of B cells and memory CD4+ T cells restored complete protection against 2,500 EID50 PR8 (Figure 4A), similar to that observed in nude hosts (Figure 2C). Thus, despite the fact that SCID hosts have defects in addition to the lack of T and B cells, adding back only naive B cells was able to phenocopy nude hosts. However, reconstitution of SCID hosts with memory CD4+ T cells and B cells from unprimed membrane Ig (mIg) Tg (Bonnie) mice expressing IgM recognizing 4-hydroxy-3-nitrophenyl acetyl (NP), but unable to secrete Ab (28), did not rescue protection (Figure 4A). These results confirm that memory CD4+ T cells’ synergy with unprimed B cells requires that they secrete IAV-specific Ab.
To confirm our hypothesis that memory CD4+ T cells could directly synergize with IAV-specific Ab to protect, we introduced convalescent serum from IAV-primed animals at 7 and 8 dpi, when virus-specific IgG can first be detected in unprimed mice challenged with IAV (ref. 29 and K.K. McKinstry et al., unpublished observations). As neutralizing or nonneutralizing Ab could potentially contribute to protection (30), we transferred serum containing 500 μg of PR8-specific IgG obtained from either PR8 (H1N1, containing neutralizing Ab) or X31 (H3N2, containing cross-reactive but not neutralizing Ab) immune mice, which alone did not protect (Figure 4B) or reduce viral titers at 9 dpi (K.K. McKinstry et al., unpublished observations). However, H1N1, but not H3N2, immune serum synergized with memory CD4+ T cells to fully protect SCID hosts (Figure 4B). This indicates that neutralizing Ab present at the time when the first IAV-specific class-switched Ab is being produced can synergize with direct antiviral activities of memory CD4+ T cells to ensure survival and clear IAV.
Memory CD4+ T cells provide superior help to B cells for Ab production compared with naive CD4+ T cells (31). Studies utilizing IAV show that CD40-dependent signals (32) and signaling lymphocytic activation molecule–associated protein (SAP) expression by CD4+ T cells (33), both associated with follicular helper CD4+ T cell (TFH) function, are critical for germinal center formation and for maximal long-lived Ab responses. Nonetheless, a T cell–independent burst of IAV-specific class-switched Ab is generated in the absence of these pathways, and dramatic differences in Ab titer in models where helper T cells lack SAP or CD40L are only observed after approximately 14 dpi (32–34). We thus tested whether protection mediated by memory CD4+ T cell synergy with B cells depended on CD40-dependent help or whether the Ab response generated in the absence of this pathway was sufficient. Memory CD4+ T cells were transferred to nude hosts, which have an intact B cell compartment, infected with IAV, and treated daily with either CD40L-blocking Ab (MR-1) or an isotype control. As expected, MR-1 treatment significantly reduced IAV-specific IgG titers at 14 dpi (Figure 4C), but viral clearance by 14 dpi (Figure 4D), weight recovery (Figure 4E), and survival (Figure 4F) were not affected. A similar result was observed in a C57BL/6 model in which nude mice receiving SAP-deficient memory CD4+ T cells recognizing an epitope of OVA (OT-II) were challenged with an otherwise lethal dose of PR8-OVAII (Supplemental Figure 3). Thus, while memory CD4+ T cells provide CD40L- and SAP-dependent help for optimal IAV-specific Ab responses, viral clearance mediated by memory CD4+ T cell synergy with B cells is independent of germinal center helper pathways. This analysis suggests that early Ab is critical for synergy with other memory CD4 T cell functions, consistent with the view that complete clearance of IAV depends on coordinated, timely action.
Memory CD4+ T cells synergize with CD8+ T cell effectors.
To investigate protection provided by memory CD4+ T cell synergy with host T cells, we administered CD8-depleting or control Ab to JHD mice prior to memory HNT cell transfer, leaving only host CD4+ T cells. We challenged with 2,500 EID50 PR8 and observed similar weight loss and survival for CD8-depleted JHD (JHD[αCD8]) hosts (Figure 5, A and B) and SCID hosts (Figure 3, A and B), suggesting that in the absence of B cells, naive CD4+ T cells do not synergize with memory CD4+ T cells to protect. Indeed, JHD(αCD8) hosts receiving memory CD4+ T cells failed to clear virus and, like SCID hosts, harbored high titers until experiments were terminated (Supplemental Figure 4), consistent with an important antiviral role for CD8+ T cells. To analyze whether CD8+ T cells act during the phase of viral clearance (days 6–10) or instead “mop up” residual virus later, we depleted CD8+ T cells from JHD mice receiving memory HNT cells at 6 or 10 dpi. Depletion at 6 dpi abrogated protection, while depletion at 10 dpi did not (Figure 5, C and D), supporting the concept that the synergy between host CD8+ T cell and donor memory CD4+ T cell responses during 6–10 dpi efficiently cleared virus in the absence of neutralizing Ab.
Figure 5. Memory CD4+ T cells synergize with CD8+ T cell effectors in the absence of B cells.
Memory HNT cells (5 × 106) were transferred to JHD hosts treated with 1 mg of isotype or CD8-depleting ab followed by infection with 2,500 EID50 PR8. (A) Survival and (B) weight loss for n = 5–10/group. (C) Survival and (D) weight loss following memory HNT cell transfer and depletion of CD8 T cells on the indicated days. n = 5–10/group. Memory HNT cells (5 × 106) were transferred to SCID hosts ± naive CD8+ T cells followed by infection with 2,500 EID50 PR8. (E) Survival and (F) weight loss for n = 5–10/group. Data representative of 2 independent experiments.
To confirm the ability of naive CD8+ T cells to complement memory CD4+ T cells to provide protection, we reconstituted SCID hosts with unprimed CD8+ T cells. Adding both naive CD8+ T cells and memory CD4+ T cells restored protection against 2,500 EID50 PR8, while CD8+ T cell transfer alone had no protective impact (Figure 5, E and F). The naive CD8+ T cell addition to SCID mice phenocopied B cell–deficient JHD mice (Figure 2). In agreement with previous studies utilizing IAV (35), we did not observe a role for CD4+ T cells in enhancing the magnitude or accelerating the kinetics of the primary CD8+ T cell response (K.K. McKinstry et al., unpublished observations), indicating that the protective synergy between memory CD4+ T cells and naive CD8+ T cells does not depend on CD4+ T cell help.
Memory CD4+ T cell select for escape mutants via a perforin-dependent mechanism.
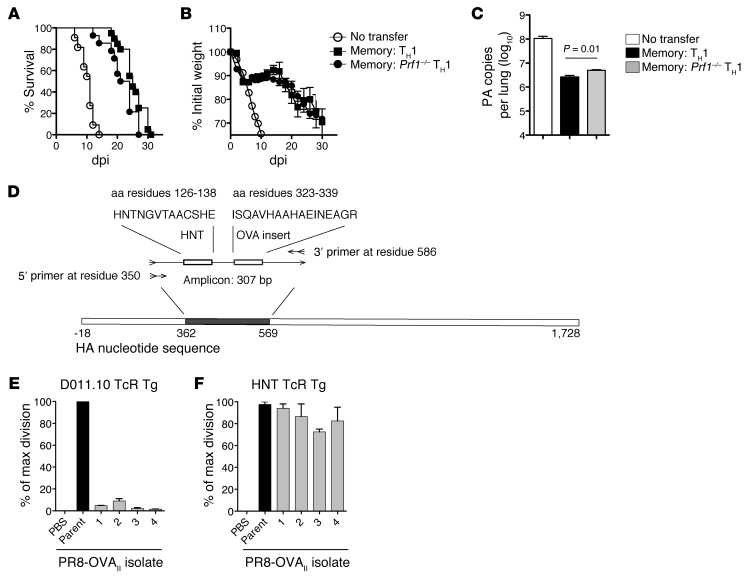
We next investigated protection mediated by memory CD4+ T cells in the absence of both B and CD8+ T cells, reasoning that we might discover mechanisms obscured in the presence of the protective synergies described above. We have identified perforin-dependent cytotoxicity as a critical protective mechanism employed by CD4+ T cell effectors during IAV infection in JHD hosts (2). To ask whether memory CD4+ T cells require this pathway for protection, we transferred Prf1–/– HNT memory cells to SCID hosts and infected them with PR8. Similar patterns of weight loss and survival were observed after transfer of either WT or Prf1–/– memory cells (Figure 6, A and B), but Prf1–/– cell recipients had significantly higher viral titers (Figure 6C). These results suggest that a perforin-dependent mechanism contributes to viral control mediated by memory CD4+ T cells but also indicate a major role for perforin-independent mechanisms.
Figure 6. Memory CD4+ T cells select for viral escape mutants through a perforin-dependent mechanism.
5 × 106 WT or Prf1–/– memory HNT cells were transferred to SCID hosts, then infected with 2,500 EID50 PR8. (A) Survival, (B) weight loss, and (C) viral titers at 9 dpi are shown for at least n = 10/group (summary of 3 separate experiments). (D) Schematic of the amplicon used for sequencing PR8 and PR8-OVAII isolates. Naive splenocytes were infected with concentrated PR8-OVAII stock or viral isolates from SCID mice receiving memory DO11.10 cells and cocultured with naive CFSE-labeled TCR Tg CD4+ T cells for 72 hours. The percentage of maximal division from triplicate cultures for each of the 4 viral isolates or stock PR8-OVAII cultured with naive (E) DO11.10 and (F) HNT cells.
Since perforin-mediated cytolytic activity is the major mechanism by which CD8+ T cells select for IAV escape mutants in the absence of other lymphocytes (36), we asked whether memory CD4+ T cells were capable of selecting for such variants and whether selection required perforin. To test this, we transferred WT or Prf1–/– memory CD4+ T cells to SCID mice and harvested lungs of PR8-infected recipients after they had lost 30% of their initial weight. PR8 stock and virus present in lung were expanded in vitro and concentrated, and the HNT coding sequence was analyzed. While expanded stock virus contained no mutations, 10 of 27 isolates obtained from mice receiving WT cells in 3 separate experiments contained an altered HNT sequence (Table 1 and summarized in Supplemental Table 1). In contrast, only 1 of 13 isolates from mice receiving Prf1–/– memory cells harbored a mutation, suggesting that monoclonal memory CD4+ T cells can exert sufficient selective pressure to drive epitope-specific escape mutants through a perforin-dependent pathway.
Table 1.
The proportion of viral isolates harboring mutations within the HNT sequence after donor cell transfer
To confirm that the emergence of escape mutants required TcR-mediated pressure, we transferred DO11.10 memory CD4+ T cells recognizing the OVAII epitope to SCID hosts and infected them with PR8-OVAII. Isolates from the lungs of these mice were amplified and sequenced as described for PR8 isolates, as the amplicon also contained the OVAII peptide insertion (Figure 5D). In a representative experiment, 3 of 4 isolates displayed a mutation in the OVAII sequence, and the remaining isolate harbored a deletion (Supplemental Table 1). Importantly, no mutations were observed in the HNT sequences (K.K. McKinstry et al., unpublished observations). We observed identical results, with mutations in the OVAII but not HNT sequences, employing a B6 model in which memory OT-II TCR Tg CD4+ T cells was transferred to Rag–/– hosts challenged with PR8-OVAII (n = 10 isolates; Supplemental Table 2).
To confirm that these mutations did in fact impair recognition by CD4+ T cells expressing the selecting TcR, we infected spleen cells with stock virus or viral isolates, added naive CFSE-labeled TCR Tg CD4+ T cells, and assessed proliferation after 4 days. When the 4 PR8-OVAII isolates from SCID mice receiving DO11.10 memory cells (Supplemental Table 1) were used to stimulate naive DO11.10 cells, virtually no division was observed (Figure 6E). However, no reduction in division was observed relative to the stock virus when the PR8-OVAII isolates were used to stimulate naive HNT cells (Figure 6F). Together, these results demonstrate that a perforin-dependent memory CD4+ T cell mechanism can drive the evolution of epitope-specific IAV escape mutants that most likely arise from the outgrowth of viral progeny that escape TcR recognition, supporting an important role for cytolytic CD4+ T cells in viral clearance.
Direct memory CD4+ T cell protection requires IFN-γ.
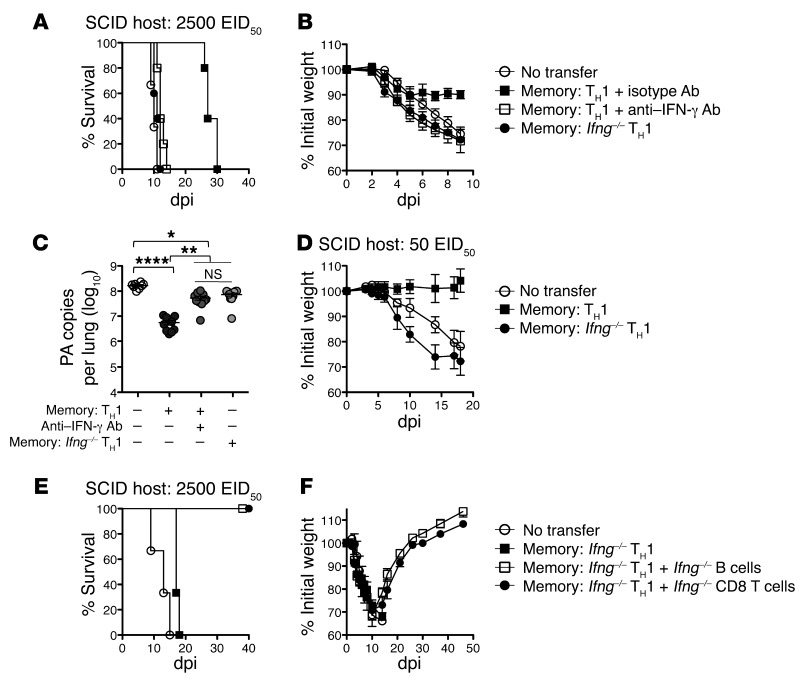
Finally, we sought to characterize additional mechanisms of viral control utilized by memory CD4+ T cells. We reasoned that in the absence of both B and CD8+ T cells, a role for IFN-γ production by CD4+ T cells might be revealed. We thus treated SCID mice receiving WT memory HNT cells daily with anti–IFN-γ Ab or isotype Ab following 2500 EID50 PR8 infection. In contrast with isotype-treated animals, which recovered during the first week, mice receiving anti–IFN-γ Ab resembled animals not receiving memory CD4+ T cells that all succumbed by 12 dpi (Figure 7, A and B). To confirm that CD4+ T cell–derived IFN-γ was critical, we transferred memory cells generated from Ifng–/– HNT cells to SCID mice and again observed no protection (Figure 7, A and B). The requirement for IFN-γ was not an artifact of TH1 polarization, as a similar lack of protection was observed utilizing TH17-polarized memory generated from Ifng–/– HNT cells and in SCID mice receiving in vivo IAV-primed CD4+ T cell memory and treated with anti–IFN-γ Ab (Supplemental Figure 5). We assessed PR8 titers at 9 dpi to determine whether memory CD4+ T cell–derived IFN-γ had an antiviral effect. While WT cells reduced titers by over 1 log, titers in mice receiving Ifng–/– memory or WT cells and anti–IFN-γ Ab were only slightly reduced compared with no-transfer controls (Figure 7C).
Figure 7. Memory CD4+ T cell protection requires IFN-γ in the absence of host lymphocytes.
5 × 106 WT or Ifng–/– memory HNT cells were transferred to unprimed SCID hosts, then infected with 2,500 EID50 PR8. Recipients of WT cells were treated with 500 μg of isotype or anti–IFN-γ neutralizing ab throughout the infection. (A) Survival, (B) weight loss, and (C) mean viral titers (horizontal bar) at 9 dpi are shown for at least n = 5/group (circles) (1 of 2 separate experiments, *P < 0.005; **P < 0.005; ****P < 0.0001). (D) SCID recipients of 5 × 106 WT or Ifng–/– memory HNT cells were challenged with 50 EID50 PR8 and weight loss determined (n = 6/group). SCID recipients of Ifng–/– memory HNT cells were also reconstituted with no additional cells, unprimed Ifng–/– B cells, or unprimed Ifng–/– CD8 T cells and (E) survival and (F) weight loss determined. n = 5/group (1 of 2 separate experiments).
We next infected SCID mice receiving Ifng–/– memory cells with lower doses of IAV. In contrast with mice receiving WT cells (Figure 3F), Ifng–/– memory CD4+ T cells could not protect against 500 EID50 PR8 (K.K. McKinstry et al., unpublished observations). More strikingly, Ifng–/– memory cells could not even protect against 50 EID50 PR8 (0.01 LD50 for WT mice), against which SCID mice receiving WT memory displayed virtually no weight loss (Figure 7D). Thus memory CD4+ T cell–mediated protection operating independently of other lymphocytes is completely dependent on IFN-γ.
Finally, we asked whether we could recapitulate our observations in nude and JHD hosts and render the requirement for IFN-γ for memory CD4+ T cell protection in SCID mice redundant by reintroducing either naive B or CD8+ T cells. We thus reconstituted SCID mice with Ifng–/– memory CD4+ T cells and either B or CD8+ T cells from unprimed Ifng–/– BALB/c mice. While B or CD8 T cells alone did not protect against 2500 EID50 PR8 (K.K. McKinstry et al., unpublished observations), cotransfer of either population together with Ifng–/– memory CD4+ T cells restored protection (Figure 7, E and F), consistent with the lack of a role for IFN-γ in protection mediated by memory CD4+ T cell synergy with B or CD8+ T cells.
In summary, by systematically deconstructing protection mediated by memory CD4+ T cells against IAV, we have identified multiple, distinct mechanisms which interact in a synergistic, additive or redundant fashion.
Discussion
While any particular animal model of infectious disease is not adequate for predicting what will occur in humans, mouse models are uniquely useful for uncovering the basic mechanisms the immune system can mobilize, and they offer the valuable opportunity for isolating individual mechanisms, defining how they operate, and investigating their interactions. Since the goal of vaccination is to develop broad and effective protection, understanding the full potential of memory CD4+ T cells to have an impact on immunity is of great importance. Through deconstruction of the protective response mediated by memory CD4+ T cells against IAV, we identify multiple pathways through which this population can provide protection. First, in the absence of other lymphocytes, memory CD4+ T cells mediate Ag-specific viral clearance that is largely dependent on IFN-γ and, to a lesser extent, perforin, but we find this response alone is not sufficiently robust to clear high-challenge doses. Second, memory CD4+ T cells synergize with naive B cells that act via secreting neutralizing Ab to improve viral clearance. Interestingly, while memory CD4+ T cells drive enhanced Ab production, the protective synergy with B cells does not depend on critical TFH-associated helper pathways. Third, an equivalent degree of protection against IAV is provided by memory CD4+ T cell synergy with CD8+ T cells during the phase of viral clearance. Either of these mechanisms is effective alone against 2,500 EID50 PR8 challenge, but when both are present, protection is seen against 10,000 EID50, indicating they are additive. Thus, our results suggest that optimal immunity to overcome high doses of rapidly mutating viruses, such as IAV, is best achieved by simultaneous responses involving Ab and CD4+ and CD8+ T cells, each of which target distinct epitopes.
While several studies have investigated the requirements for heterosubtypic immunity, a consensus on the critical cellular requirements is lacking. For example, previous studies have reached incompatible conclusions that protection depends primarily upon CD4+ and CD8+ T cells but not Ab (4, 37), on CD4+ and not CD8+ T cells or Ab (5), on CD8+ and not CD4+ T cells or Ab (3), on B cells and not CD8+ T cells (38), or that cross protection is optimal in the presence of both B and T cell responses against IAV (39). Our results serve to reconcile these seemingly contradictory results by demonstrating that distinct pathways of immunity mediated by memory CD4+ T cells can provide similar levels of protection. It is likely that variables such as the strain and dose of priming virus or differences in numbers and types of memory cells induced by distinct vaccine formulations will all determine the relative strength of the individual components of immunity against IAV and thus their potential contribution. In addition, since the size of the memory CD4+ T cell population gradually wanes, the time between priming (or boosting) and challenge is likely to have a considerable impact on the overall efficacy of memory CD4+ T cell–mediated protection, with protective efficacy tilting after long intervals toward memory CD8+ T cells that are retained at more stable numbers (40).
We believe the approach we have taken here, in which we transfer memory CD4+ T cells to a series of B cell–, T cell–, and lymphocyte-deficient hosts, is a valid one and in fact the only one capable of resolving individual components within a multilayered protective response. Such hosts do offer the possibility of introducing artifacts because they are lymphopenic, so that the cells introduced might expand to a greater extent than they would in intact mice. While that is likely to be the case, we see no evidence that this has a substantial impact on the results presented here. First, memory CD4+ T cell protection is robust in intact mice as well as lymphopenic mice when the pathways we identify are preserved. Second, the protective capacity of memory CD4+ T cells in SCID hosts reconstituted with naive CD8+ T or B cells or even IAV-specific Ab was equivalent to that in nude or JHD hosts, respectively, and less than in intact hosts, consistent with the same sets of mechanisms working in each case. Thus, the reconstruction studies suggest that the efficacy of each mechanism is not much affected by the host except for the absence of the cells in question. Moreover, since we have uncovered multiple mechanisms of memory CD4+ T cell protection that can be redundant when present together, it is clear that the details of each can only be revealed by a reductionist approach where a particular pathway can be assessed in isolation. In fact, our studies point out the impossibility of identifying and analyzing pathways of protection by conventional approaches that target mechanisms one-by-one in intact mice. A similar reductionist approach has shown that B cells are capable of protecting against IAV through multiple pathways (39). The critical contributions of CD8+ T cells have not been elucidated fully, likely because of their own ability to employ redundant protective mechanisms (R.W. Dutton et al., unpublished observations).
We have shown that memory CD4+ T cells responding to IAV control virus during the initial stages of infection through engaging broad elements of innate immunity (11). We suggest that the early capping of virus through this mechanism, which is intact in SCID hosts (11), keeps titers beneath a critical threshold until CD8+ T cells, neutralizing Ab, or both are able to synergize with CD4+ T cell–mediated functions, resulting in potent viral clearance that is largely independent of further innate defense mechanisms. We found, perhaps surprisingly, that neither the TFH pathway nor help that enhances CD8+ T cell expansion is required for enhanced memory CD4+ T cell protection through their respective synergy with B cells or CD8+ T cells. One possibility in both cases is that the effects rather than the cells synergize. In the case of B cells, this suggests either that nonfollicular B cell help plays a critical role or that the T cell—independent Ab response against IAV is sufficient to synergize with antiviral memory CD4+ T cell function to clear the virus. In the case of synergy with CD8+ T cells, the combination of antiviral activities of CD8+ T cells recognizing viral peptides in the context of MHC I with antiviral functions of memory CD4+ T cells recognizing MHC II restricted peptides could preferentially target different infected cells to more effectively clear IAV. Further experiments to evaluate the detailed requirements for synergy of memory CD4+ T cells with B and CD8+ T cells are required.
In contrast, the weaker form of protection mediated by memory CD4+ T cells that is independent of other lymphocytes may be mediated in large part by innate immunity. This would explain the critical requirement for IFN-γ in controlling IAV in SCID but not in nude, JHD, or SCID hosts reconstituted with naive B or CD8+ T cells. IFN-γ is crucial for the activation of many innate populations, including neutrophils and macrophages, both of which can contribute to protection against IAV (41, 42). Further experiments are required to determine the precise role of IFN-γ produced by memory CD4+ T cells, but regardless, our results help to reconcile contradictory results on the role of IFN-γ in protection against IAV (2, 13–19) by demonstrating its differing impact in different settings of IAV infection.
We also show that memory CD4+ T cells are capable of exerting selective pressure on IAV, albeit in an artificial setting. IAV-infected lung epithelial cells express MHC II on their surface (43), providing an appealing mechanism for explaining that direct recognition of infected cells by memory CD4+ T cells can eliminate epithelial cells harboring virus capable of producing progeny containing peptide sequences recognized by their TcR through perforin-dependent cytotoxicity while sparing cells infected with mutants that do not. The use of monoclonal CD4+ T cell memory populations allowed us to readily detect variants in the epitope seen by the transgenic TcR, but it is likely that less easily detected selection occurs under physiological circumstances. In support of this, IAV escape from a characterized human IAV CD4+ T cell epitope has been reported (44), and such viruses could represent potent threats, given their potential for escaping the multiple protective mechanisms described here. How such mutants arise in the human population is not clear, but it is possible that heterologous immunity induced by previous infection with a pathogen sharing a crossreactive CD4+ T cell epitope with IAV could, in certain individuals, result in dominance of the response by one clone, as has been shown to lead to CD8+ T cell viral escape in intact mice (45).
One might be tempted to conclude that the failure of memory CD4+ T cells to clear IAV in SCID mice was solely due to the emergence of escape mutants. However Prf1–/– memory cells, which failed to select for escape mutants, provided nearly equivalent, inadequate protection. Instead, analysis of SCID hosts receiving WT and Prf1–/– memory cells revealed extensive and increasing lung pathology and decreasing lung function during the third and fourth week after infection (K.K. McKinstry et al., unpublished observations). The high viral titers that were sustained in SCID mice were most likely possible because of the prominent and extensive proliferation of bronchial and bronchiolar epithelium observed (K.K. McKinstry et al., unpublished observations). It is possible that factors released by macrophages activated by memory CD4+ T cells (11, 46) may stimulate epithelial proliferation, setting up conditions that allow for chronic infection, increasing lung inflammation, and eventually, death of SCID mice regardless of the emergence of escape mutants.
Most current vaccines aim to generate neutralizing Ab against predicted seasonal IAV and often fail to induce or boost T cell responses (47). The ability of memory CD4+ T cells to engage in multiple mechanisms of protection that together provide potent protection even in the absence of both memory B and CD8+ T cells suggests that inducing memory CD4+ T cells through vaccination deserves strong consideration. Recent human studies correlating preexisting CD4+ T cells responding against IAV with reduced illness and viral shedding support this concept (8). Furthermore, as T cells can recognize epitopes derived from internal viral proteins that are largely conserved between IAV isolates (3), vaccines promoting the generation of CD4+ T cell memory in addition to Ab may offer more robust “universal” protection against seasonal and pandemic strains.
Methods
Mice.
BALB/c, Ifng–/– BALB/c, JHD, mIg Tg Bonnie, and TcR Tg mice were obtained from the animal breeding facility at Trudeau Institute or University of Massachusetts Medical School. Nude and SCID mice were obtained from Jackson Laboratories. Mice were at least 8 weeks old at the time of infection. Naive CD4+ T cells were obtained from 5- to 8-week-old HNT Thy1.1, HNT perforin-deficient, Prf1–/–, HNT Ifng–/–, or DO11.10 mice on a BALB/c background. The HNT TcR recognizes aa 126–138 (HNTNGVTAACSHE) of A/PuertoRico/8/34 (PR8, H1N1) HA. The DO11.10 TcR recognizes the aa 323–339 (ISQAVHAAHAEINEAGR) of ovalbumin.
Virus stocks and infections.
PR8 (H1N1) was produced in the allantoic cavity of embryonated hen eggs from stock originating at St. Jude Children’s Hospital (Memphis, Tennessee, USA) and the EID50 characterized. Influenza A/Philippines/2/82/x-79 (H3N2) originating from a stock from S. Epstein (NIH, Bethesda, Maryland, USA) and PR8-OVAII originating from a stock from P.C. Doherty (University of Melbourne, Melbourne, Australia) were similarly prepared. Mice were infected intranasally under light isoflurane anesthesia (Webster Veterinary Supply) with stated doses of virus in 50 μl PBS.
Naive cell isolation, memory CD4+ T cell generation, Ab treatments, and cell transfer.
Naive CD4+ T cells were obtained from pooled spleen and lymph nodes as previously described (21). Resulting TCR Tg cells were routinely greater than 97% TcR+ and expressed a naive phenotype (small size, CD62Lhi, CD44lo, and CD25lo). TH0, TH1, TH2, and TH17 effectors were generated as previously described (21, 27). In vitro–generated memory cells were obtained from effectors that were washed several times and rested for at least 3 days in medium free of antigen or exogenous cytokine. IAV-primed memory cells were generated as described previously (11).
In certain experiments, 5 × 107 B cells or 2 × 107 CD8+ T cells from unprimed BALB/c or Ifng–/– BALB/c donors were transferred to hosts either alone or with memory CD4+ T cells. B cells were obtained from spleens by positive MACS (Miltenyi) selection for CD19. The negative fraction was then positively selected for CD8+ T cells. The purity of MACS-isolated B and CD8+ T cell populations was confirmed by FACS analysis and was routinely more than 96%.
In certain experiments, mice were injected i.p. with 1 mg of anti-CD8–depleting Ab (TIB210; 2.43) 1 day before infection or with 500 μg on 5–6 or 11–12 dpi. Efficient depletion was confirmed by FACS analysis in control animals within each condition. Some mice were injected i.p. with 500 μg of anti–IFN-γ Ab (XMG1.2) or an isotype control (HRPN; BioXcell) or were injected i.p. with PR8- or X31-immune serum (containing 250 μg of virus-specific IgG) in 100 μl on stated days. The concentration of PR8-specific IgG in convalescent serum was determined using purified anti-H1N1–specific IgG (Chemicon International).
All cell populations were adoptively transferred in 200 μl PBS by i.v. injection. IAV infections were performed after adoptive transfer on the same day.
Determination of viral titer and sequence analysis.
Viral titers were determined by quantitation of viral RNA as described previously to amplify the polymerase (PA) gene of PR8 (27).
Stock viruses and isolates from infected lungs were grown in Madin-Darby canine kidney (MDCK) cells for 72 hours and virus concentrated by adsorption to chicken red blood cells. Viral RNA was extracted and reverse transcribed. Forward (5′-GAGCTGAGGGAGCAATTGAG-3′) and reverse (5′-TCATCACCGCCTAACAGTA-3′) primers were designed for the viral HA fragment containing the HNT epitope (OVAII epitope in the case of PR8-OVAII) and were used to generate a PCR amplicon of 307 bp. Amplicons were collected and extracted with a DNA extraction kit (QIAGEN) and submitted for sequence analysis. Virus amplicon nucleotide and aa sequences from original stock PR8, MDCK cell–expanded, and concentrated stock PR8 virus all matched the known sequence (GenBank ABO21709.1).
In vitro proliferation assay.
Naive CFSE-labeled HNT or DO11.10 cells, 1 × 106 per well, were cultured in vitro in 24-well plates (Corning) in the absence or presence of 1 × 106 T cell–depleted spleen cells infected with stock virus or viral isolates. Prior to culture with CD4+ T cells, spleen cells were cultured for 1 hour with virus at an MOI of 0.1. After 72 hours, triplicate cultures were harvested and stained with Thy1.2 Ab to identify CD4+ T cells and proliferation assessed by dilution of CFSE by FACS. The proportion of CFSE-divided cells in control cultures was used as 100% maximum division.
Flow cytometry.
Cell suspensions were washed, resuspended in FACS buffer (Sigma-Aldrich) PBS plus 0.5% BSA and 0.02% sodium azide (NaN3) (Sigma-Aldrich), and incubated on ice with 1 μg anti-FcR (2.4G2) followed by saturating concentrations of fluorochrome-labeled Ab for surface staining. FACS analysis was performed using BD FACS Scan (BD Biosciences) and FlowJo (Tree Star) analysis software.
Statistics.
The log rank test was used to test for significant differences in Kaplan-Meier survival curves. Comparisons of the proportion of mutant isolates among groups were made using the χ2 test, and a P value of less than 0.05 was considered to be statistically significant. All error bars represent SD.
Study approval.
Experimentalprotocols involving mice were approved by the Animal Care and Use Committee at the Trudeau Institute (Saranac Lake, New York, USA) or the Animal Care and Use Committee of the University of Massachusetts Medical School.
Supplementary Material
Acknowledgments
We thank L. Selin, E. Szomolanyi-Tsuda, and R. Welsh for helpful discussions and P. Adams of the Trudeau Institute for assistance with sequencing. This work was supported by NIH grants AI-46530 and NS-061014.
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J Clin Invest. 2012;122(8):2847–2856. doi:10.1172/JCI63689.
See the related Commentary beginning on page 2768.
References
- 1.Allan W, Tabi Z, Cleary A, Doherty PC. Cellular events in the lymph node and lung of mice with influenza. Consequences of depleting CD4+ T cells. J Immunol. 1990;144(10):3980–3986. [PubMed] [Google Scholar]
- 2.Brown DM, Dilzer AM, Meents DL, Swain SL. CD4 T cell-mediated protection from lethal influenza: perforin and antibody-mediated mechanisms give a one-two punch. J Immunol. 2006;177(5):2888–2898. doi: 10.4049/jimmunol.177.5.2888. [DOI] [PubMed] [Google Scholar]
- 3.Powell TJ, et al. Priming with cold-adapted influenza A does not prevent infection but elicits long-lived protection against supralethal challenge with heterosubtypic virus. J Immunol. 2007;178(2):1030–1038. doi: 10.4049/jimmunol.178.2.1030. [DOI] [PubMed] [Google Scholar]
- 4.Guo H, Santiago F, Lambert K, Takimoto T, Topham DJ. T cell-mediated protection against lethal 2009 pandemic H1N1 influenza virus infection in a mouse model. J Virol. 2011;85(1):448–455. doi: 10.1128/JVI.01812-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun K, Ye J, Perez DR, Metzger DW. Seasonal FluMist vaccination induces cross-reactive T cell immunity against H1N1 (2009) influenza and secondary bacterial infections. J Immunol. 2011;186(2):987–993. doi: 10.4049/jimmunol.1002664. [DOI] [PubMed] [Google Scholar]
- 6.Liang S, Mozdzanowska K, Palladino G, Gerhard W. Heterosubtypic immunity to influenza type A virus in mice. Effector mechanisms and their longevity. J Immunol. 1994;152(4):1653–1661. [PubMed] [Google Scholar]
- 7.Epstein SL, et al. Mechanisms of heterosubtypic immunity to lethal influenza A virus infection in fully immunocompetent, T cell-depleted, beta2-microglobulin-deficient, and J chain-deficient mice. J Immunol. 1997;158(3):1222–1230. [PubMed] [Google Scholar]
- 8.Wilkinson TM, et al. Preexisting influenza-specific CD4+ T cells correlate with disease protection against influenza challenge in humans. Nat Med. 2012;18(2):274–280. doi: 10.1038/nm.2612. [DOI] [PubMed] [Google Scholar]
- 9.Swain SL, McKinstry KK, Strutt TM. Expanding roles for CD4(+) T cells in immunity to viruses. Nat Rev Immunol. 2012;12(2):136–148. doi: 10.1038/nri3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McKinstry KK, Strutt TM, Swain SL. Hallmarks of CD4 T cell immunity against influenza. J Intern Med. 2011;269(5):507–518. doi: 10.1111/j.1365-2796.2011.02367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Strutt TM, et al. Memory CD4+ T cells induce innate responses independently of pathogen. Nat Med. 2010;16(5):558–564. doi: 10.1038/nm.2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Teijaro JR, Verhoeven D, Page CA, Turner D, Farber DL. Memory CD4 T cells direct protective responses to influenza virus in the lungs through helper-independent mechanisms. J Virol. 2010;84(18):9217–9226. doi: 10.1128/JVI.01069-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Graham MB, Dalton DK, Giltinan D, Braciale VL, Stewart TA, Braciale TJ. Response to influenza infection in mice with a targeted disruption in the interferon gamma gene. J Exp Med. 1993;178(5):1725–1732. doi: 10.1084/jem.178.5.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nguyen HH, van Ginkel FW, Vu HL, Novak MJ, McGhee JR, Mestecky J. Gamma interferon is not required for mucosal cytotoxic T-lymphocyte responses or heterosubtypic immunity to influenza A virus infection in mice. J Virol. 2000;74(12):5495–5501. doi: 10.1128/JVI.74.12.5495-5501.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun K, Torres L, Metzger DW. A detrimental effect of interleukin-10 on protective pulmonary humoral immunity during primary influenza A virus infection. J Virol. 2010;84(10):5007–5014. doi: 10.1128/JVI.02408-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wiley JA, Cerwenka A, Harkema JR, Dutton RW, Harmsen AG. Production of interferon-gamma by influenza hemagglutinin-specific CD8 effector T cells influences the development of pulmonary immunopathology. Am J Pathol. 2001;158(1):119–130. doi: 10.1016/S0002-9440(10)63950-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karupiah G, Chen JH, Mahalingam S, Nathan CF, MacMicking JD. Rapid interferon gamma-dependent clearance of influenza A virus and protection from consolidating pneumonitis in nitric oxide synthase 2-deficient mice. J Exp Med. 1998;188(8):1541–1546. doi: 10.1084/jem.188.8.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baumgarth N, Kelso A. In vivo blockade of gamma interferon affects the influenza virus-induced humoral and the local cellular immune response in lung tissue. J Virol. 1996;70(7):4411–4418. doi: 10.1128/jvi.70.7.4411-4418.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bot A, Bot S, Bona CA. Protective role of gamma interferon during the recall response to influenza virus. J Virol. 1998;72(8):6637–6645. doi: 10.1128/jvi.72.8.6637-6645.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scott B, et al. A role for non-MHC genetic polymorphism in susceptibility to spontaneous autoimmunity. Immunity. 1994;1(1):73–83. doi: 10.1016/1074-7613(94)90011-6. [DOI] [PubMed] [Google Scholar]
- 21.McKinstry KK, Golech S, Lee WH, Huston G, Weng NP, Swain SL. Rapid default transition of CD4 T cell effectors to functional memory cells. J Exp Med. 2007;204(9):2199–2211. doi: 10.1084/jem.20070041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McKinstry KK, Strutt TM, Swain SL. Regulation of CD4+ T-cell contraction during pathogen challenge. Immunol Rev. 2010;236:110–124. doi: 10.1111/j.1600-065X.2010.00921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richards KA, Chaves FA, Sant AJ. The memory phase of the CD4 T-cell response to influenza virus infection maintains its diverse antigen specificity. Immunology. 2011;133(2):246–256. doi: 10.1111/j.1365-2567.2011.03435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caton AJ, Gerhard W. The diversity of the CD4+ T cell response in influenza. Semin Immunol. 1992;4(2):85–90. [PubMed] [Google Scholar]
- 25.Hogan RJ, Zhong W, Usherwood EJ, Cookenham T, Roberts AD, Woodland DL. Protection from respiratory virus infections can be mediated by antigen-specific CD4(+) T cells that persist in the lungs. J Exp Med. 2001;193(8):981–986. doi: 10.1084/jem.193.8.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kearney ER, Pape KA, Loh DY, Jenkins MK. Visualization of peptide-specific T cell immunity and peripheral tolerance induction in vivo. Immunity. 1994;1(4):327–339. doi: 10.1016/1074-7613(94)90084-1. [DOI] [PubMed] [Google Scholar]
- 27.McKinstry KK, et al. IL-10 deficiency unleashes an influenza-specific Th17 response and enhances survival against high-dose challenge. J Immunol. 2009;182(12):7353–7363. doi: 10.4049/jimmunol.0900657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hannum LG, Haberman AM, Anderson SM, Shlomchik MJ. Germinal center initiation, variable gene region hypermutation, and mutant B cell selection without detectable immune complexes on follicular dendritic cells. J Exp Med. 2000;192(7):931–942. doi: 10.1084/jem.192.7.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mozdzanowska K, Furchner M, Zharikova D, Feng J, Gerhard W. Roles of CD4+ T-cell-independent and -dependent antibody responses in the control of influenza virus infection: evidence for noncognate CD4+ T-cell activities that enhance the therapeutic activity of antiviral antibodies. J Virol. 2005;79(10):5943–5951. doi: 10.1128/JVI.79.10.5943-5951.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carragher DM, Kaminski DA, Moquin A, Hartson L, Randall TD. A novel role for non-neutralizing antibodies against nucleoprotein in facilitating resistance to influenza virus. J Immunol. 2008;181(6):4168–4176. doi: 10.4049/jimmunol.181.6.4168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.MacLeod MK, David A, McKee AS, Crawford F, Kappler JW, Marrack P. Memory CD4 T cells that express CXCR5 provide accelerated help to B cells. J Immunol. 2011;186(5):2889–2896. doi: 10.4049/jimmunol.1002955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee BO, et al. CD40, but not CD154, expression on B cells is necessary for optimal primary B cell responses. J Immunol. 2003;171(11):5707–5717. doi: 10.4049/jimmunol.171.11.5707. [DOI] [PubMed] [Google Scholar]
- 33.Kamperschroer C, Dibble JP, Meents DL, Schwartzberg PL, Swain SL. SAP is required for Th cell function and for immunity to influenza. J Immunol. 2006;177(8):5317–5327. doi: 10.4049/jimmunol.177.8.5317. [DOI] [PubMed] [Google Scholar]
- 34.Lee BO, et al. CD4 T cell-independent antibody response promotes resolution of primary influenza infection and helps to prevent reinfection. J Immunol. 2005;175(9):5827–5838. doi: 10.4049/jimmunol.175.9.5827. [DOI] [PubMed] [Google Scholar]
- 35.Belz GT, Wodarz D, Diaz G, Nowak MA, Doherty PC. Compromised influenza virus-specific CD8(+)-T-cell memory in CD4(+)-T-cell-deficient mice. J Virol. 2002;76(23):12388–12393. doi: 10.1128/JVI.76.23.12388-12393.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Price GE, Huang L, Ou R, Zhang M, Moskophidis D. Perforin and Fas cytolytic pathways coordinately shape the selection and diversity of CD8+-T-cell escape variants of influenza virus. J Virol. 2005;79(13):8545–8559. doi: 10.1128/JVI.79.13.8545-8559.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hillaire ML, et al. Cross-protective immunity against influenza pH1N1 2009 viruses induced by seasonal influenza A (H3N2) virus is mediated by virus-specific T-cells. J Gen Virol. 2011;92(pt 10):2339–2349. doi: 10.1099/vir.0.033076-0. [DOI] [PubMed] [Google Scholar]
- 38.Nguyen HH, van Ginkel FW, Vu HL, McGhee JR, Mestecky J. Heterosubtypic immunity to influenza A virus infection requires B cells but not CD8+ cytotoxic T lymphocytes. J Infect Dis. 2001;183(3):368–376. doi: 10.1086/318084. [DOI] [PubMed] [Google Scholar]
- 39.Rangel-Moreno J, et al. B cells promote resistance to heterosubtypic strains of influenza via multiple mechanisms. J Immunol. 2008;180(1):454–463. doi: 10.4049/jimmunol.180.1.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seder RA, Ahmed R. Similarities and differences in CD4+ and CD8+ effector and memory T cell generation. Nat Immunol. 2003;4(9):835–842. doi: 10.1038/ni969. [DOI] [PubMed] [Google Scholar]
- 41.Tate MD, Deng YM, Jones JE, Anderson GP, Brooks AG, Reading PC. Neutrophils ameliorate lung injury and the development of severe disease during influenza infection. J Immunol. 2009;183(11):7441–7450. doi: 10.4049/jimmunol.0902497. [DOI] [PubMed] [Google Scholar]
- 42.Tate MD, Pickett DL, van Rooijen N, Brooks AG, Reading PC. Critical role of airway macrophages in modulating disease severity during influenza virus infection of mice. J Virol. 2010;84(15):7569–7580. doi: 10.1128/JVI.00291-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brown DM, Lee S, Garcia-Hernandez Mde L, Swain SL. Multi-functional CD4 cells expressing IFN-gamma and perforin mediate protection against lethal influenza infection. J Virol. 2012;86(12):6792–6803. doi: 10.1128/JVI.07172-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Berkhoff EG, et al. An amino acid substitution in the influenza A virus hemagglutinin associated with escape from recognition by human virus-specific CD4+ T-cells. Virus Res. 2007;126(1–2):282–287. doi: 10.1016/j.virusres.2007.02.018. [DOI] [PubMed] [Google Scholar]
- 45.Cornberg M, et al. Narrowed TCR repertoire and viral escape as a consequence of heterologous immunity. J Clin Invest. 2006;116(5):1443–1456. doi: 10.1172/JCI27804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Narasaraju T, Ng HH, Phoon MC, Chow VT. MCP-1 antibody treatment enhances damage and impedes repair of the alveolar epithelium in influenza pneumonitis. Am J Respir Cell Mol Biol. 2010;42(6):732–743. doi: 10.1165/rcmb.2008-0423OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Epstein SL, Price GE. Cross-protective immunity to influenza A viruses. Expert Rev Vaccines. 2010;9(11):1325–1341. doi: 10.1586/erv.10.123. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.