Abstract
The purpose of this paper is to highlight the endocrine signaling of Western diet, a fundamental environmental factor involved in the pathogenesis of epidemic acne. Western nutrition is characterized by high calorie uptake, high glycemic load, high fat and meat intake, as well as increased consumption of insulin- and IGF-1-level elevating dairy proteins. Metabolic signals of Western diet are sensed by the nutrient-sensitive kinase, mammalian target of rapamycin complex 1 (mTORC1), which integrates signals of cellular energy, growth factors (insulin, IGF-1) and protein-derived signals, predominantly leucine, provided in high amounts by milk proteins and meat. mTORC1 activates SREBP, the master transcription factor of lipogenesis. Leucine stimulates mTORC1-SREBP signaling and leucine is directly converted by sebocytes into fatty acids and sterols for sebaceous lipid synthesis. Over-activated mTORC1 increases androgen hormone secretion and most likely amplifies androgen-driven mTORC1 signaling of sebaceous follicles. Testosterone directly activates mTORC1. Future research should investigate the effects of isotretinoin on sebocyte mTORC1 activity. It is conceivable that isotretinoin may downregulate mTORC1 in sebocytes by upregulation of nuclear levels of FoxO1. The role of Western diet in acne can only be fully appreciated when all stimulatory inputs for maximal mTORC1 activation, i.e., glucose, insulin, IGF-1 and leucine, are adequately considered. Epidemic acne has to be recognized as an mTORC1-driven disease of civilization like obesity, type 2 diabetes, cancer and neurodegenerative diseases. These new insights into Western diet-mediated mTORC1-hyperactivity provide a rational basis for dietary intervention in acne by attenuating mTORC1 signaling by reducing (1) total energy intake, (2) hyperglycemic carbohydrates, (3) insulinotropic dairy proteins and (4) leucine-rich meat and dairy proteins. The necessary dietary changes are opposed to the evolution of industrialized food and fast food distribution of Westernized countries. An attenuation of mTORC1 signaling is only possible by increasing the consumption of vegetables and fruit, the major components of vegan or Paleolithic diets. The dermatologist bears a tremendous responsibility for his young acne patients who should be advised to modify their dietary habits in order to reduce activating stimuli of mTORC1, not only to improve acne but to prevent the harmful and expensive march to other mTORC1-related chronic diseases later in life.
Keywords: FoxO, IGF-1, Western diet, acne, androgen, insulin, leucine, mTORC1, nutrition, prevention
Introduction
Acne is an epidemic skin disease of industrialized countries, reaching prevalence rates of over 85% of teenagers.1 In the United States, acne nowadays persists after adolescence into the third decade of life in nearly half of men and women.2 This demonstrates that the environmental acne-promoting mechanisms persist after puberty and are independent of endocrine signaling of puberty. Acne has been clearly identified as a disease of Western civilization and has been closely linked to Western diet.3 Intriguingly, acne is absent in populations consuming a Paleolithic diet excluding sugar, grains and dairy protein like the diet of the Kitava islanders who exhibit low basal insulin levels compared with age-matched Europeans and do not suffer from epidemic diseases of civilization.3,4 Remarkably, a randomized placebo-controlled Australian trial confirmed that a reduction of glycemic load improved the clinical symptoms of acne, the rate of sebum excretion and free androgen index in male acne patients in the age range of 15–25 y.5-8
Epidemiologic data derived from the Nurses Health Study II and the Growing Up Today Study in the United States provided epidemiological evidence for a correlation between milk, and especially skim milk consumption, and the prevalence of acne.9-11 Moreover, positive associations between acne and the consumption of other dairy products like instant breakfast drink, sherbet, cream cheese and cottage cheese have been reported.9 The association between acne and food composition has recently been confirmed in 783 patients with acne and 502 control subjects in South Korea.12 The frequency of vegetables and fish intake was significantly higher in the control group than in the acne group. Intake of instant noodles, junk food, carbonated drinks, snacks, processed cheeses, pork, chicken, nuts and seaweed were significantly higher in acne patients than in the controls.12 Thus, the food pattern of Western diet composed of high glycemic load, high fat intake, and high dairy and meat consumption played an important role in the exacerbation of acne in South Korea. Nearly half of the male and female acne patients reported that food intake was an aggravating factor of their acne. Remarkably, in the group of food-aggravated acne patients, serum IGF-1 levels (543.9 ± 56.4 ng/mL) were significantly higher than IGF-1 levels (391.3 ± 118.2 ng/mL) in the acne group not reporting aggravation by food.12 Accumulating evidence derived from epidemiologic and controlled dietary studies allows the conclusion that especially high glycemic load diets and increased consumption of dairy proteins are the major dietary factors of Western diet promoting the development or exacerbation of acne.13-20 Although there is overwhelming evidence for the role of diet in acne, the role of food in acne is still a controversial issue.21 The major problem of this uncertainty is the lack of knowledge on signaling pathways mediated by nutrients. This paper will help to elucidate major pathways of nutrient signaling of Western diet involved in the pathogenesis of acne and highlights the central role of the nutrient-sensitive kinase mammalian target of rapamycin complex 1 (mTORC1) in mediating the effects of nutrient-derived signals in the development of acne.
mTORC1 Senses and Integrates Nutrient-Derived Signals
Recent discoveries in the field of molecular biology have established the key role of the nutrient-sensitive mammalian target of rapamycin complex 1 (mTORC1) kinase in cell regulation and cell function. mTORC1 signaling stimulates gene transcription, translation, ribosome biogenesis, protein synthesis, cell growth, cell proliferation and lipid synthesis but suppresses the mechanisms of autophagy.22-27 mTOR is a multi-domain protein of approximately 300 kDa exhibiting a protein kinase domain at its C-terminus related to phosphoinositol-3-kinases (PI3K). In mammalian cells two functionally different mTOR complexes exist: mTORC1 and mTORC2. Among other functional proteins, mTORC1 contains the important partner protein Raptor, which interacts with substrates for mTORC1-mediated phosphorylation. mTORC1 controls the G1/S transition and G2/M progression of the cell cycle.24 In contrast to mTORC2, which contains the partner protein Rictor, only mTORC1 plays a special role in sensing cellular nutrients, amino acid and energy (ATP) levels important for cell growth and proliferation. Liver kinase B1 (LKB1) and AMP-activated protein kinase (AMPK) are critical regulators of mTORC1.28 Most functions of mTORC1 are inhibited by rapamycin, a triene macrolide antibiotic synthesized by Streptomyces hygroscopicus.27
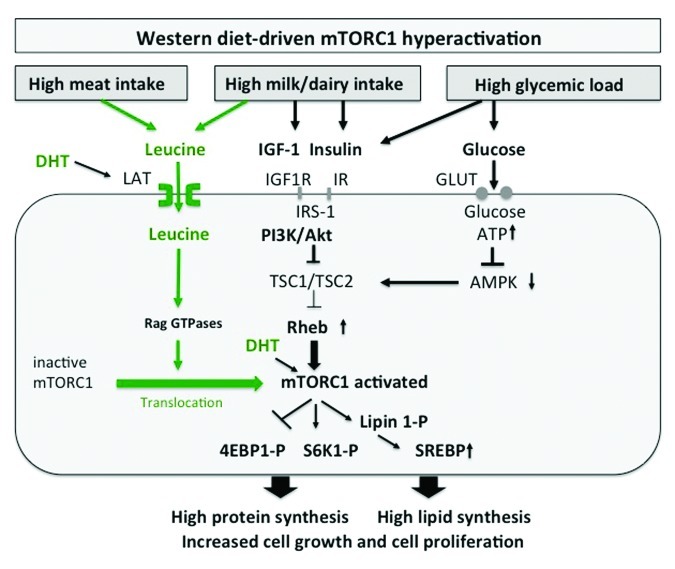
mTORC1 has to be regarded as a pivotal convergence point in cell signaling, because it integrates many intra- and extracellular signals such as growth factors (insulin, IGF-1), energy-sensing signals (glucose, the AMP/ATP-ratio regulating AMPK), and most importantly the availability of sufficient amounts of amino acids, especially the branched-chain essential amino acid (BCAA) leucine for mTORC1 activation (Fig. 1).29
Figure 1. mTORC1 signaling of Western diet in acne: Leucine, IGF-1, insulin and glucose synergistically activate mTORC1. Leucine activates mTORC1 by translocation of inactive mTORC1 to Rheb-enriched membrane compartments. High glucose intake increases insulin signaling and elevates cellular ATP levels resulting in AMPK suppression. mTORC1 activates protein synthesis via S6K1 and 4EBP1 and phosphorylates lipin 1, inducing SREBP-mediated lipogenesis. DHT increases cellular leucine uptake and directly activates mTORC1.
Recent advances in molecular biology have elucidated two parallel mechanisms of mTORC1 activation: (1) the upstream activation of the small GTPase Rheb (Ras homolog enriched in brain) by growth factor signals and high cellular energy levels and (2) the amino acid-dependent translocation of inactive mTORC1 to active Rheb localized at late endosome or lysosome compartments.30-32 The activity of Rheb is tightly regulated by the tuberous sclerosis proteins TSC1 (hamartin) and TSC2 (tuberin), which form a functional heterodimeric complex. Intriguingly, loss-of-function mutations of either the TSC1 or TSC2 gene cause the hamartoma syndrome tuberous sclerosis complex. TSC1 stabilizes TSC2, that possesses a GTPase-activating protein, which hydrolyses GTP to GDP. The TSC1/TSC2 complex provides this function to Rheb leading to inactivation of Rheb. Insulin and IGF-1, which both activate the kinase Akt (protein kinase B) as well as other growth-related kinases such as ERK and RSK phosphorylate TSC2, thereby inhibiting the function of the TSC1/TSC2 complex. This inhibition leads to activation of Rheb with final activation of mTORC1.33-35
Besides the important input of growth factor signaling on mTORC1 activation, AMPK, an essential energy sensor, plays a key role in energy-dependent mTORC1 regulation. During states of energy-deficient conditions like glucose deprivation, ATP levels fall and AMP levels rise, resulting in AMPK activation. AMPK phosphorylates TSC2 and Raptor, thereby suppressing mTORC1 activity.36,37 Abundant cellular energy provided by hypercaloric Western diet with high glycemic load thus reduces AMPK activity and stimulates mTORC1 signaling.
FoxO Proteins: Rheostats Tuning mTORC1 Activity
Acne pathogenesis has recently been linked to increased insulin/IGF-1 signaling leading to decreased nuclear levels of FoxO transcription factors which are extruded into the cytoplasm by growth-factor-activated PI3K/Akt-signaling.38,39 Although experimental direct evidence is still lacking, indirect evidence from various cell systems supports the concept that isotretinoin increases nuclear levels of FoxO1, which explains the therapeutic mechanism and the adverse effects of isotretinoin treatment.40 There is convincing evidence that other important nutrient- and growth factor-sensors, especially the FoxO transcription factors, modulate mTORC1 signaling (Fig. 2).41 Increased insulin/IGF-1 signaling and activation of the PI3K/Akt-pathway results in Akt-mediated nuclear phosphorylation of FoxO proteins, thereby promoting their extrusion from the nucleus into the cytoplasm. This FoxO shuttling mechanism functions as a molecular switch for FoxO-mediated gene regulation. Like mTORC1, FoxOs are involved in the regulation of cell proliferation, apoptosis, anti-oxidative stress responses and regulation of metabolism.42 Intriguingly, FoxOs have emerged as important rheostats that coordinate the activity of Akt and mTORC1.41 Activated FoxOs (FoxO1 and FoxO3, FoxO4) induce the expression of Sestrin3, which activates AMPK to inhibit mTORC1 in a TSC2-dependent manner.43 Moreover, AMPK has been shown to phosphorylate FoxO3 and facilitate its nuclear localization.44 It has been demonstrated that Akt-phosphorylated cytoplasmic FoxO1 is able to associate with the C terminus of TSC2 thereby dissociating the TSC1/TSC2 complex leading to activation of mTORC1.45 From theses data it becomes apparent that FoxOs intimately interact with mTORC1 and control mTORC1 signaling.
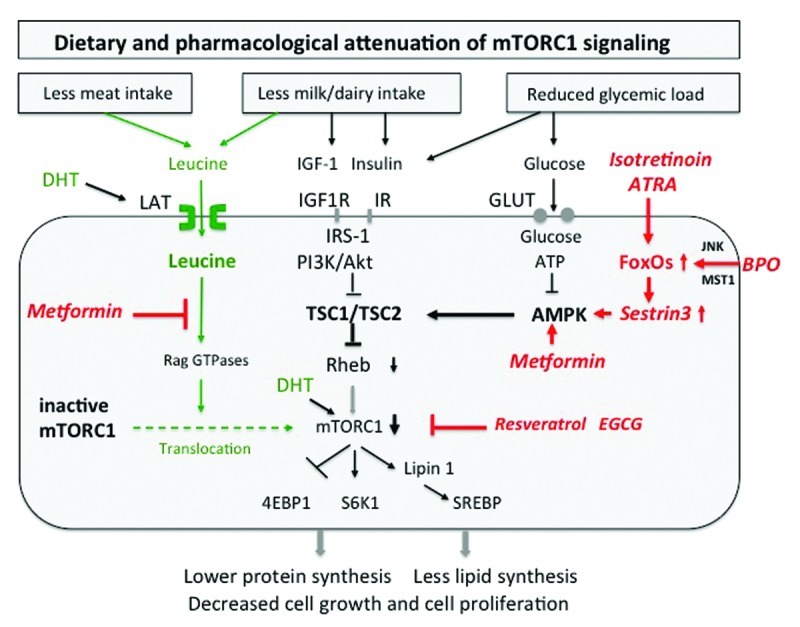
Figure 2. Attenuation of mTORC1 activity by dietary intervention. Reduction of animal protein (leucine), hyperglycemic carbohydrates (glucose) and dairy proteins (insulin/IGF-1) mitigates mTORC1 signaling. Isotretinoin inhibits mTORC1 by upregulation of FoxO-Sestrin3, which stimulates AMPK. Benzoyl peroxide (BPO) upregulates FoxO-Sestrin3-stimulated AMPK, thereby inhibiting mTORC1. Metformin inhibits mTORC1 activity by antagonizing leucine signaling and by stimulating AMPK activity. Plant-derived mTORC1 inhibitors directly downregulate mTORC1 (Abbreviations see text).
Remarkably, in response to amino acid depletion, mTORC1 activity is rapidly abolished.46 Amino acid starvation impairs binding of mTORC1 to Rheb.47 From all essential amino acids, leucine exerts the greatest effects on mTORC1 signaling.24,27,46 Recent evidence has been provided that amino acids and especially leucine promote the cellular translocation of inactive mTORC1 to lysosomal compartments enriched in activated Rheb.30,31 This spatial regulation of inactive mTORC1 by amino acids is mediated by an active Rag heterodimer and is of utmost biological importance as it explains the complete mechanism of nutrient sensing of mTORC1. Thus, mTORC1 integrates not only growth factor/energy-derived signals to Rheb, but requires parallel signaling of leucine for final mTORC1 activation by translocation of inactive mTORC1 to cell compartments enriched in activated Rheb (Fig. 1). These two synergistic major pathways of mTORC1 activation explain why either insulin/IGF-1 signaling or amino acid signaling alone is not sufficient to reach maximal mTORC1 activation. Insulin is not able to activate the mTORC1 pathway when cells are deprived of amino acids.48 Infact, recent experimental evidence confirmed that both insulin- and amino acid-signaling are required for maximal mTORC1 activity in rat liver.49
Activated mTORC1 finally phosphorylates important substrates involved in the regulation of the translational machinery, the S6 kinases (S6Ks), which phosphorylate ribosomal protein S6, and eukaryotic initiation factor (eIF) 4E-binding proteins (4E-BPs), which control the activity of the translation factor eIF-4E that binds to the 5′-cap structure of eukaryotic mRNAs, thereby facilitating ribosome recruitment. Intriguingly, the downstream target of mTORC1, S6K1, phosphorylates insulin receptor substrate protein-1 (IRS-1), mediating an important feed back mechanism, which downregulates insulin/IGF-1 signaling. This is a most important mechanism of insulin resistance, a characteristic feature of puberty, obesity, type 2 diabetes and states of hyperandrogenism.50
The Link Between Androgen- and mTORC1-Signaling
We are at the very beginning to understand the important molecular crosstalk between androgens and the mTORC1 pathway. Anabolic effects of testosterone certainly increase protein synthesis. The mTORC1 pathway is the major regulator of protein synthesis and cell growth, but the relationship between testosterone action and mTORC1 has not yet been characterized in sebaceous glands. Nevertheless, it has been shown in cultured cardiomyocytes that testosterone induced hypertrophic effects via mTORC1 signaling. Testosterone increased the phosphorylation of mTOR and its downstream targets S6K1 and 4E-BP1.51 S6K1-phosphorylation induced by testosterone was blocked by the mTORC1 antagonist rapamycin. This observation is of great importance for the understanding of insulin resistance in states of hyperandrogenism like polycystic ovary syndrome (PCOS). Testosterone-mediated mTORC1-S6K1- IRS-1 signaling provides a most critical mechanism for the induction of insulin resistance.50 On the other hand, metformin treatment of patients with PCOS reduces androgen levels and improves insulin resistance. Remarkably, metformin inhibits mTORC1 activity by antagonizing leucine-mediated mTORC1 activation as well as AMPK-mediated suppression of mTORC1 activity.52,53 The androgen-mTORC1-S6K1 pathway explains the development of insulin resistance in various syndromes associated with acne and insulin resistance.54
In the prostate of mice PI3K levels and mTORC1 activity are robustly induced by androgens during prostatic development. PI3K/mTORC1 signaling is necessary for prostatic epithelial bud invasion of surrounding mesenchyme.55 The right balance of PI3K and downstream mTORC1/mTORC2 activity plays a critical role in the regulation of prostatic epithelial morphogenesis. Future studies in humans should clarify the role of androgen-mTORC1-mediated effects on sebaceous gland morphogenesis and differentiation.
Androgen- and mTORC1-Dependent Amino Acid Uptake
High intake of BCAAs provided by high dairy protein and meat consumption may be another important mechanism stimulating sebaceous lipogenesis. Increased levels of amino acids derived from meat hydrolysis as well as dairy protein consumption are known to increase serum levels of IGF-1.56-59 IGF-1 stimulates the activity of 5α-reductase, which converts testosterone to the more potent dihydrotestosterone (DHT).60 DHT has recently been shown to stimulate increased uptake of BCAAs in an mTORC1-dependent process, which could be inhibited by pre-treatment of muscle cells with the mTORC1 inhibitor rapamycin.61 It is thus conceivable that DHT promotes BCAA-uptake by sebaceous glands, a process that delivers leucine as an important precursor for sebaceous lipogenesis. In this regard it is of special concern that male adolescents in the fitness and bodybuilding environment consume high amounts (60–80 g/d) of leucine-rich whey- or casein-based protein concentrates to gain muscle mass, a procedure which is often associated with the development of acne (Table 1).62,63 Experimental studies are necessary to investigate the possible role of DHT for leucine uptake of sebaceous glands. Combined androgen abuse with high dairy protein intake may operate in a synergistic fashion increasing sebaceous gland growth and proliferation. Both increased mTORC1-dependent leucine uptake for leucine-dependent lipid synthesis as well as androgen- and leucine-stimulated mTORC1-activation may stimulate sebocyte growth, proliferation and SREBP-mediated lipid synthesis. Thus, increased consumption of leucine-rich proteins provided by Western diet appears as a new dietary factor promoting sebaceous lipogenesis.
Table 1. Leucine-rich proteins (ref. 106).
| Protein source | Leucine content (g/100 g protein) |
|---|---|
| Whey protein concentrate |
14% |
| Cow milk protein (mostly casein) |
10% |
| Egg protein |
8.5% |
| Muscle protein |
8% |
| Soy protein isolate |
8% |
| Wheat protein | 7% |
The Link Between mTORC1 and Comedogenesis
Comedogenesis is regarded as the primary process in the pathogenesis of acne and is induced by increased proliferation and retention of acroinfundibular androgen-dependent keratinocytes.64 Recently, the PI3K/Akt/mTORC1 pathway has been shown to stimulate keratinocyte proliferation.65 In keratinocyte cultures of high cell density, which imitate states of hyperproliferation, isotretinoin decreased keratinocyte proliferation.66 This finding can now be well explained by FoxO1-mediated inhibition of mTORC1.45 Further evidence points to the role of mTORC1 signaling for keratinocyte proliferation because genetic excision of TSC1 activated mTORC1 signaling in keratinocytes.65 Loss-of-function of either the TSC1 (harmatin) or TSC2 (tuberin) gene, upstream inhibitory regulators of mTORC1, leads to persistent activation of mTORC1 resulting in the hamartoma syndrome tuberosus sclerosis complex.67 Intriguingly, folliculocystic and collagen hamartoma of tuberous sclerosis complex with multiple comedones and keratin-containing cysts lined by infundibular epithelium have recently been described.68 These findings support the view that increased mTORC1 signaling of acroinfundibular keratinocytes may be involved in comedogenesis.
mTORC1: Central Activator of Lipogenesis
Although mTORC1 mediates central pathways in cell metabolism of all mammalian cells, studies investigating mTORC1 signaling in sebocytes are not yet available. However, by means of translational research important deductions for sebocyte biology can be drawn. mTORC1 is a crucial regulatory node controlling protein biosynthesis and cell growth and proliferation. However, cell growth not only affords the synthesis of new proteins but also requires substantial amounts of lipids for the maintenance of membrane compartments absolutely necessary for appropriate cell function. It is thus not surprising that mTORC1 signaling has been linked to lipid biosynthesis.69 The key master transcription factor of most lipid synthesizing enzymes for fatty acid and sterol biosynthesis is the transcription factor SREBP (sterol regulatory element-binding factor). The SREBP family is comprised of three isoforms: SREBP-1a, SREBP-1c, and SREBP-2. Previous work shows that mTORC1 positively regulates the activity of SREBP-1.70,71 It has most recently been demonstrated that mTORC1 regulates SREBP by controlling the nuclear entry of lipin 1, a phosphatidic acid phosphatase. Nutrient-activated, and especially amino acid-activated, mTORC1 phosphorylates lipin1, which is retained in the cytoplasm and allows promotor binding of SREBP in the nucleus (Fig. 3). However, in the absence of nutrients and amino acids mTORC1 activity is suppressed. Lipin 1 enters the nucleus and displaces SREBP from its promoter sites of lipid synthesizing target genes. SREBP migrates than to the nuclear lamina.72 Glucose and amino acid deprivation, which inhibits mTORC1 activity, promoted nuclear accumulation of lipin 1 and thus suppressed SREBP signaling. On the other hand, cytoplasmatic retention of lipin 1 with increased SREBP activity was positively promoted by activated mTORC1, which is stimulated by availability of glucose, insulin, IGF-1 and amino acids, especially leucine (Fig. 3).72 These new insights clearly underline the relationship between nutrient- and amino acid-mediated mTORC1 signaling and SREBP-induced lipogenesis which most likely applies to sebaceous gland lipogenesis.
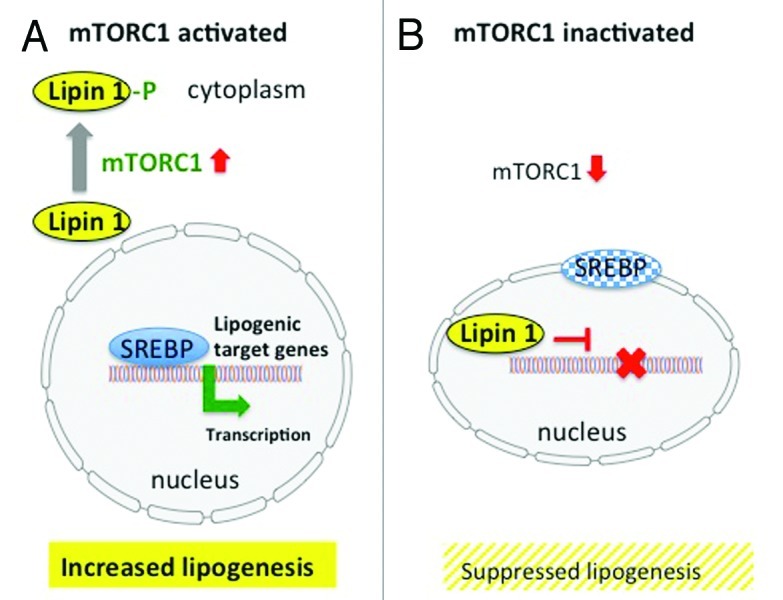
Figure 3. (A) mTORC1-SREBP-mediated lipogenesis. Activated mTORC1 phosphorylates lipin1, which resides in the cytoplasm and allows nuclear SREBP binding to lipogenic target genes resulting in increased lipogenesis. (B) Inactivation of mTORC1 results in nuclear entry of lipin 1, which disrupts SREBP binding to target genes, thus suppressing lipogenesis (modified according to Peterson et al.72
Leucine: Precursor of Sebaceous Lipids
The Western diet, enriched in meat and dairy proteins, provides high and persitantly increasing amounts of leucine. From 1950 to 2010, the annual per capita intake of leucine by consumption of animal-derived proteins has triplicated in Germany. Leucine not only contributes to the synthesis of muscle proteins but most importantly can be converted into lipids (fatty acids and cholesterol) and stored in adipose tissue.73 Adipose tissue efficiently converts BCAAs carbon skeletons into newly synthesized fatty acids, a process that is stimulated by insulin.73 Remarkably, sebaceous glands like adipocytes are able to take up and convert leucine into their major sebum lipid classes.74,75 In this regard, the leucine-enriched Western diet may have two major effects on sebaceous lipogenesis: (1) to increase leucine-stimulated mTORC1/SREBP signaling thus driving the genetic program of sebogenesis and (2) to provide leucine as a structural lipid precursor for de novo sebaceous lipid synthesis.
Western Diet and mTORC1-Stimulated T Cell Activity
T cell infiltrates are found in early acne lesions, which are associated with increased synthesis of interleukin-1α of acne-prone sebaceous follicles.76 Thus, the question arises whether Western diet-mediated mTORC1-overactivity affects T-cell homeostasis and the regulation of inflammatory immune responses in acne. In fact, mTORC1 signaling has recently been appreciated to play a fundamental role in the regulation of T-cell homeostasis and mTORC1 has been linked to T-cell differentiation, function and metabolism.77 mTORC1 integrates signals in the immune microenvironment and programs the generation of CD4+ effector vs. regulatory T cells, the generation of CD8+ effector vs. memory cells, T cell trafficking, and T cell activation vs. anergy. Thus, mTORC1 provides a direct link between T cell metabolism and T cell function.78 Remarkably, the tumor suppressor TSC1 established a quiescence program in naive T cells by controlling cell size, cell cycle entry and responses to stimulation of the T cell antigen receptor. TSC1-deficient T cells exhibited higher mTORC1 activity, which was essential for the disruption of immune homeostasis.79 TSC1-dependent control of mTORC1 is crucial in actively maintaining quiescence of naive T cells to facilitate adaptive immune functions.79 Thus, stimulation of T cell mTORC1 activity by exaggerated insulin/IGF-1 and leucine signaling of Western diet may disturb T-cell homeostasis and promote deviations in T cell signaling involved in the pathogenesis of acne.
mTORC1-Mediated Pro-inflammatory Signaling of Keratinocytes
Augmented mTORC1 signaling not only activates T cells but also appears to be involved in pro-inflammatory signaling of keratinocytes. In primary human keratinocytes treated with TNFα, mTORC1 activated pro-inflammatory NFκB signaling, whereas the mTORC1 inhibitor rapamycin inhibited TNFα-induced IκB degradation, thus reducing the transcriptional activity of NFκB.80 In primary human keratinocytes, TNFα-mediated activation of mTORC1 and pro-inflammatory NFκB signaling resulted in increased transcription of pro-inflammatory cytokines TNFα, IL-6, IL-8 IL-17, IL-20, IL-22 and IL-23.80 Intriguingly, IKKβ, a major downstream kinase in the TNFα-signaling pathway, has been demonstrated to physically interact with and phosphorylate TSC1, thereby suppressing TSC1, which results in mTORC1 activation.81 TNFα/IKKβ/TSC1/Rheb-mediated activation of mTORC1 is thus a most conceivable mechanism for TNFα-mediated keratinocyte proliferation in autoinflammatory disorders associated with acne and increased systemic TNFα levels like PAPA syndrome.54 Indeed, treatment with TNFα-antagonist showed benefical clinical effects in patients with PAPA syndrome.82-84
The Role of Leucine in T Cell Activation
While research of the last decades has primarily focused on the role of cytokine and hormonal signals in guiding T cell responses, the fundamental aspect of cellular energy metabolism and nutrients that regulate T cell function and differentiation has been neglected.85 Most recently, the pivotal role of mTORC1 in T cell metabolism affecting T cell function and differentiation has been appreciated.78 The metabolic demands of T cells are extraordinarily high and an increase in T cell metabolism has been recognized as a pivotal contribution to T cell activation.86,87 The transcription factors KLF2 and FoxO have been implicated in regulating T cell metabolism.78 Whereas quiescent T cells are in a resting state of metabolism characterized by catabolism driven by autophagy, activated T cells have high demands for adequate amounts of the essential components for protein, amino acids, lipid, and DNA biosynthesis involving mTORC1 function.
Leucine plays a fundamental role in T cell activation and function.88 Notably, the leucine-antagonist N-acetylleucine amide (NALA) inhibited T cell function and T cell receptor (TCR) engagement in the presence of NALA and promoted T cell anergy.89,90 NALA inhibited leucine-induced S6K phosphorylation and was capable of inhibiting amino acid-mTORC1 signaling in Jurkat cells, caused cell cycle arrest at G1 concomitant with the inhibition of S6K activation and inhibition of p27 degradation.89 In as much as a lack of leucine inhibits mTORC1 activation, these latter findings are consistent with the observation that TCR engagement during rapamycin-mediated mTORC1 inhibition promoted anergy.91 Similarly, the glucose analog 2-deoxyglucose (2DG) inhibited mTORC1 function most likely via the AMPK pathway and promoted T cell anergy.92 Furthermore, the AMPK activator AICAR inhibited mTORC1 and T cell function and mitigated experimental autoimmune encephalomyelitis.78 Intriguingly, regulatory T cells can inhibit T cell function by expressing amino acid degrading enzymes that deplete the environment of essential amino acids, which are important for mTORC1 function.93
These observations clearly demonstrate that hyperalimentation and especially increased consumption of leucine-rich animal proteins, predominately milk proteins and meat, drive mTORC1-mediated mechanisms of T cell activation and inflammation which may all promote the development of acne.
Glucocorticoids Inhibit mTORC1 Signaling
Glucocorticoids are given during initial stages of severe flares of conglobate acne. Recently, the molecular crosstalk between glucocorticoid receptor (GR) and mTORC1 signaling has been elaborated.94 A well known adverse effect of prolonged systemic glucocorticoid treatment is muscle atrophy. In skeletal muscle, direct target genes of the GR signaling involve the protein REDD1 (regulated in development and DNA damage responses) and the transcription factor KLF15 (Krüppel-like factor-15). Both inhibit mTORC1 activity, although via distinct mechanisms. The REDD1 gene is activated at the promotor level by ligand-bound GR and is transcriptionally induced under stress conditions like hypoxia (via HIF1α), which appears necessary for the downregulation of mTORC1 signaling during stress conditions.95 REDD1 functions upstream of TSC2 and Rheb in order to downregulate mTORC1 signaling in response to glucocorticosteroids.95-97
KLF15 upregulates gene expression of branched-chain aminotransferase-2 (BCAT2), a mitochondrial enzyme, catalyzing the first step in the catabolism of BCAAs.98 The glucocorticoid-driven GR-KLF15-BCAT2 axis may negatively modulate the intracellular availability of BCAAs resulting in a negative impact on mTORC1 function in skeletal muscle. Glucocorticoid-mediated downregulation of mTORC1 is not only a superb explanation for glucocorticoid-induced muscle atrophy, but also for skin atrophy after long-term systemic or topical glucocorticoid use. However, long-term use of glucocorticoids induces insulin resistence of adipose tissue and promotes the development of obesity, which is associated with hyperleucinemia. Under these conditions high insulin and leucin plasma levels may promote mTORC1 signaling of the peripheral sebaceous follicles thus promoting the development of acne.
Western Diet Stimulates All Major mTORC1 Activation Pathways
The Western diet stimulates all three major pathways important for mTORC1 activation (Fig. 1). The Western diet provides abundant energy, glucose, and fat to suppress AMPK activity increasing mTORC1 signaling (Table 2). The high glycemic load increases glucose availability and stimulates increased glucose-dependent insulin signaling. High intake of insulinotropic food has been a matter of concern for more than a decade.99 Despite of all efforts in prevention the total yearly consumption of sugar still increases worldwide. Highly glycemic and insulinemic foods are ubiquitous elements of the Western diet and comprised 47.7% of the per capita energy intake in the United States in the year 2002.100,101 Today, the proportion of insulinotropic food will be much higher and is further driven by expanding activities of multiple fast food distributors.
Table 2. Mechanisms of mTORC1 activation by Western diet.
| Compound of Western diet | Mechanisms of mTORC1 activation |
|---|---|
| High total calories ( = high energy) |
Reduced activity of AMPK |
| High glycemic load ( = high energy) |
Reduced activity of AMPK Increased insulin signaling |
| High fat intake ( = high energy) |
Reduced activity of AMPK |
| High alcohol intake ( = high energy) |
Reduced activity of AMPK |
| High dairy protein intake ( = high leucine) |
Increased insulin/IGF-1 signaling and leucine-mediated mTORC1 activation |
| High meat intake ( = high leucine) | Leucine- and IGF-1-mediated mTORC1 activation |
Milk proteins significantly contribute to high insulin/IGF-1 signaling of Western diet. Mammalian milk has to be regarded as an endocrine signaling system that upregulates mTORC1 activity by increasing insulin secretion, hepatic IGF-1 secretion, and mTORC1-mediated β-cell proliferation for neonatal growth requirements.102 Milk consumption not only stimulates the somatotropic axis but also activates incretin signaling by enteral stimulation of glucose-dependent insulinotropic polypeptide (GIP).57,103 Milk’s excessive insulinotropic activity is characterized by milk’s high insulinemic index.104 Notably, increased daily intake of milk but not meat significantly raised basal insulin- and IGF-1 serum levels and increased insulin resistance in 8-y old boys.105 Milk-induced insulin resistance can be explained by increased mTORC1/S6K1-mediated IRS-1 phosphorylation.50 Epidemiological data in adults clearly confirmed the correlation between increased dairy protein consumption and raised IGF-1 serum levels.58,59
Most importantly, to achieve the physiological requirements for adequate growth, milk proteins provide highest amounts of leucine, the most effective, essential amino acid required for mTORC1 activation. Whey proteins have thus to be regarded as life starter proteins that not surprisingly contain the highest amount of leucine (14%), followed by casein (10%), the major protein constituent of cow milk and cheese (Table 1).106 For comparison, 100 g of rump steak contains approximately 2.4 g leucine comparable to 100 g of Gouda cheese (2.4 g), which is in strong contrast to 100 g white cabbage (0.056 g), or 100 g apple (0.016 g). To reach the leucine intake provided by 100 g Gouda cheese or steak, 4.2 kg white cabbage or 100 apples could be consumed (Tables 3 and 4). These simple calculations exemplify the extreme differences in leucine amounts provided by an animal meat/dairy protein-based diet in comparison to a vegetarian or vegan diet. Thus, the increased consumption of meat and dairy proteins, staples of Western diet, provides abundant amounts of leucine for mTORC1 activation. In comparison to meat, milk proteins offer two major signals for mTORC1 activation, insulin/IGF-1 as well as high leucine.49 It is most critical that the mTORC1-activating “system milk” is frequently combined with hyperglycemic carbohydrates or pure sugar (milk plus cornflakes, milk chocolate or milk ice), a combination which potentiates mTORC1 activation.
Table 3. Leucine-enriched animal-derived foods.
| Food | Leucine (mg/100 g food) |
|---|---|
| Beef (rump steak) |
2,369 |
| Gouda cheese (40% fat) |
2,359 |
| Coalfish, cooked |
1,883 |
| Broiler, cooked |
1,806 |
| Curd cheese (20% fat) |
1,290 |
| Yoghurt (3.5% fat) |
410 |
| Cow milk (1.5% fat) | 381 |
Source: German Nutrient Database, BLS-version 3.01.
Table 4. Plant-derived foods with low leucine content.
| Food | Leucine (mg/100 g food) |
|---|---|
| Corn (cooked) |
394 |
| Wheat (cooked) |
274 |
| Rice (cooked) |
219 |
| Broccoli (cooked) |
193 |
| Cauliflower (cooked) |
185 |
| Potato (cooked) |
124 |
| White cabbage (cooked) |
56 |
| Tomato |
38 |
| Apple | 16 |
Source: German Nutrient Database, BLS-version 3.01.
Adolescents exhibit the highest protein intake in comparison to young children or elderly individuals. Dietary survey data of USA in 2004—which will be much higher today—demonstrated that protein intake averaged 56 ± 14 g/d in young children, increased to a high of approximately 91 ± 22 g/d in adults aged 19–30 y, and decreased to approximately 66 ± 17 g/d in the elderly.107 Adolescents and young adults of Western countries consume the highest amounts of total protein. These data correlate with acne prevalence showing a climax during puberty but persisting into the third decade of life.2 High intake of glucose, total energy, insulinotropic and IGF1-raising food and total intake of animal proteins increasing the availability of leucine will alltogether maximize mTORC1 signaling (Table 2).
Signaling of Puberty Superimposed by Signaling of Western Diet
During puberty, nutrient-mediated mTORC-1 activation overlaps with puberty-driven (IGF-1/androgen)-mTORC1 activation, thus promoting epidemic acne. It is important to realize that acne coincides with the growth phase of puberty induced by increased pituitary secretion of growth hormone (GH) and GH-mediated hepatic secretion of IGF-1, which is intimately involved in the pathogenesis of acne.108 IGF-1 is a strong stimulator of sebaceous lipogenesis and upregulates the PI3K/Akt and mTORC1 pathway resulting in increased expression of SREBP-1, the key transcription factor of most lipid synthesizing enzymes.109 IGF-1 mediated activation of the PI3K/Akt pathway results in nuclear extrusion of FoxO transcription factors, recently linked to acne pathogenesis.38,39 IGF-1 is also related to increased androgen signaling, as IGF1- stimulates adrenal and gonadal androgen synthesis and increases 5α-reductase activity, the responsible enzyme for the conversion of testosterone to the 10-fold more potent DHT.60,108 Intriguingly, subjects with Laron syndrome and congenital IGF-1 deficiency due to loss-of-function mutations of GH receptor exhibit short stature and do not develop acne or clinical signs of hyperandrogenism, unless substituted with high doses of recombinant IGF-1.110 Remarkably, the transcriptional activity of the androgen receptor itself is also linked to insulin/IGF-1 signaling. IGF-1 stimulates androgen receptor transactivation by nuclear extrusion of the androgen receptor cosuppressor FoxO1 from the androgen receptor complex.111,112 Thus, there is substantial evidence for the role of insulin/IGF-1 signaling in the pathogenesis of acne, an IGF-1- and androgen-dependent disease. Both, IGF1- and androgen-signals are known to activate mTORC1, which is necessary for growth in puberty. However, it appears that normal puberty in non-Westernized populations does not lead to the development of acne. There is no acne in adolescent Kitava during puberty who live on a Paleolithic diet and do not consume hyperinsulinemic, IGF-1-raising food like hyperglycemic carbohydrates and dairy proteins.3,4 Thus, there appears to be a threshold for mTORC1-mediated acne which is clearly exceeded under conditions of Western life style and nutrition.
Anti-Acne Agents Inhibit Over-Activated Nutrient-Stimulated mTORC1
Isotretinoin
Oral isotretinoin (13-cis-retinoic acid), the most powerful sebum suppressive anti-acne agent, is intracellularly isomerized to all-trans-retinoic acid (ATRA). ATRA/retinoic acid receptor (RAR) signaling induces in a secondary response increased expression of FoxO transcription factors.40 FoxOs have been recognized as interacting partners of the mTORC1 pathway.41 FoxO1, FoxO3 and FoxO4 inhibit mTORC1 signaling by increasing the expression of the AMPK activator Sestrin3 (Fig. 2).41,43-45 Furthermore, isotretinoin has been shown to reduce serum IGF-1 concentrations.113 Thus, isotretinoin inhibits mTORC1 by increased FoxO-Sestrin3-AMPK-signaling and decreased IGF-1-PI3K/Akt signaling. Isotretinoin promotes sebaceous gland hypotrophy and inhibits the G1/S checkpoint of the cell cycle.114 Indirect evidence links isotretinoin’s mode of action to FoxO-mediated inhibition of mTORC1, which induces autophagy leading to sebaceous gland apoptosis or hypotrophy. Infact, both mTORC1 inhibition and isotretinoin lead to cell cycle arrest at the G1/S transition.24,114 Moreover, isotretinoin, downregulated SREBP1 expression in human sebocytes.109,114 In accordance, mTORC1 has been linked to SREBP-regulated lipid metabolism.69-72 It is thus conceivable that hyperactivated mTORC1 signaling induced by Western diet is attenuated by isotretinoin-FoxO1-mediated suppression of mTORC1 resulting in autophagy and/or apotosis of sebocytes with decreased SREBP signaling.
Metformin
It should be emphasized that subjects exhibiting genetic variants featuring increased expression and responsiveness of certain components of the somatotropic axis (GH, GHR, IGF-1 and IGF1R) and/or reduced activity of FoxO transcription factors will show increased susceptibility for acne and mTORC1 activation.115 Prototypically, obese women with polycystic ovary syndrome (PCOS) exhibit signs of hyperandrogenism, acne, insulin resistance and increased insulin and IGF-1 serum levels.54 Treatment of PCOS with the anti-diabetic drug metformin improves overweight, reduces insulin resistance and clinical signs of hyperandrogenism including acne.116 Metformin is known to activate AMPK resulting in mTORC1 inhibition.28,37 Recently, a second AMPK-independent mechanism of metformin-mediated mTORC1-inhibition has been identified. Metformin inhibited leucine-induced translocation of inactive mTORC1 to Rheb-enriched lysosomal membrane compartments, thereby attenuating mTORC1 activity (Fig. 2).52 Metformin is thus an effective dual mTORC1 inhibitor, reduces overweight, insulin resistance (via mTORC1/S6K1-driven IRS-phosphorylation) and hyperandrogenism. Morover, metformin counterbalances the adverse effects of Western diet mediated by high insulin/IGF-1 as well as increased leucine signaling. Metformin (C4H11N5; molar mass 129.1) appears to function as a competitive inhibitor of leucine (C6H13NO2; molar mass 131.2) in the Rag GTPase-dependent process of mTORC1 activation. Notably, the usual daily dose of metformin (2 g/d) is in the range of 2 g leucine derived from daily consumption of 100 g meat or cheese. PCOS is associated with an increased risk of cancer. Intriguingly, recent evidence points to cancer-protective effects of metformin treatment.117-121
Benzoyl peroxide
Benzoyl peroxide (BPO), the classical external anti-acne agent commonly applied in excessive concentrations between 3 and 10% induces high cellular levels of hydrogen peroxide (H2O2), which is able to cross membrane compartments. Across species, high oxidative stress associated with the generation of reactive oxygen species (ROS) like hydrogen peroxide is known to activate nuclear FoxO transcription factors by stimulation of the oxidative stress inducible kinases, Jun N-terminus kinase (JNK) and ST20-like protein kinase 1 (MST1), which phosphorylate specific FoxO sites resulting in their nuclear accumulation.41 Intriguingly, FoxO1, FoxO3 and FoxO4 activated by oxidative stress induce Sestrin3 transcriptionally. Sestrin 3 activates AMPK and thereby inhibits mTORC1.41 Thus, topical BPO-treatment may increase FoxO transcriptional activities, which finally inhibit mTORC1.
Resveratrol
Resveratrol, a polyphenolic flavonoid from grapes and red wine, downregulates PI3K/Akt/mTORC1 signaling.122-126 Intriguingly, resveratrol directly inhibits PI3K by targeting the class IA PI3K ATP-binding site in a competitive and reversible fashion.127 PI3K and mTOR belong to the same family of PI3K-related lipid kinases. Thus, there is convincing evidence for the role of resveratrol as a direct and indirect inhibitor of mTORC1 (Fig. 2).
These recent insights imply that resveratrol may exert therapeutic effects in the treatment of acne. Resveratrol has been shown to inhibit the proliferation of Propionibacterium acnes.128 Recently, it has been reported that topical treatment of facial acne vulgaris in 20 patients with a resveratrol-containing gel (0.01% weight/volume) significantly reduced the number of microcomedones, papules and pustules compared with vehicle control.129
Epigallocatechin-3-gallate
The specific green tea catechin, epigallocatechin-3-gallate (EGCG), is regarded as the active anti-inflammatory and anti-proliferative compound of green tea extracts.130-133 EGCG inhibited type I collagen expression in keloid fibroblasts by inhibition of the PI3K/Akt/mTORC1 signaling pathway.134 EGCG has been proven to function as an ATP-competitive inhibitor of both PI3K and mTORC1, respectively (Fig. 2).135
Topical 2% green tea lotion was effective in the treatment of mild-to-moderate acne vulgaris.136 After 6 weeks, the mean total lesion count and mean severity index of acne showed significant reductions of 58% and 39%, respectively.136 Furthermore, a 3% green tea emulsion significantly reduced sebum production in 10 healthy male volunteers after 8 weeks of treatment.137 These data provide preliminary evidence for the effectiveness of natural plant-derived mTORC1 inhibitors in the treatment of acne and underline the role of mTORC1 signaling in the pathogenesis of acne.
Acne and Prostate Cancer Related to mTORC1 Signaling?
An epidemiologic association between the prevalence of severe long-lasting acne and increased risk of prostate cancer later in life has been reported.138 This association may depend on a life long over-stimulation of mTORC1-signaling, which may promote tumorigenesis.139,140 L-type amino acid transporters such as LAT1 and LAT3 mediate the uptake of essential amino acids. LAT3 is most effective in leucine transport. Prostate cancer cells coordinate the expression of LAT1 and LAT3 to maintain sufficient levels of leucine needed for mTORC1 signaling and cell growth. Inhibiting LAT function was sufficient to decrease cell growth and mTORC1 signaling in prostate cancer cells. These cells maintained levels of amino acid influx through androgen receptor-mediated regulation of LAT3 expression, and ATF4 regulation of LAT1 expression after amino acid deprivation.140 Anti-androgenic hormone therapy in prostate cancer may thus reduce cellular leucine uptake and leucine-mediated mTORC1 activation. Anti-androgens used in acne treatment may exert similar effects on cellular leucine influx, which should be investigated in future experimental studies.
Acne: An mTORC1-Driven Disease of Western Civilization
Accumulating evidence implies that nutrient-activated mTORC1 signaling plays a pivotal role in the pathogenesis of acne. Moreover, acne should be linked to other mTORC1-driven diseases of civilization like obesity, type 2 diabetes, metabolic syndrome, cancer and neurodegenerative diseases (Fig. 4).141-148 The common underlying pathogenic factor of these apparently unrelated diseases appears to be nutrient-mediated over-activation of mTORC1 leading to increased cellular growth, cell proliferation, tumorigenic stimulation, endoplasmic reticulum stress and deranged cell protein homeostasis.
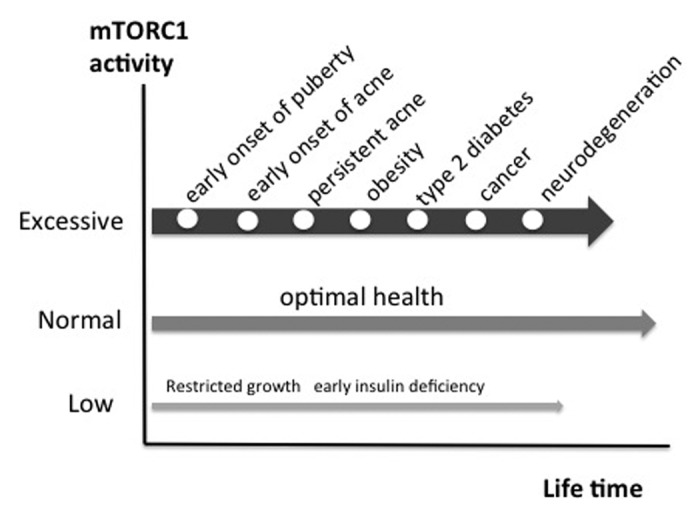
Figure 4. The march of mTORC1-driven diseases of civilization. Persistent over-activation of mTORC1 promotes chronic diseases of civilization. Epidemic acne is a visible indicator disease of excessive mTORC1 signaling increasing the risk for subsequent chronic diseases of civilization.
In this regard it is frightening to realize that more than 85% of adolescents of Western countries exhibit acne, whereas individuals of non-Western populations like the Kitava are not affected by this disease and other mTORC1-driven diseases of civilization.3,149 This implies that the majority of our Western population is living with over-activated mTORC1 signaling, a major pathogenic factor, which probably may pave the way for the development of other serious diseases of civilization (Fig. 4).
Conclusion and Future Perspectives
Epidemic acne of Westernized societies should be considered as a visible model disease of exaggerated mTORC1 signaling promoted by the Western diet. Dermatologists should not needlessly waste time with controversial discussions concerning isolated food components in the pathogenesis of acne but should realize the emerging whole network of exaggerated mTORC1 signaling mediated by Western diet. The most important task of preventive dermatology will be the reduction of mTORC1. All three major stimulatory pathways of mTORC1 activation have to be attenuated. Dietary intervention in acne should thus (1) decrease total energy, glucose and fat intake, (2) diminish insulin/IGF-1 signaling predominantly mediated by high dairy protein consumption, and (3) should limit the total leucine uptake predominantly provided by increased animal protein intake including meat and dairy proteins. This comprehensive dietary strategy can only be achieved by higher consumption of vegetables and fruit and reduction of animal-derived food. Indeed, diets enriched in vegetables and fruits, vegan diet as wells as Paleolithic diet (excluding sugar, hyperglycemic grains and dairy) have all been demonstrated to improve insulin sensitivity in type 2 diabetes, and metabolic syndrome and showed preventive effects in the development of Alzheimer disease.120,147,150-155 According to the recent Korean acne diet study, the frequency of vegetables and fish intake was significantly higher in the acne-free control group than in the acne group consuming more hyperglycemic carbohydrates, processed meat and dairy products.12 Paleolithic diet regimens come close to the goal of attenuated mTORC1 signaling but have to consider that unlimited total protein intake may overstimulate leucine-mediated mTORC1 activation. Vegetable-accentuated diets provide less mTORC1 activating signals and additionally contain natural plant-derived mTORC1-inhibitors like resveratrol, EGCG, curcumin, genestein, and indole-3-carbinol monomers, precursors of 3,3′-diindolylmethane.
The dermatologist should take responsibility for dietary education and intervention of his acne patients and should initiate first measures in correcting a harmful nutritional pathway of over-activated mTORC1 signaling with long-term adverse effects. A deeper understanding of diet-mediated mTORC1 signaling will help to appreciate the statement of Hippocrates of Kós who said about 2,400 y ago, “Your diet should be your medicine, and your medicine should be your diet.”
Glossary
Abbreviations:
- Akt
Akt kinase (protein kinase B)
- AMP
adenosine monophosphate
- AMPK
AMP-activated protein kinase
- ATP
adenosine triphosphate
- ATRA
all-trans-retinoic acid
- BCAA
branched-chain amino acid
- BCAT2
branched-chain aminotransferase-2
- BPO
benzoyl peroxide
- 2DG
2-desoxyglucose
- EGCG
epigallocatechin-3-gallate
- FoxO
forkhead box class O transcription factor
- DHT
dihydrotestosterone
- 4E-BP
eukaryotic initiation factor (eIF) 4E-binding protein
- GH
growth hormone
- GHR
growth hormone receptor
- GIP
glucose-dependent insulinotropic polypeptide
- GLUT
glucose transporter protein
- GR
glucocorticoid receptor
- GDP
guanosine diphosphate
- GTP
guanosine triphosphate
- IGF
insulin-like growth factor
- IGF1R
IGF-1 receptor
- IKK
inhibitor of kappa light chain gene enhancer in B cells
- JNK
Jun N-terminus kinase
- IL
interleukin
- IRS
insulin receptor substrate
- KLF
Krüppel-like factor
- LAT
L-type amino acid transporter
- LKB
liver kinase B
- mTOR
mammalian target of rapamycin
- MST1
STE20-like protein kinase 1
- NALA
N-acetylleucine amide
- NFκB
nuclear factor kappa B
- PCOS
polycystic ovary syndrome
- PI3K
phosphoinositol-3 kinase
- Rag
Ras-related GTP-binding protein
- Raptor
regulatory associated protein of mTOR
- REDD1
regulated in development and DNA damage responses
- Rheb
Ras homolog enriched in brain
- Rictor
rapamycin-insensitive companion of mTOR
- RSK
ribosomal S6 kinase
- S6K
p70 S6 kinase
- SREBP
sterol regulatory element-binding protein
- TCR
T cell receptor
- TNF
tumor necrosis factor
- TOR
target of rapamycin
- TSC
tuberous sclerosis complex
- TSC1
hamartin
- TSC2
tuberin
Note
This article is dedicated to Professor Otto Braun-Falco on the occasion of his 90th birthday.
Footnotes
Previously published online: www.landesbioscience.com/journals/dermatoendocrinology/article/19828
References
- 1.James WD. Clinical practice. Acne. N Engl J Med. 2005;352:1463–72. doi: 10.1056/NEJMcp033487. [DOI] [PubMed] [Google Scholar]
- 2.Collier CN, Harper JC, Cafardi JA, Cantrell WC, Wang W, Foster KW, et al. The prevalence of acne in adults 20 years and older. J Am Acad Dermatol. 2008;58:56–9. doi: 10.1016/j.jaad.2007.06.045. [DOI] [PubMed] [Google Scholar]
- 3.Cordain L, Lindeberg S, Hurtado M, Hill K, Eaton SB, Brand-Miller J. Acne vulgaris: a disease of Western civilization. Arch Dermatol. 2002;138:1584–90. doi: 10.1001/archderm.138.12.1584. [DOI] [PubMed] [Google Scholar]
- 4.Lindeberg S, Eliasson M, Lindahl B, Ahrén B. Low serum insulin in traditional Pacific Islanders--the Kitava Study. Metabolism. 1999;48:1216–9. doi: 10.1016/S0026-0495(99)90258-5. [DOI] [PubMed] [Google Scholar]
- 5.Smith RN, Mann NJ, Braue A, Mäkeläinen H, Varigos GA. A low-glycemic-load diet improves symptoms in acne vulgaris patients: a randomized controlled trial. Am J Clin Nutr. 2007;86:107–15. doi: 10.1093/ajcn/86.1.107. [DOI] [PubMed] [Google Scholar]
- 6.Smith RN, Braue A, Varigos GA, Mann NJ. The effect of a low glycemic load diet on acne vulgaris and the fatty acid composition of skin surface triglycerides. J Dermatol Sci. 2008;50:41–52. doi: 10.1016/j.jdermsci.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 7.Smith RN, Mann NJ, Braue A, Mäkeläinen H, Varigos GA. The effect of a high-protein, low glycemic-load diet versus a conventional, high glycemic-load diet on biochemical parameters associated with acne vulgaris: a randomized, investigator-masked, controlled trial. J Am Acad Dermatol. 2007;57:247–56. doi: 10.1016/j.jaad.2007.01.046. [DOI] [PubMed] [Google Scholar]
- 8.Smith R, Mann NJ, Mäkeläinen H, Roper J, Braue A, Varigos G. A pilot study to determine the short-term effects of a low glycemic load diet on hormonal markers of acne: a nonrandomized, parallel, controlled feeding trial. Mol Nutr Food Res. 2008;52:718–26. doi: 10.1002/mnfr.200700307. [DOI] [PubMed] [Google Scholar]
- 9.Adebamowo CA, Spiegelman D, Danby FW, Frazier AL, Willett WC, Holmes MD. High school dietary dairy intake and teenage acne. J Am Acad Dermatol. 2005;52:207–14. doi: 10.1016/j.jaad.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 10.Adebamowo CA, Spiegelman D, Berkey CS, Danby FW, Rockett HH, Colditz GA, et al. Milk consumption and acne in adolescent girls. Dermatol Online J. 2006;12:1–12. [PubMed] [Google Scholar]
- 11.Adebamowo CA, Spiegelman D, Berkey CS, Danby FW, Rockett HH, Colditz GA, et al. Milk consumption and acne in teenaged boys. J Am Acad Dermatol. 2008;58:787–93. doi: 10.1016/j.jaad.2007.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jung JY, Yoon MY, Min SU, Hong JS, Choi YS, Suh DH. The influence of dietary patterns on acne vulgaris in Koreans. Eur J Dermatol. 2010;20:768–72. doi: 10.1684/ejd.2010.1053. [DOI] [PubMed] [Google Scholar]
- 13.Spencer EH, Ferdowsian HR, Barnard ND. Diet and acne: a review of the evidence. Int J Dermatol. 2009;48:339–47. doi: 10.1111/j.1365-4632.2009.04002.x. [DOI] [PubMed] [Google Scholar]
- 14.Melnik B. [Acne vulgaris. Role of diet] Hautarzt. 2010;61:115–25. doi: 10.1007/s00105-009-1831-0. [DOI] [PubMed] [Google Scholar]
- 15.Danby FW. Nutrition and acne. Clin Dermatol. 2010;28:598–604. doi: 10.1016/j.clindermatol.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 16.Melnik BC. Evidence for acne-promoting effects of milk and other insulinotropic dairy products. Nestle Nutr Workshop Ser Pediatr Program. 2011;67:131–45. doi: 10.1159/000325580. [DOI] [PubMed] [Google Scholar]
- 17.Bowe WP, Joshi SS, Shalita AR. Diet and acne. J Am Acad Dermatol. 2010;63:124–41. doi: 10.1016/j.jaad.2009.07.043. [DOI] [PubMed] [Google Scholar]
- 18.Veith WB, Silverberg NB. The association of acne vulgaris with diet. Cutis. 2011;88:84–91. [PubMed] [Google Scholar]
- 19.Danby FW. New, relevant information and innovative interventions in the management of acne. G Ital Dermatol Venereol. 2011;146:197–210. [PubMed] [Google Scholar]
- 20.Danby FW. Acne: Diet and acneigenesis. Indian Dermatol Online J. 2011;2:2–5. doi: 10.4103/2229-5178.79851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bowers J. Diet & acne. Role of food remains controversial. Dermatology World. 2011;2011:31–4. [Google Scholar]
- 22.Inoki K, Ouyang H, Li Y, Guan KL. Signaling by target of rapamycin proteins in cell growth control. Microbiol Mol Biol Rev. 2005;69:79–100. doi: 10.1128/MMBR.69.1.79-100.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhaskar PT, Hay N. The two TORCs and Akt. Dev Cell. 2007;12:487–502. doi: 10.1016/j.devcel.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 24.Wang X, Proud CG. Nutrient control of TORC1, a cell-cycle regulator. Trends Cell Biol. 2009;19:260–7. doi: 10.1016/j.tcb.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 25.Sengupta S, Peterson TR, Sabatini DM. Regulation of the mTOR complex 1 pathway by nutrients, growth factors, and stress. Mol Cell. 2010;40:310–22. doi: 10.1016/j.molcel.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suzuki T, Inoki K. Spatial regulation of the mTORC1 system in amino acids sensing pathway. Acta Biochim Biophys Sin (Shanghai) 2011;43:671–9. doi: 10.1093/abbs/gmr066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang X, Proud CG. mTORC1 signaling: what we still don’t know. J Mol Cell Biol. 2011;3:206–20. doi: 10.1093/jmcb/mjq038. [DOI] [PubMed] [Google Scholar]
- 28.Shaw RJ. LKB1 and AMP-activated protein kinase control of mTOR signalling and growth. Acta Physiol (Oxf) 2009;196:65–80. doi: 10.1111/j.1748-1716.2009.01972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Avruch J, Long X, Ortiz-Vega S, Rapley J, Papageorgiou A, Dai N. Amino acid regulation of TOR complex 1. Am J Physiol Endocrinol Metab. 2009;296:E592–602. doi: 10.1152/ajpendo.90645.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sancak Y, Peterson TR, Shaul YD, Lindquist RA, Thoreen CC, Bar-Peled L, et al. The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science. 2008;320:1496–501. doi: 10.1126/science.1157535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sancak Y, Bar-Peled L, Zoncu R, Markhard AL, Nada S, Sabatini DM. Ragulator-Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell. 2010;141:290–303. doi: 10.1016/j.cell.2010.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goberdhan DCI. Intracellular amino acid sensing and mTORC1-regulated growth: new ways to block an old target? Curr Opin Investig Drugs. 2010;11:1360–7. [PMC free article] [PubMed] [Google Scholar]
- 33.Inoki K, Li Y, Zhu T, Wu J, Guan KL. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat Cell Biol. 2002;4:648–57. doi: 10.1038/ncb839. [DOI] [PubMed] [Google Scholar]
- 34.Manning BD, Tee AR, Logsdon MN, Blenis J, Cantley LC. Identification of the tuberous sclerosis complex-2 tumor suppressor gene product tuberin as a target of the phosphoinositide 3-kinase/akt pathway. Mol Cell. 2002;10:151–62. doi: 10.1016/S1097-2765(02)00568-3. [DOI] [PubMed] [Google Scholar]
- 35.Tee AR, Fingar DC, Manning BD, Kwiatkowski DJ, Cantley LC, Blenis J. Tuberous sclerosis complex-1 and -2 gene products function together to inhibit mammalian target of rapamycin (mTOR)-mediated downstream signaling. Proc Natl Acad Sci U S A. 2002;99:13571–6. doi: 10.1073/pnas.202476899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–90. doi: 10.1016/S0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- 37.Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, et al. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30:214–26. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Melnik BC. FoxO1 - the key for the pathogenesis and therapy of acne? J Dtsch Dermatol Ges. 2010;8:105–14. doi: 10.1111/j.1610-0387.2010.07344.x. [DOI] [PubMed] [Google Scholar]
- 39.Melnik BC. The role of transcription factor FoxO1 in the pathogenesis of acne vulgaris and the mode of isotretinoin action. G Ital Dermatol Venereol. 2010;145:559–71. [PubMed] [Google Scholar]
- 40.Melnik BC. Isotretinoin and FoxO1: A scientific hypothesis. Dermatoendocrinol. 2011;3:141–65. doi: 10.4161/derm.3.3.15331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hay N. Interplay between FOXO, TOR, and Akt. Biochim Biophys Acta. 2011;1813:1965–70. doi: 10.1016/j.bbamcr.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gross DN, Wan M, Birnbaum MJ. The role of FOXO in the regulation of metabolism. Curr Diab Rep. 2009;9:208–14. doi: 10.1007/s11892-009-0034-5. [DOI] [PubMed] [Google Scholar]
- 43.Chen CC, Jeon SM, Bhaskar PT, Nogueira V, Sundararajan D, Tonic I, et al. FoxOs inhibit mTORC1 and activate Akt by inducing the expression of Sestrin3 and Rictor. Dev Cell. 2010;18:592–604. doi: 10.1016/j.devcel.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Greer EL, Oskoui PR, Banko MR, Maniar JM, Gygi MP, Gygi SP, et al. The energy sensor AMP-activated protein kinase directly regulates the mammalian FOXO3 transcription factor. J Biol Chem. 2007;282:30107–19. doi: 10.1074/jbc.M705325200. [DOI] [PubMed] [Google Scholar]
- 45.Cao Y, Kamioka Y, Yokoi N, Kobayashi T, Hino O, Onodera M, et al. Interaction of FoxO1 and TSC2 induces insulin resistance through activation of the mammalian target of rapamycin/p70 S6K pathway. J Biol Chem. 2006;281:40242–51. doi: 10.1074/jbc.M608116200. [DOI] [PubMed] [Google Scholar]
- 46.Hara K, Yonezawa K, Weng QP, Kozlowski MT, Belham C, Avruch J. Amino acid sufficiency and mTOR regulate p70 S6 kinase and eIF-4E BP1 through a common effector mechanism. J Biol Chem. 1998;273:14484–94. doi: 10.1074/jbc.273.23.14484. [DOI] [PubMed] [Google Scholar]
- 47.Long X, Ortiz-Vega S, Lin Y, Avruch J. Rheb binding to mammalian target of rapamycin (mTOR) is regulated by amino acid sufficiency. J Biol Chem. 2005;280:23433–6. doi: 10.1074/jbc.C500169200. [DOI] [PubMed] [Google Scholar]
- 48.Nobukuni T, Joaquin M, Roccio M, Dann SG, Kim SY, Gulati P, et al. Amino acids mediate mTOR/raptor signaling through activation of class 3 phosphatidylinositol 3OH-kinase. Proc Natl Acad Sci U S A. 2005;102:14238–43. doi: 10.1073/pnas.0506925102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dennis MD, Baum JI, Kimball SR, Jefferson LS. Mechanisms involved in the coordinate regulation of mTORC1 by insulin and amino acids. J Biol Chem. 2011;286:8287–96. doi: 10.1074/jbc.M110.209171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zick Y. Ser/Thr phosphorylation of IRS proteins: a molecular basis for insulin resistance. Sci STKE. 2005;2005:pe4. doi: 10.1126/stke.2682005pe4. [DOI] [PubMed] [Google Scholar]
- 51.Altamirano F, Oyarce C, Silva P, Toyos M, Wilson C, Lavandero S, et al. Testosterone induces cardiomyocyte hypertrophy through mammalian target of rapamycin complex 1 pathway. J Endocrinol. 2009;202:299–307. doi: 10.1677/JOE-09-0044. [DOI] [PubMed] [Google Scholar]
- 52.Kalender A, Selvaraj A, Kim SY, Gulati P, Brûlé S, Viollet B, et al. Metformin, independent of AMPK, inhibits mTORC1 in a rag GTPase-dependent manner. Cell Metab. 2010;11:390–401. doi: 10.1016/j.cmet.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hardie DG. Role of AMP-activated protein kinase in the metabolic syndrome and in heart disease. FEBS Lett. 2008;582:81–9. doi: 10.1016/j.febslet.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 54.Chen W, Obermayer-Pietsch B, Hong JB, Melnik BC, Yamasaki O, Dessinioti C, et al. Acne-associated syndromes: models for better understanding of acne pathogenesis. J Eur Acad Dermatol Venereol. 2011;25:637–46. doi: 10.1111/j.1468-3083.2010.03937.x. [DOI] [PubMed] [Google Scholar]
- 55.Ghosh S, Lau H, Simons BW, Powell JD, Meyers DJ, De Marzo AM, et al. PI3K/mTOR signaling regulates prostatic branching morphogenesis. Dev Biol. 2011;360:329–42. doi: 10.1016/j.ydbio.2011.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wheelhouse NM, Stubbs AK, Lomax MA, MacRae JC, Hazlerigg DG. Growth hormone and amino acid supply interact synergistically to control insulin-like growth factor-I production and gene expression in cultured ovine hepatocytes. J Endocrinol. 1999;163:353–61. doi: 10.1677/joe.0.1630353. [DOI] [PubMed] [Google Scholar]
- 57.Rich-Edwards JW, Ganmaa D, Pollak MN, Nakamoto EK, Kleinman K, Tserendolgor U, et al. Milk consumption and the prepubertal somatotropic axis. Nutr J. 2007;6:28. doi: 10.1186/1475-2891-6-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Norat T, Dossus L, Rinaldi S, Overvad K, Grønbaek H, Tjønneland A, et al. Diet, serum insulin-like growth factor-I and IGF-binding protein-3 in European women. Eur J Clin Nutr. 2007;61:91–8. doi: 10.1038/sj.ejcn.1602494. [DOI] [PubMed] [Google Scholar]
- 59.Crowe FL, Key TJ, Allen NE, Appleby PN, Roddam A, Overvad K, et al. The association between diet and serum concentrations of IGF-I, IGFBP-1, IGFBP-2, and IGFBP-3 in the European Prospective Investigation into Cancer and Nutrition. Cancer Epidemiol Biomarkers Prev. 2009;18:1333–40. doi: 10.1158/1055-9965.EPI-08-0781. [DOI] [PubMed] [Google Scholar]
- 60.Horton R, Pasupuletti V, Antonipillai I. Androgen induction of steroid 5 α-reductase may be mediated via insulin-like growth factor-I. Endocrinology. 1993;133:447–51. doi: 10.1210/en.133.2.447. [DOI] [PubMed] [Google Scholar]
- 61.Hamdi MM, Mutungi G. Dihydrotestosterone stimulates amino acid uptake and the expression of LAT2 in mouse skeletal muscle fibres through an ERK1/2-dependent mechanism. J Physiol. 2011;589:3623–40. doi: 10.1113/jphysiol.2011.207175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Melnik B, Jansen T, Grabbe S. Abuse of anabolic-androgenic steroids and bodybuilding acne: an underestimated health problem. J Dtsch Dermatol Ges. 2007;5:110–7. doi: 10.1111/j.1610-0387.2007.06176.x. [DOI] [PubMed] [Google Scholar]
- 63.Melnik BC. Androgen abuse in the community. Curr Opin Endocrinol Diabetes Obes. 2009;16:218–23. doi: 10.1097/MED.0b013e32832afdfe. [DOI] [PubMed] [Google Scholar]
- 64.Plewig G, Fulton JE, Kligman AM. Cellular dynamics of comedo formation in acne vulgaris. Arch Dermatol Forsch. 1971;242:12–29. doi: 10.1007/BF00595286. [DOI] [PubMed] [Google Scholar]
- 65.Squarize CH, Castilho RM, Bugge TH, Gutkind JS. Accelerated wound healing by mTOR activation in genetically defined mouse models. PLoS One. 2010;5:e10643. doi: 10.1371/journal.pone.0010643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schroeder M, Zouboulis CC. All-trans-retinoic acid and 13-cis-retinoic acid: pharmacokinetics and biological activity in different cell culture models of human keratinocytes. Horm Metab Res. 2007;39:136–40. doi: 10.1055/s-2007-961813. [DOI] [PubMed] [Google Scholar]
- 67.Rosner M, Hanneder M, Siegel N, Valli A, Fuchs C, Hengstschläger M. The mTOR pathway and its role in human genetic diseases. Mutat Res. 2008;659:284–92. doi: 10.1016/j.mrrev.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 68.Torrelo A, Hadj-Rabia S, Colmenero I, Piston R, Sybert VP, Hilari-Carbonell H, et al. Folliculocystic and collagen hamartoma of tuberosus sclerosis complex. J Am Acad Dermatol. 2011;66:617–21. doi: 10.1016/j.jaad.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 69.Porstmann T, Santos CR, Lewis C, Griffiths B, Schulze A. A new player in the orchestra of cell growth: SREBP activity is regulated by mTORC1 and contributes to the regulation of cell and organ size. Biochem Soc Trans. 2009;37:278–83. doi: 10.1042/BST0370278. [DOI] [PubMed] [Google Scholar]
- 70.Düvel K, Yecies JL, Menon S, Raman P, Lipovsky AI, Souza AL, et al. Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Mol Cell. 2010;39:171–83. doi: 10.1016/j.molcel.2010.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li S, Brown MS, Goldstein JL. Bifurcation of insulin signaling pathway in rat liver: mTORC1 required for stimulation of lipogenesis, but not inhibition of gluconeogenesis. Proc Natl Acad Sci U S A. 2010;107:3441–6. doi: 10.1073/pnas.0914798107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Peterson TR, Sengupta SS, Harris TE, Carmack AE, Kang SA, Balderas E, et al. mTOR complex 1 regulates lipin 1 localization to control the SREBP pathway. Cell. 2011;146:408–20. doi: 10.1016/j.cell.2011.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rosenthal J, Angel A, Farkas J. Metabolic fate of leucine: a significant sterol precursor in adipose tissue and muscle. Am J Physiol. 1974;226:411–8. doi: 10.1152/ajplegacy.1974.226.2.411. [DOI] [PubMed] [Google Scholar]
- 74.Wheatley VR. Cutaneous lipogenesis. Major pathways of carbon flow and possible interrelationships between the epidermis and sebaceous glands. J Invest Dermatol. 1974;62:245–56. doi: 10.1111/1523-1747.ep12676798. [DOI] [PubMed] [Google Scholar]
- 75.Cassidy DM, Lee CM, Laker MF, Kealey T. Lipogenesis in isolated human sebaceous glands. FEBS Lett. 1986;200:173–6. doi: 10.1016/0014-5793(86)80533-6. [DOI] [PubMed] [Google Scholar]
- 76.Jeremy AH, Holland DB, Roberts SG, Thomson KF, Cunliffe WJ. Inflammatory events are involved in acne lesion initiation. J Invest Dermatol. 2003;121:20–7. doi: 10.1046/j.1523-1747.2003.12321.x. [DOI] [PubMed] [Google Scholar]
- 77.Pierdominici M, Vacirca D, Delunardo F, Ortona E. mTOR signaling and metabolic regulation of T cells: new potential therapeutic targets in autoimmune diseases. Curr Pharm Des. 2011;17:3888–97. doi: 10.2174/138161211798357809. [DOI] [PubMed] [Google Scholar]
- 78.Powell JD, Delgoffe GM. The mammalian target of rapamycin: linking T cell differentiation, function, and metabolism. Immunity. 2010;33:301–11. doi: 10.1016/j.immuni.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yang K, Neale G, Green DR, He W, Chi H. The tumor suppressor Tsc1 enforces quiescence of naive T cells to promote immune homeostasis and function. Nat Immunol. 2011;12:888–97. doi: 10.1038/ni.2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Young CN, Koepke JI, Terlecky LJ, Borkin MS, Boyd Savoy L, Terlecky SR. Reactive oxygen species in tumor necrosis factor-alpha-activated primary human keratinocytes: implications for psoriasis and inflammatory skin disease. J Invest Dermatol. 2008;128:2606–14. doi: 10.1038/jid.2008.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lee DF, Kuo HP, Chen CT, Hsu JM, Chou CK, Wei Y, et al. IKK β suppression of TSC1 links inflammation and tumor angiogenesis via the mTOR pathway. Cell. 2007;130:440–55. doi: 10.1016/j.cell.2007.05.058. [DOI] [PubMed] [Google Scholar]
- 82.Edrees AF, Kaplan DL, Abdou NI. Pyogenic arthritis, pyoderma gangrenosum, and acne syndrome (PAPA syndrome) associated with hypogammaglobulinemia and elevated serum tumor necrosis factor-alpha levels. J Clin Rheumatol. 2002;8:273–5. doi: 10.1097/00124743-200210000-00009. [DOI] [PubMed] [Google Scholar]
- 83.Cortis E, De Benedetti F, Insalaco A, Cioschi S, Muratori F, D’Urbano LE, et al. Abnormal production of tumor necrosis factor (TNF) -- alpha and clinical efficacy of the TNF inhibitor etanercept in a patient with PAPA syndrome [corrected] J Pediatr. 2004;145:851–5. doi: 10.1016/j.jpeds.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 84.Tofteland ND, Shaver TS. Clinical efficacy of etanercept for treatment of PAPA syndrome. J Clin Rheumatol. 2010;16:244–5. doi: 10.1097/RHU.0b013e3181e969b9. [DOI] [PubMed] [Google Scholar]
- 85.Pearce EL. Metabolism in T cell activation and differentiation. Curr Opin Immunol. 2010;22:314–20. doi: 10.1016/j.coi.2010.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fox CJ, Hammerman PS, Thompson CB. Fuel feeds function: energy metabolism and the T-cell response. Nat Rev Immunol. 2005;5:844–52. doi: 10.1038/nri1710. [DOI] [PubMed] [Google Scholar]
- 87.Jones RG, Thompson CB. Revving the engine: signal transduction fuels T cell activation. Immunity. 2007;27:173–8. doi: 10.1016/j.immuni.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 88.Fumarola C, La Monica S, Guidotti GG. Amino acid signaling through the mammalian target of rapamycin (mTOR) pathway: Role of glutamine and of cell shrinkage. J Cell Physiol. 2005;204:155–65. doi: 10.1002/jcp.20272. [DOI] [PubMed] [Google Scholar]
- 89.Hidayat S, Yoshino K, Tokunaga C, Hara K, Matsuo M, Yonezawa K. Inhibition of amino acid-mTOR signaling by a leucine derivative induces G1 arrest in Jurkat cells. Biochem Biophys Res Commun. 2003;301:417–23. doi: 10.1016/S0006-291X(02)03052-8. [DOI] [PubMed] [Google Scholar]
- 90.Zheng Y, Delgoffe GM, Meyer CF, Chan W, Powell JD. Anergic T cells are metabolically anergic. J Immunol. 2009;183:6095–101. doi: 10.4049/jimmunol.0803510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Powell JD, Lerner CG, Schwartz RH. Inhibition of cell cycle progression by rapamycin induces T cell clonal anergy even in the presence of costimulation. J Immunol. 1999;162:2775–84. [PubMed] [Google Scholar]
- 92.Cham CM, Driessens G, O’Keefe JP, Gajewski TF. Glucose deprivation inhibits multiple key gene expression events and effector functions in CD8+ T cells. Eur J Immunol. 2008;38:2438–50. doi: 10.1002/eji.200838289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cobbold SP, Adams E, Farquhar CA, Nolan KF, Howie D, Lui KO, et al. Infectious tolerance via the consumption of essential amino acids and mTOR signaling. Proc Natl Acad Sci U S A. 2009;106:12055–60. doi: 10.1073/pnas.0903919106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shimizu N, Yoshikawa N, Ito N, Maruyama T, Suzuki Y, Takeda S, et al. Crosstalk between glucocorticoid receptor and nutritional sensor mTOR in skeletal muscle. Cell Metab. 2011;13:170–82. doi: 10.1016/j.cmet.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 95.Brugarolas J, Lei K, Hurley RL, Manning BD, Reiling JH, Hafen E, et al. Regulation of mTOR function in response to hypoxia by REDD1 and the TSC1/TSC2 tumor suppressor complex. Genes Dev. 2004;18:2893–904. doi: 10.1101/gad.1256804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.DeYoung MP, Horak P, Sofer A, Sgroi D, Ellisen LW. Hypoxia regulates TSC1/2-mTOR signaling and tumor suppression through REDD1-mediated 14-3-3 shuttling. Genes Dev. 2008;22:239–51. doi: 10.1101/gad.1617608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang H, Kubica N, Ellisen LW, Jefferson LS, Kimball SR. Dexamethasone represses signaling through the mammalian target of rapamycin in muscle cells by enhancing expression of REDD1. J Biol Chem. 2006;281:39128–34. doi: 10.1074/jbc.M610023200. [DOI] [PubMed] [Google Scholar]
- 98.Gray S, Wang B, Orihuela Y, Hong EG, Fisch S, Haldar S, et al. Regulation of gluconeogenesis by Krüppel-like factor 15. Cell Metab. 2007;5:305–12. doi: 10.1016/j.cmet.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cordain L, Eades MR, Eades MD. Hyperinsulinemic diseases of civilization: more than just Syndrome X. Comp Biochem Physiol A Mol Integr Physiol. 2003;136:95–112. doi: 10.1016/S1095-6433(03)00011-4. [DOI] [PubMed] [Google Scholar]
- 100.Gerrior S, Bente L.Nutrient Content of the U.S. Food Supply, 1909-99: A Summary Report. U.S. Department of Agriculture, Center for Nutrition Policy and Promotion. Home Economics Report 2002; No. 55. [Google Scholar]
- 101.Cordain L. Implications for the role of diet in acne. Semin Cutan Med Surg. 2005;24:84–91. doi: 10.1016/j.sder.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 102.Melnik BC. Milk signalling in the pathogenesis of type 2 diabetes. Med Hypotheses. 2011;76:553–9. doi: 10.1016/j.mehy.2010.12.017. [DOI] [PubMed] [Google Scholar]
- 103.Nilsson M, Holst JJ, Björck IM. Metabolic effects of amino acid mixtures and whey protein in healthy subjects: studies using glucose-equivalent drinks. Am J Clin Nutr. 2007;85:996–1004. doi: 10.1093/ajcn/85.4.996. [DOI] [PubMed] [Google Scholar]
- 104.Hoyt G, Hickey MS, Cordain L. Dissociation of the glycaemic and insulinaemic responses to whole and skimmed milk. Br J Nutr. 2005;93:175–7. doi: 10.1079/BJN20041304. [DOI] [PubMed] [Google Scholar]
- 105.Hoppe C, Mølgaard C, Vaag A, Barkholt V, Michaelsen KF. High intakes of milk, but not meat, increase s-insulin and insulin resistance in 8-year-old boys. Eur J Clin Nutr. 2005;59:393–8. doi: 10.1038/sj.ejcn.1602086. [DOI] [PubMed] [Google Scholar]
- 106.Millward DJ, Layman DK, Tomé D, Schaafsma G. Protein quality assessment: impact of expanding understanding of protein and amino acid needs for optimal health. Am J Clin Nutr. 2008;87:1576S–81S. doi: 10.1093/ajcn/87.5.1576S. [DOI] [PubMed] [Google Scholar]
- 107.Fulgoni VL., 3rd Current protein intake in America: analysis of the National Health and Nutrition Examination Survey, 2003-2004. Am J Clin Nutr. 2008;87:1554S–7S. doi: 10.1093/ajcn/87.5.1554S. [DOI] [PubMed] [Google Scholar]
- 108.Melnik BC, Schmitz G. Role of insulin, insulin-like growth factor-1, hyperglycaemic food and milk consumption in the pathogenesis of acne vulgaris. Exp Dermatol. 2009;18:833–41. doi: 10.1111/j.1600-0625.2009.00924.x. [DOI] [PubMed] [Google Scholar]
- 109.Smith TM, Gilliland K, Clawson GA, Thiboutot D. IGF-1 induces SREBP-1 expression and lipogenesis in SEB-1 sebocytes via activation of the phosphoinositide 3-kinase/Akt pathway. J Invest Dermatol. 2008;128:1286–93. doi: 10.1038/sj.jid.5701155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ben-Amitai D, Laron Z. Effect of insulin-like growth factor-1 deficiency or administration on the occurrence of acne. J Eur Acad Dermatol Venereol. 2011;25:950–4. doi: 10.1111/j.1468-3083.2010.03896.x. [DOI] [PubMed] [Google Scholar]
- 111.Fan W, Yanase T, Morinaga H, Okabe T, Nomura M, Daitoku H, et al. Insulin-like growth factor 1/insulin signaling activates androgen signaling through direct interactions of Foxo1 with androgen receptor. J Biol Chem. 2007;282:7329–38. doi: 10.1074/jbc.M610447200. [DOI] [PubMed] [Google Scholar]
- 112.Ma Q, Fu W, Li P, Nicosia SV, Jenster G, Zhang X, et al. FoxO1 mediates PTEN suppression of androgen receptor N- and C-terminal interactions and coactivator recruitment. Mol Endocrinol. 2009;23:213–25. doi: 10.1210/me.2008-0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Karadag AS, Ertugrul DT, Tutal E, Akin KO. Short-term isotretinoin treatment decreases insulin-like growth factor-1 and insulin-like growth factor binding protein-3 levels: does isotretinoin affect growth hormone physiology? Br J Dermatol. 2010;162:798–802. doi: 10.1111/j.1365-2133.2009.09618.x. [DOI] [PubMed] [Google Scholar]
- 114.Nelson AM, Gilliland KL, Cong Z, Thiboutot DM. 13-cis Retinoic acid induces apoptosis and cell cycle arrest in human SEB-1 sebocytes. J Invest Dermatol. 2006;126:2178–89. doi: 10.1038/sj.jid.5700289. [DOI] [PubMed] [Google Scholar]
- 115.Melnik B. Acne and genetics. In: Zouboulis CC, Katsabas AD, Kligman AM (eds) Acne vulgaris and Rosacea: Pathogenesis and Treatment. Spinger, Heidelberg, 2012; In press. [Google Scholar]
- 116.Pasquali R, Gambineri A. Insulin-sensitizing agents in women with polycystic ovary syndrome. Fertil Steril. 2006;86(Suppl 1):S28–9. doi: 10.1016/j.fertnstert.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 117.Dowling RJ, Zakikhani M, Fantus IG, Pollak M, Sonenberg N. Metformin inhibits mammalian target of rapamycin-dependent translation initiation in breast cancer cells. Cancer Res. 2007;67:10804–12. doi: 10.1158/0008-5472.CAN-07-2310. [DOI] [PubMed] [Google Scholar]
- 118.Yang Y. Metformin for cancer prevention. Front Med. 2011;5:115–7. doi: 10.1007/s11684-011-0112-3. [DOI] [PubMed] [Google Scholar]
- 119.Li D. Metformin as an antitumor agent in cancer prevention and treatment. J Diabetes. 2011;3:320–7. doi: 10.1111/j.1753-0407.2011.00119.x. [DOI] [PubMed] [Google Scholar]
- 120.McCarty MF. mTORC1 activity as a determinant of cancer risk--rationalizing the cancer-preventive effects of adiponectin, metformin, rapamycin, and low-protein vegan diets. Med Hypotheses. 2011;77:642–8. doi: 10.1016/j.mehy.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 121.Bo S, Ciccone G, Rosato R, Villois P, Appendino G, Ghigo E, et al. Cancer mortality reduction and metformin: a retrospective cohort study in type 2 diabetic patients. Diabetes Obes Metab. 2012;14:23–9. doi: 10.1111/j.1463-1326.2011.01480.x. [DOI] [PubMed] [Google Scholar]
- 122.Marques FZ, Markus MA, Morris BJ. Resveratrol: cellular actions of a potent natural chemical that confers a diversity of health benefits. Int J Biochem Cell Biol. 2009;41:2125–8. doi: 10.1016/j.biocel.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 123.Zhou H, Luo Y, Huang S. Updates of mTOR inhibitors. Anticancer Agents Med Chem. 2010;10:571–81. doi: 10.2174/187152010793498663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Jiang H, Shang X, Wu H, Gautam SC, Al-Holou S, Li C, et al. Resveratrol downregulates PI3K/Akt/mTOR signaling pathways in human U251 glioma cells. J Exp Ther Oncol. 2009;8:25–33. [PMC free article] [PubMed] [Google Scholar]
- 125.Brito PM, Devillard R, Nègre-Salvayre A, Almeida LM, Dinis TC, Salvayre R, et al. Resveratrol inhibits the mTOR mitogenic signaling evoked by oxidized LDL in smooth muscle cells. Atherosclerosis. 2009;205:126–34. doi: 10.1016/j.atherosclerosis.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 126.Lin JN, Lin VC, Rau KM, Shieh PC, Kuo DH, Shieh JC, et al. Resveratrol modulates tumor cell proliferation and protein translation via SIRT1-dependent AMPK activation. J Agric Food Chem. 2010;58:1584–92. doi: 10.1021/jf9035782. [DOI] [PubMed] [Google Scholar]
- 127.Fröjdö S, Cozzone D, Vidal H, Pirola L. Resveratrol is a class IA phosphoinositide 3-kinase inhibitor. Biochem J. 2007;406:511–8. doi: 10.1042/BJ20070236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Docherty JJ, McEwen HA, Sweet TJ, Bailey E, Booth TD. Resveratrol inhibition of Propionibacterium acnes. J Antimicrob Chemother. 2007;59:1182–4. doi: 10.1093/jac/dkm099. [DOI] [PubMed] [Google Scholar]
- 129.Fabbrocini G, Staibano S, De Rosa G, Battimiello V, Fardella N, Ilardi G, et al. Resveratrol-containing gel for the treatment of acne vulgaris: a single-blind, vehicle-controlled, pilot study. Am J Clin Dermatol. 2011;12:133–41. doi: 10.2165/11530630-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 130.Reuter J, Wölfle U, Weckesser S, Schempp C. Which plant for which skin disease? Part 1: Atopic dermatitis, psoriasis, acne, condyloma and herpes simplex. J Dtsch Dermatol Ges. 2010;8:788–96. doi: 10.1111/j.1610-0387.2010.07496.x. [DOI] [PubMed] [Google Scholar]
- 131.Fowler JF, Jr., Woolery-Lloyd H, Waldorf H, Saini R. Innovations in natural ingredients and their use in skin care. J Drugs Dermatol. 2010;9(Suppl):S72–81, quiz s82-3. [PubMed] [Google Scholar]
- 132.Reuter J, Merfort I, Schempp CM. Botanicals in dermatology: an evidence-based review. Am J Clin Dermatol. 2010;11:247–67. doi: 10.2165/11533220-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 133.Liao S. The medicinal action of androgens and green tea epigallocatechin gallate. Hong Kong Med J. 2001;7:369–74. [PubMed] [Google Scholar]
- 134.Zhang Q, Kelly AP, Wang L, French SW, Tang X, Duong HS, et al. Green tea extract and (-)-epigallocatechin-3-gallate inhibit mast cell-stimulated type I collagen expression in keloid fibroblasts via blocking PI-3K/AkT signaling pathways. J Invest Dermatol. 2006;126:2607–13. doi: 10.1038/sj.jid.5700472. [DOI] [PubMed] [Google Scholar]
- 135.Van Aller GS, Carson JD, Tang W, Peng H, Zhao L, Copeland RA, et al. Epigallocatechin gallate (EGCG), a major component of green tea, is a dual phosphoinositide-3-kinase/mTOR inhibitor. Biochem Biophys Res Commun. 2011;406:194–9. doi: 10.1016/j.bbrc.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 136.Elsaie ML, Abdelhamid MF, Elsaaiee LT, Emam HM. The efficacy of topical 2% green tea lotion in mild-to-moderate acne vulgaris. J Drugs Dermatol. 2009;8:358–64. [PubMed] [Google Scholar]
- 137.Mahmood T, Akhtar N, Khan BA, Khan HM, Saeed T. Outcomes of 3% green tea emulsion on skin sebum production in male volunteers. Bosn J Basic Med Sci. 2010;10:260–4. doi: 10.17305/bjbms.2010.2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sutcliffe S, Giovannucci E, Isaacs WB, Willett WC, Platz EA. Acne and risk of prostate cancer. Int J Cancer. 2007;121:2688–92. doi: 10.1002/ijc.23032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Nardella C, Carracedo A, Alimonti A, Hobbs RM, Clohessy JG, Chen Z, et al. Differential requirement of mTOR in postmitotic tissues and tumorigenesis. Sci Signal. 2009;2:ra2. doi: 10.1126/scisignal.2000189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wang Q, Bailey CG, Ng C, Tiffen J, Thoeng A, Minhas V, et al. Androgen receptor and nutrient signaling pathways coordinate the demand for increased amino acid transport during prostate cancer progression. Cancer Res. 2011;71:7525–36. doi: 10.1158/0008-5472.CAN-11-1821. [DOI] [PubMed] [Google Scholar]
- 141.Shaw RJ, Cantley LC. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature. 2006;441:424–30. doi: 10.1038/nature04869. [DOI] [PubMed] [Google Scholar]
- 142.Dann SG, Selvaraj A, Thomas G. mTOR Complex1-S6K1 signaling: at the crossroads of obesity, diabetes and cancer. Trends Mol Med. 2007;13:252–9. doi: 10.1016/j.molmed.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 143.Mieulet V, Lamb RF. Tuberous sclerosis complex: linking cancer to metabolism. Trends Mol Med. 2010;16:329–35. doi: 10.1016/j.molmed.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 144.Proud CG. mTOR signalling in health and disease. Biochem Soc Trans. 2011;39:431–6. doi: 10.1042/BST0390431. [DOI] [PubMed] [Google Scholar]
- 145.Melnik BC. Milk--the promoter of chronic Western diseases. Med Hypotheses. 2009;72:631–9. doi: 10.1016/j.mehy.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 146.Melnik BC. Permanent impairment of insulin resistance from pregnancy to adulthood: the primary basic risk factor of chronic Western diseases. Med Hypotheses. 2009;73:670–81. doi: 10.1016/j.mehy.2009.04.047. [DOI] [PubMed] [Google Scholar]
- 147.Melnik BC, John SM, Schmitz G. Over-stimulation of insulin/IGF-1 signaling by western diet may promote diseases of civilization: lessons learnt from Laron syndrome. Nutr Metab (Lond) 2011;8:41. doi: 10.1186/1743-7075-8-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Melnik BC. Milk signalling in the pathogenesis of type 2 diabetes. In: Lewis BS, Flugelman MY, Halon DA (eds) Coronary artery disease: 2011 update. Proceedings oft he 9th International Congress on Coronary Artery Disease. Medimond International Proceedings, Bologna 2011, pp17-22. [Google Scholar]
- 149.Lindeberg S. Food and Western disease. Health and nutrition from an evolutionary persepctive. Wiley-Blackwell, Oxford, 2010. [Google Scholar]
- 150.Yu R, Woo J, Chan R, Sham A, Ho S, Tso A, et al. Relationship between dietary intake and the development of type 2 diabetes in a Chinese population: the Hong Kong Dietary Survey. Public Health Nutr. 2011:1–9. doi: 10.1017/S136898001100053X. [DOI] [PubMed] [Google Scholar]
- 151.Gu Y, Nieves JW, Stern Y, Luchsinger JA, Scarmeas N. Food combination and Alzheimer disease risk: a protective diet. Arch Neurol. 2010;67:699–706. doi: 10.1001/archneurol.2010.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Klonoff DC. The beneficial effects of a Paleolithic diet on type 2 diabetes and other risk factors for cardiovascular disease. J Diabetes Sci Technol. 2009;3:1229–32. doi: 10.1177/193229680900300601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Jönsson T, Granfeldt Y, Ahrén B, Branell UC, Pålsson G, Hansson A, et al. Beneficial effects of a Paleolithic diet on cardiovascular risk factors in type 2 diabetes: a randomized cross-over pilot study. Cardiovasc Diabetol. 2009;8:35. doi: 10.1186/1475-2840-8-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Frassetto LA, Schloetter M, Mietus-Synder M, Morris RC, Jr., Sebastian A. Metabolic and physiologic improvements from consuming a paleolithic, hunter-gatherer type diet. Eur J Clin Nutr. 2009;63:947–55. doi: 10.1038/ejcn.2009.4. [DOI] [PubMed] [Google Scholar]
- 155.Seneff S, Wainwright G, Mascitelli L. Nutrition and Alzheimer’s disease: the detrimental role of a high carbohydrate diet. Eur J Intern Med. 2011;22:134–40. doi: 10.1016/j.ejim.2010.12.017. [DOI] [PubMed] [Google Scholar]