Abstract
Basal and adaptive β-cell regeneration capacity declines with old age, but the underlying molecular mechanisms remain incompletely understood. Poly (adenosine diphosphate [ADP]-ribose) polymerase 1 (PARP-1) is considered a multifunctional enzyme and transcription factor that regulates pancreatic β-cell death, regeneration and insulin secretion. We analyzed the capacity of β-cell regeneration in 2-month-old (young) and 12-month-old (old) wild-type (WT) and PARP-1−/− mice before and after low-dose streptozotocin (STZ), a stimulus of β-cell regeneration and the underlying mechanism. Before STZ administration, young WT and PARP-1−/− mice showed similar β-cell proliferation. By contrast, old WT but not old PARP-1−/− mice showed severely restricted β-cell proliferation. In further assessment of the adaptive β-cell regeneration capacity with age, we observed that with a single low dose of STZ, young WT and PARP-1−/− mice showed a similar increase in β-cell proliferation, with few changes in old WT mice. Surprisingly, adaptive β-cell proliferation capacity was significantly higher in old PARP-1−/− mice than old WT mice after STZ administration. The ability of β-cell mass to expand was associated with increased levels of the regenerating (Reg) genes RegI and RegII but not RegIV. Therefore, PARP-1 is a key regulator in β-cell regeneration with advancing age in mice.
INTRODUCTION
In mice, β-cell mass expands to match the increased demand for insulin in obesity, pregnancy and diabetes (1,2). Types 1 and 2 diabetes are caused by insulin secretion that is insufficient to match insulin demand. Inadequate insulin secretion in diabetes is caused in part by loss of β-cell mass or abnormalities in β-cell function (3,4). Basal β-cell regeneration capacity varies by age (5). Two independent groups showed a decrease in adaptive β-cell proliferation capacity with age (6,7). As well, a recent study of adults in China showed that the prevalence of diabetes varied by age: 3.2%, 11.5% and 20.4% among people aged 20 to 39, 40 to 59, and ≥60 years, respectively (8). Recent data suggest that age is a key factor in basal and adaptive β-cell regeneration capacity.
Studies of various animal models have suggested the involvement of poly (adenosine diphosphate [ADP]-ribose) polymerase 1 (PARP-1) in multiple physiological processes (9), including β-cell death and regeneration (10). PARP-knockout (PARP−/−) mice are protected against streptozotocin (STZ)-induced diabetes (11,12), whereby PARP overactivation may participate in the pathophysiological process of β-cell death and diabetes. In addition, PARP inhibitors ameliorated surgically induced diabetes in rats, which induced the regeneration of pancreatic β cells (13).
PARP inhibitors enhanced the transcription of the regenerating (Reg) gene by inhibiting the autopoly (ADP- ribosyl)ation of PARP (14). The Reg gene is expressed in pancreatic, liver, gastric and intestinal cells for proliferation or differentiation (15). The Reg protein family plays an important role in β-cell regeneration and/or survival and has a definite growth-stimulating effect on β cells, and Reg protein expression may parallel islet physiology (16,17).
However, the mechanisms of islet β-cell regeneration in old mice are not yet known. In the present study, we compared basal and adaptive β-cell regeneration capacity in wild-type (WT) and PARP-1−/− mice aged 2 months (young) and 12 months (old) before and after STZ administration and sought to determine the the underlying mechanism. We found that in PARP-1−/− mice the expression of RegI and RegII proteins was associated with resistance to age-dependent decline in β-cell proliferation.
MATERIALS AND METHODS
Reagents
The antibodies against insulin, glucagon, cleaved PARP-1 and all secondary antibodies were from Cell Signaling Technology (Danvers, MA, USA). Antibodies to RegI, RegII and RegIV were from Santa Cruz Biotechnology (Santa Cruz, CA, USA) and 5-bromo-2′-deoxyuridine (BrdU) monoclonal antibody and STZ were from Sigma (Sigma-Aldrich, St. Louis, MO, USA). The universal colorimetric PARP-1 assay kit was from GENMED (Wyoming, MI, USA). The enzyme-linked immunosorbent assay Mouse Insulin kit was from Millipore (Billerica, MA, USA). The One-Touch glucose meter ACCU-CHEK® Advantage was from Roche (Indianapolis, IN, USA). Other chemicals were purchased from Beyotime (Shanghai, China).
Animals
Male WT mice and PARP-1−/− mice, 4–5 wks old, were purchased from the Jackson Laboratory. The mice were fed normal chow and had free access to water. The temperature of the rearing room was 22°C ± 1°C. In the study, young groups began at 2 months old, and old groups were fed until they were 12 months old. All animal experiments were in accordance with the US National Institutes of Health Guide for the Care and Use of Laboratory Animals (18) and the Care and Use of Laboratory Animals Committee of Shandong University.
Low-Dose STZ Administration
Mice were injected with or without a single low dose (90 mg/kg) of STZ and then given BrdU, 1 mg/mL, in drinking water continuously until they were killed after 14 d.
Immunohistochemistry
The pancreases from each group were dissected, fixed in 4% paraformaldehyde and paraffin embedded. Sections (5 μm) were stained with hematoxylin and eosin (H&E). For immunostaining, sections were incubated overnight at 4°C with primary antibodies against insulin, glucagon, BrdU, RegI, Reg II or Reg IV, then a suitable secondary antibody at 37°C for 30 min. Images were obtained with use of a laser-scanning confocal microscope and data were representative of 4 to 5 animals per group.
β-Cell Proliferation Analysis
Total β-cell proliferation was determined in triple-stained pancreatic sections by counting all DAPI/insulin/BrdU copositive cells, and total β-cell size was determined in DAPI/insulin copositive cells of all islets in five sections per animal. The β-cell proliferation rate was calculated by the product of (DAPI/insulin/BrdU copositive cells)/(DAPI/insulin copositive cells). Results were averaged from five animals per group, each of which represented five sections per animal.
β-Cell Mass Analysis
The pancreas from each animal was weighed and processed for histological analysis. Sections triple stained with DAPI/insulin/glucagon for β cells and α cells were examined by use of a laser-scanning confocal microscope. β-cell mass was analyzed in five sections per animal. The cross-sectional areas of β cells and pancreas were determined by use of Adobe Photoshop. The β-cell mass per pancreas was calculated as the ratio of the area of β cells/total tissue and weight of the pancreas from at least four animals for each group.
Determination of Glucose Tolerance and Blood Insulin
After a 12-h fast, mice underwent measurement of blood glucose levels in venous blood from the tail vein by use of a glucose meter. Blood insulin levels were measured by the use of enzyme-linked immunosorbent assay. Then, glucose solution (2 mg glucose/g body weight) was injected intraperitoneally into mice, and blood glucose was measured at 15, 30, 60 and 120 min after injection. Blood insulin levels were measured at 5, 15, 30, and 60 min after injection. Each value was the mean of 5 animals per group.
Measurement of PARP-1 Activity
The PARP-1 activity of pancreatic islets was determined by the universal colorimetric PARP-1 assay kit according to the manufacturer’s instructions.
The color reaction was measured at 405 nm by use of a spectrophotometer. The activity was expressed as micro-moles of p-nitrophenol formed per minute per milligram of PARP-1 protein.
Western Blot Analysis
Pancreatic islets were isolated as previously described (7). Protein expression was assayed with islet lysates with equal amounts of protein. Lysates were resolved by SDS-PAGE, transferred to polyvinylidene difluoride membranes for immunoblotting. The membranes were incubated with specific antibodies against β-actin, cleaved PARP-1, RegI, Reg II and Reg IV. Each value was the mean of three independent experiments.
Statistical Analysis
All data are presented as mean ± standard deviation (SD) from at least triplicate experiments. Groups were evaluated by Student t test (SPSS 17.0). p < 0.05 was considered statistically significant.
RESULTS
Improved Glucose Homeostasis in Aged PARP-1−/− Mice
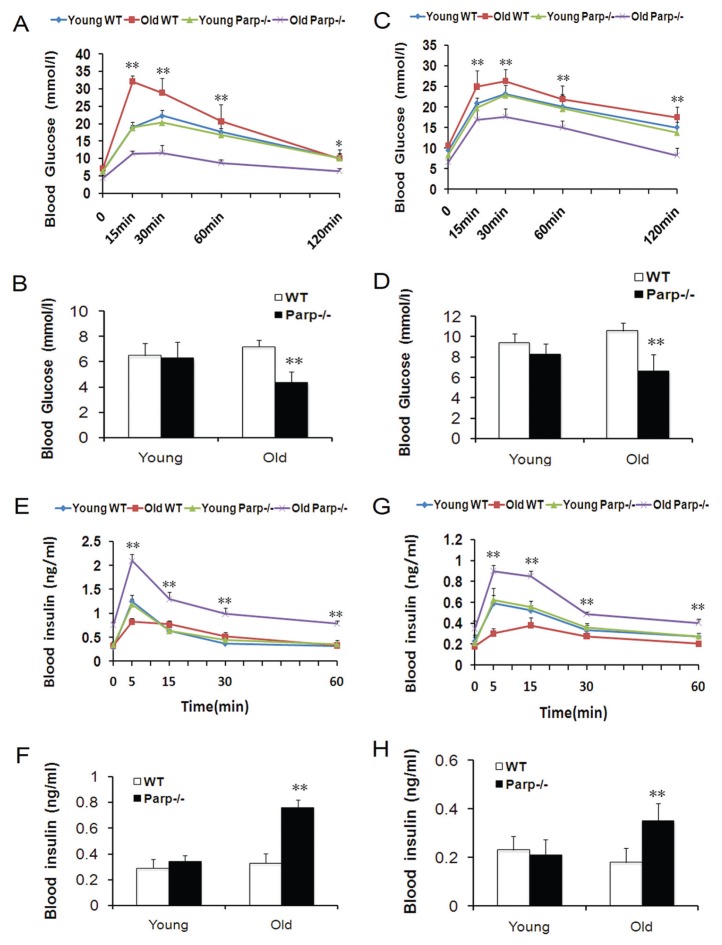
To ascertain whether PARP-1 plays a role in glucose homeostasis associated with age, we investigated young (2-month-old) and old (12-month-old) WT mice and PARP-1−/− mice. Blood glucose level was significantly higher for old WT than old PARP-1−/− mice after glucose administration at 15, 30, 60 (p < 0.01) and 120 min (p < 0.05; Figure 1A). Insulin release in response to glucose administration was significantly higher in old PARP-1−/− than in old WT mice, with a 2.75-fold difference in old PARP-1−/− mice and a 2.48-fold difference in old WT mice at 5 min after injection (Figure 1E). Basal blood glucose level and blood insulin level did not differ between young WT and young PARP-1−/− mice in the fasting state (6.48 ± 0.99 versus 6.27 ± 1.29 mmol/L for blood glucose; 0.29 ± 0.07 versus 0.34 ± 0.05 ng/mL for blood insulin) (Figures 1B, F) but were significantly different between old WT and old PARP-1−/− mice in the fasting state (7.18 ± 0.54 versus 4.37 ± 0.83 mmol/L for blood glucose; 0.33 ± 0.07 versus 0.76 ± 0.06 ng/mL for blood insulin) (p < 0.01; Figures 1B, F).
Figure 1.
Glucose homeostasis in young (2-month-old) and old (12-month-old) WT and PARP-1−/− mice. Intraperitoneal glucose tolerance test in young and old mice (n = 4–5) before (A) and after (C) a single administration of low-dose (90 mg/kg) STZ. Mice fasted overnight before testing, and levels of blood glucose were measured before and after glucose challenge (2 mg glucose/g body weight). *p < 0.05, **p < 0.01 versus old PARP-1−/− mice. Insulin secretion by determining blood insulin levels in the glucose tolerance test before (E) and after (G) STZ. **p < 0.01 versus old WT mice. Blood glucose levels of young and old mice (n = 4–5) after overnight fasting before (B) and after (D) STZ. Blood insulin levels of young and old mice (n = 4–5) after overnight fasting before (F) and after (H) STZ. **p < 0.01 versus old WT mice.
We used low-dose STZ to further test whether glucose homeostasis restricted with advanced age is associated with PARP-1. After low-dose STZ, blood glucose levels of old WT mice remained >17 mmol/L at 120 min. By contrast, levels for young mice and old PARP-1−/− mice after low-dose STZ reached baseline by the end of the test (Figure 1C). Blood glucose levels were significantly higher for old WT mice than old PARP-1−/− mice after low-dose STZ at all times (p < 0.01; Figure 1C). Five minutes after glucose loading, insulin levels were significantly higher in old PARP-1−/− than in old WT mice, with a 2.56-fold difference in old PARP-1−/− mice and a 1.69-fold difference in old WT mice (p < 0.01; Figure 1G). The peak of insulin secretion in the early phase was at the 5-min point after glucose loading in old PARP-1−/− mice, but at the 15-min point in old WT mice. In contrast, the insulin secretory response in young PARP-1−/− mice was similar to that of young WT mice (Figure 1G). After low-dose STZ, basal blood glucose levels were increased and blood insulin levels were decreased in all groups compared with before STZ (Figures 1D, H). Similarly, basal blood glucose levels and insulin levels were significantly different between old WT mice and old PARP-1−/− mice in the fasting state (10.58 ± 0.8 versus 6.63 ± 1.6 mmol/L for blood glucose; 0.18 ± 0.06 versus 0.35 ± 0.07 ng/mL for blood insulin) (p < 0.01; Figures 1D, H).
Decreased Islet Sizes of Old WT Mice after Low-Dose STZ Treatment
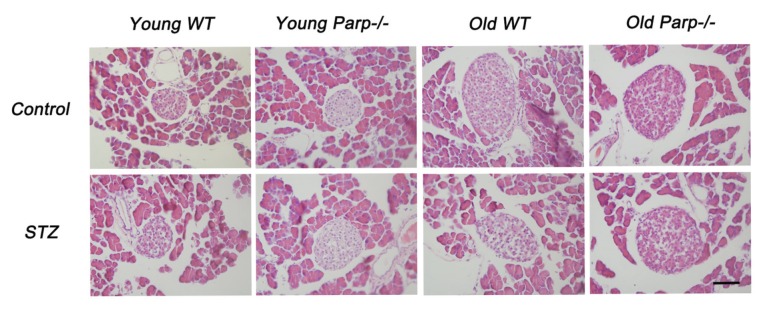
Recent studies demonstrated that physiological and pathological states can affect islet sizes and architecture. Increased islet sizes (>10,000 μm2) were observed in increased metabolic demand models, such as diabetes and pregnancy (2). We found a large number of small islets in the pancreas (<2,000 μm2) of young WT and PARP-1−/− mice and a similar frequency of larger islets (Figure 2). Old WT and PARP-1−/− mice showed markedly increased islets (>10,000 μm2). Interestingly, after low-dose STZ, islet sizes did not differ among young WT mice, young PARP-1−/− mice and old PARP-1−/− mice (Figure 2). In contrast, low-dose STZ treatment significantly decreased islet sizes of old WT mice.
Figure 2.
Islet morphology of young and old mice before and after a low-dose STZ treatment as judged by staining with H&E. Results are representative of 5–6 slides spanning the whole pancreas of each mouse for at least 4 mice per group. Bar = 50 μm.
Increased Adaptive Expansion of β-Cell Mass in Old PARP-1−/− Mice
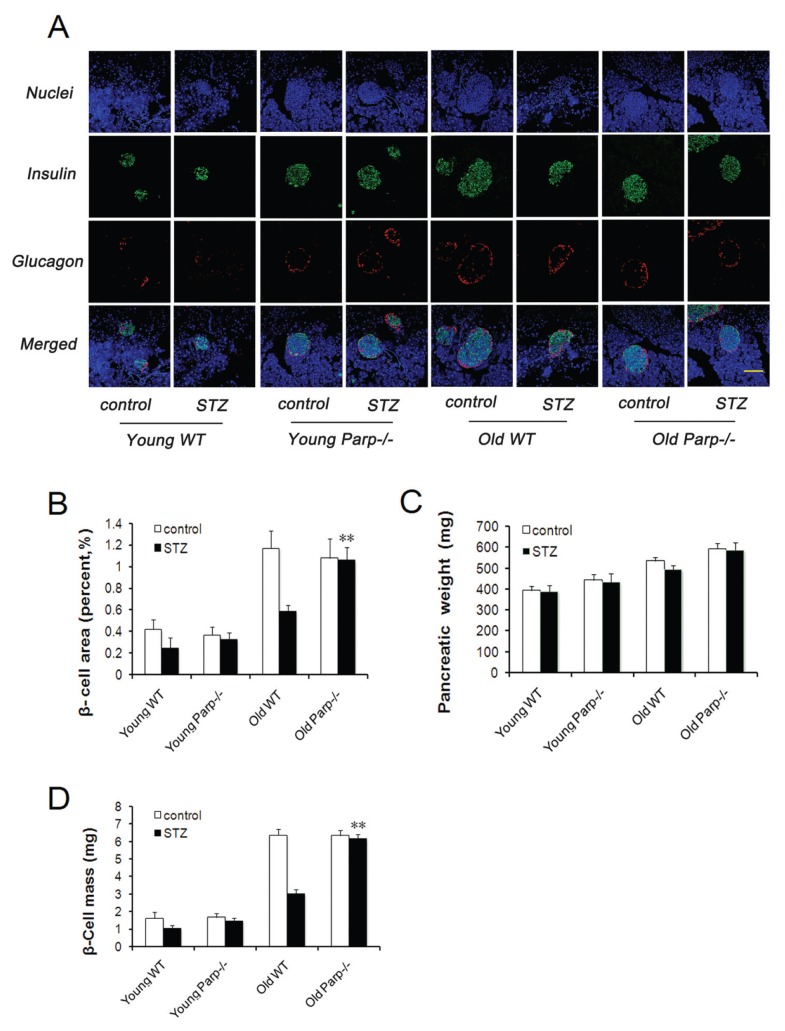
We quantified the change in β-cell mass in WT and PARP-1−/− mice. Pancreatic islets showed no change in structure, as shown by antiinsulin and anti -glucagon antibody staining in all groups before and after low-dose STZ (Figure 3A). β-Cell area did not differ significantly between young WT and PARP-1−/− mice (0.42% ± 0.09% versus 0.37% ± 0.07%) and was not changed substantially after low-dose STZ (0.25% ± 0.09% versus 0.33% ± 0.06%) (Figure 3B). In contrast, β-cell area was higher in old PARP-1−/− mice than in old WT mice before and after low-dose STZ (1.08% ± 0.18% versus 1.17% ± 0.16% before STZ; 1.06% ± 0.12% versus 0.59% ± 0.05% after STZ, respectively, p < 0.01) (Figure 3B). Pancreatic weight did not differ before or after low-dose STZ in all groups (Figure 3C). Pancreatic β-cell mass did not differ between young WT and PARP-1−/− mice before or after STZ treatment. In contrast, β-cell mass was significantly higher in old PARP-1−/− mice than old WT mice after STZ injection (6.16 ± 0.23 versus 3.00 ± 0.25 mg, p < 0.01) (Figure 3D).
Figure 3.
Adaptive expansion of β-cell mass in young and old mice. (A) Immunofluorescence analysis of representative images of pancreatic sections from young and old mice before and after low-dose STZ treatment and stained with antiinsulin (green) and anti-glucagon (red) antibodies. Results are representative of 5–6 sections of the whole pancreas of each mouse (n = 4–5). (B) Quantification of the insulin-expressing cell area in the pancreas of young and old mice before and after low-dose STZ treatment (n = 4–5). **p < 0.01 versus old WT mice. (C) Pancreas weights of WT and PARP-1−/− mice before and after low-dose STZ (n = 9–12). (D) β-cell mass calculated by β-cell area and pancreas weight (n = 9–12). **p < 0.01 versus old WT mice. Bar = 100 μm.
β-Cell Regenerative Capacity Decreases in Old WT but Not Old PARP-1−/− Mice
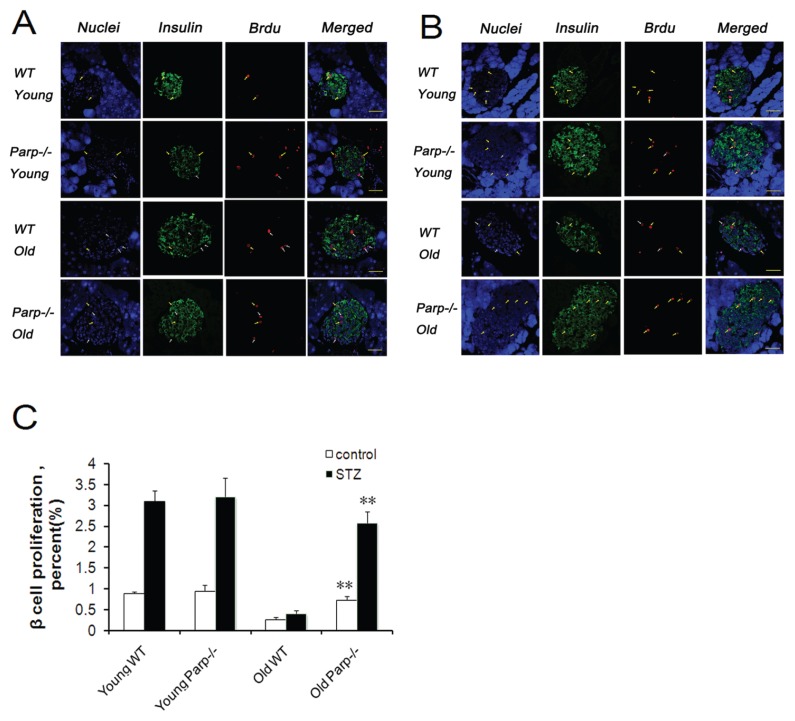
β-Cell proliferation was assessed by BrdU antibody staining (Figures 4A, B). Young WT and PARP-1−/− mice showed no significant difference in BrdU-positive nuclei in islets (Figures 4A, C). However, the proportion of BrdU-positive nuclei was higher in old PARP-1−/− mice than in old WT mice (0.73% ± 0.08% versus 0.27% ± 0.04%, p < 0.01). Similar results for β-cell proliferation were obtained after STZ treatment (Figures 4B, C). The proportion of BrdU- positive nuclei in the islets was significantly increased in both young WT and PARP-1−/− mice, with no difference between the two groups. In contrast, the proportion of BrdU-positive nuclei was significantly higher in old PARP-1−/− than old WT mice (2.58% ± 0.27% versus 0.41% ± 0.07%, p < 0.01).
Figure 4.
Basal and adaptive β-cell regeneration capacity in WT and PARP-1−/− mice. Representative sections of pancreatic sections immunostained with antibodies against insulin (green) and BrdU (red) and counterstained with DAPI (blue) before (A) and after (B) STZ (n = 4–5). (C) Proportion of BrdU-positive β cells of young and old mice before and after STZ injection. Values were averaged from 5 slides for each mouse, with 4–5 mice in each group.**p < 0.01 versus old WT mice. Yellow arrows = insulin and BrdU copositive cells; white arrows = BrdU-labeled and non–insulin-labeled cells. Bar = 50 μm.
Higher Expression of Cleaved PARP-1 and PARP-1 Activity in the Pancreas of Old WT Mice before and after STZ Treatment
The islets of old WT mice showed higher levels of cleaved PARP-1 protein and PARP-1 activity than those of young WT mice, which was detected by Western blot (Figures 5A, B) and the universal colorimetric PARP-1 assay kit (Figure 5C), respectively. Furthermore, similar results were observed after low-dose STZ (Figures 5A–C).
Figure 5.
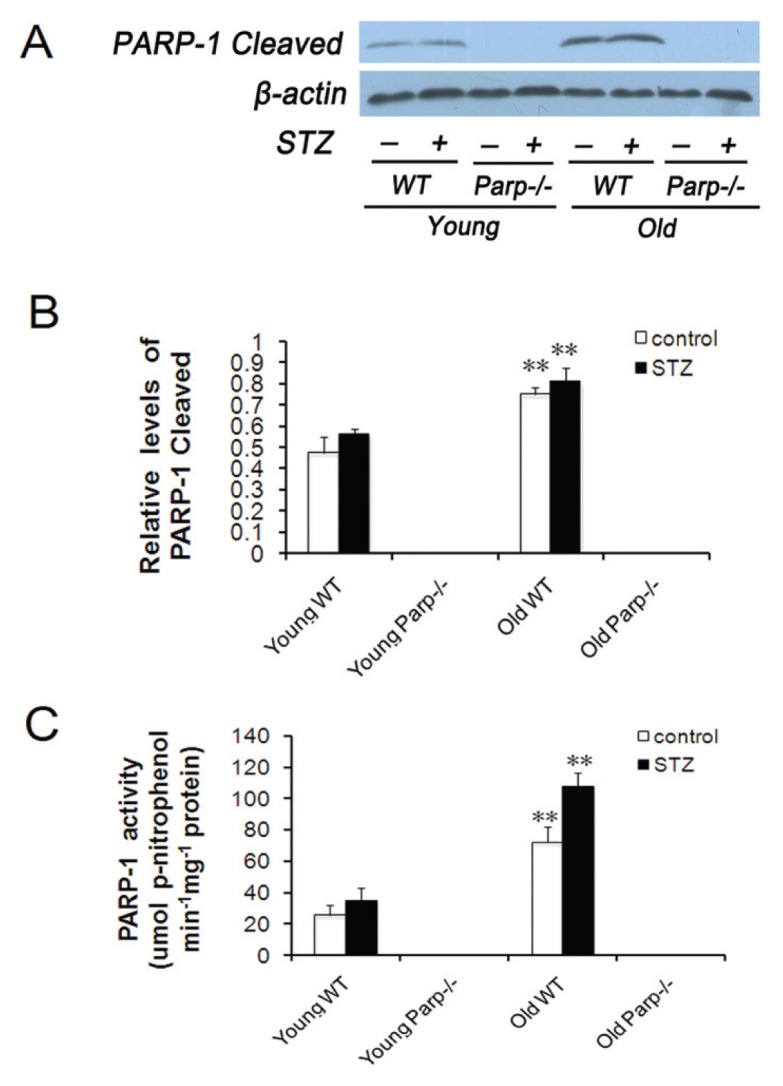
Expression of cleaved PARP-1 and PARP-1 activity in the pancreas of WT mice before and after low-dose STZ treatment. (A) Levels of cleaved PARP-1 protein expression in the islets isolated from young and old mice by Western blot analysis. (B) Analysis of immunoblot of cleaved PARP-1 protein normalized to β-actin. Values were representative of islets from 4–5 mice per group. **p < 0.01 versus young WT mice. (C) The PARP-1 activity of pancreatic islets was determined with the universal colorimetric PARP-1 assay kit, and results were expressed as micromoles of p-nitrophenol formed per minute per milligram of PARP-1 protein. Values were representative of islets from 4–5 mice per group. **p < 0.01 versus young WT mice.
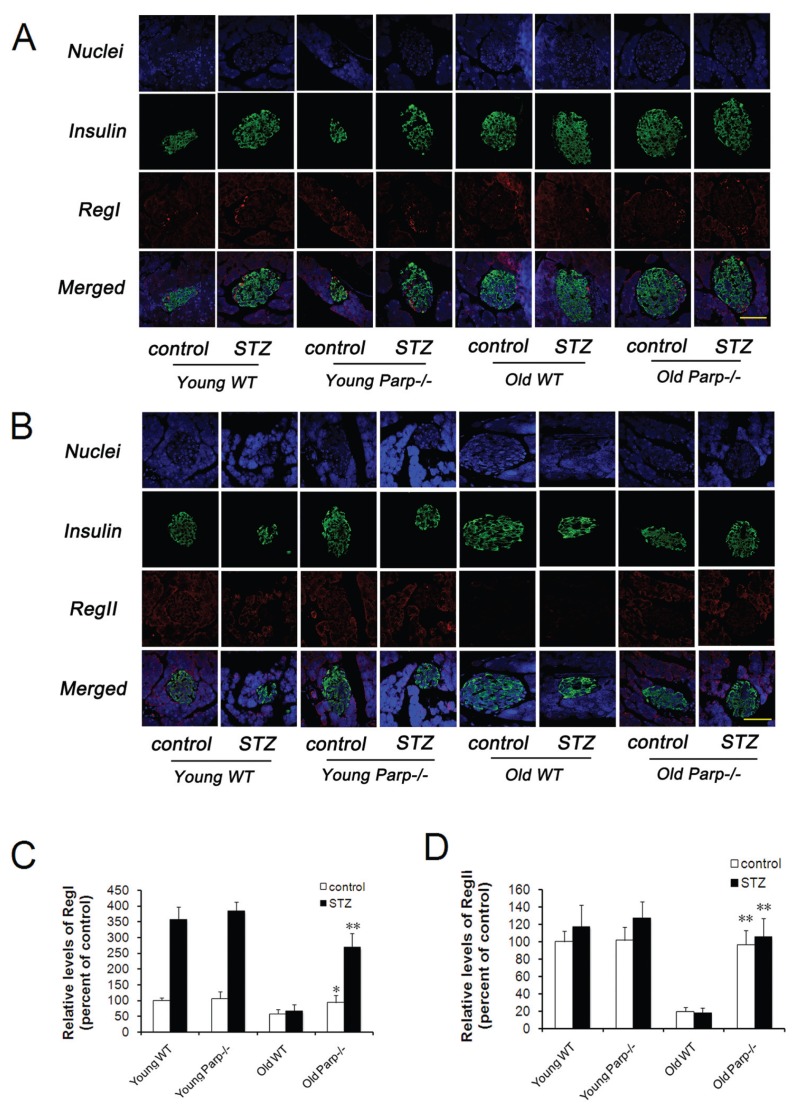
Increased Expression of RegI and RegII in the Pancreas of Young Mice and Old PARP-1−/− Mice after STZ Treatment
Immunohistochemical analysis of mouse pancreas from young and old WT and PARP-1−/− mice showed that RegI strongly bound to the periphery of the islets, whereas RegII predominantly stained the exocrine pancreas and gave a weak signal in the islets (Figures 6A, B). After STZ treatment, the level of pancreatic RegI was increased 3.5-, 3.6-, 1.1-and 2.9-fold in young WT, young PARP-1−/−, old WT and old PARP-1−/− mice, respectively (Figure 6C). Young WT and PARP-1−/− mice did not differ in expression of RegI before or after STZ treatment. In contrast, RegI expression was significantly higher in old PARP-1−/− than old WT mice (93.7% ± 21.9% versus 58.4% ± 12.8% before STZ treatment, p < 0.05; 269.0% ± 43.7% versus 66.7% ± 21.2% after STZ treatment, p < 0.01) (Figure 6C). The levels of RegII were slightly increased after STZ treatment in each group except for the old WT mice group (Figure 6D). The expression of RegII was similar between young WT and PARP-1−/− mice regardless of STZ treatment. However, RegII expression was significantly higher for old PARP-1−/− mice than WT mice (96.7% ± 16.3% versus 19.5% ± 4.8% before STZ treatment, p < 0.01; 105.3% ± 21.8% versus 17.8% ± 6.1% after STZ treatment, p < 0.01).
Figure 6.
RegI and RegII levels in the pancreas of young and old WT and PARP-1−/− mice before and after low-dose STZ treatment. (A) Double staining for insulin (green) and RegI (red) or (B) stained with RegII (red) and counterstained with DAPI (blue) (n = 4–5). Bar = 50 μm. (C) Quantitative analysis of RegI protein expressed as fold increase over the control group (young WT mice before low-dose STZ treatment) (n = 5). *p < 0.05 versus old WT mice (control group). **p < 0.01 versus old WT mice (STZ group). (D) Quantitative analysis of RegII protein expressed as fold increase over control group (young WT mice before a low-dose STZ treatment) (n = 5). **p < 0.01 versus old WT mice.
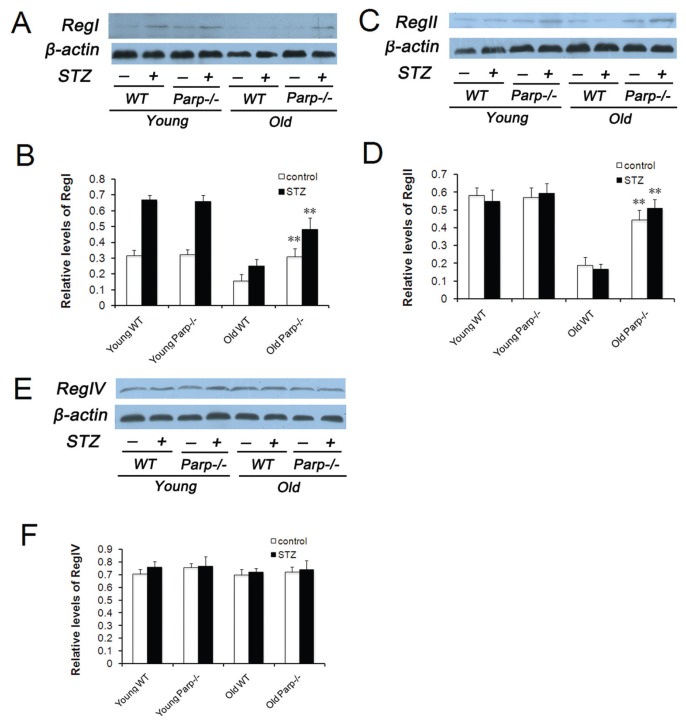
Similar results were observed with the use of Western blot analysis (Figures 7A–D).
Figure 7.
Expression of RegI, RegII and Reg IV protein in the pancreas before and after low-dose STZ treatment. Levels of RegI (A), RegII (C) and Reg IV (E) expression in the islets isolated from young and old mice by Western blot analysis. Analysis of immunoblot of RegI (B), RegII (D) and Reg IV (F) normalized to β-actin. Values were representative of islets from 4–5 mice per group. **p < 0.01 versus old WT mice.
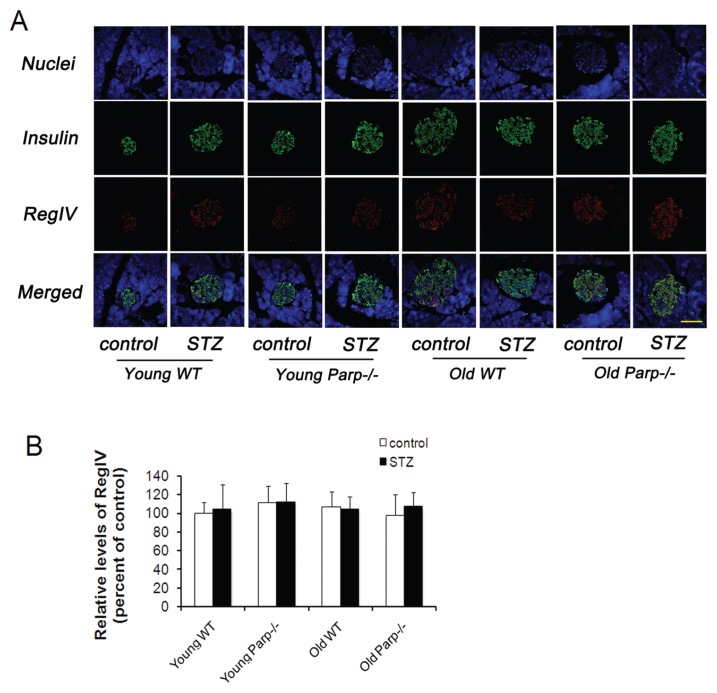
Levels of RegIV Were Not Changed after STZ Treatment
Double staining for the islet hormones insulin and RegIV showed that RegIV gave a strong signal in the islets in each group (Figure 8A). RegIV expression was found in almost all β cells; however, only a small number of insulin-negative islet cells were stained for RegIV. RegIV expression was similar in young and old mouse groups regardless of STZ treatment (Figure 8B).
Figure 8.
RegIV levels in the pancreas of young and old WT mice and PARP-1−/− mice before and after low-dose STZ treatment. (A) Double staining for insulin (green) and RegIV (red), counterstained with DAPI (blue) (n = 4–5). Bar = 50 μm. (B) Quantitative analysis of RegIV protein expressed as fold increase over the control group (young WT mice before low-dose STZ treatment) (n = 5).
Furthermore, islets from each group did not show significant differences in expression of RegIV before or after STZ treatment, which was detected by Western blot analysis (Figures 7E, F).
DISCUSSION
Basal and adaptive β-cell regeneration capacity declines with old age, but the underlying molecular mechanisms remain incompletely understood. PARP-1 regulates pancreatic β-cell death, regeneration and insulin secretion. We analyzed β-cell regeneration with age by examining young and old WT and PARP-1−/− mice before and after low-dose STZ to stimulate β-cell regeneration. Basal β-cell regenerative capacity decreased in old WT but not old PARP-1−/− mice. Adaptive β-cell regeneration capacity induced by low-dose STZ was better in PARP-1−/− than WT mice. Finally, RegI and RegII proteins were significantly expressed in old PARP-1−/− mice before or after low-dose STZ, which suggests that regenerative capacity in old PARP-1−/− mice is closely related to Reg protein expression. PARP-1/Reg may be a key regulator in β-cell regeneration in old mice.
Many experiments have evaluated whether age plays a role in the proliferative capacity of pancreatic β cells by using distinct models of endogenous β-cell regeneration: low-dose STZ administration, high-fat diet, treatment with the glucagon-like peptide 1 analog exendin-4 and partial pancreatectomy (5–7). In all models (5–7), young mice could regenerate β cells, whereas old mice did not show a similar plasticity, which is similar to rats (19,20). In recent years, the prevalence of type 2 diabetes among elderly people has increased worldwide (8,21,22). Therefore, old human patients could have poor capacity to regenerate β-cell mass to fulfill the need. Consistent with these data, our results showed that glucose homeostasis was associated with age in mice, with old PARP-1−/− mice showing better glucose homeostasis than old WT mice.
PARP-1 is involved in the pathophysiological features of many age-related diseases, such as chronic obstructive pulmonary disease (23), atherosclerotic lesions (24) and diabetes (25,26), by regulating DNA integrity, transcription, inflammation, cell regeneration and cell death. The extent of β-cell death and dysfunction was markedly reduced in PARP−/− mice (11,12), and administration of PARP inhibitors induced the regeneration of β cells (13,27). We observed restricted β-cell mass expansion in old WT but not old PARP-1−/− mice. Low-dose STZ is a robust stimulus of β-cell regeneration that is well tolerated and does not cause diabetes (7). We used low-dose STZ to further test whether β-cell regeneration capacity is restricted in old PARP-1−/− mice. As expected, STZ administration stimulated β-cell proliferation in old PARP-1−/− but not old WT mice. BrdU labeling revealed a similar result. Therefore, loss of PARP-1 resulted in resistance to age-dependent decreases in β-cell proliferation in mice.
The first Reg gene was isolated from a regenerating islet-derived cDNA library in rats (15). Reg family proteins act on β cells and other cells as autocrine or paracrine growth factors (16,28–33). In mice, RegI and RegII proteins expressed in regenerating islets and pancreatic exocrine tissues (15,34,35). RegIIIδ (islet neogenesis-associated protein), expressed predominantly in exocrine pancreas, enhances the secretion of insulin and the transcription of many islet genes in controlling β-cell neogenesis and islet metabolism (36,37). RegIIIα, RegIIIβ and RegI-IIγ are expressed weakly in pancreas and strongly in the intestinal tract; however, in a recent study, RegIIIα and RegIIIβ were activated in pancreatic-specific insulin-like growth factor I (IGF-I) gene–deficient and diabetic mice and participated in islet regeneration after β-cell damage (38). RegIV, the latest member of the Reg family to be identified, plays an important role in the progression of colorectal cancer (39). Moreover, PARP inhibitors may enhance and stabilize the DNA–protein complex for Reg gene transcription in β cells by inhibiting autopoly (ADP-ribosyl)ation of PARP (14,17). Consistent with these data, our results showed old PARP-1−/− mice with significantly higher expression of RegI and RegII in islets than WT mice before or after STZ treatment. However, levels of RegIV were not changed further in each group after STZ treatment. Therefore, PARP-1−/− mice are resistant to an age-dependent decrease in β-cell proliferation by upregulating RegI and RegII gene expression.
CONCLUSION
In summary, our study demonstrated that the ability to expand β-cell mass decreases with age in WT mice but not in PARP-1−/− mice. Furthermore, the age- dependent decrease in basal and adaptive regenerative capacity of β cells is associated with the expression of PARP-1/Reg family proteins. These findings might therefore represent a potential target in efforts to improve β-cell regeneration in treating diabetes in elderly patients. However, further studies are needed to understand the latent mechanisms of PARP-1/Reg family proteins in regulating β-cell regeneration in aged animal models.
ACKNOWLEDGMENTS
The study is supported by the National 973 Basic Research program of China (No. 2009CB521904) and grants from the National Natural Science Foundation of China (No. 81170275) and State Program of National Natural Science Foundation of China for Innovative Research Group (81021001).
Footnotes
Online address: http://www.molmed.org
DISCLOSURE
The authors declare that they have no competing interests as defined by Molecular Medicine, or other interests that might be perceived to influence the results and discussion reported in this paper.
REFERENCES
- 1.Hughes E, Huang C. Participation of Akt, menin, and p21 in pregnancy-induced beta-cell proliferation. Endocrinology. 2011;152:847–55. doi: 10.1210/en.2010-1250. [DOI] [PubMed] [Google Scholar]
- 2.Kim A, et al. Islet architecture: a comparative study. Islets. 2009;1:129–36. doi: 10.4161/isl.1.2.9480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444:840–6. doi: 10.1038/nature05482. [DOI] [PubMed] [Google Scholar]
- 4.Butler AE, et al. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52:102–10. doi: 10.2337/diabetes.52.1.102. [DOI] [PubMed] [Google Scholar]
- 5.Teta M, Long SY, Wartschow LM, Rankin MM, Kushner JA. Very slow turnover of beta-cells in aged adult mice. Diabetes. 2005;54:2557–67. doi: 10.2337/diabetes.54.9.2557. [DOI] [PubMed] [Google Scholar]
- 6.Rankin MM, Kushner JA. Adaptive beta-cell proliferation is severely restricted with advanced age. Diabetes. 2009;58:1365–72. doi: 10.2337/db08-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tschen SI, Dhawan S, Gurlo T, Bhushan A. Age-dependent decline in beta-cell proliferation restricts the capacity of beta-cell regeneration in mice. Diabetes. 2009;58:1312–20. doi: 10.2337/db08-1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang W, et al. Prevalence of diabetes among men and women in China. N Engl J Med. 2010;362:1090–101. doi: 10.1056/NEJMoa0908292. [DOI] [PubMed] [Google Scholar]
- 9.Rouleau M, Patel A, Hendzel MJ, Kaufmann SH, Poirier GG. PARP inhibition: PARP1 and beyond. Nat Rev Cancer. 10:293–301. doi: 10.1038/nrc2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takasawa S, Okamoto H. Pancreatic beta-cell death, regeneration and insulin secretion: roles of poly(ADP-ribose) polymerase and cyclic ADP-ribose. Int J Exp Diabetes Res. 2002;3:79–96. doi: 10.1080/15604280214485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pieper AA, et al. Poly(ADP-ribose) poly-merase-deficient mice are protected from strepto-zotocin-induced diabetes. Proc Natl Acad Sci USA. 1999;96:3059–64. doi: 10.1073/pnas.96.6.3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Masutani M, et al. Poly(ADP-ribose) polymerase gene disruption conferred mice resistant to streptozotocin-induced diabetes. Proc Natl Acad Sci USA. 1999;96:2301–4. doi: 10.1073/pnas.96.5.2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yonemura Y, et al. Amelioration of diabetes mellitus in partially depancreatized rats by poly(ADP-ribose) synthetase inhibitors. Evidence of islet B-cell regeneration. Diabetes. 1984;33:401–4. doi: 10.2337/diab.33.4.401. [DOI] [PubMed] [Google Scholar]
- 14.Akiyama T, et al. Activation of Reg gene, a gene for insulin-producing beta -cell regeneration: poly (ADP-ribose) polymerase binds Reg promoter and regulates the transcription by autopoly(ADP-ribosyl)ation. Proc Natl Acad Sci USA. 2001;98:48–53. doi: 10.1073/pnas.240458597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Terazono K, et al. A novel gene activated in regenerating islets. J Biol Chem. 1988;263:2111–4. [PubMed] [Google Scholar]
- 16.Zhang YW, Ding LS, Lai MD. Reg gene family and human diseases. World J Gastroenterol. 2003;9:2635–41. doi: 10.3748/wjg.v9.i12.2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okamoto H, Takasawa S. Recent advances in the Okamoto model: the CD38-cyclic ADP-ribose signal system and the regenerating gene protein (Reg)-Reg receptor system in beta-cells. Diabetes. 2002;51(Suppl 3):S462–73. doi: 10.2337/diabetes.51.2007.s462. [DOI] [PubMed] [Google Scholar]
- 18.Institute of Laboratory Animal Resources; Commission on Life Sciences; National Research Council. Guide for the Care and Use of Laboratory Animals. Washington (DC): National Academy Press; 1996. [cited 2010 Jan 1]. Available from: http://www.nap.edu/openbook.php?record_id=5140. [Google Scholar]
- 19.Tanigawa K, et al. Effect of aging on B-cell function and replication in rat pancreas after 90% pancreatectomy. Pancreas. 1997;15:53–9. doi: 10.1097/00006676-199707000-00008. [DOI] [PubMed] [Google Scholar]
- 20.Montanya E, Nacher V, Biarnes M, Soler J. Linear correlation between beta-cell mass and body weight throughout the lifespan in Lewis rats: role of beta-cell hyperplasia and hypertrophy. Diabetes. 2000;49:1341–6. doi: 10.2337/diabetes.49.8.1341. [DOI] [PubMed] [Google Scholar]
- 21.Lerman-Garber I, Rosales-Calderon M. Changes in glucose tolerance in elderly [in Spanish] Rev Invest Clin. 2010;62:312–7. [PubMed] [Google Scholar]
- 22.Mehta R, del-Moral ME, Aguilar-Salinas CA. Epidemiology of diabetes in the elderly [in Spanish] Rev Invest Clin. 2010;62:305–11. [PubMed] [Google Scholar]
- 23.Weseler AR, Geraets L, Moonen HJ, et al. Poly (ADP-ribose) polymerase-1-inhibiting flavonoids attenuate cytokine release in blood from male patients with chronic obstructive pulmonary disease or type 2 diabetes. J Nutr. 2009;139:952–7. doi: 10.3945/jn.108.102756. [DOI] [PubMed] [Google Scholar]
- 24.Perrotta I, et al. iNOS induction and PARP-1 activation in human atherosclerotic lesions: an immunohistochemical and ultrastructural approach. Cardiovasc Pathol. 2010;20:195–203. doi: 10.1016/j.carpath.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 25.Shevalye H, Maksimchyk Y, Watcho P, Obrosova IG. Poly(ADP-ribose) polymerase-1 (PARP-1) gene deficiency alleviates diabetic kidney disease. Biochim Biophys Acta. 20101802:1020–7. doi: 10.1016/j.bbadis.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sodhi RK, Singh N, Jaggi AS. Poly(ADP-ribose) polymerase-1 (PARP-1) and its therapeutic implications. Vascul Pharmacol. 2010;53:77–87. doi: 10.1016/j.vph.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 27.Yonemura Y, et al. Induction of islet B-cell regeneration in partially pancreatectomized rats by poly(ADP-ribose) synthetase inhibitors. Int J Pancreatol. 1988;3:73–82. doi: 10.1007/BF02788225. [DOI] [PubMed] [Google Scholar]
- 28.Sasaki Y, et al. REG1A expression is an independent factor predictive of poor prognosis in patients with breast cancer. Ann Surg Oncol. 2008;15:3244–51. doi: 10.1245/s10434-008-0137-2. [DOI] [PubMed] [Google Scholar]
- 29.Hayashi K, et al. REG I enhances chemo-and radiosensitivity in squamous cell esophageal cancer cells. Cancer Sci. 2008;99:2491–5. doi: 10.1111/j.1349-7006.2008.00980.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhong B, et al. Reg-II is an exocrine pancreas injury-response product that is up- regulated by keratin absence or mutation. Mol Biol Cell. 2007;18:4969–78. doi: 10.1091/mbc.E07-02-0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gurr W, Shaw M, Li Y, Sherwin R. RegII is a beta-cell protein and autoantigen in diabetes of NOD mice. Diabetes. 2007;56:34–40. doi: 10.2337/db06-0669. [DOI] [PubMed] [Google Scholar]
- 32.Nata K, et al. Molecular cloning, expression and chromosomal localization of a novel human REG family gene, REG III. Gene. 2004;340:161–70. doi: 10.1016/j.gene.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 33.Gu Z, et al. Reg IV: a promising marker of hormone refractory metastatic prostate cancer. Clin Cancer Res. 2005;11:2237–43. doi: 10.1158/1078-0432.CCR-04-0356. [DOI] [PubMed] [Google Scholar]
- 34.Unno M, et al. Production and characterization of Reg knockout mice: reduced proliferation of pancreatic beta-cells in Reg knockout mice. Diabetes. 2002;51(Suppl 3):S478–83. doi: 10.2337/diabetes.51.2007.s478. [DOI] [PubMed] [Google Scholar]
- 35.Okamoto H. The Reg gene family and Reg proteins: with special attention to the regeneration of pancreatic beta-cells. J Hepatobiliary Pancreat, Surg. 1999;6:254–62. doi: 10.1007/s005340050115. [DOI] [PubMed] [Google Scholar]
- 36.Dungan KM, Buse JB, Ratner RE. Effects of therapy in type 1 and type 2 diabetes mellitus with a peptide derived from islet neogenesis associated protein (INGAP) Diabetes Metab Res Rev. 2009;25:558–65. doi: 10.1002/dmrr.999. [DOI] [PubMed] [Google Scholar]
- 37.Barbosa H, et al. Islet Neogenesis Associated Protein (INGAP) modulates gene expression in cultured neonatal rat islets. Regul Pept. 2006;136:78–84. doi: 10.1016/j.regpep.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 38.Lu Y, et al. Activation of the Reg family genes by pancreatic-specific IGF-I gene deficiency and after streptozotocin-induced diabetes in mouse pancreas. Am J Physiol Endocrinol Metab. 2006;291:E50–8. doi: 10.1152/ajpendo.00596.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rafa L, et al. REG4 acts as a mitogenic, motility and pro-invasive factor for colon cancer cells. Int J Oncol. 2010;36:689–98. doi: 10.3892/ijo_00000544. [DOI] [PubMed] [Google Scholar]