Abstract
The rapid increase in the prevalence of obesity is a priority for investigators from across numerous disciplines, including biology, nutritional science, and public health and policy. In this paper, we systematically examine the premise that common dietary obesity is an addictive disorder, based on the criteria for addiction described in the Diagnostic and Statistical Manual (DSM) of Mental Disorders of the American Psychiatric Association, version IV, and consider the consequences of such a reclassification of obesity for public policy. Specifically, we discuss evidence from both human and animal studies investigating the effects of various types and amounts of food and the food environment in obese individuals. Neurobiological studies have shown that the hedonic brain pathways activated by palatable food overlap considerably with those activated by drugs of abuse and suffer significant deficits after chronic exposure to high-energy diets. Furthermore, food as a stimulus can induce the sensitization, compulsion and relapse patterns observed in individuals who are addicted to illicit drugs. The current food environment encourages these addictive-like behaviors where increased exposure through advertisements, proximity and increased portion sizes are routine. Taking lessons from the tobacco experience, it is clear that reclassifying common dietary obesity as an addictive disorder would necessitate policy changes (e.g., regulatory efforts, economic strategies, and educational approaches). These policies could be instrumental in addressing the obesity epidemic, by encouraging the food industry and the political leadership to collaborate with the scientific and medical community in establishing new and more effective therapeutic approaches.
Introduction
Obesity is a complex condition that constitutes a risk factor for a myriad of health problems such as cardiovascular disease, diabetes mellitus, osteoarthritis, and some cancers. The etiology of accelerated weight gain is multi-factorial and possible contributors include metabolic processes, the thrifty genotype, increased availability of high-energy palatable food as part of the current food environment and sedentary lifestyles. However, there is general consensus that a key root cause of the obesity epidemic is hyperphagia, which we could define as overeating or eating beyond one’s energy needs on a chronic basis. Attempts to address this complex problem at the level of basic science, clinical practice and social policy have had limited success. Further understanding of why increasing numbers of people consume more food than needed for energy balance, as well as why it is so difficult for people to adhere to low-calorie prescriptions despite obesity’s known adverse effects, is clearly needed.
An important area of research that has gained attention over the last decade is the investigation of the relationship between hyperphagia and addiction. With technological advancements in measuring brain activity in animals and humans, researchers have instigated the hypothesis that overeating may be an addictive behavior [1]. Food, as an addictive substance, has been held responsible in craving, binge eating, and weight gain[2, 3]. In addition, addiction to food may be influenced by many environmental factors including: increased availability to high energy dense foods, exposure to food advertisements, the decreased cost of high fat, high carbohydrate foods, a lifestyle that encourages food to be consumed quickly and often (snacking), and the role of food in today’s Western societies and other cultures worldwide. This review explores evidence that substantiates or challenges the plausibility of obesity as an outcome of food addiction. The current understanding and criteria for addiction are presented as well as evidence from epidemiological data, as well as animal and human models. This evaluation may help assess if obesity merits classification as a brain disorder and may have an important impact on current societal views of the disease, therapeutic approaches and the food industry [4].
Neurobiology of Hyperphagia versus Substance Abuse
Deciphering the precise definition of hyperphagia and hedonic eating as a behavior is of utmost importance in identifying the underlying neurobiology. Is it essential, for example, to demonstrate that in hedonic eating there is no amount of physical energy expenditure that could balance the energy intake due to food intake in excess of homeostatic need? As weight increases, the energy requirements also increase, so hedonic eating may need to be defined with respect to intake above homeostatic need, and this has not been consistently and precisely measured or shown. When comparing obese individuals with and without binge eating disorder (matched for BMI) using an operant task those with and without BED “worked” for the same amount of food when hungry [5]. When fed, the obese without BED reduced their “working for food” by 30% while those with BED appeared sensitized and increased responding for food by 40%. Therefore, there are individuals whose obesity is not “driven” by hyperphagia under all circumstances. Nevertheless, “working for food” is just one parameter of potentially measuring food addiction.
Another study demonstrated a relationship between psychoactive “drug” effects of a high energy dense food and reported desire to consume more of that food in a subject pool of mostly lean individuals suggesting that potential addiction to food can be dissociated from weight gain and not necessarily lead to obesity [6]. A few methodological challenges to address here include the fact that several studies on human subjects rely on self-report to assess a subject as satiated. Furthermore, it is not a given that eating in response to energy needs does not carry any hedonic component; hedonic vs. homeostatic may be a distinction rooted in academic tradition but could be a more complex and integrated process in reality. In any case, the question of homeostasis should be brought to bear in relation to hyperphagia. There is clearly a need for more precise work in this area. One example of a study trying to distinguish between homeostatic and hedonic eating reported that levels of ghrelin and endocannabinoids are activated by hedonic eating in satiated subjects, suggesting that endogenous signals of reward are linked to hedonic but not homeostatic eating [7].
When discussing whether hyperphagia that leads to obesity is an addictive behavior, it is helpful to compare the neurobiological mechanisms that respond to both palatable food intake and drugs of abuse. The primary pathway that responds to both food and drugs of abuse is the midbrain dopamine system, particularly in the nucleus accumbens (also referred to as the ventral striatum). These neurons have been repeatedly shown to release dopamine in response to almost all drugs of abuse [8, 9]. Food intake has shown a similar effect on dopamine but the impact is 3 to 5 times lower compared to drugs of abuse [10–12]. At the same time, withdrawal from drugs like morphine, alcohol, and psycho-stimulants leads to specific withdrawal symptoms that are associated with an aversive state and attenuated dopamine release while chronic food deprivation has the same effect [13–15]. However, the effects of the dopamine system on eating also involve multiple other neurochemical pathways including the opioid system [16–18], the cannabinoid receptors [19–22], and effects on nicotinic receptors [23, 24], all of which also respond to drugs of abuse. This large overlap between the action of abusable drugs and food necessitates the exploration of possible mechanisms that may lead to food becoming an abusable substance.
A neurobiology similar to drugs of abuse indicates that sensitization to food reward is also a possibility
Sensitization refers to the amplification of an effect from the same amount of the drug with intermittent repeated exposure to the drug. Food-induced behavioral sensitization has been shown in food-restriction rodent models [25]. In a study of overweight and obese children, a certain sub-population of the children had a sensitized response to palatable food prior to habituation to the food. This may indicate that at least a portion of the population is susceptible to food sensitization similarly to that seen with drug abuse [26]. In a second human study, Davis et al. found that obese humans had a higher sensitivity to reward than normal weight controls. However, in this study they also found that this sensitivity was linked to a depressive (or anhedonic) state indicating that other alterations in dopamine signaling may be occurring [27]. Cross-sensitization occurs when repeated exposure to one drug causes increases in a specific behavior following exposure to a second drug. In a model of intermittent access to sucrose, cross-sensitization to amphetamine occurs following three weeks of intermittent access to a 10% sucrose solution [28, 29]. Taken together these studies indicate that food can have similar sensitization effects as drugs of abuse. Therefore, based on the similarities between the neurobiological and basic sensitization responses to food and abusable drugs, a closer look at how food as an addictive substance fits into the current clinical definition of substance dependence is warranted.
In elucidating the similarities in the neurobiology of drugs of abuse and palatable food, it is perhaps useful to point out that dietary obesity may be linked to defects in systems other than reward circuits that may work synergistically or independently to induce hyperphagia. Some obese persons may apparently eat more because of specific defects in satiation or hunger, such as the high prevalence of melanocortin-4 receptor mutations leading to lack of satiation [30] or polymorphisms in the leptin, leptin receptor gene or CCK gene resulting in changes in snacking frequency or meal size [31]. Furthermore, obese people may have altered sweet taste hedonics [32] or feature reduced sensory thresholds for sweetness [33], suggesting that they may regulate reward by increasing intake in order to arrive at the same total reward. Interestingly, such a taste defect could work synergistically with reward deficits. Such examples indicate that alterations in reward per se are not necessary to cause obesity, although they may be sufficient in the majority of the population. This in turn suggests that analysis of individual cases rather than populations is required to establish whether food addiction contributes to obesity and introduce a therapeutic intervention.
Definition of Addictive Disorders
The DSM-IV categorizes disorders related to the use of a drug of abuse, the side effects of a medication, and toxin exposure as substance-related disorders. Substance dependence or, simply, addiction is the maladaptive pattern of substance use, leading to clinically significant impairment or distress, as manifested by three (or more) of the seven-point criteria, occurring at any time in the same 12-month period. Repetition in many behaviors can cause physiological alterations in the brain that parallel changes in drug dependent individuals [1, 34]. Hyperphagia could be profiled as one such behavior. Ifland proposed that consumption of refined foods such as sugar, flour, salt and certain fats parallels the criteria defined for addiction in DSM-IV [35]. According to Holden, overeating does appear to be an addiction potentially governing a person’s life [36]. Below we explore how each individual criterion for substance-dependent disorders applies to the case of food addiction.
Criterion 1: Tolerance, as demonstrated by a need for markedly increased amounts of the substance to achieve the desired effect or a markedly diminished effect with continued use of the same amount of the substance
From a neurobiological perspective, the development of tolerance is at least partially due to the down regulation of central dopamine signaling as the result of repeated exposure to substances that activate this pathway. Attenuated dopamine signaling has been shown in individuals who are addicted to opiates, alcohol, cocaine and methamphetamine [15, 37–39] [9] and others. In addition, by increasing D2 receptor density in the nucleus accumbens, researchers have been able to decrease the self-administration of alcohol and cocaine in rodents [40]. In obesity, several studies have shown that depletions in dopamine signaling are present in animals and humans that are hyperphagic. In animal models, we have shown that dopamine signaling is diminished in the nucleus accumbens of a number of genetic and dietary models of obesity [41–44]. In humans, lower levels of D2 receptor density in the striatum are correlated with increased BMI [45].
The transgenerational effects of obesity indicate that mechanisms that might result in hyperphagia are passed from parents to their children. Genetic studies have shown that the Taq1 A1 allele of the DRD2 gene which codes for the D2 receptor has been shown to be linked to diminished reward in alcoholics and has recently been shown to be associated with obesity [46, 47]. Furthermore, maternal diet studies have shown that mothers that have been fed a high-fat diet produce offspring that are also hyperphagic [48–50]. While the mechanisms that drive this hyperphagia are still unknown, it is possible that the obesogenic maternal environment inhibits the development of the central dopamine system of the offspring causing them to consume more to receive the normal rewarding levels of food.
Indirect evidence points to the development of tolerance in humans. Epidemiological studies indicate that the rise in obesity rates is linked to the rise in the per capita consumption of proposed addictive foods such as refined carbohydrates [51]. This association may mean that dietary obesity results in increased intake because the effect of the rewarding properties of these foods has decreased. More direct evidence of the development of tolerance in humans has also been shown. In a double-blind, randomized study of overweight women, the authors reported a reduction in the intensity of mitigating negative emotions over repeated consumption of the same quantity of the carbohydrate rich meal [52]. However, this was a self-report study. More quantitative studies are needed to determine the extent to which tolerance to the rewarding effects of food develops in such patients.
Ingesting larger quantities of particular foods than was intended is considered a form of compulsive behavior known as bingeing and has been shown to be a consistent self reported trait amongst a group of overweight/obese subjects [35]. One could argue that a mismatch between the intention to consume a particular portion size and the actual ingestion of a bigger portion size could be partly explained by defects in satiation and/or desensitization to the reward value of certain foods. Both mechanisms would be the closest to the tolerance effect observed with drugs of abuse. Common binge foods reported in the literature are typically energy dense, highly palatable foods rich in sugar and/or fat [35, 42, 53, 54]. The underlying neuronal as well as behavioral patterns with bingeing of such foods have shown parallel patterns to drugs of abuse in animals [53, 55]. An extension of this association is the observation that binge eaters have a recurrent release of DA in the brain indicative of tolerance as in drug dependence [56]. Behavioral signs triggered by bingeing also coincide with signs of drug tolerance as demonstrated in rat models where increases in sugar intake are seen as early as during the first hour of feeding [57] and followed by subsequent larger meals [54, 57, 58]. Similar observations on binge properties of fat have also been reported [53]. In obese non-binging patients, the mismatch between intention and ingestion could be less pronounced, yet still sufficient to lead to chronic tolerance of increased palatable food intake and weight gain [5].
Behavioral sensitization to a substance and the development of tolerance seem to be contradictory characteristics of addiction since sensitization refers to the enhancement of the behavior associated with intermittent exposure to a substance and tolerance refers to the need to take more of a substance to get the same effect. However, in a system where dopamine is depleted due to repeated exposure to food and the development of tolerance, behavioral sensitization might also be seen because it may require less dopamine release to bind super-sensitive postsynaptic receptors and initiate the behavioral response to dopamine activation. Alternately, there may be a time-dependent adaptation in the central dopamine system of a hyperphagic individual where there is initially increased sensitivity to the reward, but over time and continued exposure, the system is blunted resulting in the development of tolerance. While recent evidence indicates that in rodents predisposed to dietary obesity, the dopamine signal is diminished very early in life [42], normal weight animals exposed to high-energy food as adults also developed hyperphagia and the same central dopamine deficit [41].
Criterion 2: Withdrawal, as manifested by either a characteristic withdrawal syndrome for the substance or consumption of the same (or a closely related) substance to relieve or avoid withdrawal symptoms
Withdrawal syndromes vary between different drugs of abuse, but it is often characterized by psychological changes including increases in anxiety and aggression along with physiological changes such as a drop in body temperature following the removal of the addictive substance. Reports on neurochemical changes in animals associated with withdrawal from a high sugar [53] and high-fat diet [59] being analogous to those induced by withdrawal from drugs are convincing. In addition, in obesity-prone rats given free choice of high-fat/high-sugar food versus normal lab chow anxiety was increased compared to obesity-resistant controls following the removal of the palatable food [60]. Psychological expressions of withdrawal such as depression, anxiety, and agitation were reported by human subjects who attempted to restrict/limit intake of refined foods [35]. Some suggest psychological withdrawal is more prominent than physical withdrawal in case of food addiction [35], while others believe that eating itself does not produce withdrawal effects in humans [61]. Withdrawal symptoms have not been systematically studied in obese humans who have reduced their intake of highly palatable foods.
Criterion 3: The substance is often taken in larger amounts or over a longer period than was intended
As described in Criterion 1, binge eating is when a person consumes more food than they normally would in a short period of time. After periods of abstinence from sugar, rats seem more likely to increase consumption after sugar becomes available again, as compared to before the sugar was abstained [29].
Associations from human population wide data between fast food and soft drink consumption and rising rates of obesity are strong [35]. Escalations in the per capita consumption of currently proposed addictive foods like refined carbohydrates in the last few decades have been significant [51]. Further, most studies on portion size and energy intake document that when larger portion sizes are presented to individuals, they consume more energy [62]. In the current food environment, the ease of access to food also appears to be a contributor of energy intake, with greater availability resulting in greater energy consumption [63]. Large portions and easy access to food are a particular problem when eating out because restaurant meals typically combine the problems of large portion, high energy density, and variety [64]. When restaurant entrées feature a reduced energy density, overall energy intake is smaller [65].
Both non-obese and obese humans report that variety [66], palatability [67, 68] and taste preferences are major impediments to adoption of healthier eating habits. It should also be noted that overweight and obese individuals typically show preferences for high fat and high energy density foods [69, 70] and that the psychological construct disinhibition [71] (which is an indicator of the extent to which individuals are willing to overeat highly palatable foods opportunistically) is one of the strongest predictors of BMI and weight gain [72]. Additionally, there is a positive association of dietary variety with body fatness when the variety comes from high-energy density items, but a negative association when the variety came from low-energy-dense items (especially vegetables) [64].
Children and adults appear to react differently to variety as sensory-specific satiety is primarily product-specific in children, whereas in adults it is transferred to uneaten foods with sensory characteristics similar to those of the consumed food (such as “sweet”, “sour” and “fatty”), which are all less liked post-meal [73]. Similar differences between children and adult are reported for sensory-specific desire and this might have implications for planning meal compositions for children versus adults.
Criterion 4: There is a persistent desire or unsuccessful efforts to cut down or control substance use
An important question when considering the potentially addictive properties of food is whether the concepts of self-control and relapse are relevant to obesity. Is the cycle of abstinence, withdrawal, and reinstatement that characterizes drug relapse analogous to the occurrence of yo-yo dieting? Dieting is a pervasive phenomenon in the United States for at least a few decades now, however a significant proportion of the population struggles with weight loss and long-term weight maintenance [74, 75]. Over 70% of adults in the U.S. are estimated to use dieting strategies such as exercise, decreased fat intake, reduced meal size and reduced caloric intake at least once in four years [76, 77]. Weight cycling, or repeated episodes of dieting and weight loss followed by weight regain, increases the risk of overeating and obesity and is associated with significant psychological distress, morbidity and mortality [78–80]. Important predictors of weight relapse, usually defined as a weight regain of ≥ 5%, include an inability to successfully maintain weight loss over extended periods of time (> 1–2 years), high levels of dietary disinhibition leading to loss of control over eating, and high levels of depression [81]. Of clinical concern, rarely do individuals recover from weight relapse, even when considering those individuals who regain minor amounts of weight [75]. These unsuccessful attempts to control overeating, despite an individual’s persistent desire to diet and lose weight, parallels chronic relapse observed in addicts. With reason, investigators are continuing to advance the hypothesis that highly palatable foods, particularly processed foods “fortified” with refined carbohydrates, refined sweeteners, fat, salt, and caffeine, have addictive qualities [35].
With regard to the issue of diet control and weight relapse, a significant amount of research has focused on restrained eaters, or chronic dieters who are exaggeratedly preoccupied with body image, voluntary restrict food intake, and exhibit paradoxical counter-regulatory (disinhibitory) eating behavior in response to aversive contexts [71, 82–84]. Interestingly, there are a number of shared behavioral characteristics between individuals who suffer addiction and individuals who exhibit dietary restraint and a high tendency towards disinhibition, two factors that significantly elevate risk of obesity [85]. For instance, negative affect (e.g. anger, anxiety, depression), stress, and drug-related cues are only a few of many factors documented to trigger addiction relapse in humans [86–88] and in animals [89]. Correspondingly, copious studies have documented situational risk factors that reduce the ability of restrained eaters to control eating behavior, leading to overeating, including high calorie food pre-loads, alcohol, cognitive load, stress, emotional suppression or distress, and negative affect [82, 83, 90–101].
Among the most notable features of addiction is the sense of intense craving, which has been linked with anticipation of reward and preoccupation with a substance in models of relapse [102]. This approach may contribute in understanding why some individuals have difficulty self-regulating eating behavior. Anticipatory, or “appetitive,” reward refers to one’s expectation of the rewarding effects of a substance, as opposed to the reward experienced after the actual receipt of a substance. Repeated use of a substance increases the individual’s anticipation, craving, or “wanting,” of the substance as well as the motivation to seek out and take drugs of abuse. Supportive preclinical and human studies of addiction have demonstrated alterations in dopamine response patterns from the prospect of receiving a reward, such that dopamine release increases in the anticipatory phase before a substance is consumed [103, 104]. Likewise, animal studies have demonstrated increased dopamine response in anticipation of natural rewards, including novel food items [105, 106] and sucrose [107], providing support for shared neural substrates for food and drug craving.
Intense cravings for palatable foods and the tendency towards overeating are hypothesized to be cue controlled [108–110]. The cue reactivity paradigm, which has frequently been used to study neural and behavioral responses of addicts, has consistently shown that drug, drug-paired, and withdrawal-paired cues elicit cravings and often reinstate addictive behavior [111, 112]. For instance, withdrawal from cocaine is associated with subjective reports of craving that can be exacerbated by drug-related environmental cues [113]. Likewise, it has been shown that deprivation of a palatable food (e.g. chocolate) increases craving and overconsumption of food in restrained, but not in unrestrained, eaters [114, 115]. Moreover, human neuroimaging studies have demonstrated that cravings for food and drugs share neural substrates, including the hippocampus, insula, and caudate [110]. Additionally, obese subjects show selective activation within the dorsal striatum in response to pictures of high-caloric foods [116], the same region involved in cue-induced drug craving [117]. Obese individuals, when presented with highly palatable foods, demonstrated increased activation of the limbic system, including the orbital-frontal and prefrontal cortices, anterior cingulate cortex, insula, striatum, hippocampus, and amygdala [118]. Similarly, obese participants showed greater activation in the gustatory cortex and somatosensory regions in response to both anticipation and receipt of a milkshake. Participants showing the greatest activation in anticipatory and consummatory reward gained significantly more weight at a 1-year follow up [119]. Accordingly, it has been proposed that variations in reward processing differentially influences self-control, whereby heightened reactivity in response to tempting foods may be a risk factor for overeating and obesity [59, 119–121] [110, 122].
One might wonder how, in addition to food preferences, associations between highly palatable food cues and reward processes persist and increase the risk for overeating and obesity. Why do cues for pizza and milkshakes trigger cravings? Why are these cravings irresistibly intense for some individuals and not others? In drug addiction, it is hypothesized that the intensity and persistence of drug cravings associated with relapse involve substance-induced neuroadaptations in reward-related learning and memory processes, particularly in the mesocorticolimbic dopamine system [123]. Drug-seeking and drug taking are theorized to be motivated by expectancy-based associative learning, a process that sensitizes the brain reward pathways to trigger cravings in response to cues [124]. Briefly, the conditioning process entails the repeated pairing of a rewarding substance (unconditioned stimulus, UCS) with a neutral set of environmental stimuli (conditioned stimulus, CS), which will result in the latter acquiring the motivational properties of UCS, thus serving as a cue. In the brain, this association induces a cascade of biological events that initiate plasticity, via modified gene expression, in brain reward systems so that connections between these pertinent networks of neurons are sensitized to substance-associated stimuli [125, 126]. For instance, as a result of learning-induced plasticity, ventral tegmental dopaminergic neurons will show a greater response to a conditioned stimulus than to the reward itself once a predictable association is formed, indicating a stable shift in reward pathway activity as a result of learning [127]. This evidence strongly suggests that, after repeated exposure to palatable foods, the hedonic value ascribed by anticipation, or subjective “wanting” of a palatable food, may result in cravings.
Furthermore, mounting evidence indicates that chronic drug use results in aberrant reward processing due to long-term changes in dopamine-regulated signaling. Neuroplasticity factors located in striatal regions that are implicated in these drug-induced neuroadaptations include cyclic AMP response binding protein (CREB), brain derived neurotrophic factor (BDNF), delta FosB, and extracellular signal-regulated kinase (ERK), among others [123, 128]. For instance, repeated exposure to cocaine, morphine, alcohol, and tetrahydrocannabinol (THC), robustly induced the accumulation of the transcription factor delta FosB in the nucleus accumbens core and caudate putamen of rats, which is associated with down regulation of dopamine signaling [129, 130]. Correspondingly, if compulsive overeating is an addictive disorder or if particular foods have addictive qualities, it is likely that alterations of neuroplasticity factors associated with dopamine in striatal reward regions would be observed in response to overeating or particular qualities of food.
Importantly, several lines of evidence are supportive of the notion that palatable food exposure and food-motivated experiences can induce enduring addiction-like brain-based changes that increase the risk for overeating. For instance, acute early-life exposure of mice to a palatable high-fat diet induced neuroadaptations in dopamine signaling that persist to adulthood, including significant alterations in phospho-dopamine, CREB, delta FosB, cyclic adenosine monophosphate regulated phosphoprotein with a molecular mass of 32 kDA (DARPP-32), and cyclin-dependent kinase 5 [131]. Additionally, overexpression of delta FosB in bitransgenic mice has been shown to augment food-reinforced instrumental performance and progressive ratio responding [132], as well as to downregulate levels of dopamine, phosphorylated CREB, BDNF, and DARPP-32 in the nucleus accumbens [129]. Importantly, the biochemical effects of delta FosB overexpression are normalized after chronic exposure to a high-fat diet [129]. In addition, inhibition of protein kinase A or protein synthesis has been shown to interfere with food-motivated instrumental responding [133, 134], and the presence of a conditioned cue signaling food reward has been shown to increase ERK2 activation in the nucleus accumbens, while inhibition of ERK2 disrupts food reward responding [135].
In sum, these data indicate that food and food-cue induced alterations in gene expression within reward neurocircuitry have a similar impact on incentive-motivational effects as drugs of abuse. However, despite the distinction between “liking’ and wanting” at the behavioral and physiological level [136–141], one should note that to-date there has not been decisive evidence that food and drug “liking” are processed by brain mechanisms distinct from those processing food and drug “wanting”. Reduced “liking” and “wanting” of low-calorie foods in normal rats after chronic high-fat feeding suggest a role for ontogenetic effects of the obese state on reward functions, while similar differences between inbred obesity-prone and resistant rats prior to obesity suggest a genetic component [142].
Criterion 5: A great amount of time is spent in activities necessary to obtain the substance (e.g., visiting multiple doctors or driving long distances), use the substance (e.g., chain-smoking), or recover from its effects
The first part of this criterion may not apply well to food related addiction since availability is not a limiting factor in our current food environment [35]. However, the association between overeating and drug addiction can be employed in our effort to understand food-seeking behavior. As compared to non-dieters, dieters have demonstrated a significant increase in the number of food and dieting-related thoughts [143]. Thoughts of food and fantasizing about favorite foods, especially while dieting (refraining from specific foods) increase in individuals with increasing BMIs. This is seen most often in those who have tried multiple times to lose weight, or go through weight cycles [144, 145].
What could explain the increase in food seeking behavior? In the United States between 1970 and 2000, the percentage of dietary energy coming from fat decreased, while rates of obesity rose dramatically [146]. This suggests that there are factors other than fat consumption, which have contributed to the trend of spending more time seeking or thinking about palatable food. Recent short-term studies have shown that consumption of foods that are high on the glycemic index (GI) (foods that take a shorter time to digest) may increase hunger and encourage overeating, as compared to lower GI foods. Increased consumption of high GI foods leads to less satiety, and therefore an increase in overall caloric consumption from high GI foods. Relating this to Criteria V, we can conclude that by consuming foods that are less filling, more time is spent obtaining food, and eating it to achieve the same level of satiety as consuming low GI foods.
Fiber is one example of a low GI food that is consumed much less today than in the mid 20th century [147]. Refined sugar consumption increased in the later part of the century, and fiber intake remained low. Recent studies have shown that individuals who consume diets with high amounts of fiber have lower waist circumference and lower BMI than those with decreased amounts of fiber in their diets [148]. This is significant, again because it leads to the conclusion that because a diet low in fiber is less filling (low GI) the individual has to seek other sources of food to feel satisfied.
Additionally, exposure to “high risk” foods, such as those high in fat and carbohydrates, induce cravings and time spent seeking palatable foods. In an obesogenic food environment, cravings are increased due to repeated exposure to these food options. In addition, studies have shown a higher response to external food exposure and higher cravings in women than in men [149]. Women had an increased reaction to environmental food cues, including the sight, smell and anticipation of food, which led to cravings, specifically for high fat foods [149]. The high fat in ketogenic diets and the induction of a starvation-like state due to low glucose may also increase food seeking and food-centered behavior and may partly explain the lack of success of low-carbohydrate diets for certain individuals in the long term.
Finally, the inverse relationship of alcohol dependence and BMI may be further indicative that perhaps food seeking could actually compete with drug seeking [150]. Reports of alcohol abuse or dependence after bariatric surgery [151] also reinforce this notion.
Criterion 6: Important social, occupational, or recreational activities are given up or reduced because of substance use
Social isolation is frequently seen in overweight/obese individuals of all age groups [152–154]. Additionally, the proposition that binge eaters socially marginalize themselves comes from the association between stigmatizing events, psychological distress and binge eating [35].
The social stigma associated with obesity further contributes to social isolation. Particularly young professionals who are committed to a healthy lifestyle and exercise are likely to possess negative associations and bias toward obese individuals [155]. These attitudes are very hard to reverse [156]. Anti-fat bias and weight discrimination may contribute to unhealthy lifestyle behaviors and reduced quality of life for many obese individuals who are at high risk for chronic disease. Furthermore, many aspects of today’s environment are non-friendly for obese individuals with frequent incidents related to size of chairs in public places, airplanes and the workplace, perception of obese individuals as lazy etc. All these factors and stereotypes may encourage the withdrawal from socially acceptable and rewarding activities in personal and professional life.
Criterion 7: The substance use is continued despite knowledge of having a persistent or recurrent physical or psychological problem that is likely to have been caused or exacerbated by the substance (e.g., current cocaine use despite recognition of cocaine-induced depression, or continued drinking despite recognition that an ulcer was made worse by alcohol consumption)
The persistence of behaviors associated with hyperphagia and weight gain is convincingly reflected in the systematic failure of commercial weight loss programs to produce sustained results. Although millions of Americans are enrolled in such regimens on a daily basis, these programs are typically associated with high costs, high attrition rates, and a high probability of regaining 50% or more of lost weight in 1 to 2 years [157].
Compliance to dietary advice for weight management is overall quite poor [35, 158]. People are aware of the health- related problems such as cardiovascular disease, diabetes mellitus that come with obesity. Besides, the quality of life is also adversely affected with weight gain and in extreme cases it also affects activities of daily living. Even if this involves medical advice to lose weight, compliance has been shown to be unsatisfactory. Studies have reported that the mean daily energy exceeded the prescribed diet by 40–50% in obese diabetic subjects [159]. It seems that most people are unable or do not wish to give up preferred foods [160]. This pattern can also relate to the availability and low cost of palatable high-energy food.
Additionally, weight loss maintenance rates are low. Once a significant amount of weight has been lost, the 6 months after the weight loss is the most likely time for the person to regain the weight [161]. However, subjects who received post-diet support through internet groups or in-person support sessions regained less weight than those who did not receive support.
Overweight individuals who undergo bariatric surgery also face challenges after the surgery to follow a stricter diet and food intake regime. Behavioral non-compliance rates of postoperative patients have been reported high—with lack of exercise and snacking as the top two behaviors of non-compliance [162]. The patients who underwent treatment for obesity were fully aware of the implications of staying at an unhealthy weight, and of increased risks resulting from non-compliance after the surgery. Furthermore, patients with a substance abuse history appear to be those who moderate their eating the most and lower their BMI following bariatric surgery [163]. However, compliance with the postoperative regimen appears to be counteracted by an increased risk of addictive behaviors that complement hyperphagia [151, 164].
The preponderance of the evidence from animal and human literature suggests that common dietary obesity satisfy all DSM criteria for an addictive disorder (table 1). Although there are obese individuals who can gain weight through defects in satiety and genetic predisposition alone (i.e. congenital leptin deficiency), they do not seem to represent the vast majority of obese patients; and, although, there are eating disorders where the addiction potential for food is greater than that of common dietary obesity, the average rate of hyperphagia in most obese patients is sufficient to induce significant weight gain; only a moderate increase in daily caloric intake would be enough for such an effect over time. The addictive potential in obesity is most likely mediated by the blunting of the CNS response to palatable food through the midbrain dopamine pathways, which induces an increase in food intake to compensate. However, there is still a relative paucity of studies on whether and how the obese brain could be sensitized to palatable food, on whether “liking” and “wanting” is a meaningful distinction at the neuroanatomical and neurochemical level and on the role of cue-induced learning in obesity. Further methodological questions relate to whether hyperphagia and hedonic eating should be defined as distinct from homeostatic eating that strictly addresses the need for energy balance or constitute parts of the same integrating process.
Table 1.
Common Dietary Obesity and DSM IV Criteria for Addictive Disorders. Three of the 7 criteria need to be met for diagnosis.
| DSM IV Criteria | Animal Model | Humans | Evidence | |
|---|---|---|---|---|
| 1 | Tolerance | √ | √ | Food Binging, Hyperphagia, Delayed Satiety |
| 2 | Withdrawal | √ | √ | Hypofuctioning Brain Dopamine System, Opiate Withdrawal-Like Symptoms, Psychological and Physical Dependence |
| 3 | Use more than intended in longer periods of time | √ | √ | Hyperphagia, Change of Eating Patterns and Meal Frequency (Snacking), Negative Experience Triggers, Cue- Induced Behaviors, Larger Portion Size, Proximity to Food Sources, Lower Cost of High- Energy Foods |
| 4 | Attempts to cut back | √ | Dietary Restraint Participation in Weight Loss Programs |
|
| 5 | Spend time in the pursuit/use/recovery of the substance | √ | √ | Anticipation and Preoccupation, Cravings, Food Thoughts, Increased Brain Dopamine Levels in Response to Anticipation and Consumption, Negative Experience Triggers, Cue-Induced Behaviors, Change of Eating Patterns and Meal Frequency, Increase in Habitual (vs. Physical) Hunger |
| 6 | Missed important activities | √ | √ | Social & Occupational Activities Given Up, Social Marginalization, Psychological Distress, Discrimination |
| 7 | Persistent behavior in spite of knowledge of consequences | √ | √ | Lack of Diet Compliance, Failure to Achieve Long-Term Weight Loss, Hyperphagia Resistant to Aversive Cues |
Potential Policy Effects of Re-classification
As obesity-related health issues increase around the world, no nation has yet effectively managed to bring the obesity epidemic under control. By using what we have learned from public health triumphs of the past century such as school vaccinations, smoking bans, and seatbelt regulations, government regulation and legislation seem to be key players [165]. Policy actions that have been suggested include: advertising and marketing pressure, changing the image of healthy foods, ensuring access to and availability of healthier food, school and worksite initiatives to create a supportive environment, economic benefits/subsidies that facilitate healthier food choices; heavy taxation on unhealthy foods, reduction of fat, free (particularly added) sugars and salt in manufactured products, and strict nutrition labeling regulations. Based on the number of different levels and organizations that would be involved in implementing policy changes this widespread, one theme that is apparent is that the action needs to be intersectoral requiring strong internationally coordinated efforts.
While our review of the literature indicates that common dietary obesity could be the result of a person’s addiction to high-energy palatable foods, policy recommendations for obesity and non-communicable disease prevention from both the CDC and the WHO that have been published in the last decade do little to take this into account. The WHO strategy was published in 2004 and is a broad overview of how member nations can work to address the increased burden of non-communicable diseases in cultures where unhealthy diets, poor overall nutrition and physical inactivity have become the norm. In these guidelines, nothing is said about the potential that an unhealthy diet may not be easily changed due to addictive aspects of hyperphagia. Furthermore, while they do recommend using interventions like taxation on high-energy food, they do not discuss more strict strategies like limiting the access of children to such food [166]. In the CDC recommendations, published in 2009, they hint at the need to protect the public from unhealthy foods including a subset of strategies designed to support healthy food and beverage choices. This strategy includes reducing access to the unhealthy foods through removal from schools and public facilities, decreasing portion sizes and limiting availability of sugar-sweetened beverages [167]. However, the assumption here is that by simply changing the ratio of healthy and unhealthy food, people will eat the healthy food. Unfortunately, it is clear with drugs of abuse that limiting access to an abusable substance does not inhibit the abuser from finding ways to acquire that substance. Therefore, if excessive intake of certain foods were the result of an addictive behavior, limiting access to high-energy palatable food would not be enough to provide protection against the development of obesity. Alternative policies would need to be put in place to discourage consumption of high-energy foods.
One area of policy-making that we may garner insight from to address addictive behavior associated with obesity involves the curbing of tobacco usage and, consequently, nicotine addiction. It is widely accepted that addiction to smoking results from nicotine dependence, and not other ingredients in cigarettes, and that prolonged tobacco usage has detrimental health consequences, including increased risk of cancers, cardiovascular disease, respiratory disease, infectious diseases, as well as reproductive complications. The arduous campaign to end smoking was set in motion in 1952, when studies first began linking smoking to lung cancer [168], leading to a steady decline in the rate of smoking in the US, from 42% in 1965 to approximately 21% at present [169]. After decades of litigation and regulatory efforts, economic strategies, educational approaches, and clinical interventions, the Center for Disease Control (CDC) deemed the war on tobacco to be one of the greatest public health achievements in the United States. With this accomplishment, obesity researchers are placing a strong emphasis on identifying the successful elements of tobacco policy, as well as understanding how lessons learned from failures may inform innovative strategies to combat common dietary obesity [170, 171].
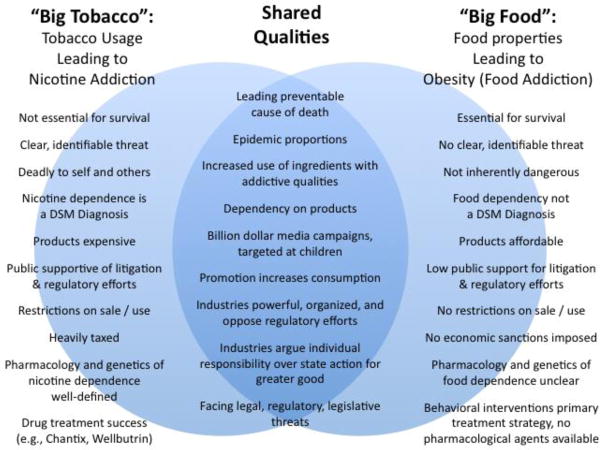
A number of recent reviews have compared and contrasted the tobacco and obesity epidemics, focusing on shared features that may provide clues to how we can control food addiction and dependence (Figure 1) [168, 170–175]. First and most certainly, obesity and tobacco usage are both leading causes of preventable death. Based on nationally representative health surveys and disease-specific mortality statistics in 2005, tobacco smoking was responsible for an estimated 467,000 deaths, or 1 in every 5 individuals, and overweight and obesity were responsible for an estimated 216,000 deaths, or 1 in every 10 individuals [176]. Worse, researchers estimate obesity will soon overtake tobacco use as the leading cause of death, likely because: (1) the public largely does not perceive a significant threat [174], (2) there is low public support for policies aimed at curbing obesity (e.g., reducing television watching, taxing sugar-sweetened beverages [174], (3) there is skepticism owing to the stigmatization of obesity as a personal weakness or moral failure [177], and (4) the rate of adults receiving primary obesity prevention is very low (2.6%) [178].
Figure 1.
Venn Diagram comparing tobacco and food industry and regulatory policy.
Secondly, both “Big Tobacco” and “Big Food” are lucrative and efficient industries that are global in scope and have significant political sway [168, 175]. These industries rely on similar tactics to redirect blame, such as emphasizing personal responsibility, spreading misinformation and doubt, and employing lobbyists, lawyers, and trade organizations to resist state regulation [168, 172]. For instance, support for the regulation of food is complicated by the arguments, heavily endorsed by the food industry, that personal weakness and deficient levels of physical activity are accountable for the obesity epidemic, and that there are no good or bad foods, creating doubt that changes should be made to food products [168]. In all likelihood, in consideration of the idea that certain types and amounts of food are addictive, the food industry would respond by emphasizing individual restraint and funding research that would create doubt about the plausibility of food addiction, among other similar tactics used by the tobacco industry. This underscores the necessity for funding food dependency awareness campaigns and anti-obesity messages to educate the public, and to establish programs to prevent or control obesity.
Lastly, the tobacco and food industries share a history of investing heavily in media campaigns that influence product preference and choice, and lead to increased sales and consumption [170]. The Federal Trade Commission (FTC) Cigarette Report (issued in 2009) indicates that the five major cigarette manufacturers in the US spent in excess of $12.49 billion on advertising and promotion in 2006. Evidence has repeatedly shown that tobacco promotion fosters positive attitudes and beliefs towards tobacco use, and pro-tobacco marketing and media strongly increases the risk of tobacco initiation in children and adolescents [179–181]. Importantly, the tobacco industry now submits to considerable regulatory measures, which reduce the scope and amount of tobacco advertisements (e.g., banning television and radio ads), limit the sale of tobacco products to adults 18 years and older, and require warning labels on all tobacco product packaging [182]. Increasing evidence indicates similar regulatory measures would benefit the obesity epidemic. In the case of food, another FTC report (issued in 2008) indicated that 44 major food and beverage companies spent $1.6 billion in 2006 to advertise their products to children under 17 ($870 million on children under 12, and approximately $1 billion to adolescents aged 12–17), using a variety of mediums including television, internet, in-store advertising, and product packaging. Notably, numerous studies have demonstrated a positive association between food advertising and obesity [183–185]. For instance, the frequency of food advertising per hour is positively associated with the proportion of overweight children [183]. Further, exposure to food advertisements has been shown to increase consumption of energy-dense snacks in children regardless of weight status, though the greatest consumption was observed in obese and overweight children [185]. At issue, food products account for approximately 60% of television ads run during Saturday morning cartoons [186], and one study estimated that children are exposed to as many as 12 television food advertisements per hour; among the food advertised, the majority of products are energy-dense, nutrient-poor, and positively associated with obesity [175]. One may note that cue exposure increases risk of substance initiation, use and relapse in addiction. Anticipation for food reward, which is connected to palatable food preferences, can trigger cravings for food and lead an individual to eat energy-dense food, even when not hungry. Thus, following the same logic applied to tobacco advertising, it is highly plausible that limiting the exposure of children and adults to food advertisements depicting energy-dense foods, foods with sugar, fat, and salt additives, and foods with low nutritional value, would significantly reduce the rates of hyperphagia leading to obesity.
One additional strategy credited with tobacco control success is the levying of taxes upon tobacco products. According to the WHO, raising taxes is among the most effective measures to reduce the demand for tobacco products and to encourage individuals to quit [187]. Furthermore, these tax revenues can be applied to prevention and intervention programs, such as quit lines and clinical cessation services. Taxation, by increasing the expense of cigarettes, is particularly among groups with low purchasing power, such as adolescents and the poor, as these groups are more sensitive to changes in cost [187]. Contrary to cigarettes, the average cost of high-energy dense food is very low and availability is high, whereas the cost of healthier food, like fruits and vegetables, is high but availability is variable (e.g., supermarket deserts). One controversial movement gaining support calls for an increase of taxes on sugar-sweetened beverages, because these drinks are linked with weight gain, poor nutrition, and replacement of healthier calories with empty calories – all major factors contributing to the obesity epidemic [168]. Studies demonstrate that, like taxes on tobacco, high taxes on sugar-sweetened beverages decrease consumption. In fact, the trade publication Beverage Digest estimated in 2008 that a 6.8% tax increase on soft drinks produces a 7.8% drop in consumption, and a comprehensive review of 160 studies on price elasticity is supportive of these findings, estimating that an increase in soft drink price by 10% would produce a 8–10% drop in consumption, equating to about 1 penny per ounce [188, 189].
It is also vital to consider the numerous elements unique to the obesity epidemic that may require novel policy strategies. Most evident is the fact that food is essential for survival, whereas tobacco use is viewed as a dispensable, or even recreational, activity [168, 174]. It is also clear that food is not inherently dangerous, and thus there are no restrictions on the sale or use of food products. Another difference is vested in the fact that the term “food” encompasses an enormous number of products that are produced by thousands of companies around the world: ranging from small-time farmers to restaurants to multinational agribusinesses [168].
In the same vein, one of the greatest challenges to the food addiction/food dependence hypothesis is the ambiguity cloud surrounding what it is about food that constitutes a threat. Is dependence due to increasing caloric density of foods? Is it the unhealthful food additives, such as fat, salt or sugar? In contrast to the obesity epidemic, the threats associated with tobacco products (e.g., nicotine addiction, carcinogens) are clear cut, and so opponents of the tobacco industry can more easily frame the issue of threat to the public by advertising hazardous nature of tobacco products and the immediacy of harm from tobacco usage. In the case of obesity and food dependence, advances in scientific evidence are crucial. Expressly, an avenue of research critical for gaining public support of obesity policy is the identification of the precise dietary components, as well as the types and amounts of food, that can produce biochemical and behavioral changes specific to addiction.
Furthermore, research that clarifies the psychological, pharmacological and genetic bases of food dependency would improve drug treatment options for obesity. At present, a considerable amount of research has refined our understanding of the mechanisms underlying nicotine dependence, and as a result, a number of successful pharmacological treatments have been developed to control tobacco (e.g., varenicline, bupropion) [190]. On the contrary, anti-obesity drugs, such as orlistat, rimonabant, and sibutramine, have produced unexceptional results (placebo-subtracted weight losses < 5%), and trials have been plagued with high rates of attrition owing to psychiatric or gastrointestinal side-effects that cause subjects to discontinue these medications [191]. It is only in the case of binge eating disorder and anorexia that preliminary results indicate that drugs like baclofen and topiramate are more effective in reducing binge eating, craving and weight gain [192, 193]. A notable historical exception to the lack of results with anti-obesity drugs is a now controlled substance (d-amphetamine), which was used over the counter as weight loss medicine until the early 70s. Interestingly, amphetamine is a dopaminergic drug that induces massive DA release in the brain. Reframing the obesity issue from the addiction perspective may encourage the development of novel human and animal laboratory paradigms, which would provide mechanistic insight into how individuals can become dependent on food.
In order for policy changes to be effective, implementation of the policies must also be possible. The Policy Options for Responding to the Growing Challenge of Obesity Research Project (PorGrow) evaluates a broad range of policy options by exhaustive interviews with a wide range of stakeholders ranging from food industry representatives, journalists, caterers, consumer organizations etc. The need for ‘downstream’ interventions such as educational measures designed to enhance skills at the individual level to make appropriate health choices and to put them into practice was readily accepted. However, ‘upstream’ policy measures, designed to increase the opportunities to make healthier choices or restrict the counteracting influences, were not so readily accepted by some stakeholders, particularly those in the private sector [194]. Thus modeling the anti-tobacco framework, moving the onus from the individual to society, faces strong opposition from stakeholders.
Insurance companies play a large role in the creation of future policies to help curb the obesity epidemic; however, before suggesting changes to existing insurance policies, we must first look at current plans to understand what is offered now, and under what conditions. Our review of standard insurance plans offered in various parts of the country found that typically, insurance companies will help cover the treatment of those who are morbidly obesity, but have limited reimbursement policies available for prevention of obesity, weight management programs for overweight and obesity individuals, or maintenance programs for those of a healthy weight. Additionally, the standards for being considered for these types of programs to reduce weight are set very high—only those with a BMI above 40 will receive coverage for weight loss surgery or being enrolled in a weight loss program. In an analysis of plans in Pennsylvania, it was found that out of the 16 insurers who responded to the survey, all 16 would cover bariatric surgery if the BMI was high enough, and if co-morbidities were present. This same study found that 9 of the 16 insurers would cover individual dietary counseling, but the majority of these programs also required a co-morbidity to be present. The study found that very few insurers offered coverage of such services as physical activity programs, commercial programs, and group dieting counseling [157]. Another example of a standard insurance policy in Massachusetts offers an online service to help track weight and diet—but also requires a diagnosis of morbid obesity to be considered for further counseling and treatment.
By covering the cost of treatment of the morbidly obese instead of working on programs to cover costs of prevention to those who fall into the category of overweight or obese, insurance companies will end up paying more in the long run for additional treatments, as potentially more and more individuals fall into the morbidly obese category. As current studies show, the effect of modest weight loss drastically decreases co-morbidities in overweight and obese individuals. Insurance policies will need to be adjusted to accommodate patients by creating plans that support these modest weight-loss focused programs. Due to the personal nature of one’s weight and attempts at weight loss, insurance companies often hesitate to cover issues that are considered to be caused by an individual’s behavior. As more research is conducted on the causes of obesity, it is becoming clear that there are multiple levels of issues that lead to obesity. By taking a similar approach to this issue as tobacco or drug policies, it will become necessary to reconsider the criteria necessary for an individual to be considered for a weight loss program.
Conclusion
Through this review, we have identified and discussed the overlaps between drug use and overeating. In addition to the alterations in brain neurochemistry, we have also presented the external cues i.e. our food system/environment that are drivers for overeating. Evolutionarily we were not prepared to cope with the ready access to a wide variety of drugs [195]. Paralleling this to food and the historical outlook of the human species as hunter-gatherers, it is no surprise that struggles to cope with the ready availability and easy access to food have been largely unsuccessful. Just like drug abuse, obesity is a disease of civilization. We can perhaps draw lessons from the experiences of the tobacco industry. Smoking was subjected to culture and social pressures only when it was labeled a social illness moving the model of blame from personal to communal. The weighing in of scientific evidence was supported by media efforts to bring awareness among people forcing the tobacco industry to step down. These power plays between science and industry indeed can be applied to food addiction and its role in obesity. To our opinion, political parties and lawmakers in different countries often see obesity as a personal issue, or one linked to support or influence and feedback from the food industry. Science recognizes obesity as a multi-factorial complex disease and yet again scientific evidence often stands weak against the food industry [168].
Putting forth the other side of the story, there is no support from the human literature for the hypothesis of sugar addiction [4]. Quoting Benton, “if sugar addiction has played a major in the increase in obesity, fasting should increase food cravings predominantly for sweet items; cravings should occur after an overnight fast; withdrawal symptoms should prevent a decline in the preference for sucrose; the obese should prefer sucrose containing rather than other palatable foods or find sweetness particularly attractive; sucrose containing rather than other food items should predispose to obesity. These predictions have in common that on no occasion was the behavior predicted by the addiction model supported by human studies.” Additionally, the synopsis of a recent symposium on food addiction concluded that “even highly palatable food is not addictive in and of itself. Rather, it is the manner in which the food is presented (i.e. intermittently) and consumed (i.e. repeated, intermittent “gorging”) that appears to entrain the addiction-like process” [2]. Finally, there are indeed instances where persistently elevated dopaminergic stimulation promotes the development and maintenance of addictive behaviors, such as in Parkinson’s disease patients who show a significant vulnerability to gambling when under medication that upregulates central dopamine systems [196]. However, in view of the commonalities in the brain reward circuitries active on ingestion by drug and food, the drug addiction model presents new vistas for understanding the neurochemistry initiated by palatable high-energy food and how it is compromised when the drive to eat for reward overcomes the energy balance.
Overall, the thesis of our review is not that each and every obese patient is suffering from food addiction. It is rather that common dietary obesity satisfies the DSM criteria and, therefore, the potential for addictive behavior associated with food should always come into consideration by the treating physician or dietician. The response of the patient to such an approach would further determine whether the therapeutic intervention should primarily address addictive behavior or metabolic imbalances for successful long-term weight loss.
The last word of this article would have to address the future. We presented some, we believe, overwhelming evidence that obesity shares crucial characteristics with addictive disorders and should be classified as such as it appears to satisfy all relevant DSM criteria. However, whether this is going to happen in one of the future editions of the DSM is an open question. It is primarily the role of the psychiatric community to make the decision and their resistance to reclassification may not necessarily stem from lack of scientific evidence. Concerns about the dietary obese being stigmatized as “addicts” and about psychiatric care being overwhelmed by the addition of millions of new patients may feature prominently in the concerns of the clinical community and not unjustifiably. These issues are real and serious and should be addressed but maybe should not be left to stand in the way of the appropriate treatment of the dietary obese. At the very least, we propose that training of physicians, dieticians, nurses, physician assistants and other health care professionals who treat obese patients in Addiction Medicine should feature prominently in medical education priorities. Such training should also expand to include representatives of hospital administration and medical insurance. Literacy in addiction treatment is a prerequisite for the health care community to properly address obesity-related addictive behaviors regardless of DSM reclassification. As for the latter, only time and the evolution of the obesity epidemic will show whether any delay in classifying dietary obesity as an addictive disorder is a right or wrong decision.
Highlights.
DSM-IV Criteria 1–7 for addictive disorders are applicable to dietary obesity.
Reclassification of obesity would significantly affect the health care system.
Anti-tobacco policies can be evaluated and reapplied in the context of obesity.
Acknowledgments
Part of the experimental evidence presented in this article was made possible through the generous support of the National Institutes of Health (DK065872, ARRA 3R01DK065872, F31DA023760) and a Smith Family Foundation Award of Excellence in Biomedical Research (ENP).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Barry D, Clarke M, Petry NM. Obesity and Its Relationship to Addictions: Is Overeating a Form of Addictive Behavior? American Journal on Addictions. 2009;18:439–51. doi: 10.3109/10550490903205579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Corwin RL, Grigson PS. Symposium Overview. Food Addiction: Fact or Fiction? J Nutr. 2009 doi: 10.3945/jn.108.097691. jn.108.097691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Riva G, Bacchetta M, Cesa G, Conti S, Castelnuovo G, Mantovani F, et al. Is Severe Obesity a Form of Addiction?: Rationale, Clinical Approach, and Controlled Clinical Trial. CyberPsychology & Behavior. 2006;9:457–79. doi: 10.1089/cpb.2006.9.457. [DOI] [PubMed] [Google Scholar]
- 4.Benton D. The plausibility of sugar addiction and its role in obesity and eating disorders. Clinical Nutrition. doi: 10.1016/j.clnu.2009.12.001. In Press, Corrected Proof. [DOI] [PubMed] [Google Scholar]
- 5.Nasser JA, Evans SM, Geliebter A, Pi-Sunyer FX, Foltin RW. Use of an operant task to estimate food reinforcement in adult humans with and without BED. Obesity (Silver Spring) 2008;16:1816–20. doi: 10.1038/oby.2008.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nasser JA, Bradley LE, Leitzsch JB, Chohan O, Fasulo K, Haller J, et al. Psychoactive effects of tasting chocolate and desire for more chocolate. Physiology & behavior. 2011;104:117–21. doi: 10.1016/j.physbeh.2011.04.040. [DOI] [PubMed] [Google Scholar]
- 7.Monteleone P, Piscitelli F, Scognamiglio P, Monteleone AM, Canestrelli B, Di Marzo V, et al. Hedonic Eating Is Associated with Increased Peripheral Levels of Ghrelin and the Endocannabinoid 2-Arachidonoyl-Glycerol in Healthy Humans: A Pilot Study. The Journal of clinical endocrinology and metabolism. 2012 doi: 10.1210/jc.2011-3018. [DOI] [PubMed] [Google Scholar]
- 8.Koob GF, Bloom FE. Cellular and molecular mechanisms of drug dependence. Science. 1988;242:715–23. doi: 10.1126/science.2903550. [DOI] [PubMed] [Google Scholar]
- 9.Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A. 1988;85:5274–8. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wise RA. Brain reward circuitry: insights from unsensed incentives. Neuron. 2002;36:229–40. doi: 10.1016/s0896-6273(02)00965-0. [DOI] [PubMed] [Google Scholar]
- 11.Schultz W. Getting formal with dopamine and reward. Neuron. 2002;36:241–63. doi: 10.1016/s0896-6273(02)00967-4. [DOI] [PubMed] [Google Scholar]
- 12.Hernandez L, Hoebel BG. Food reward and cocaine increase extracellular dopamine in the nucleus accumbens as measured by microdialysis. Life Sci. 1988;42:1705–12. doi: 10.1016/0024-3205(88)90036-7. [DOI] [PubMed] [Google Scholar]
- 13.Pothos EN, Creese I, Hoebel BG. Restricted eating with weight loss selectively decreases extracellular dopamine in the nucleus accumbens and alters dopamine response to amphetamine, morphine, and food intake. J Neurosci. 1995;15:6640–50. doi: 10.1523/JNEUROSCI.15-10-06640.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pothos EN, Hernandez L, Hoebel BG. Chronic food deprivation decreases extracellular dopamine in the nucleus accumbens: implications for a possible neurochemical link between weight loss and drug abuse. Obes Res. 1995;3 (Suppl 4):525S–9S. doi: 10.1002/j.1550-8528.1995.tb00222.x. [DOI] [PubMed] [Google Scholar]
- 15.Pothos E, Rada P, Mark GP, Hoebel BG. Dopamine microdialysis in the nucleus accumbens during acute and chronic morphine, naloxone-precipitated withdrawal and clonidine treatment. Brain Res. 1991;566:348–50. doi: 10.1016/0006-8993(91)91724-f. [DOI] [PubMed] [Google Scholar]
- 16.Will MJ, Pratt WE, Kelley AE. Pharmacological characterization of high-fat feeding induced by opioid stimulation of the ventral striatum. Physiology & behavior. 2006;89:226–34. doi: 10.1016/j.physbeh.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 17.Zuberi AR, Townsend L, Patterson L, Zheng H, Berthoud HR. Increased adiposity on normal diet, but decreased susceptibility to diet-induced obesity in mu-opioid receptor-deficient mice. Eur J Pharmacol. 2008;585:14–23. doi: 10.1016/j.ejphar.2008.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Will MJ, Franzblau EB, Kelley AE. Nucleus accumbens mu-opioids regulate intake of a high-fat diet via activation of a distributed brain network. J Neurosci. 2003;23:2882–8. doi: 10.1523/JNEUROSCI.23-07-02882.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Need AB, Davis RJ, Alexander-Chacko JT, Eastwood B, Chernet E, Phebus LA, et al. The relationship of in vivo central CB1 receptor occupancy to changes in cortical monoamine release and feeding elicited by CB1 receptor antagonists in rats. Psychopharmacology (Berl) 2006;184:26–35. doi: 10.1007/s00213-005-0234-x. [DOI] [PubMed] [Google Scholar]
- 20.Duarte C, Alonso R, Bichet N, Cohen C, Soubrie P, Thiebot MH. Blockade by the cannabinoid CB1 receptor antagonist, rimonabant (SR141716), of the potentiation by quinelorane of food-primed reinstatement of food-seeking behavior. Neuropsychopharmacology. 2004;29:911–20. doi: 10.1038/sj.npp.1300370. [DOI] [PubMed] [Google Scholar]
- 21.Melis T, Succu S, Sanna F, Boi A, Argiolas A, Melis MR. The cannabinoid antagonist SR 141716A (Rimonabant) reduces the increase of extra-cellular dopamine release in the rat nucleus accumbens induced by a novel high palatable food. Neurosci Lett. 2007;419:231–5. doi: 10.1016/j.neulet.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 22.Sinnayah P, Jobst EE, Rathner JA, Caldera-Siu AD, Tonelli-Lemos L, Eusterbrock AJ, et al. Feeding induced by cannabinoids is mediated independently of the melanocortin system. PLoS One. 2008;3:e2202. doi: 10.1371/journal.pone.0002202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taraschenko OD, Rubbinaccio HY, Maisonneuve IM, Glick SD. 18-methoxycoronaridine: a potential new treatment for obesity in rats? Psychopharmacology (Berl) 2008;201:339–50. doi: 10.1007/s00213-008-1290-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bellinger L, Cepeda-Benito A, Wellman PJ. Meal patterns in male rats during and after intermittent nicotine administration. Pharmacol Biochem Behav. 2003;74:495–504. doi: 10.1016/s0091-3057(02)01033-x. [DOI] [PubMed] [Google Scholar]
- 25.Le Merrer J, Stephens DN. Food-induced behavioral sensitization, its cross-sensitization to cocaine and morphine, pharmacological blockade, and effect on food intake. J Neurosci. 2006;26:7163–71. doi: 10.1523/JNEUROSCI.5345-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Epstein LH, Robinson JL, Temple JL, Roemmich JN, Marusewski A, Nadbrzuch R. Sensitization and habituation of motivated behavior in overweight and non-overweight children. Learn Motiv. 2008;39:243–55. doi: 10.1016/j.lmot.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davis C, Strachan S, Berkson M. Sensitivity to reward: implications for overeating and overweight. Appetite. 2004;42:131–8. doi: 10.1016/j.appet.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 28.Avena NM, Hoebel BG. A diet promoting sugar dependency causes behavioral cross-sensitization to a low dose of amphetamine. Neuroscience. 2003;122:17–20. doi: 10.1016/s0306-4522(03)00502-5. [DOI] [PubMed] [Google Scholar]
- 29.Avena NM. Examining the addictive-like properties of binge eating using an animal model of sugar dependence. Exp Clin Psychopharmacol. 2007;15:481–91. doi: 10.1037/1064-1297.15.5.481. [DOI] [PubMed] [Google Scholar]
- 30.Tao YX. The melanocortin-4 receptor: physiology, pharmacology, and pathophysiology. Endocrine reviews. 2010;31:506–43. doi: 10.1210/er.2009-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Krom M, van der Schouw YT, Hendriks J, Ophoff RA, van Gils CH, Stolk RP, et al. Common genetic variations in CCK, leptin, and leptin receptor genes are associated with specific human eating patterns. Diabetes. 2007;56:276–80. doi: 10.2337/db06-0473. [DOI] [PubMed] [Google Scholar]
- 32.Bartoshuk LM, Duffy VB, Hayes JE, Moskowitz HR, Snyder DJ. Psychophysics of sweet and fat perception in obesity: problems, solutions and new perspectives. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 2006;361:1137–48. doi: 10.1098/rstb.2006.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pepino MY, Finkbeiner S, Beauchamp GK, Mennella JA. Obese women have lower monosodium glutamate taste sensitivity and prefer higher concentrations than do normal-weight women. Obesity (Silver Spring) 2010;18:959–65. doi: 10.1038/oby.2009.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Volkow ND, Wang GJ, Fowler JS, Telang F. Overlapping neuronal circuits in addiction and obesity: evidence of systems pathology. Philosophical Transactions of the Royal Society B: Biological Sciences. 2008;363:3191–200. doi: 10.1098/rstb.2008.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ifland JR, Preuss HG, Marcus MT, Rourke KM, Taylor WC, Burau K, et al. Refined food addiction: A classic substance use disorder. Medical hypotheses. 2009;72:518–26. doi: 10.1016/j.mehy.2008.11.035. [DOI] [PubMed] [Google Scholar]
- 36.Holden C. ADDICTION: ‘Behavioral’ Addictions: Do They Exist? Science. 2001;294:980–2. doi: 10.1126/science.294.5544.980. [DOI] [PubMed] [Google Scholar]
- 37.Volkow ND, Chang L, Wang GJ, Fowler JS, Ding YS, Sedler M, et al. Low level of brain dopamine D2 receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. Am J Psychiatry. 2001;158:2015–21. doi: 10.1176/appi.ajp.158.12.2015. [DOI] [PubMed] [Google Scholar]
- 38.Fowler JS, Volkow ND, Wang GJ, Gatley SJ, Logan J. [(11)]Cocaine: PET studies of cocaine pharmacokinetics, dopamine transporter availability and dopamine transporter occupancy. Nucl Med Biol. 2001;28:561–72. doi: 10.1016/s0969-8051(01)00211-6. [DOI] [PubMed] [Google Scholar]
- 39.Bowirrat A, Oscar-Berman M. Relationship between dopaminergic neurotransmission, alcoholism, and Reward Deficiency syndrome. Am J Med Genet B Neuropsychiatr Genet. 2005;132B:29–37. doi: 10.1002/ajmg.b.30080. [DOI] [PubMed] [Google Scholar]
- 40.Thanos PK, Michaelides M, Umegaki H, Volkow ND. D2R DNA transfer into the nucleus accumbens attenuates cocaine self-administration in rats. Synapse. 2008;62:481–6. doi: 10.1002/syn.20523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Geiger BM, Haburcak M, Avena NM, Moyer MC, Hoebel BG, Pothos EN. Deficits of mesolimbic dopamine neurotransmission in rat dietary obesity. Neuroscience. 2009;159:1193–9. doi: 10.1016/j.neuroscience.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Geiger BM, Behr GG, Frank LE, Caldera-Siu AD, Beinfeld MC, Kokkotou EG, et al. Evidence for defective mesolimbic dopamine exocytosis in obesity-prone rats. Faseb J. 2008;22:2740–6. doi: 10.1096/fj.08-110759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fulton S, Pissios P, Manchon RP, Stiles L, Frank L, Pothos EN, et al. Leptin regulation of the mesoaccumbens dopamine pathway. Neuron. 2006;51:811–22. doi: 10.1016/j.neuron.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 44.Pothos EN, Sulzer D, Hoebel BG. Plasticity of quantal size in ventral midbrain dopamine neurons: possible implications for the neurochemistry of feeding and reward. Appetite. 1998;31:405. doi: 10.1006/appe.1998.0210. [DOI] [PubMed] [Google Scholar]
- 45.Wang GJ, Volkow ND, Logan J, Pappas NR, Wong CT, Zhu W, et al. Brain dopamine and obesity. Lancet. 2001;357:354–7. doi: 10.1016/s0140-6736(00)03643-6. [DOI] [PubMed] [Google Scholar]
- 46.Stice E, Spoor S, Bohon C, Small DM. Relation between obesity and blunted striatal response to food is moderated by TaqIA A1 allele. Science. 2008;322:449–52. doi: 10.1126/science.1161550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ponce G, Jimenez-Arriero MA, Rubio G, Hoenicka J, Ampuero I, Ramos JA, et al. The A1 allele of the DRD2 gene (TaqI A polymorphisms) is associated with antisocial personality in a sample of alcohol-dependent patients. Eur Psychiatry. 2003;18:356–60. doi: 10.1016/j.eurpsy.2003.06.006. [DOI] [PubMed] [Google Scholar]
- 48.Gregorio BM, Souza-Mello V, Carvalho JJ, Mandarim-de-Lacerda CA, Aguila MB. Maternal high-fat intake predisposes nonalcoholic fatty liver disease in C57BL/6 offspring. Am J Obstet Gynecol. 2010;203:495, e1–8. doi: 10.1016/j.ajog.2010.06.042. [DOI] [PubMed] [Google Scholar]
- 49.Ainge H, Thompson C, Ozanne SE, Rooney KB. A systematic review on animal models of maternal high fat feeding and offspring glycaemic control. Int J Obes (Lond) 2011;35:325–35. doi: 10.1038/ijo.2010.149. [DOI] [PubMed] [Google Scholar]
- 50.Purcell RH, Sun B, Pass LL, Power ML, Moran TH, Tamashiro KL. Maternal stress and high-fat diet effect on maternal behavior, milk composition, and pup ingestive behavior. Physiol Behav. 2011;104:474–9. doi: 10.1016/j.physbeh.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Putnam JJA. Food Consumption, Prices, and Expenditures, 1970–97. Statistical Bulletin. 1999;(SB-965) [Google Scholar]
- 52.Spring B, Schneider K, Smith M, Kendzor D, Appelhans B, Hedeker D, et al. Abuse potential of carbohydrates for overweight carbohydrate cravers. Psychopharmacology. 2008;197:637–47. doi: 10.1007/s00213-008-1085-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Avena NM, Rada P, Hoebel BG. Sugar and Fat Bingeing Have Notable Differences in Addictive-like Behavior. J Nutr. 2009 doi: 10.3945/jn.108.097584. jn.108.097584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Avena N, Rada P, Hoebel B. Evidence for sugar addiction: Behavioral and neurochemical effects of intermittent, excessive sugar intake. Neurosci Biobehav Rev. 2008;32:20–39. doi: 10.1016/j.neubiorev.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hajnal A, Norgren R. Repeated access to sucrose augments dopamine turnover in the nucleus accumbens. Neuroreport. 2002;13:2213–6. doi: 10.1097/00001756-200212030-00010. [DOI] [PubMed] [Google Scholar]
- 56.Wang GJ, Volkow ND, Thanos PK, Fowler JS. Similarity between obesity and drug addiction as assessed by neurofunctional imaging: a concept review. J Addict Dis. 2004;23:39–53. doi: 10.1300/J069v23n03_04. [DOI] [PubMed] [Google Scholar]
- 57.Colantuoni C, Schwenker J, McCarthy J, Rada P, Ladenheim B, Cadet JL, et al. Excessive sugar intake alters binding to dopamine and mu-opioid receptors in the brain. Neuroreport. 2001;12:3549–52. doi: 10.1097/00001756-200111160-00035. [DOI] [PubMed] [Google Scholar]
- 58.Rada P, Avena NM, Hoebel BG. Daily bingeing on sugar repeatedly releases dopamine in the accumbens shell. Neuroscience. 2005;134:737–44. doi: 10.1016/j.neuroscience.2005.04.043. [DOI] [PubMed] [Google Scholar]
- 59.Lutter M, Nestler EJ. Homeostatic and Hedonic Signals Interact in the Regulation of Food Intake. J Nutr. 2009 doi: 10.3945/jn.108.097618. jn.108.097618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pickering C, Alsio J, Hulting AL, Schioth HB. Withdrawal from free-choice high-fat high-sugar diet induces craving only in obesity-prone animals. Psychopharmacology (Berl) 2009;204:431–43. doi: 10.1007/s00213-009-1474-y. [DOI] [PubMed] [Google Scholar]
- 61.Rogers PJ, Smit HJ. Food Craving and Food “Addiction”: A Critical Review of the Evidence From a Biopsychosocial Perspective. Pharmacology Biochemistry and Behavior. 2000;66:3–14. doi: 10.1016/s0091-3057(00)00197-0. [DOI] [PubMed] [Google Scholar]
- 62.Wansink BvIK. Portion size me: downsizing our consumption norms. J Am Diet Assoc. 2007 Jul;107:1103–6. doi: 10.1016/j.jada.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 63.Harnack LJ, Jeffery RW, Boutelle KN. Temporal trends in energy intake in the United States: an ecologic perspective. Am J Clin Nutr. 2000;71:1478–84. doi: 10.1093/ajcn/71.6.1478. [DOI] [PubMed] [Google Scholar]
- 64.McCrory MA, Saltzman E, Rolls BJ, Roberts SB. The energy density-palatability interrelationship as a determinant of voluntary energy intake from individual foods in identical twins consuming covertly manipulated high- and low-fat diets. 1999 (submitted) [Google Scholar]
- 65.Rolls BJ. The relationship between dietary energy density and energy intake. Physiology & behavior. 2009;97:609–15. doi: 10.1016/j.physbeh.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McCrory MA, Fuss P, ES, Roberts SB. Dietary determinants of energy intake and weight regulation in healthy adults. J Nutr. 2000;130:276S–9S. doi: 10.1093/jn/130.2.276S. [DOI] [PubMed] [Google Scholar]
- 67.Glanz K, Basil M, Maibach E, Goldberg J, Snyder D. Why Americans eat what they do: Taste, nutrition, cost, convenience, and weight control concerns as influences on food consumption. J Am Diet Assoc. 1998;98:1118–26. doi: 10.1016/S0002-8223(98)00260-0. [DOI] [PubMed] [Google Scholar]
- 68.Lloyd HM, Paisley CM, Mela DJ. Barriers to the adoption of reduced-fat diets in a UK population. J Am Diet Assoc. 1995;95:316–22. doi: 10.1016/s0002-8223(95)00082-8. [DOI] [PubMed] [Google Scholar]
- 69.Drewnowski A, Kurth CL, Rahaim JE. Taste preferences in human obesity: environmental and familial factors. Am J Clin Nutr. 1991;54:634–41. doi: 10.1093/ajcn/54.4.635. [DOI] [PubMed] [Google Scholar]
- 70.Mela DJ, Sacchetti DA. Sensory preferences for fats: relationships with diet and body composition. Am J Clin Nutr. 1991;53:908–15. doi: 10.1093/ajcn/53.4.908. [DOI] [PubMed] [Google Scholar]
- 71.Stunkard AJ, Messick S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J Psychosom Res. 1985;29:71–83. doi: 10.1016/0022-3999(85)90010-8. [DOI] [PubMed] [Google Scholar]
- 72.Hays NP, Bathalon GP, McCrory MA, Roubenoff R, Lipman R, Roberts SB. Eating behavior correlates of adult weight gain and obesity in healthy women aged 55–65 y. Am J Clin Nutr. 2001;75:476–83. doi: 10.1093/ajcn/75.3.476. [DOI] [PubMed] [Google Scholar]
- 73.Olsen A, Ritz C, Hartvig DL, Moller P. Comparison of sensory specific satiety and sensory specific desires to eat in children and adults. Appetite. 2011;57:6–13. doi: 10.1016/j.appet.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 74.Williamson DF, Serdula MK, Anda RF, Levy A, Byers T. Weight loss attempts in adults: goals, duration, and rate of weight loss. Am J Public Health. 1992;82:1251–7. doi: 10.2105/ajph.82.9.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Phelan S, Hill JO, Lang W, Debello JR, Wing RR. Recovery from relapse among successful weight maintainers. Am J Clin Nutr. 2003;78:1079–84. doi: 10.1093/ajcn/78.6.1079. [DOI] [PubMed] [Google Scholar]
- 76.Savage JS, Birch LL. Patterns of weight control strategies predict differences in women’s 4-year weight gain. Obesity (Silver Spring) 2010;18:513–20. doi: 10.1038/oby.2009.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.French SA, Jeffery RW, Murray D. Is dieting good for you?: Prevalence, duration and associated weight and behaviour changes for specific weight loss strategies over four years in US adults. Int J Obes Relat Metab Disord. 1999;23:320–7. doi: 10.1038/sj.ijo.0800822. [DOI] [PubMed] [Google Scholar]
- 78.Petroni ML, Villanova N, Avagnina S, Fusco MA, Fatati G, Compare A, et al. Psychological distress in morbid obesity in relation to weight history. Obes Surg. 2007;17:391–9. doi: 10.1007/s11695-007-9069-3. [DOI] [PubMed] [Google Scholar]
- 79.Marchesini G, Cuzzolaro M, Mannucci E, Dalle Grave R, Gennaro M, Tomasi F, et al. Weight cycling in treatment-seeking obese persons: data from the QUOVADIS study. Int J Obes Relat Metab Disord. 2004;28:1456–62. doi: 10.1038/sj.ijo.0802741. [DOI] [PubMed] [Google Scholar]
- 80.Brownell KD, Rodin J. Medical, Metabolic, and Psychological Effects of Weight Cycling. Arch Intern Med. 1994;154:1325–30. [PubMed] [Google Scholar]
- 81.Wing RR, Tate DF, Gorin AA, Raynor HA, Fava JL. A self-regulation program for maintenance of weight loss. N Engl J Med. 2006;355:1563–71. doi: 10.1056/NEJMoa061883. [DOI] [PubMed] [Google Scholar]
- 82.Ruderman AJ. Dysphoric mood and overeating: A test of restraint theory’s disinhibition hypothesis. J Abnorm Psychol. 1985;94:78–85. doi: 10.1037//0021-843x.94.1.78. [DOI] [PubMed] [Google Scholar]
- 83.Herman CP, Polivy J. Anxiety, restraint, and eating behavior. J Abnorm Psychol. 1975;84:666–72. [PubMed] [Google Scholar]
- 84.Herman CP, Polivy J. A boundary model for the regulation of eating. Res Publ Assoc Res Nerv Ment Dis. 1984;62:141–56. [PubMed] [Google Scholar]
- 85.Chaput JP, Leblanc C, Perusse L, Despres JP, Bouchard C, Tremblay A. Risk Factors for Adult Overweight and Obesity in the Quebec Family Study: Have We Been Barking Up the Wrong Tree[quest] Obesity. 2009;17:1964–70. doi: 10.1038/oby.2009.116. [DOI] [PubMed] [Google Scholar]
- 86.Shiffman S. Reflections on smoking relapse research. Drug and Alcohol Review. 2006;25:15–20. doi: 10.1080/09595230500459479. [DOI] [PubMed] [Google Scholar]
- 87.MURAVEN #160, Mark COLLINS, Lorraine R, NIENHAUS, et al. ETATS-UNIS. Washington, DC: American Psychological Association; 2002. Self-control and alcohol restraint: An initial application of the self-control strength model. [DOI] [PubMed] [Google Scholar]
- 88.Cohen S, Lichtenstein E. Perceived stress, quitting smoking, and smoking relapse. Health Psychology. 1990;9:466–78. doi: 10.1037//0278-6133.9.4.466. [DOI] [PubMed] [Google Scholar]
- 89.Shaham Y, Stewart J. Stress reinstates heroin-seeking in drug-free animals: An effect mimicking heroin, not withdrawal. Psychopharmacology. 1995;119:334–41. doi: 10.1007/BF02246300. [DOI] [PubMed] [Google Scholar]
- 90.Baucom DH, Aiken PA. Effect of depressed mood on eating among obese and nonobese dieting and nondieting persons. Journal of Personality and Social Psychology. 1981;41:577–85. doi: 10.1037//0022-3514.41.3.577. [DOI] [PubMed] [Google Scholar]
- 91.Baumeister RF, Heatherton TF, Tice DM. Losing control: How and why people fail at self-regulation. San Diego: Academic; 1994. [Google Scholar]
- 92.Herman CP, Polivy J, Lank CN, Heatherton TF. Anxiety, hunger, and eating behavior. J Abnorm Psychol. 1987;96:264–9. doi: 10.1037//0021-843x.96.3.264. [DOI] [PubMed] [Google Scholar]
- 93.Cools J, Schotte DE, McNally RJ. Emotional arousal and overeating in restrained eaters. J Abnorm Psychol. 1992;101:348–51. doi: 10.1037//0021-843x.101.2.348. [DOI] [PubMed] [Google Scholar]
- 94.Boon B, Stroebe W, Schut H, Ijntema R. Ironic processes in the eating behaviour of restrained eaters. British Journal of Health Psychology. 2002;7:1–10. doi: 10.1348/135910702169303. [DOI] [PubMed] [Google Scholar]
- 95.HEATHERTON #160, HERMAN FT, POLIVY PC, et al. ETATS-UNIS. Washington, DC: American Psychological Association; 1991. Effects of physical threat and ego threat on eating behavior. [DOI] [PubMed] [Google Scholar]
- 96.Haynes C, Lee MD, Yeomans MR. Interactive effects of stress, dietary restraint, and disinhibition on appetite. Eating Behaviors. 2003;4:369–83. doi: 10.1016/j.eatbeh.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 97.Herman PCJP. Handbook of self-regulation: Research, theory, and applications. New York, NY: Guilford Press; 2004. The self-regulation of eating: Theoretical and practical problems; p. 574. [Google Scholar]
- 98.Vohs KD, Heatherton TF. Self-regulatory failure: A resource-depletion approach. Psychological Science. 2000;11:249–54. doi: 10.1111/1467-9280.00250. [DOI] [PubMed] [Google Scholar]
- 99.Muraven M, Baumeister RF. Self-regulation and depletion of limited resources: Does self-control resemble a muscle? Psychological Bulletin. 2000;126:247–59. doi: 10.1037/0033-2909.126.2.247. [DOI] [PubMed] [Google Scholar]
- 100.Westenhoefer J, Broeckmann P, Münch AK, Pudel V. Cognitive control of eating behaviour and the disinhibition effect. Appetite. 1994;23:27–41. doi: 10.1006/appe.1994.1032. [DOI] [PubMed] [Google Scholar]
- 101.Ward A, Mann T. Don’t mind if I do: Disinhibited eating under cognitive load. Journal of Personality and Social Psychology. 2000;78:753–63. doi: 10.1037//0022-3514.78.4.753. [DOI] [PubMed] [Google Scholar]
- 102.Koob GF, Moal ML. Drug Abuse: Hedonic Homeostatic Dysregulation. Science. 1997;278:52–8. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- 103.Kiyatkin EA, Wise RA, Gratton A. Drug- and behavior-associated changes in dopamine-related electrochemical signals during intravenous heroin self-administration in rats. Synapse. 1993;14:60–72. doi: 10.1002/syn.890140109. [DOI] [PubMed] [Google Scholar]
- 104.Gratton A, Wise RA. Drug- and behavior-associated changes in dopamine-related electrochemical signals during intravenous cocaine self-administration in rats. J Neurosci. 1994;14:4130–46. doi: 10.1523/JNEUROSCI.14-07-04130.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ljungberg T, Apicella P, Schultz W. Responses of monkey dopamine neurons during learning of behavioral reactions. J Neurophysiol. 1992;67:145–63. doi: 10.1152/jn.1992.67.1.145. [DOI] [PubMed] [Google Scholar]
- 106.Apicella P, Ljungberg T, Scarnati E, Schultz W. Responses to reward in monkey dorsal and ventral striatum. Exp Brain Res. 1991;85:491–500. doi: 10.1007/BF00231732. [DOI] [PubMed] [Google Scholar]
- 107.Kosobud AE, Harris GC, Chapin JK. Behavioral associations of neuronal activity in the ventral tegmental area of the rat. J Neurosci. 1994;14:7117–29. doi: 10.1523/JNEUROSCI.14-11-07117.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jansen A. A learning model of binge eating: cue reactivity and cue exposure. Behav Res Ther. 1998;36:257–72. doi: 10.1016/s0005-7967(98)00055-2. [DOI] [PubMed] [Google Scholar]
- 109.Gilhooly CH, Das SK, Golden JK, McCrory MA, Dallal GE, Saltzman E, et al. Food cravings and energy regulation: the characteristics of craved foods and their relationship with eating behaviors and weight change during 6 months of dietary energy restriction. Int J Obes (Lond) 2007;31:1849–58. doi: 10.1038/sj.ijo.0803672. [DOI] [PubMed] [Google Scholar]
- 110.Pelchat ML, Johnson A, Chan R, Valdez J, Ragland JD. Images of desire: food-craving activation during fMRI. NeuroImage. 2004;23:1486–93. doi: 10.1016/j.neuroimage.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 111.Carter BL, Tiffany ST. Meta-analysis of cue-reactivity in addiction research. Addiction. 1999;94:327–40. [PubMed] [Google Scholar]
- 112.Tiffany ST. A cognitive model of drug urges and drug-use behavior: role of automatic and nonautomatic processes. Psychol Rev. 1990;97:147–68. doi: 10.1037/0033-295x.97.2.147. [DOI] [PubMed] [Google Scholar]
- 113.Grimm JW, Hope BT, Wise RA, Shaham Y. Neuroadaptation: Incubation of cocaine craving after withdrawal. Nature. 2001;412:141–2. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fedoroff IC, Polivy J, Herman CP. The effect of pre-exposure to food cues on the eating behavior of restrained and unrestrained eaters. Appetite. 1997;28:33–47. doi: 10.1006/appe.1996.0057. [DOI] [PubMed] [Google Scholar]
- 115.Fedoroff I, Polivy J, Herman CP. The specificity of restrained versus unrestrained eaters’ responses to food cues: general desire to eat, or craving for the cued food? Appetite. 2003;41:7–13. doi: 10.1016/s0195-6663(03)00026-6. [DOI] [PubMed] [Google Scholar]
- 116.Rothemund Y, Preuschhof C, Bohner G, Bauknecht HC, Klingebiel R, Flor H, et al. Differential activation of the dorsal striatum by high-calorie visual food stimuli in obese individuals. NeuroImage. 2007;37:410–21. doi: 10.1016/j.neuroimage.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 117.Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, et al. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J Neurosci. 2006;26:6583–8. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Stoeckel LE, Weller RE, Cook EW, 3rd, Twieg DB, Knowlton RC, Cox JE. Widespread reward-system activation in obese women in response to pictures of high-calorie foods. NeuroImage. 2008;41:636–47. doi: 10.1016/j.neuroimage.2008.02.031. [DOI] [PubMed] [Google Scholar]
- 119.Stice E, Spoor S, Ng J, Zald DH. Relation of obesity to consummatory and anticipatory food reward. Physiol Behav. 2009;97:551–60. doi: 10.1016/j.physbeh.2009.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tetley A, Brunstrom J, Griffiths P. Individual differences in food-cue reactivity. The role of BMI and everyday portion-size selections. Appetite. 2009;52:614–20. doi: 10.1016/j.appet.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 121.Stice E, Spoor S, Bohon C, Veldhuizen MG, Small DM. Relation of reward from food intake and anticipated food intake to obesity: a functional magnetic resonance imaging study. J Abnorm Psychol. 2008;117:924–35. doi: 10.1037/a0013600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Roefs A, Herman CP, MacLeod CM, Smulders FTY, Jansen A. At first sight: How do restrained eaters evaluate high-fat palatable foods? Appetite. 2005;44:103–14. doi: 10.1016/j.appet.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 123.Thomas MJ, Kalivas PW, Shaham Y. Neuroplasticity in the mesolimbic dopamine system and cocaine addiction. Br J Pharmacol. 2008;154:327–42. doi: 10.1038/bjp.2008.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Koob GF. The neurobiology of addiction: a neuroadaptational view relevant for diagnosis. Addiction. 2006;101 (Suppl 1):23–30. doi: 10.1111/j.1360-0443.2006.01586.x. [DOI] [PubMed] [Google Scholar]
- 125.Robinson TE, Berridge KC. The psychology and neurobiology of addiction: an incentive-sensitization view. Addiction. 2000;95 (Suppl 2):S91–117. doi: 10.1080/09652140050111681. [DOI] [PubMed] [Google Scholar]
- 126.Nestler EJ. Molecular mechanisms of drug addiction. Neuropharmacology. 2004;47 (Suppl 1):24–32. doi: 10.1016/j.neuropharm.2004.06.031. [DOI] [PubMed] [Google Scholar]
- 127.Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–9. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- 128.Everitt BJ, Wolf ME. Psychomotor Stimulant Addiction: A Neural Systems Perspective. J Neurosci. 2002;22:3312–20. doi: 10.1523/JNEUROSCI.22-09-03312.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Teegarden SL, Nestler EJ, Bale TL. Delta FosB-mediated alterations in dopamine signaling are normalized by a palatable high-fat diet. Biol Psychiatry. 2008;64:941–50. doi: 10.1016/j.biopsych.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Perrotti LI, Weaver RR, Robison B, Renthal W, Maze I, Yazdani S, et al. Distinct patterns of DeltaFosB induction in brain by drugs of abuse. Synapse. 2008;62:358–69. doi: 10.1002/syn.20500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Teegarden SL, Scott AN, Bale TL. Early life exposure to a high fat diet promotes long-term changes in dietary preferences and central reward signaling. Neuroscience. 2009;162:924–32. doi: 10.1016/j.neuroscience.2009.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Olausson P, Jentsch JD, Tronson N, Neve RL, Nestler EJ, Taylor JR. DeltaFosB in the nucleus accumbens regulates food-reinforced instrumental behavior and motivation. J Neurosci. 2006;26:9196–204. doi: 10.1523/JNEUROSCI.1124-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hernandez PJ, Sadeghian K, Kelley AE. Early consolidation of instrumental learning requires protein synthesis in the nucleus accumbens. Nat Neurosci. 2002;5:1327–31. doi: 10.1038/nn973. [DOI] [PubMed] [Google Scholar]
- 134.Baldwin AE, Sadeghian K, Holahan MR, Kelley AE. Appetitive instrumental learning is impaired by inhibition of cAMP-dependent protein kinase within the nucleus accumbens. Neurobiol Learn Mem. 2002;77:44–62. doi: 10.1006/nlme.2000.4002. [DOI] [PubMed] [Google Scholar]
- 135.Shiflett MW, Martini RP, Mauna JC, Foster RL, Peet E, Thiels E. Cue-elicited reward-seeking requires extracellular signal-regulated kinase activation in the nucleus accumbens. J Neurosci. 2008;28:1434–43. doi: 10.1523/JNEUROSCI.2383-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Berridge KC, Robinson TE, Aldridge JW. Dissecting components of reward: ‘liking’, ‘wanting’, and learning. Curr Opin Pharmacol. 2009;9:65–73. doi: 10.1016/j.coph.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Robinson TE, Berridge KC. Review. The incentive sensitization theory of addiction: some current issues. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 2008;363:3137–46. doi: 10.1098/rstb.2008.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–91. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- 139.Kaplan JM, Seeley RJ, Grill HJ. A behavioral probe of the growth of intake potential during the inter-meal interval in the rat. Behav Neurosci. 1994;108:353–61. [PubMed] [Google Scholar]
- 140.Breslin PA, Spector AC, Grill HJ. A quantitative comparison of taste reactivity behaviors to sucrose before and after lithium chloride pairings: a unidimensional account of palatability. Behav Neurosci. 1992;106:820–36. doi: 10.1037//0735-7044.106.5.820. [DOI] [PubMed] [Google Scholar]
- 141.Kaplan JM, Spector AC, Grill HJ. Ingestion rate as an independent variable in the behavioral analysis of satiation. Am J Physiol. 1990;258:R662–71. doi: 10.1152/ajpregu.1990.258.3.R662. [DOI] [PubMed] [Google Scholar]
- 142.Shin AC, Townsend RL, Patterson LM, Berthoud HR. “Liking” and “wanting” of sweet and oily food stimuli as affected by high-fat diet-induced obesity, weight loss, leptin, and genetic predisposition. American journal of physiology” Regulatory, integrative and comparative physiology. 2011;301:R1267–80. doi: 10.1152/ajpregu.00314.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Jones N, Rogers PJ. Preoccupation, food, and failure: An investigation of cognitive performance deficits in dieters. International Journal of Eating Disorders. 2003;33:185–92. doi: 10.1002/eat.10124. [DOI] [PubMed] [Google Scholar]
- 144.Wadden TA, Neiberg RH, Wing RR, Clark JM, Delahanty LM, Hill JO, et al. Four-Year Weight Losses in the Look AHEAD Study: Factors Associated With Long-Term Success. Obesity (Silver Spring) 2011 doi: 10.1038/oby.2011.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Delahanty LM, Meigs JB, Hayden D, Williamson DA, Nathan DM. Psychological and behavioral correlates of baseline BMI in the diabetes prevention program (DPP) Diabetes Care. 2002;25:1992–8. doi: 10.2337/diacare.25.11.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Willett WC. The great fat debate: total fat and health. J Am Diet Assoc. 2011;111:660–2. doi: 10.1016/j.jada.2011.03.029. [DOI] [PubMed] [Google Scholar]
- 147.Gross LS, Li L, Ford ES, Liu S. Increased consumption of refined carbohydrates and the epidemic of type 2 diabetes in the United States: an ecologic assessment. Am J Clin Nutr. 2004;79:774–9. doi: 10.1093/ajcn/79.5.774. [DOI] [PubMed] [Google Scholar]
- 148.Du H, van der AD, Boshuizen HC, Forouhi NG, Wareham NJ, Halkjaer J, et al. Dietary fiber and subsequent changes in body weight and waist circumference in European men and women. Am J Clin Nutr. 2010;91:329–36. doi: 10.3945/ajcn.2009.28191. [DOI] [PubMed] [Google Scholar]
- 149.Burton PJ, Smit HJ, Lightowler H. The influence of restrained and external eating patterns on overeating. Appetite. 2007;49:191–7. doi: 10.1016/j.appet.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 150.Kleiner KD, Gold MS, Frost-Pineda K, Lenz-Brunsman B, Perri MG, Jacobs WS. Body mass index and alcohol use. J Addict Dis. 2004;23:105–18. doi: 10.1300/J069v23n03_08. [DOI] [PubMed] [Google Scholar]
- 151.Ertelt TW, Mitchell JE, Lancaster K, Crosby RD, Steffen KJ, Marino JM. Alcohol abuse and dependence before and after bariatric surgery: a review of the literature and report of a new data set. Surgery for Obesity and Related Diseases. 2008;4:647–50. doi: 10.1016/j.soard.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 152.Ludwig DS. Childhood Obesity -- The Shape of Things to Come. N Engl J Med. 2007;357:2325–7. doi: 10.1056/NEJMp0706538. [DOI] [PubMed] [Google Scholar]
- 153.Strauss RS, Pollack HA. Social Marginalization of Overweight Children. Arch Pediatr Adolesc Med. 2003;157:746–52. doi: 10.1001/archpedi.157.8.746. [DOI] [PubMed] [Google Scholar]
- 154.Sarlio-Lahteenkorva S, Lahelma E. The association of body mass index with social and economic disadvantage in women and men. Int J Epidemiol. 1999;28:445–9. doi: 10.1093/ije/28.3.445. [DOI] [PubMed] [Google Scholar]
- 155.Chambliss HO, Finley CE, Blair SN. Attitudes toward obese individuals among exercise science students. Med Sci Sports Exerc. 2004;36:468–74. doi: 10.1249/01.mss.0000117115.94062.e4. [DOI] [PubMed] [Google Scholar]
- 156.Ciao AC, Latner JD. Reducing Obesity Stigma: The Effectiveness of Cognitive Dissonance and Social Consensus Interventions. Obesity (Silver Spring) 2011 doi: 10.1038/oby.2011.106. [DOI] [PubMed] [Google Scholar]
- 157.Tsai AG, Wadden TA. Systematic review: an evaluation of major commercial weight loss programs in the United States. Ann Intern Med. 2005;142:56–66. doi: 10.7326/0003-4819-142-1-200501040-00012. [DOI] [PubMed] [Google Scholar]
- 158.Smith DE, Wing RR. Diminished weight loss behavioral compliance during repeated diets in obese patients with type II diabetes. Health Psychol. 1991;10:378–83. doi: 10.1037//0278-6133.10.6.378. [DOI] [PubMed] [Google Scholar]
- 159.Zilli F, Croci M, Tufano A, Caviezel F. The compliance of hypocaloric diet in type 2 diabetic obese patients: a brief-term study. Eat Weight Disord. 2000 Dec;5:217–22. doi: 10.1007/BF03354449. [DOI] [PubMed] [Google Scholar]
- 160.Morreale SJ, Schwartz NE. Helping Americans eat right: developing practical and actionable public nutrition education messages based on the ADA Survey of American Dietary Habits. J Am Diet Assoc. 1995;95:305–8. doi: 10.1016/S0002-8223(95)00078-X. [DOI] [PubMed] [Google Scholar]
- 161.Svetkey LP, Stevens VJ, Brantley PJ, Appel LJ, Hollis JF, Loria CM, et al. Comparison of strategies for sustaining weight loss: the weight loss maintenance randomized controlled trial. JAMA. 2008;299:1139–48. doi: 10.1001/jama.299.10.1139. [DOI] [PubMed] [Google Scholar]
- 162.Elkins G, Whitfield P, Marcus J, Symmonds R, Rodriguez J, Cook T. Noncompliance with behavioral recommendations following bariatric surgery. Obes Surg. 2005;15:546–51. doi: 10.1381/0960892053723385. [DOI] [PubMed] [Google Scholar]
- 163.Heinberg LJ, Ashton K. History of substance abuse relates to improved postbariatric body mass index outcomes. Surg Obes Relat Dis. 2010;6:417–21. doi: 10.1016/j.soard.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 164.Suzuki J, Haimovici F, Chang G. Alcohol Use Disorders After Bariatric Surgery. Obes Surg. 2010 doi: 10.1007/s11695-010-0346-1. [DOI] [PubMed] [Google Scholar]
- 165.Sacks G, Swinburn B, Lawrence M. Obesity Policy Action framework and analysis grids for a comprehensive policy approach to reducing obesity. Obes Rev. 2009;10:76–86. doi: 10.1111/j.1467-789X.2008.00524.x. [DOI] [PubMed] [Google Scholar]
- 166.WHO. The global burden of disease: 2004 update. World Health Organization; 2004. [Google Scholar]
- 167.Khan LK, Sobush K, Keener D, Goodman K, Lowry A, Kakietek J, et al. Recommended community strategies and measurements to prevent obesity in the United States. MMWR Recomm Rep. 2009;58:1–26. [PubMed] [Google Scholar]
- 168.BROWNELL KD, WARNER KE. The Perils of Ignoring History: Big Tobacco Played Dirty and Millions Died. How Similar Is Big Food? Milbank Quarterly. 2009;87:259–94. doi: 10.1111/j.1468-0009.2009.00555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.WHO. mpower: A Policy Package to Reverse the Tobacco Epidemic. World Health Organization; 2008. [Google Scholar]
- 170.Mercer SL, Green LW, Rosenthal AC, Husten CG, Khan LK, Dietz WH. Possible lessons from the tobacco experience for obesity control. Am J Clin Nutr. 2003;77:1073S–82S. doi: 10.1093/ajcn/77.4.1073S. [DOI] [PubMed] [Google Scholar]
- 171.Yach D, McKee M, Lopez AD, Novotny T. Improving diet and physical activity: 12 lessons from controlling tobacco smoking. BMJ. 2005;330:898–900. doi: 10.1136/bmj.330.7496.898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Chopra M, Darnton-Hill I. Tobacco and obesity epidemics: not so different after all? BMJ. 2004;328:1558–60. doi: 10.1136/bmj.328.7455.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Engelhard CL, Garson A, Jr, Dorn S. Reducing obesity: policy strategies from the tobacco wars. Methodist Debakey Cardiovasc J. 2009;5:46–50. doi: 10.14797/mdcj-5-4-46. [DOI] [PubMed] [Google Scholar]
- 174.Klein JD, Dietz W. Childhood obesity: the new tobacco. Health Aff (Millwood) 2010;29:388–92. doi: 10.1377/hlthaff.2009.0736. [DOI] [PubMed] [Google Scholar]
- 175.Knai C, Lobstein T, McKee M. Childhood obesity: the case for binding international legislation. Eur J Public Health. 2005;15:559–61. doi: 10.1093/eurpub/cki215. [DOI] [PubMed] [Google Scholar]
- 176.Danaei G, Ding EL, Mozaffarian D, Taylor B, Rehm J, Murray CJ, et al. The preventable causes of death in the United States: comparative risk assessment of dietary, lifestyle, and metabolic risk factors. PLoS Med. 2009;6:e1000058. doi: 10.1371/journal.pmed.1000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Cuttler L, Whittaker JL, Kodish ED. Pediatric obesity policy: the danger of skepticism. Arch Pediatr Adolesc Med. 2003;157:722–4. doi: 10.1001/archpedi.157.8.722. [DOI] [PubMed] [Google Scholar]
- 178.Lutfiyya MN, Nika B, Ng L, Tragos C, Won R, Lipsky MS. Primary prevention of overweight and obesity: an analysis of national survey data. J Gen Intern Med. 2008;23:821–3. doi: 10.1007/s11606-008-0581-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Wellman RJ, Sugarman DB, DiFranza JR, Winickoff JP. The extent to which tobacco marketing and tobacco use in films contribute to children’s use of tobacco: a meta-analysis. Arch Pediatr Adolesc Med. 2006;160:1285–96. doi: 10.1001/archpedi.160.12.1285. [DOI] [PubMed] [Google Scholar]
- 180.DiFranza JR, Wellman RJ, Sargent JD, Weitzman M, Hipple BJ, Winickoff JP. Tobacco promotion and the initiation of tobacco use: assessing the evidence for causality. Pediatrics. 2006;117:e1237–48. doi: 10.1542/peds.2005-1817. [DOI] [PubMed] [Google Scholar]
- 181.Slater SJ, Chaloupka FJ, Wakefield M, Johnston LD, O’Malley PM. The impact of retail cigarette marketing practices on youth smoking uptake. Arch Pediatr Adolesc Med. 2007;161:440–5. doi: 10.1001/archpedi.161.5.440. [DOI] [PubMed] [Google Scholar]
- 182.Giovino GA. The tobacco epidemic in the United States. Am J Prev Med. 2007;33:S318–26. doi: 10.1016/j.amepre.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 183.Lobstein T, Dibb S. Evidence of a possible link between obesogenic food advertising and child overweight. Obesity Reviews. 2005;6:203–8. doi: 10.1111/j.1467-789X.2005.00191.x. [DOI] [PubMed] [Google Scholar]
- 184.Goris JM, Petersen S, Stamatakis E, Veerman JL. Television food advertising and the prevalence of childhood overweight and obesity: a multicountry comparison. Public Health Nutrition. 2009:1–10. doi: 10.1017/S1368980009992850. First View. [DOI] [PubMed] [Google Scholar]
- 185.Halford JC, Gillespie J, Brown V, Pontin EE, Dovey TM. Effect of television advertisements for foods on food consumption in children. Appetite. 2004;42:221–5. doi: 10.1016/j.appet.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 186.Gamble M, Cotugna N. A Quarter Century of TV Food Advertising Targeted at Children. American Journal of Health Behavior. 1999;23:261–7. [Google Scholar]
- 187.WHO. WHO Report on the Global Tobacco Epidemic, 2009: Implementing smoke-free environments. World Health Organization; 2009. [Google Scholar]
- 188.Brownell KD, Frieden TR. Ounces of prevention--the public policy case for taxes on sugared beverages. N Engl J Med. 2009;360:1805–8. doi: 10.1056/NEJMp0902392. [DOI] [PubMed] [Google Scholar]
- 189.Andreyeva T, Long MW, Brownell KD. The impact of food prices on consumption: a systematic review of research on the price elasticity of demand for food. Am J Public Health. 2010;100:216–22. doi: 10.2105/AJPH.2008.151415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Benowitz NL. Clinical pharmacology of nicotine: implications for understanding, preventing, and treating tobacco addiction. Clin Pharmacol Ther. 2008;83:531–41. doi: 10.1038/clpt.2008.3. [DOI] [PubMed] [Google Scholar]
- 191.Padwal RS, Majumdar SR. Drug treatments for obesity: orlistat, sibutramine, and rimonabant. Lancet. 2007;369:71–7. doi: 10.1016/S0140-6736(07)60033-6. [DOI] [PubMed] [Google Scholar]
- 192.Guardia D, Rolland B, Karila L, Cottencin O. GABAergic and glutamatergic modulation in binge eating: therapeutic approach. Current pharmaceutical design. 2011;17:1396–409. doi: 10.2174/138161211796150828. [DOI] [PubMed] [Google Scholar]
- 193.Aigner M, Treasure J, Kaye W, Kasper S. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for the pharmacological treatment of eating disorders. The world journal of biological psychiatry : the official journal of the World Federation of Societies of Biological Psychiatry. 2011;12:400–43. doi: 10.3109/15622975.2011.602720. [DOI] [PubMed] [Google Scholar]
- 194.Millstone E, Lobstein T. The PorGrow project: overall cross-national results, comparisons and implications. Obes Rev. 2007;8 (Suppl 2):29–36. doi: 10.1111/j.1467-789X.2007.00356.x. [DOI] [PubMed] [Google Scholar]
- 195.Nesse RM. Evolution And Addiction. Addiction. 2002;97:470–1. doi: 10.1046/j.1360-0443.2002.00086.x. [DOI] [PubMed] [Google Scholar]
- 196.Dagher A, Robbins TW. Personality, addiction, dopamine: insights from Parkinson’s disease. Neuron. 2009;61:502–10. doi: 10.1016/j.neuron.2009.01.031. [DOI] [PubMed] [Google Scholar]