Abstract
Hormones associated with pregnancy and parturition have been implicated in facilitating the onset of maternal behavior via reductions in neophobia, anxiety, and stress responsiveness. To determine whether the onset of paternal behavior has similar associations in biparental male California mice (Peromyscus californicus), we compared paternal responsiveness, neophobia (novel-object test), and anxiety-like behavior (elevated plus maze, EPM) in isolated virgins (housed alone), paired virgins (housed with another male), expectant fathers (housed with pregnant pairmate), and new fathers (housed with pairmate and pups). Corticotropin-releasing hormone (CRH) and Fos immunoreactivity (IR) were quantified in brain tissues following exposure to a predator-odor stressor or under baseline conditions. New fathers showed lower anxiety-like behavior than expectant fathers and isolated virgins in EPM tests. In all housing conditions, stress elevated Fos-IR in the hypothalamic paraventricular nucleus (PVN). Social isolation reduced overall (baseline and stress-induced) Fos- and colocalized Fos/CRH-IR, and increased overall CRH-IR, in the PVN. In the central nucleus of the amygdala, social isolation increased stress-induced CRH-IR and decreased stress-induced activation of CRH neurons. Across all housing conditions, paternally behaving males displayed more anxiety-related behavior than nonpaternal males in the EPM, but showed no differences in CRH- or Fos-IR. Finally, the latency to engage in paternal behavior was positively correlated with the latency to approach a novel object. These results suggest that being a new father does not reduce anxiety, neophobia, or neural stress responsiveness. Low levels of neophobia, however, were associated with, but not necessary for paternal responsiveness.
Keywords: Paternal behavior, stress, CRH, biparental, anxiety, neophobia
1. Introduction
Maternal behavior in lactating rats is associated with reduced fearfulness, anxiety, and stress responsiveness [1–7]. In contrast to lactating dams, virgin female rats avoid and are fearful of unfamiliar pups due to the aversive properties of the infants' odors and vocalizations [1,8,9]. As would be expected, inhibiting the neural circuitry mediating fear and avoidance, as well as the neural pathways processing olfactory stimuli, facilitates the onset of maternal behavior in virgin female rats [8,10–12]. Under natural conditions, inhibition of the fear/avoidance circuitry is most likely mediated by the high estrogen:progesterone ratio and the elevated central concentrations of prolactin and oxytocin found during the peripartum and postpartum periods [9,12–14].
Maternal hormones also serve to downregulate stress reactivity in postpartum females. In several species, the hormonal and neuronal changes occurring at parturition and lactation are accompanied by profoundly reduced behavioral and neural responses to stress. Compared to virgin females, for example, lactating female rats show a blunted stress-induced activation of the hypothalamo-pituitary-adrenal (HPA) axis, manifest in reduced synthesis and release of corticotropin-releasing hormone (CRH) from the paraventricular nucleus of the hypothalamus, adrenocorticotropic hormone (ACTH) from the anterior pituitary, and glucocorticoids from the adrenal glands [6,15–21]. These changes are accompanied by attenuated levels of anxiety and fear in comparison to virgin females [1–6,22]. The function of lactational stress hyporesponsiveness is unknown. However, as anxiety and neophobia are associated with pup-avoidance behaviors in virgin females (1,9,11,23], it is likely that lactational hyporesponsiveness facilitates the onset of maternal behavior.
Spontaneous paternal behavior by males occurs in approximately 6% of mammalian species, including humans [9,24]. Little is known about the neuronal or endocrine mechanisms underlying the onset and maintenance of paternal behavior, or its associations with stress responsiveness. The recent literature on the mechanistic basis of paternal behavior has implicated the bed nucleus of the stria terminalis (BnST), lateral habenula, medial amygdala, nucleus accumbens, and, most prominently, the medial preoptic area (mPOA) [25–29]. These findings correspond, in part, to findings in female rats, which showed that the BnST, mPOA, and nucleus accumbens are important for the expression of maternal behavior [12] in response to visual, auditory, and olfactory cues from pups. Similarly, several of the hormones and neuropeptides that influence the expression of maternal behavior have also been implicated in activating (estrogen, prolactin, oxytocin) or inhibiting (progesterone) paternal behavior [30–34].
It is unknown whether these similarities in the neural and hormonal mechanisms controlling maternal and paternal behavior extend to the relationship between reduced stress responsiveness and the onset of parental behavior. In females, the onset of maternal behavior requires the suppression of fear and avoidance responses when exposed to pup sounds and odors [12]. Once the females are attracted to pup stimuli, maternal responses are intimated. Similar mechanisms could be employed in males. Initial studies of the monogamous, biparental California mouse (Peromyscus californicus) suggest that paternal experience is associated with changes in anxiety-related behavior [35] and behavioral responses to acute stress [36], but does not markedly affect the corticosterone response to acute stress [36]; however, no studies have attempted to elucidate the role that anxiety and stress responsiveness may play in the onset and maintenance of paternal behavior, and it is unknown whether engaging in paternal behavior is either a consequence or a cause of reduced behavioral and neural stress responses.
We therefore tested two hypotheses, which are not mutually exclusive:
-
1)
Parental status influences stress responsiveness and emotionality in males. Specifically, fatherhood reduces neophobia, anxiety-like behavior, and neural responses to stress.
-
2)
Individual differences in stress responsiveness and/or emotionality are associated with individual differences in paternal responsiveness. Specifically, males that show spontaneous paternal behavior toward a foster pup exhibit less neophobia, less anxiety-related behavior, and smaller neural responses to stress, as compared to males that do not behave paternally.
To test these hypotheses, we characterized behavioral responses to a foster pup, neophobia, anxiety-like behavior, and neural stress responsiveness in males that were housed (1) with a pregnant/lactating pairmate and pups, (2) with a female pairmate that was pregnant for the first time (3) with a same-sex pairmate, or (4) individually. This design allowed us to disentangle effects of fatherhood from potential effects of copulatory experience and cues from a pregnant female, as well as effects of social housing in general, on behavioral and neural stress responsiveness. We performed paternal-behavior, novel-object, and elevated-plus-maze tests to characterize paternal responsiveness, neophobia, and anxiety, respectively, and predator-urine exposure was used as an ethologically relevant stressor to characterize behavioral and neural (CRH, Fos) responses to stress.
2. Methods
2.1 Animals
We used 70 male and 36 female California mice, descendants of males and females that were purchased as adults from the Peromyscus Genetic Stock Center (University of South Carolina, Columbia, SC, USA). Mice were housed in 44 × 24 × 20 cm polycarbonate cages containing aspen shavings for bedding and cotton wool for nesting material, with food (Purina Rodent Chow 5001) and water available ad libitum. Lighting was on a 14:10 cycle, with lights on from 0500h to 1900h, room temperature at 18–26°C, and humidity at 60–70%. At 27–33 days of age, prior to the birth of the next litter of siblings, animals were ear-punched for identification, removed from their parents' cage, and housed in groups of four same-sex, age-matched cagemates until the start of the study. Animals were inspected daily, and cages and water bottles were changed once per week. Beginning at the time of pair formation or isolation (see Section 2.2), mice were weighed twice per week to monitor health and pregnancies and to habituate the animals to handling. At least 2 days elapsed between cage-changing and any experimental procedures. All procedures were conducted in accordance with the Guide for the Care and Use of Laboratory Animals and were reviewed and approved by the University of California, Riverside (UCR) Institutional Animal Care and Use Committee. UCR is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care.
2.2 Experimental design
At 105.4 ± 2.1 (mean ± SE) days of age, male California mice were randomly assigned to four housing conditions. New fathers (N=18) were housed with a female pairmate and underwent data collection following the birth of their first litter; expectant fathers (N=18) were housed with a female pairmate and underwent data collection when the female was pregnant with their first litter; paired virgins (N=16) were housed with an unrelated male; and isolated virgins (N=18) were housed alone for the duration of the study. Each experimental animal underwent a series of behavioral tests (see Sections 2.3–2.5 below) at intervals of 24–48 h. First, each male was exposed to a foster pup in a paternal-behavior test, followed 29–31 h later by exposure to a novel object, and subsequently, 24–26 h later by an elevated-plus-maze (EPM) test. Approximately 19 h following EPM tests, animals were perfused transcardially and brains were collected (see Section 2.6 below for details). Within each housing condition, approximately half of the males were euthanized following exposure to an acute stressor, and the other half were left undisturbed prior to perfusion.
Mice underwent the experimental procedures in cohorts consisting of age-matched males in the new father, expectant father, paired virgin, and isolated virgin conditions. Female pairmates of males in the new father group gave birth to litters of 1–3 pups 41.6 ± 2.2 (mean ± SE) days following pair formation (litter size for this species typically ranges from 1 to 4 pups; [37]). We did not attempt to standardize the number of pups remaining in each litter, due to the small range of litter sizes. New fathers underwent their first behavioral test, the paternal-behavior test, 2–3 days following the birth of their first litter of pups. The start of behavioral testing for all other groups was based on the number of days from pair formation to the onset of testing in the new father group (44.3 ± 2.1 days). Animals were weighed twice per week beginning 3.3 ± 2.0 days following pair formation (new fathers, expectant fathers, and paired virgins) or isolation (isolated virgins). Neither mean age at the time of the paternal-behavior test (146.5 ± 2.2 days) nor duration of the period of biweekly weighing (38.5 ± 1.9 days) differed among the four housing conditions (age at paternal test: F[3,69]=2.039, P=0.117; duration of weighing period: F[3,69]=0.731, P=0.537).
Female pairmates of new fathers and expectant fathers were euthanized after the males were removed from their cages for perfusion; their uterine horns were dissected out, and wet weights were obtained. Females were classified as pregnant following visual identification of embryos within uterine embryo sacs. Pregnant females had uterine horns weighing a total of 2.4 ± 0.6g, compared to a previously identified nonpregnant weight of 0.001 ± 0.0g (t[22]=2.141, P=0.044) typically found in virgin females (unpub data). The specific stage of pregnancy, however, was not determined. Males in the expectant father condition whose mates were not confirmed as pregnant were omitted from data analysis.
2.3 Paternal-behavior tests
Paternal-behavior tests were carried out as described previously [26,27]. Briefly, the tests were performed between 1000h and 1200h, during the light phase, in which California mice typically engage in a high degree of paternal behavior [38]. Each male was removed from its home cage and placed in a clean cage containing bedding and cotton nesting material. The mouse, in its cage, was then carried to a separate room and allowed to habituate for 10 min. An unrelated pup, age 1–4 days, was placed in the corner of the cage furthest from the male. Mice were videotaped for the duration of the 10-min test, after which the male and pup were immediately returned to their respective home cages. In five tests (N=3 isolated virgin males, 2 paired virgin males), experimental males attacked stimulus pups. These tests were terminated immediately following initiation of the attacks, and the pups were euthanized with pentobarbital (ca. 200–300 mg/kg ip; Fatal-Plus, Vortech Pharmaceuticals, Dearborn, MI, USA).
For paternal-behavior tests as well as all other behavioral tests, behavioral measures were scored from videotapes by an observer blind to housing condition, using the event-recorder program JWatcher [39]. For paternal-behavior tests, latencies to approach the foster pup, huddle the foster pup, and engage in paternal behavior (i.e., lick, huddle, or mouth-carry the foster pup), and duration of time spent mouth-carrying, sniffing, licking, huddling the pup, in kyphosis and nest-building were scored as previously described [26,27].
2.4 Novel-object tests
Novel-object tests took place in the colony room between 1630h and 1830h, nearing the start of the dark phase, when the mice begin to increase their physical activity (unpub data). Each male's cagemate(s) were removed from the home cage, and 5 min later a stainless steel, wire-mesh tea-ball (diameter: 8 cm) was placed in the corner of the cage furthest from the mouse. Behavioral responses to the novel object were recorded on videotape for the duration of the 5-min test. Immediately following testing, the male was reunited with its cagemate(s) in the home cage. Behavioral parameters scored included latency to approach to within 2 cm of the novel object, as well as duration of time spent sniffing and touching the novel object, and duration of rearing behavior.
2.5 Elevated-plus-maze (EPM) tests
EPM tests were conducted between 1630h and 1830h in an unfamiliar room. The EPM apparatus was constructed from dark, opaque polycarbonate material and consisted of two open arms (51 × 9 cm) and two closed arms (51 × 9 × 20 cm), raised 1 m above the floor. A lamp containing a 60W bulb was placed directly above the center of the apparatus to provide even illumination to all arms. Each experimental male was removed from its home cage in the colony room, carried in a small container to the unfamiliar room, and then placed in the center of the maze, facing an open arm as per the protocol of Walf and Frye [40]. Mice were videotaped in the EPM for 5 min, after which they were reunited with their cagemates. Behaviors scored in JWatcher included number and duration of open-arm, closed-arm and EPM center entries; duration of time spent immobile; and number of fecal boli expelled and head dips over the edges of the open arms. It should be noted that the EPM test has not been validated as a measure of anxiety-like behavior in California mice; therefore, results must be interpreted with caution.
2.6 Predator-urine exposure
At 1000–1100 h, animals assigned to the stress condition, along with their cagemate(s), were carried in their home cage to an unfamiliar room and allowed to habituate for 2 h. A plastic cup containing a cotton ball soaked with 1 mL coyote urine (PredatorPee.com, Lexington Outdoors, Robbinston, ME, USA) was placed in a corner of the cage for 5 min and then removed; this procedure elicits rapid and marked elevations in plasma corticosterone concentrations in California mice [36]. Animals in the undisturbed condition were left undisturbed in their home cage in the colony room. One hour following the end of predator-odor exposure for stressed mice, or between 1200h and 1300h for undisturbed mice, males were deeply anesthetized with pentobarbital (ca. 200–300 mg/kg, ip), followed by transcardial perfusion with 0.1M phosphate-buffered saline and later, 4% paraformaldehyde. This time point was utilized as Fos, CRH, and Fos/CRH expression are regularly assessed 1–2 h following stress exposure [41–44]. Brains were processed for immunohistochemistry as described below (Section 2.7).
2.7 Fos and CRH immunohistochemistry
Immunohistochemistry was performed as described previously [26,27]. Briefly, brains were sliced into 30um coronal sections on a cryostat and collected in five series. Brain slices in one series were double-stained for Fos and CRH. All slices from the series were incubated overnight with rabbit-anti-Fos antibody (sc-253, 1:5000, Santa Cruz Biotechnology, Santa Cruz, CA, USA), followed by donkey-anti-rabbit second antibody (1:1500; Jackson ImmunoResearch Laboratories, West Grove, PA, USA), then stained with 3,3'-diaminobenzidine (DAB) and ammonium nickel sulfate in order to mark Fos-positive cells as blue-black in color. To achieve Fos and CRH colocalization, the brain slices were later incubated overnight with goat-anti-CRH (1:500; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), followed by the donkey-anti-goat second antibody (1:1500; Jackson ImmunoResearch, West Grove, PA, USA) and stained with DAB, but without ammonium nickel sulfate. This resulted in a brown cytoplasmic staining that was distinguishable from the blue-black nuclear Fos staining. Individual brain sections were then mounted on gelatin/chrome-alum-coated glass slides, dehydrated, cleared in ethanol and xylene, embedded in entellan (EMS, Hatfield, PA, USA), and coverslipped.
2.8 Quantification of immunoreactivity
Due to the absence of a P. californicus brain atlas, the Mouse Brain in Stereotaxic Coordinates [45] for the neuroanatomically similar C57BL/J6 mouse was used to locate relevant brain areas [26]. Bregma levels in the text refer to levels specified in the atlas, and not actual Bregma levels in P. californicus. For the quantification of Fos-, CRH- and colocalized Fos/CRH-immunoreactivity (Fos-IR, CRH-IR, and Fos/CRH-IR, respectively), brain areas were selected based on their known functions and associations with stress responsiveness and anxiety [46,47]: the bed nucleus of the stria terminalis (BnST, mouse Bregma level +0.14), paraventricular nucleus of the hypothalamus (PVN, mouse Bregma level −0.82), and central nucleus of the amygdala (CeA, mouse Bregma level −1.46).
Standardized digital photographs of each brain area, as well as a millimeter scale, were taken at a magnification of 20× with a digital camera (Canon EOS-40D) mounted on a microscope (Leica Leitz DMRB). Using image analysis software (GNU Image Manipulation Program, Version 2.6, GIMP), a grid of lines equivalent to 0.2 × 0.2mm was placed in each photograph so that it contained either all or the majority of immunoreactive neurons in the selected area. Numbers of Fos-positive (but not CRH-positive) neurons as well as numbers of CRH-positive and Fos/CRH-colocalized neurons within the square were counted manually by an observer blind to identity, housing condition, and stress condition of the mice. Data for Fos- and CRH-positive cells are expressed as counts/200μm2, while data for Fos/CRH-colocalized neurons are expressed as the percentage of Fos-positive CRH neurons out of the total number of CRH neurons/200μm2.
The BnST and CeA showed higher densities of CRH-IR than the PVN, such that the vast majority of neurons counted in these two regions possessed either CRH-IR or Fos/CRH colocalization. Analysis of colocalization in the PVN was therefore based on the quantification of Fos-immunoreactive cells within CRH cell bodies, and for the CeA and BnST, colocalized cells were defined as those in which Fos-positive cells appeared to be immersed in CRH-rich regions.
2.9 Statistical analyses
The litters of two of the new fathers died before the end of data collection; in these cases, we used only those data that were collected prior to the death of the pups. Data from these males were comparable to those from other new fathers. In the expectant father group, three of the female pairmates gave birth prior to the completion of behavioral tests and brain collection. For the males in these pairs, we used only those data that were collected before their pairmates gave birth. Again, data from these males were comparable to those from other expectant fathers.
Behavioral and immunohistochemical data were analyzed using the Statistical Package for the Social Sciences (SPSS) v. 16.0 (IBM Corporation, Armonk, NY, USA). Variables were tested for normality using Levene's test for homogeneity of variance and the Skewness-Kurtosis test. Behavioral data were not normally distributed even after transformation (due to large numbers of zero values) and therefore were analyzed using nonparametric tests. Behavioral scores from paternal-behavior, novel-object, and EPM tests were compared among males in the four housing conditions using Kruskal-Wallis tests, and significant (P<0.0230; see below) effects were followed by nonparametric post hoc pairwise comparisons [49].
To characterize paternal responsiveness in the paternal-behavior test, for each male we calculated a composite score of total time spent engaging in licking, huddling, kyphosis, nest-building, and mouth-carrying (i.e., full paternal behavior). Each male spent either <1.0% or >20.0% of the 10-min test engaging in full paternal behavior (see Results Section 3.1). Therefore, we categorized each male as being “paternal” or “nonpaternal” based on whether or not it engaged in full paternal behavior for more than 20% of the paternal-behavior test. Mann-Whitney tests were used to compare behavior of paternal and nonpaternal males (pooled across all four housing conditions) in novel-object and EPM tests.
Numbers of Fos- and CRH-positive cells, and the percentage of CRH neurons colocalized with Fos (Fos/CRH-positive cells) in the PVN, BnST and CeA, were transformed as necessary to improve normality (see Tables 4 and 5 for transformations used). These data were then compared among animals using 2-way ANOVAs with either (1) stress (stressed, undisturbed) and housing condition (new fathers, expectant fathers, paired virgins, isolated virgins) or (2) stress and paternal responsiveness (paternal, nonpaternal) as factors. Significant main effects of housing condition were followed by Tukey's HSD post hoc tests.
Associations between paternal responsiveness, neophobia, and anxiety were quantified from correlations of paternal-behavior, novel-object, and EPM test results using Spearman's rho. The relationship between the neural response to stress and paternal responsiveness was examined by correlating stress-induced Fos-IR in the PVN and colocalized Fos/CRH-IR in the CeA with several measures of paternal behavior (see Section 3.5 below) using Spearman's rho.
Because we performed multiple statistical tests on closely related data, our Type I error rate for the entire experiment may exceed the nominal 5% alpha level. Therefore, we performed a positive false discovery rate (pFDR) analysis of our P-values using the QVALUE package (Version 1.1; [49]) for R (Version 2.8.0; R Core Development Team, 2008), allowing for 5% false significant results (pFDR = 0.05). Based on this analysis, a more appropriate and conservative alpha level for significance is α = 0.0230.
3. Results
3.1 Paternal-behavior tests
Results of paternal-behavior tests are summarized in Table 1. Males in the four housing conditions tended to differ in the latency to huddle the foster pup, as new fathers and expectant fathers tended to huddle the pup much more quickly than did both isolated and paired virgins; however, this difference was not significant after we controlled for multiple comparisons. Latency to approach the foster pup and latency to engage in paternal behavior (any one of licking, huddling, or mouth-carrying) did not differ among the four housing conditions. Furthermore, time spent huddling, licking, or sniffing the pup did not differ among housing conditions (see Table 1). Kyphosis, mouth-carrying, and nest-building occurred too infrequently to permit statistical analysis.
Table 1.
Behavioral responses (median, range) to a foster pup among male California mice in four housing conditions.
| Isolated virgins N=18 | Paired virgins N=16 | Expectant fathers N=18 | New fathers N=18 | Kruskal-Wallis P | |
|---|---|---|---|---|---|
| Paternal behavior – latency (s) | 118.2 15.9 – 600.0 |
32.7 6.5 – 600.0 |
42.2 10.6 – 600.0 |
22.7 9.4 – 600.0 |
0.082 |
| Approach pup – latency (s) | 26.6 4.9 – 273.6 |
23.1 2.1 – 600.0 |
20.9 1.6 – 600.0 |
17.5 2.7 – 600.0 |
0.848 |
| Huddle pup – latency (s) | 149.7 51.7 – 600.0 |
271.3 21.6 – 600.0 |
83.3 23.0 – 600.0 |
54.6 23.7 – 600.0 |
0.029 |
| Sniff pup – duration (s) | 23.9 0.0 – 78.0 |
15.8 0.0 – 71.0 |
27.9 0.0 – 116.0 |
7.4 0.0 – 58.0 |
0.094 |
| Lick pup – duration (s) | 298.6 0.0 – 510.2 |
225.5 0.0 – 575.4 |
267.7 0.0 – 525.3 |
307.6 0.0 – 527.4 |
0.951 |
| Huddle pup – duration (s) | 45.2 0.0 – 147.2 |
16.2 0.0 – 231.8 |
55.3 0.0 – 443.9 |
88.4 0.0 – 319.3 |
0.116 |
| Composite score for paternal behavior – duration (s)a | 346.9 0.0 – 584.1 |
306.5 0.0 – 591.6 |
419.5 0.0 – 587.8 |
442.6 0.0 – 581.2 |
0.515 |
Total time spent engaging in licking the pup, huddling the pup, mouth-carrying the pup, kyphosis, and nest-building
A composite score of full paternal behavior, compiled from durations of licking, huddling, nest-building, kyphosis and mouth-carrying, did not differ among new fathers, expectant fathers, paired virgins, and isolated virgins (see Table 1). We did, however, find a nonsignificant decrease in the proportions of males that were categorized as paternally responsive (i.e., spent ≥20% of the 10-min test engaging in paternal behavior; see Section 2.9) from new fathers (16/18, 88.9%) to expectant fathers (15/18, 83.3%) to isolated virgins (13/18, 72.2%) and finally to paired virgins (10/16, 62.5%).
By definition, paternal males (N=54) had significantly higher composite scores for full paternal behavior than nonpaternal (N=16) males. Not surprisingly, paternal and nonpaternal males also showed robust differences in the latency to approach the pup, huddle the pup, and engage in paternal behavior; and the duration of time spent sniffing, licking, and huddling the pup (P≤0.001).
3.2 Novel-object tests
New fathers, expectant fathers, paired virgins, and isolated virgins did not differ in the latency to approach the novel object or time spent touching or sniffing the novel object (Table 2). Housing condition did, however, influence the duration of time spent rearing during the novel-object test (χ2=14.601. df=3, P=0.029), generally considered to be a measure of exploratory behavior [50]. Post hoc pairwise comparisons revealed that new fathers showed a nonsignificant tendency to spend more time rearing than isolated virgins (Z=−1.783, P=0.075), but did not differ from expectant fathers (Z=−1.189, P=0.234) or paired virgins (Z=0.466, P=0.641). Rearing behavior did not differ among the remaining housing conditions.
Table 2.
Comparisons of behavioral responses (median, range) to a novel object among housing conditions and between paternally responsive and nonpaternally responsive male California mice.
| Isolated virgins N=18 | Paired virgins N=16 | Expectant fathers N=17 | New fathers N=17 | Kruskal-Wallis Pa | Paternal N=52 | Nonpaternal N=16 | Mann-Whitney Pb | |
|---|---|---|---|---|---|---|---|---|
| Approach object – latency (s) | 31.4 2.6 – 300.0 |
23.8 2.3 – 300.0 |
11.7 2.1 – 300.0 |
23.8 6.0 – 235.6 |
0.166 | 23.7 2.1 – 300.0 |
31.0 2.6 – 300.0 |
0.426 |
| Sniff object – duration (s) | 12.3 0.0 – 178.0 |
38.0 0.0 – 167.6 |
50.9 0.0 – 191.2 |
43.6 3.1 – 147.8 |
0.239 | 39.1 0.0 – 191.2 |
8.8 0.0 – 122.5 |
0.088 |
| Touch object – duration (s) | 0.1 0.0 – 171.2 |
19.0 0.0 – 186.5 |
38.9 0.0 – 232.1 |
56.2 0.0 – 222.4 |
0.240 | 33.7 0.0 – 232.1 |
6.9 0.0 – 154.1 |
0.243 |
| Rear – duration (s) | 0.0 0.0 – 0.9 |
0.2 0.0 – 2.6 |
0.5 0.0 – 5.2 |
0.5 0.0 – 8.0 |
0.002 | 0.2 0.0 – 8.0 |
0.1 0.0 – 6.6 |
0.921 |
P-values from Kruskal-Wallis tests comparing isolated virgins, paired virgins, expectant fathers, and new fathers
P-values from Mann-Whitney U tests comparing paternal and nonpaternal males
Bold indicates P-values that are statistically significant following pFDR procedure (see Methods).
Paternal and nonpaternal animals showed no significant differences in their behavioral responses to the novel object. Interestingly, however, paternal males showed a nonsignificant tendency to sniff the novel object for longer durations than nonpaternal males (Mann-Whitney test, Z=−1.708, P=0.088; see Table 2).
3.3 Elevated-plus-maze tests
Male California mice tended to fall and/or jump off the open arms of the EPM, resulting in 14 animals (4 isolated virgins, 2 paired virgins, 4 expectant fathers, 4 new fathers) being omitted from statistical analyses. A further five animals (1 isolated virgin, 2 expectant fathers, 2 new fathers) remained completely immobile for extended durations in the center or open arms of the EPM (>40% of testing time), and were thus eliminated from analyses of EPM data. It should be noted that two new fathers and one expectant father were not subjected to EPM tests due to other factors described above section (see Section 2.9), and data from one paired virgin male were omitted due to technical issues with the video recording. The final analyses used data from 13/18 isolated virgins, 13/16 paired virgins, 11/18 expectant fathers, and 10/18 new fathers.
New fathers, expectant fathers, paired virgins and isolated virgins differed in the frequency of open-arm entries (χ2=9.894, P=0.019) and head dips (χ2=11.514, P=0.009), and in the time spent in the center of the EPM (χ2=11.243, P=0.010)(Table 3). Post hoc pairwise comparisons showed that new fathers spent significantly more time in the center of the maze than expectant fathers (Z=−2.464, P=0.014), paired virgins (Z=2.482, P=0.013), and isolated virgins (Z=−2.727, P=0.006). Both new fathers and paired virgins performed a greater number of head dips, considered to be an index of exploratory behavior [51], as compared to isolated virgins (Z=−2.759, P=0.006; Z=−2.127, P=0.003, respectively) but not expectant fathers (Z=−1.656, P=0.098; Z=−1.536, P=0.125, respectively). Finally, new fathers and paired virgins entered the open arms of the EPM at greater frequencies than expectant fathers (Z=−2.254, P=0.024; Z=−2.320, P=0.020, respectively) and isolated virgins (new fathers only: Z=−2.110, P=0.035).
Table 3.
Comparisons of behaviors (median, range) performed in an elevated plus maze among housing conditions and between paternally and nonpaternally responsive male California mice.
| Isolated virgins N=13 | Paired virgins N=13 | Expectant fathers N=11 | New fathers N=10 | Kruskal-Wallis Pa | Paternal N=35 | Nonpaternal N=12 | Mann-Whitney Pb | |
|---|---|---|---|---|---|---|---|---|
| % Time in closed arms | 0.510 0.228 – 0.995 |
0.303 0.146 – 0.928 |
0.455 0.199 – 0.745 |
0.387 0.230 – 0.534 |
0.060 | 0.345 0.199 – 0.995 |
0.354 0.146 – 0.622 |
0.329 |
| % Time in center | 0.168 0.004 – 0.368 |
0.177 0.070 – 0.404 |
0.185 0.121 – 0.360 |
0.261 0.192 – 0.376 |
0.010 | 0.192 0.004 – 0.404 |
0.176 0.026 – 0.282 |
0.232 |
| % Time in open arms | 0.320 0 – 0.713 |
0.490 0 – 0.722 |
0.355 0.096 – 0.568 |
0.385 0.096 – 0.452 |
0.076 | 0.378 0 – 0.713 |
0.473 0.095 – 0.722 |
0.045 |
| % Time immobile | 0.048 0 – 0.346 |
0.006 0 – 0.143 |
0.004 0 – 0.176 |
0 0 – 0.103 |
0.272 | 0.004 0 – 0.196 |
0.005 0 – 0.349 |
0.969 |
| Head-dips (no.) | 4 0 – 14 |
11 0 – 44 |
4 0 – 32 |
15.5 0 – 33 |
0.009 | 5 0 – 33 |
9.5 0 – 44 |
0.346 |
| Entries into closed arms (no.) | 14 2 – 42 |
16 6 – 23 |
15 7 – 38 |
17.5 8 – 24 |
0.810 | 15 2 – 38 |
17.5 3 – 42 |
0.608 |
| Entries into center (no.) | 27 1 – 68 |
35 6 – 53 |
28 16 – 50 |
38.5 19 – 45 |
0.253 | 29 1 – 50 |
35.5 4 – 68 |
0.179 |
| Entries into open arms (no.) | 12 0 – 27 |
20 0 – 31 |
11 5 – 20 |
20.5 8 – 28 |
0.019 | 12 0 – 28 |
21 2 – 31 |
0.023 |
| Fecal pellets (no.) | 4 0 – 11 |
3 0 – 13 |
0 0 – 8 |
0.5 0 – 11 |
0.204 | 1 0 – 13 |
0.5 0 – 8 |
0.369 |
| Total entries (no.) | 27 2 – 69 |
35 6 – 53 |
29 18 – 49 |
38 19 – 45 |
0.270 | 31 2 – 49 |
36 5 – 69 |
0.160 |
Bold indicates P-values that are statistically significant following pFDR procedure (see Methods).
Paternal (N=35) and nonpaternal (N=12) animals differed in the number of open-arm entries. Interestingly, nonpaternal males had a greater number of entries into the open arms of the EPM (Z=−2.273, P=0.023), and showed a non-significant tendency to spend more time in the open arms (P=0.045), than paternal males. Paternal and nonpaternal males did not differ statistically in any other behavior in the EPM.
3.4 Predator-urine stress-induced Fos and CRH expression in stress-related brain regions
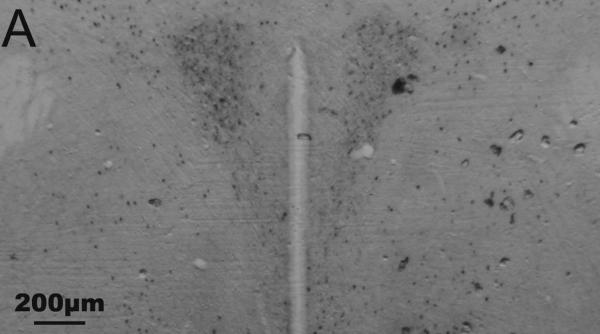
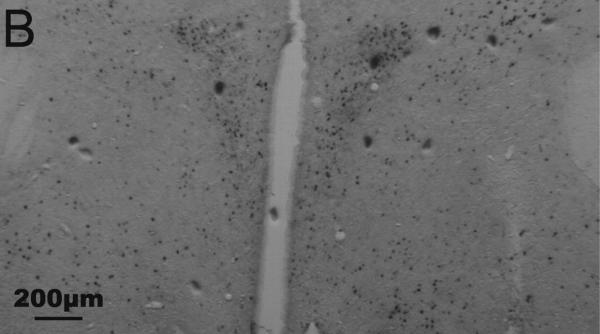
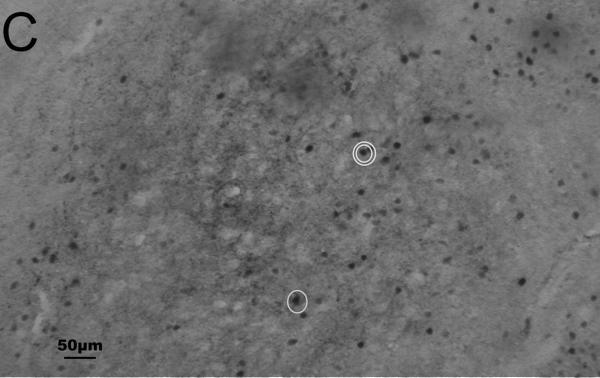
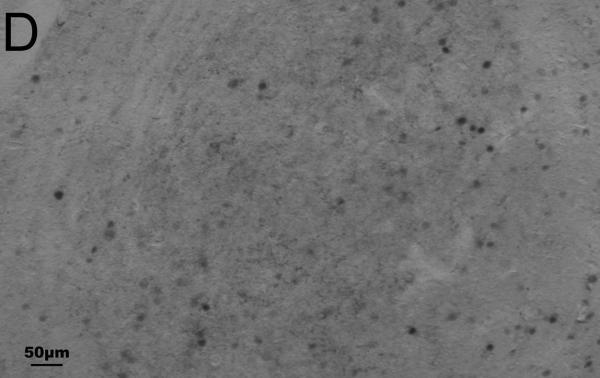
Representative photomicrographs of Fos and CRH staining in the PVN and CeA are shown in Fig. 1. Results are summarized in Tables 4 and 5.
Fig. 1.
Representative photomicrographs of immunohistochemical staining of Fos and corticotropin-releasing hormone (CRH) in the paraventricular nucleus of the hypothalamus (A, B) and central nucleus of the amygdala (C, D) of undisturbed (A, C) and stressed (B, D) male California mice. Fos is stained blue-black (nuclear staining); CRH is stained reddish brown (cytoplasmic staining). A, B: equivalent of mouse Bregma −0.82 mm; C, D: equivalent of mouse Bregma −1.46 mm. Figs. A and B were taken at 2.5× magnification; Figs. C and D were taken at 10× magnification. Fos/CRH colocalized cells are circled once, and Fos-only-immunoreactive cells are circled twice in C. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Table 4.
Numbers (mean ± SE) of Fos-IR and CRH-IR neurons, and percentage of Fos/CRH-IR neurons, in the hypothalamic paraventricular nucleus (PVN), bed nucleus of the stria terminalis (BnST), and central nucleus of the amygdala (CeA) of male California mice either following 5-minute exposure to a predator-urine stressor or under undisturbed conditions.
| Isolated virgins | Paired virgins | Expectant fathers | New fathers | 2-Way ANOVA P-values | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Undisturb. (N=10) | Stressed (N=7–8) | Undisturb. (N=5) | Stressed (N=5–7) | Undisturb. (N=6) | Stressed (N=6–7) | Undisturb. (N=7–9) | Stressed (N=6–7) | Among groups | Stressed vs. undisturbed | Group × stress | |
| PVN - Fos-IRa,b | 13.2 ± 3.6 | 20.3 ± 4.3 | 18.8 ± 5.1 | 41.0 ± 4.3 | 22.5 ± 4.7 | 38.3 ± 4.7 | 23.1 ± 3.8 | 31.8 ± 4.7 | 0.001 | <0.001 | 0.596 |
| PVN - CRH-IRa,b | 19.5 ± 2.3 | 16.9 ± 2.7 | 10.6 ± 3.2 | 8.9 ± 2.7 | 12.0 ± 3.0 | 10.0 ± 3.0 | 10.6 ± 2.4 | 11.7 ± 3.0 | 0.011 | 0.409 | 0.918 |
| PVN - Fos/CRH-IRc | 32.0 ± 7.1 | 38.4 ± 8.4 | 62.7 ± 10.0 | 68.0 ± 8.4 | 57.1 ± 9.1 | 63.5 ± 9.1 | 60.9 ± 7.4 | 56.8 ± 9.1 | 0.003 | 0.566 | 0.907 |
| BnST - Fos-IRa | 1.43 ± 0.24 | 0.96 ± 0.29 | 1.85 ± 34 | 1.88 ± 0.34 | 1.47 ± 0.31 | 2.43 ± 0.29 | 1.79 ± 0.29 | 1.51 ± 0.31 | 0.048 | 0.784 | 0.083 |
| BnST - CRH-IRa,b | 4.5 ± 1.2 | 8.3 ± 1.4 | 4.6 ± 1.7 | 2.2 ± 1.7 | 6.0 ± 1.5 | 4.0 ± 1.4 | 5.3 ± 1.4 | 3.5 ± 1.5 | 0.322 | 0.620 | 0.147 |
| BnST - Fos/CRH-IRc,d | 75.3 ± 4.8 | 55.6 ± 5.7 | 76.6 ± 6.8 | 79.8 ± 6.8 | 75.6 ± 6.2 | 77.1 ± 6.2 | 75.0 ± 5.7 | 85.8 ± 6.2 | 0.079 | 0.898 | 0.125 |
| CeA - CRH-IRa,b | 5.0 ± 1.7 | 11.6 ± 1.9 | 9.6 ± 2.4 | 4.0 ± 2.4 | 7.7 ± 2.2 | 3.7 ± 2.0 | 10.6 ± 1.9 | 6.9 ± 2.0 | 0.350 | 0.147 | 0.008 |
| CeA - Fos/CRH -IRc | 79.4 ± 5.6 | 51.7 ± 6.2 | 64.6 ± 7.9 | 81.5 ± 7.9 | 68.5 ± 7.2 | 84.2 ± 6.7 | 69.5 ± 6.2 | 78.7 ± 6.7 | 0.341 | 0.474 | 0.002 |
Total number of immunoreactive neurons in a 0.2 × 0.2 mm square
Square-root transformed prior to analysis (non-transformed data are shown for clarity)
Percent of Fos-positive CRH-containing neurons in a 0.2 × 0.2 mm square
Transformed by raising to the 2.5 power prior to analysis (non-transformed data are shown for clarity)
Bold indicates P-values that are statistically significant following pFDR procedure (see Methods).
Table 5.
Number (mean ± SE) of Fos-IR and CRH-IR neurons, and percentage of Fos/CRH-IR neurons, in the hypothalamic paraventricular nucleus (PVN), bed nucleus of the stria terminalis (BnST), and central nucleus of the amygdala (CeA) of paternally responsive and nonpaternally responsive male California mice either following 5-minute exposure to a predator-urine stressor or under undisturbed conditions.
| Paternal | Nonpaternal | 2-Way ANOVA P-values | |||||
|---|---|---|---|---|---|---|---|
| Undisturbed (N=23–25) | Stressed (N=18–20) | Undisturbed (N=5) | Stressed (N=6–8) | Paternal vs. nonpaternal | Stressed vs. undisturbed | Paternal × stress interaction | |
| PVN - Fos-IRa,b | 18.6 ± 1.9 | 28.8 ± 3.2 | 20.8 ± 4.0 | 41.5 ± 6.3 | 0.081 | 0.001 | 0.356 |
| PVN - CRH-IRa | 13.7 ± 1.5 | 13.8 ± 2.0 | 14.6 ± 4.4 | 7.8 ± 2.0 | 0.310 | 0.181 | 0.169 |
| PVN - Fos/CRH-IRc | 49.7 ± 4.8 | 51.7 ± 5.9 | 56.2 ± 10.6 | 67.2 ± 9.2 | 0.171 | 0.422 | 0.573 |
| BnST - Fos-IRa | 2.8 ± 0.4 | 4.2 ± 1.1 | 3.2 ± 0.6 | 2.8 ± 1.0 | 0.879c | 0.859 | 0.413 |
| BnST - CRH-IRa,b | 5.3 ± 0.8 | 4.6 ± 1.0 | 4.0 ± 1.3 | 5.0 ± 1.1 | 0.890 | 0.770 | 0.380 |
| BnST - Fos/CRH-IRc,d | 74.5 ± 2.8 | 71.2 ± 6.2 | 79.9 ± 6.0 | 68.7 ± 5.0 | 0.729 | 0.432 | 0.176 |
| CeA - CRH-IRa,b | 8.0 ± 1.2 | 6.3 ± 1.3 | 7.6 ± 2.2 | 8.7 ± 1.9 | 0.608 | 0.763 | 0.371 |
| CeA - Fos/CRH-IRc | 73.4 ± 3.3 | 75.5 ± 4.8 | 64.6 ± 10.2 | 64.6 ± 8.8 | 0.132 | 0.875 | 0.874 |
Total number of immunoreactive neurons in a 0.2 × 0.2 mm square
Square-root transformed prior to analysis (non-transformed data are shown for clarity)
Percent of Fos-positive CRH-containing neurons in a 0.2 × 0.2 mm square
Transformed by raising to the 2.5 power prior to analysis (non-transformed data are shown for clarity)
Bold indicates P-values that are statistically significant following pFDR procedure (see Methods).
Paraventricular nucleus of the hypothalamus (PVN)
As expected, numbers of Fos-IR neurons in the PVN were higher in stressed mice than in undisturbed mice (main effect of stress: F[1,48]=18.874, P<0.001, see Table 4). For the stressed and undisturbed conditions combined, numbers of Fos-IR neurons in the PVN differed significantly among males in the four housing conditions (main effect of housing condition: F[3,48]=6.067, P=0.001): paired virgins, expectant fathers, and new fathers all had higher numbers of Fos-IR neurons in the PVN compared to isolated virgins (Tukey's HSD test, P=0.002, P=0.002, P=0.0.01, respectively). We did not, however, find a significant interaction between housing condition and stress. Paternal males showed a nonsignificant tendency to exhibit lower Fos-IR in the PVN than nonpaternal males (F[1,52]=3.158, P=0.081); again, however, no interaction with stress was found (see Table 5).
Numbers of CRH-IR neurons in the PVN showed a significant main effect of housing condition (F[3,48]=4.106, P=0.011). Post hoc tests revealed that expectant fathers and paired virgins had lower overall CRH-IR for the stressed and undisturbed conditions combined than isolated virgins (P=0.053, P=0.008, respectively). CRH-IR in the PVN did not differ between stressed and undisturbed animals or between paternal and nonpaternal animals, and no significant interactions were found between stress and housing condition or stress and paternal responsiveness (see Tables 4 and 5).
A main effect of housing condition was found in the percentage of CRH neurons colocalized with Fos in the PVN (F[3,48]=5.431, P=0.003). New fathers, expectant fathers, and paired virgins showed greater activation of CRH neurons than isolated virgins (P=0.016, P=0.019, P=0.003, respectively) for the stressed and undisturbed conditions combined. Again, Fos/CRH-IR in the PVN did not differ between stressed and undisturbed animals or between paternal and nonpaternal animals (see Tables 4 and 5).
Central nucleus of the amygdala (CeA)
Numbers of animals expressing Fos-IR (excluding Fos/CRH-colocalization) in the CeA were too low to permit statistical analysis. For numbers of CRH- and colocalized Fos/CRH-IR neurons in the CeA, no main effects of stress or housing condition were observed; however, significant interactions between stress and housing condition were found (CRH-IR: F[3,48]=4.451, P=0.008; Fos/CRH-IR: F[3,48]=5.637, P=0.002). One-way ANOVAs revealed that among isolated virgins, stressed mice exhibited significantly more CRH-IR cells (F[1,16]=8.312, P=0.011) and a significantly lower percentage of colocalized Fos/CRH-IR cells (F[1,16]=10.796, P=0.005) in the CeA than undisturbed mice. In contrast, new fathers, expectant fathers, and paired virgins showed the opposite pattern, although these trends were not statistically significant. Again, no differences were found between paternal and nonpaternal animals (see Table 5).
Bed nucleus of the stria terminalis (BnST)
Analyses of numbers of Fos-IR and CRH-IR neurons, and percentages of colocalized Fos/CRH-IR neurons in the BnST, revealed no significant effects of stress, housing condition, or paternal responsiveness (see Tables 4 and 5).
3.5 Correlational analyses
Results of correlational analyses involving male mice from all four housing conditions are summarized in Table 6. To evaluate associations between paternal behavior and emotionality, we performed correlational analyses using the behavioral parameters that we considered the best measures of neophobia (novel-object test: time spent sniffing and latency to approach the novel object), anxiety (EPM test: total time spent in the open arms) and paternal responsiveness (paternal-behavior test: latency to engage in paternal behavior, and duration of time spent licking the foster pup and in full paternal behavior). We found a significant positive relationship between the latency to engage in paternal behavior and the latency to approach a novel object for all animals analyzed together (r=0.372, P=0.0018, N=68). When we performed correlational analyses for each housing condition individually, we found statistically significant positive correlations between latency to engage in paternal behavior and latency to approach a novel object for new fathers (r=0.723, P=0.001, N=17) and for paired virgins (r=0.590, P=0.016, N=16), but not for expectant fathers or isolated virgins. No other significant associations between paternal behavior and neophobia or anxiety were found for all four housing conditions combined or for any of the housing conditions analyzed individually.
Table 6.
Spearman correlation coefficients for comparisons of behavior in paternal-behavior tests with behavior in novel-object and elevated-plus-maze (EPM) tests, and with number of Fos-IR neurons in the paraventricular nucleus of the hypothalamus (PVN) and percentage of colocalized Fos/CRH neurons in the central nucleus of the amygdala (CeA) per 200um2. All analyses used male California mice from all four housing conditions (isolated virgins, paired virgins, expectant fathers, and new fathers). Analyses involving Fos-IR and Fos/CRH-IR utilized only the subset of animals in each housing condition that were exposed to a predator-urine stressor,
| Lick pup - duration | Full paternal behavior - duration | Paternal behavior - latency | |
|---|---|---|---|
| Approach object – latency (N=68) | −0.129 | −0.202 | 0.372a |
| Sniff object – duration (N=68) | 0.193 | 0.223 | −0.192 |
| In open arms of EPM – duration (N=47) | −0.191 | −0.261 | 0.070 |
| Fos-IR in PVN (N=26) | −0.231 | −0.253 | 0.218 |
| Fos/CRH-IR in CeA (N=27) | 0.148 | 0.162 | −0.191 |
P = 0.0018
To evaluate possible associations between paternal behavior and stress responsiveness, we compared numbers of Fos-IR PVN neurons and the percentage of colocalized Fos/CRH-IR CeA neurons in response to stress with paternal behavior (latency to engage in paternal behavior, and duration of time spent licking the foster pup and in full paternal behavior); results are summarized in Table 6. No significant correlations were found between paternal responsiveness and stress-induced Fos-IR in the PVN or the percentage of Fos/CRH-colocalized neurons in the CeA among stressed animals from all four housing conditions combined. When analyzed separately, expectant fathers showed a strong negative correlation between the number of Fos-IR PVN neurons and duration of time spent performing full paternal behavior (r=−0.886, P=0.019, N=6). No other significant relationships were found between measures of stress responsiveness and paternal behavior for individual treatment groups (results not shown).
4. Discussion
Reductions in neophobia, anxiety, and stress responsiveness have been implicated in facilitating the onset of maternal behavior in female rodents. In this study, we attempted to determine whether levels of neophobia, anxiety, and stress responsiveness are similarly associated with the degree of paternal responsiveness or housing condition in biparental, monogamous, male California mice. We hypothesized that neophobia, anxiety-like behavior, and neural stress responsiveness would be (1) lower in new fathers than in nonfathers, and (2) lower in paternally responsive males as compared to nonpaternally responsive males. Overall, we found little evidence in support of either hypothesis, although we did find evidence that individual differences in latency to engage in paternal behavior may be associated with individual differences in generalized neophobia (as indicated by behavioral responses to a novel object).
Effects of housing condition
In the current study, no differences were found in several common measures of paternal behavior among males in the four housing conditions. Latency to engage in paternal behavior and time spent huddling and licking the foster pup were similar in isolated virgin males, paired virgin males, expectant fathers, and new fathers. In contrast, Gubernick and Nelson [33] found that new fathers had shorter latencies to approach foster pups than both expectant fathers and paired virgins, a finding that was replicated in a recent study in our lab [26]. In the present study, however, we did find that the proportion of paternally behaving males decreased non-significantly from new fathers to expectant fathers to isolated virgins to paired virgins. Moreover, both new and expectant fathers tended to have shorter latencies to huddle the foster pup than isolated and paired virgins. Overall, these results are consistent with previous findings that new fathers behave more paternally than nonfathers, although not as robustly as previously described [26,52,53]. New fathers may exhibit parturition-induced behavioral modifications as observed in females (see [7] for review) that are likely mediated by pheromonal cues from their pregnant pairmates [54]. It is unknown why fathers and virgin males express different levels of paternal behavior, at least in the literature. Some possibilities that have been explored include the role of the pregnant pairmate in modulating the male's neurobiology and behavior [54,55], the role of prior paternal experience [35,56], and the role of hormones and neuropeptides such as prolactin and oxytocin [33,57]. There is currently not sufficient evidence supporting any of these hypotheses [58].
Similar to the present findings, Bardi et al. [35] and Lambert et al. [56] found no differences in paternal responsiveness between new fathers and virgin males, including virgin males that either had or had not previously interacted with pups. The high level of paternal behavior observed in virgins in the current study, along with that observed by Bardi et al. [35] and Lambert et al. [56], contrasts strikingly with findings by Gubernick and colleagues [52,53], in which more than half of adult, pair-housed virgin males and expectant fathers ignored or attacked foster pups. The California mice used by Gubernick and colleagues were likely genetically closer to the wild-type population than our animals, as their studies were performed on ninth-generation descendants of wild-caught California mice [57], in contrast to animals in our colony (and that of Bardi et al., [35] and Lambert et al., [56]) that are descendants of animals captured between 1979 and 1987. Another possible contributing factor is the time of day at which paternal-behavior tests were performed, as California mice engage in greater durations of paternal behavior during the light phase of the cycle than during the dark phase [38]. Neither Gubernick and colleagues [52,53], nor Bardi et al. [35] and Lambert et al. [56] specified the time at which paternal-behavior tests were performed; it is therefore difficult to conclude if this could provide an explanation for the differences in findings.
Neophobia, as assessed on the basis of behavioral responses to a novel, wire-mesh tea-ball, did not differ significantly among males in the four housing conditions. Correspondingly, a prior study in our lab found that behavioral and neural (Fos) responses to the same novel wire-mesh ball did not differ across housing conditions (new fathers, expectant fathers, and paired virgins) in male California mice unless a foster pup was present inside the ball [26]. Recently, Bardi et al. [35] found no differences in neophobic responses to a wooden half-log stimulus in California mice among fathers, pup-exposed paired virgin males, and pup-naïve paired virgin males. Taken together, these data suggest that neophobia and fearfulness in males of this species are not strongly influenced by social housing conditions or reproductive status. In contrast, avoidance of foster pups and longer latencies to engage in maternal behavior in virgin female rats, as compared to lactating females, have been attributed to virgins' increased fearfulness and neophobia [1,9]. A possible explanation for this difference in findings could lie in the absence of distinct elevations in centrally acting hormones/neuropeptides, such as oxytocin and prolactin, in new fathers comparable to those occurring in lactating dams [14,21,59,60–65]. While oxytocin and prolactin likely work in concert to mediate the reductions in fear, anxiety and stress responsiveness observed in lactating females [21,64,66], it is yet to be determined whether elevated levels of plasma prolactin found in California mouse fathers [33] potentially perform a similar central function. No differences in peripheral oxytocin levels were found in new fathers compared to nonfathers [57], and effects of fatherhood on central expression of oxytocin or prolactin have not been described (but see [26]).
In EPM tests, paired virgin males and new fathers had greater frequencies of entries into the open arms and performed more head-dips than isolated virgin males and expectant fathers, indicating reduced anxiety and increased exploration, respectively, in the paired virgins and new fathers [40,67,68]. These data are difficult to interpret: we had expected new fathers and expectant fathers to exhibit similar behavioral profiles, as males in both of these groups had prior sexual experience, engagement in a pair-bond, and exposure to a pregnant female, whereas paired virgins and isolated virgins had experienced none of these. Rats in the late stages of pregnancy exhibit higher levels of anxiety-like behavior in the elevated plus maze than lactating and virgin females [69–71]; thus, it is possible that anxiety levels increase during the prepartum period in new fathers as well as new mothers in biparental species. The finding that isolated virgin males showed more anxiety-like behavior in the EPM than paired virgins is consistent with previous findings that social isolation increases anxiety and enhances behavioral stress responses in rodents [72–74], and suggests that male-male relationships can have beneficial effects on emotionality in this monogamous species. In sum, the results of the EPM test suggest that long-term social relationships in general may reduce anxiety in male California mice, but that anxiety-like behavior may increase during the prepartum period. It should be noted that acute separation from their pups might have increased anxiety in new fathers, who otherwise may have shown lower anxiety than nonfathers. This is unlikely as the same procedures have been carried out in rat dams, which still exhibit reduced anxiety compared to virgin females [3]. It should also be noted, however, that the EPM has not previously been validated as an anxiety test in California mice, and as this species is considered to be semi-arboreal [75], it is possible that confinement in an EPM is not a stressful or anxiogenic experience.
The results from the current study did not support our hypothesis that fatherhood blunts the neural response to stress. New fathers did not show significant differences in the Fos, CRH, or colocalized Fos/CRH response to predator odor in the PVN, BnST or CeA in comparison to expectant fathers or paired virgins. Virgin males that were individually housed, however, showed elevated CRH in the CeA and reduced activation of CRH neurons in the CeA in response to stress, as compared to the other housing conditions. Furthermore, overall numbers of Fos-positive cells were reduced, numbers of CRH-positive cells increased, and activation of CRH neurons decreased in the PVN of stressed and undisturbed isolated virgins compared to the other housing conditions. Typically, stressors such as immobilization and predator odor elicit increased activation of CRH neurons in the PVN, as well as increased Fos and CRH mRNA in the PVN, BnST, amygdala, and hippocampus [43,76–78]. It is likely that prolonged social isolation was a form of chronic stress that caused a dysregulation of the HPA axis and its central regulatory mechanisms in our isolated virgin males [79–87]. Together, these findings suggest that social isolation functions as a chronic stressor in the California mouse, a highly social species, and that isolation stress increases central expression of CRH while downregulating neuronal activation. Exposure to acute stress in the presence of a familiar conspecific, meanwhile, likely preserved normal functioning of the HPA system in new fathers, expectant fathers, and paired virgins.
Individual differences in paternal responsiveness
We had anticipated that paternally behaving males, regardless of housing condition, would show less neophobic behavior (e.g., reduced latencies to approach the novel object), less anxiety-like behavior (e.g., increased entries into and increased time spent in the open arms of the EPM), and reduced stress responsiveness (e.g., less Fos-IR and Fos/CRH colocalization in the PVN and CeA following exposure to predator urine), as compared to nonpaternally behaving males. Contrary to these predictions, we found that individual differences in paternal responsiveness were not associated with differences in neophobic behaviors or in the neural response to stress. These results suggest that reductions in generalized fearfulness and neophobia are not necessary for the display of paternal responsiveness in California mice. This does not rule out the possibility that some associations do exist between paternal responsiveness and neophobia, however, as we found that, for all animals, the latency to approach the novel object during novel-object tests correlated positively with the latency to engage in paternal behavior in paternal-behavior tests. Correspondingly, maternally experienced female rats characterized by high novelty-seeking behavior were also found to have shorter latencies to engage in maternal behavior and longer durations of maternal behavior towards foster pups (although not towards their own pups) than maternally experienced females characterized by less novelty-seeking behavior [88].
Unexpectedly, we found evidence of reduced anxiety-related behavior in nonpaternal males compared to paternal males. Compared to paternally responsive males, nonpaternal males made significantly more entries into, and tended to spend more time in, the open arms of the EPM, typically thought to signify low anxiety [40,67]. In contrast, lactating female rats (exposed to their own pups) were found to exhibit reduced anxiety-related behavior in the EPM when compared to virgin females (exposed to no pups; [3,22,89]), although animals in these studies were not compared on the basis of individual differences in maternal responsiveness. Interestingly, both pup-sensitized virgins and lactating female rats were found to spend more time in and perform more entries into the open arms of the EPM than ovariectomized females [90]. Bardi et al. [35] found reduced numbers of interrupted grooming sequences, described as indicating low stress-related behavior, in paternally experienced male California mice and “adequate parents” (males that behaved paternally) as compared to both paternally inexperienced animals and “inadequate parents” (males that attacked pups). These findings from Pereira et al. [90] and Bardi et al. [35] suggest that animals that behave parentally, as well as animals that have parental experience, show dampened behavioral stress responsiveness. This appears to contradict our findings, which suggested that nonpaternal males exhibited less anxiety-related behavior in EPM tests. One possible explanation is that because EPM data from numerous mice were omitted, as these individuals fell or jumped off the EPM during testing, our behavioral results might have been skewed in favor of housing conditions or behavioral phenotypes in which animals were less likely to fall off the maze. It is also possible that, as mentioned above, entries into and time spent in the open arms of the EPM are not appropriate measures of anxiety in this species. Future studies are needed to further clarify the relationship between paternal responsiveness and anxiety in male California mice.
We did not find any significant relationships between paternal responsiveness and stress-induced neuronal activation in the PVN or CeA. A recent study in male California mice showed that composite scores of paternal behavior correlated negatively with Fos immunoreactivity in the PVN in response to 10 min of pup-exposure [56], suggesting that paternal responsiveness may be associated with reduced neural stress responses to an unfamiliar pup stimulus. It should be noted, though, that in the Lambert et al. [56] study, more than one pup-exposure event occurred over a period of several days, and this could have influenced stress responsiveness in ways that a single pup-exposure does not. These data point to a relationship between decreased paternal responsiveness and increased activation of anxiety-related brain areas following exposure to a pup. These findings, in conjunction with the results of the present study, suggest that behavioral responses to a foster pup correlate with neural responses to the same stimulus and are inversely associated with anxiety induced by the pup, but do not correlate with neural activation or anxiety levels in response to other, non-pup-related stimuli.
5. Conclusions
We conclude that paternal responsiveness in the biparental California mouse, unlike maternal responsiveness in female rats, is not associated with systematic alterations or modifications of the stress and anxiety systems. Furthermore, anxiety, as assessed on the basis of an elevated-plus-maze test, was not lower in paternally responsive males as compared to nonpaternally responsive males. Nonetheless, rapidity to approach a novel object was positively correlated with rapidity to engage in paternal behavior, suggesting that attraction to novelty, typically indicative of reduced fearfulness, may play a role in the initiation of paternal behavior. Finally, the findings in this study point to a lack of homology between the mechanisms underlying the onset of maternal and paternal behavior in species of rodents.
Research Highlights
-
-
Associations were found between latency to paternal behavior and to approach the novel object.
-
-
New fathers were less anxious than expectant fathers and isolated virgins
-
-
Paternal males were more anxious than nonpaternal males
-
-
Stress elevated Fos in the hypothalamic paraventricular nucleus in all animals
-
-
Social isolation, and not fatherhood, modulated neuroendocrine stress responsiveness
Acknowledgements
The authors would like to thank Leslie Karpinski, Jim Sinclair, and John Kitasako for their excellent caretaking of the mice, Dr. Akiko Sato for veterinary care, Dr. Antoon Ploeg and Scott Edwards for the use of and assistance with the microscope/camera set-up, and Dr. Manuela Martins-Green for use of her environmental chamber. We would also like to thank Marian Antonious, Kristine Bersalona, Joseph Chang, Julia Cho, Michael Feldmeier, Dylan McCreary, Kevin Measor, and Vanessa Yang for their assistance in the lab; and Breanna Harris, Dr. Kristine Kaiser, Juan Pablo Perea-Rodriguez, and three anonymous reviewers for comments on an earlier draft of the manuscript. This research was supported in part by NIH grant MH087806.
Abbreviations
- CRH
corticotropin-releasing hormone
- PVN
paraventricular nucleus of the hypothalamus
- BnST
bed nucleus of the stria terminalis
- CeA
central nucleus of the amygdala
- CRH-IR
corticotropin-releasing hormone immunoreactiv(e)(ity)
- Fos-IR
Fos immunoreactiv(e)(ity)
- Fos/CRH-IR
colocalized Fos and CRH immunoreactiv(e)(ity)
- ACTH
adrenocorticotropic hormone
- CORT
corticosterone
- EPM
elevated plus maze
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Fleming AS, Luebke C. Timidity prevents the virgin female rat from being a good mother: Emotionality differences between nulliparous and parturient females. Physiol Behav. 1981;27:863–8. doi: 10.1016/0031-9384(81)90054-8. [DOI] [PubMed] [Google Scholar]
- [2].Hard E, Hansen S. Reduced fearfulness in the lactating rat. Physiol Behav. 1985;35:641–3. doi: 10.1016/0031-9384(85)90155-6. [DOI] [PubMed] [Google Scholar]
- [3].Lonstein JS. Reduced anxiety in postpartum rats requires recent physical interactions with pups, but is independent of suckling and peripheral sources of hormones. Horm Behav. 2005;47:241–55. doi: 10.1016/j.yhbeh.2004.11.001. [DOI] [PubMed] [Google Scholar]
- [4].Toufexis DJ, Rochford J, Walker CD. Lactation-induced reduction in rats' acoustic startle is associated with changes in noradrenergic neurotransmission. Behav Neurosci. 1999b;113:176–84. doi: 10.1037//0735-7044.113.1.176. [DOI] [PubMed] [Google Scholar]
- [5].Windle RJ, Wood S, Shanks N, Perks P, Gonde GL, da Costa APC, et al. Endocrine and behavioural responses to noise stress: comparison of virgin and lactating female rats during non-disrupted maternal activity. J Neuroendocrinol. 1997;9:407–14. doi: 10.1046/j.1365-2826.1997.00587.x. [DOI] [PubMed] [Google Scholar]
- [6].Kinsley CH, Lambert KG. Reproduction-induced neuroplasticity: natural behavioral and neuronal alterations associated with the production and care of offspring. J Neuroendocrinol. 2008;20:515–25. doi: 10.1111/j.1365-2826.2008.01667.x. [DOI] [PubMed] [Google Scholar]
- [7].Lonstein JS. Regulation of anxiety during the postpartum period. Front Neuroendocrinol. 2007;28:115–41. doi: 10.1016/j.yfrne.2007.05.002. [DOI] [PubMed] [Google Scholar]
- [8].Fleming AS, Rosenblatt JS. Olfactory regulation of maternal behavior in rats: II. Effects of peripherally induce anosmia and lesions of the lateral olfactory tract in pup-induced virgins. J Comp Physiol Psych. 1974;86:233–46. doi: 10.1037/h0035936. [DOI] [PubMed] [Google Scholar]
- [9].Numan M, Insel TR. The Neurobiology of Parental Behavior. Springer-Verlag; New York: 2003. [Google Scholar]
- [10].Fleming AS, Vaccarino F, Tambosso L, Chee P. Vomeronasal and olfactory system modulation of maternal behavior in the rat. Science. 1979;203:372–4. doi: 10.1126/science.760196. [DOI] [PubMed] [Google Scholar]
- [11].Fleming AS, Vaccarino F, Luebke C. Amygdaloid inhibition of maternal behavior in the nulliparous female rat. Physiol Behav. 1980;25:731–43. doi: 10.1016/0031-9384(80)90377-7. [DOI] [PubMed] [Google Scholar]
- [12].Numan M. Motivational systems and the neural circuitry of maternal behavior in the rat. Dev Psychobiol. 2007;491:12–21. doi: 10.1002/dev.20198. DOI 10.1002/dev.20198. [DOI] [PubMed] [Google Scholar]
- [13].Bridges RS, Robertson MC, Shiu RP, Friesen HG, Stuer AM, Mann PE. Endocrine communication between conceptus and mother: placental lactogen stimulation of maternal behavior. Neuroendocrinology. 1996;64:57–64. doi: 10.1159/000127098. [DOI] [PubMed] [Google Scholar]
- [14].Bridges R, Rigero B, Byrnes E, Yang L, Walker A. Central infusions of the recombinant human prolactin receptor antagonist, S179D-PRL, delay the onset of maternal behavior in steroid-primed, nulliparous female rats. Endocrinology. 2001;142:730–9. doi: 10.1210/endo.142.2.7931. [DOI] [PubMed] [Google Scholar]
- [15].da Costa APC, Ma X, Ingram CD, Lightman SL, Aguilera G. Hypothalamic and amygdaloid corticotropin-releasing hormone (CRH) and CRH receptor-1 mRNA expression in the stress-hyporesponsive late pregnant and early lactating rat. Mol Brain Res. 2001;91:119–30. doi: 10.1016/s0169-328x(01)00137-1. [DOI] [PubMed] [Google Scholar]
- [16].Deschamps S, Woodside B, Walker C-D. Pups' presence eliminates the stress hyporesponsiveness of early lactating females to a psychological stress representing a threat to the pups. J Neuroendocrinol. 2003;15:486–97. doi: 10.1046/j.1365-2826.2003.01022.x. [DOI] [PubMed] [Google Scholar]
- [17].Lightman SL. Alterations in hypothalamic-pituitary responsiveness during lactation. Ann NY Acad Sci. 1992;652:340–6. doi: 10.1111/j.1749-6632.1992.tb34365.x. [DOI] [PubMed] [Google Scholar]
- [18].Shanks N, Kusnecov A, Pezzone M, Berkun J, Rabin BS. Lactation alters the effects of conditioned stress on immune function. Am J Physiol Regul Integr Comp Physiol. 1997;41:R16–25. doi: 10.1152/ajpregu.1997.272.1.R16. [DOI] [PubMed] [Google Scholar]
- [19].Shanks N, Windle RJ, Perks P, Wood S, Ingram CD, Lightman SL. The hypothalamic-pituitary-adrenal axis response to endotoxin is attenuated during lactation. J Neuroendocrinol. 1999;11:857–65. doi: 10.1046/j.1365-2826.1999.00400.x. [DOI] [PubMed] [Google Scholar]
- [20].Toufexis DJ, Tesolin S, Huang N, Walker CD. Altered pituitary sensitivity to corticotropin-releasing factor and arginine vasopressin participates in the stress hyporesponsiveness of lactation in the rat. J Neuroendocrinol. 1999a;11:757–64. doi: 10.1046/j.1365-2826.1999.00381.x. [DOI] [PubMed] [Google Scholar]
- [21].Slattery DA, Neumann ID. No stress please! Mechanisms of stress hyporesponsiveness of the maternal brain. J. Physiol. 2008;586:377–85. doi: 10.1113/jphysiol.2007.145896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Wartella J, Amory E, Lomas LM, Macbeth A, McNamara I, Stevens L, et al. Single or multiple reproductive experiences attenuate neurobehavioral stress and fear responses in the female rat. Physiol Behav. 2003;79:373–81. doi: 10.1016/s0031-9384(03)00150-1. [DOI] [PubMed] [Google Scholar]
- [23].Numan M, Numan MJ, English JB. Excitotoxic amino acid injections into the medial amygdala facilitate maternal behavior in virgin female rats. Horm Behav. 1993;27:56–81. doi: 10.1006/hbeh.1993.1005. [DOI] [PubMed] [Google Scholar]
- [24].Kleiman DG, Malcolm J. The evolution of male parental investment in mammals. In: Gubernick DJ, Klopfer PH, editors. Parental Care in Mammals. Plenum Press; New York: 1981. pp. 347–87. [Google Scholar]
- [25].Bester-Meredith JK, Martin PA, Marler CA. Manipulations of vasopressin alter aggression differently across testing conditions in monogamous and nonmonogamous Peromyscus mice. Aggress Behav. 2005;31:189–99. [Google Scholar]
- [26].de Jong TR, Chauke M, Harris BN, Saltzman W. From here to paternity: neural correlates of the onset of paternal behavior in California mice (Peromyscus californicus) Horm Behav. 2009;56:220–31. doi: 10.1016/j.yhbeh.2009.05.001. [DOI] [PubMed] [Google Scholar]
- [27].de Jong TR, Measor KR, Chauke M, Harris BN, Saltzman W. Brief pup exposure induces Fos expression in the lateral habenula and serotonergic caudal dorsal raphe nucleus of paternally experienced male California mice (Peromyscus californicus) Neuroscience. 2010;169:1094–1104. doi: 10.1016/j.neuroscience.2010.06.012. [DOI] [PubMed] [Google Scholar]
- [28].Kirkpatrick B, Kim JW, Insel TR. Limbic system fos expression associated with paternal behavior. Brain Res. 1994;658:112–8. doi: 10.1016/s0006-8993(09)90016-6. [DOI] [PubMed] [Google Scholar]
- [29].Lee AW, Brown RE. Comparison of medial preoptic, amygdala, and nucleus accumbens lesions on parental behavior in California mice (Peromyscus californicus) Physiol Behav. 2007;92:617–28. doi: 10.1016/j.physbeh.2007.05.008. [DOI] [PubMed] [Google Scholar]
- [30].Trainor BC, Marler CA. Testosterone promotes paternal behaviour in a monogamous mammal via conversion to oestrogen. Proc Biol Sci. 2002;269:823–9. doi: 10.1098/rspb.2001.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Trainor BC, Bird IM, Alday NA, Schlinger BA, Marler CA. Variation in aromatase activity in the medial preoptic area and plasma progesterone is associated with the onset of paternal behavior. Neuroendocrinology. 2003;78:36–44. doi: 10.1159/000071704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Schneider JS, Stone MK, Wynne-Edwards KE, Horton TH, Lydon J, O'Malley B, Levine JE. Progesterone receptors mediate male aggression toward infants. Proc Natl Acad Sci U S A. 2003;100:2951–56. doi: 10.1073/pnas.0130100100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Gubernick DJ, Nelson RJ. Prolactin and paternal behavior in the biparental California mouse, Peromyscus californicus. Horm Behav. 1989;23:203–10. doi: 10.1016/0018-506x(89)90061-5. [DOI] [PubMed] [Google Scholar]
- [34].Wynne-Edwards KE. Hormonal changes in mammalian fathers. Horm Behav. 2000;40:139–45. doi: 10.1006/hbeh.2001.1699. [DOI] [PubMed] [Google Scholar]
- [35].Bardi M, Franssen CL, Hampton JE, Shea EA, Fanean AP, Lambert KG. Paternal experience and stress responses in California mice (Peromyscus californicus) Comp Med. 2011;61:20–30. [PMC free article] [PubMed] [Google Scholar]
- [36].Chauke M, Malisch JL, Robinson C, de Jong TR, Saltzman W. Effects of reproductive status on behavioral and endocrine responses to acute stress in a biparental rodent, the California mouse (Peromyscus californicus) Horm Behav. 2011;60:128–38. doi: 10.1016/j.yhbeh.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Gubernick DJ. Reproduction in the California mouse, Peromyscus californicus. J Mammal. 1988;69:857–60. [Google Scholar]
- [38].Wright SL, Brown RE. The importance of paternal care on pup survival and pup growth in Peromyscus californicus when required to work for food. Behav Processes. 2002;60:41–52. doi: 10.1016/s0376-6357(02)00101-8. [DOI] [PubMed] [Google Scholar]
- [39].Blumstein DT, Daniel JC. Quantifying Behavior the J Watcher Way. Sinauer Associates, Inc.; Sunderland, MA, USA: 2007. [Google Scholar]
- [40].Walf AA, Frye CA. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Protoc. 2007;2:322–8. doi: 10.1038/nprot.2007.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Hand GA, Hewitt CB, Fulk LJ, Stock HS, Carson JA, Davis JM, Wilson MA. Differential release of corticotropin-releasing hormone (CRH) in the amygdala during different types of stressors. Brain Res. 2002;949:122–30. doi: 10.1016/s0006-8993(02)02972-4. [DOI] [PubMed] [Google Scholar]
- [42].Mulders WHAM, Meek J, Schmidt ED, Hafmans TGM, Cools AR. The hypothalamic paraventricular nucleus in two types of Wistar rats with different stress responses. II. Differential Fos-expression. Brain Res. 1995;689:61–70. doi: 10.1016/0006-8993(95)00546-3. [DOI] [PubMed] [Google Scholar]
- [43].Rotllant D, Nadal R, Armario A. Differential effects of stress and amphetamine administration on Fos-like protein expression in corticotropin releasing factor-neurons of the rat brain. Dev Neurobiol. 2007;67:702–14. doi: 10.1002/dneu.20345. [DOI] [PubMed] [Google Scholar]
- [44].Yokoyama C, Sasaki K. Regional expressions of Fos-like immunoreactivity in rat cerebral cortex after stress; restraint and intraperitoneal lipopolysaccharide. Brain Res. 1999;816:267–75. doi: 10.1016/s0006-8993(98)00927-5. [DOI] [PubMed] [Google Scholar]
- [45].Franklin KBJ, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. Compact 3rd ed. Elsevier, Inc; 2008. [Google Scholar]
- [46].Dielenberg RA, Hunt GE, McGregor IS. `When a rat smells a cat': The distribution of Fos immunoreactivity in rat brain following exposure to a predatory odor. Neuroscience. 2001;104:1085–97. doi: 10.1016/s0306-4522(01)00150-6. [DOI] [PubMed] [Google Scholar]
- [47].Kovacs KJ. c-Fos as a transcription factor: a stressful (re)view from a functional map. Neurochem Int. 1998;33:287–97. doi: 10.1016/s0197-0186(98)00023-0. [DOI] [PubMed] [Google Scholar]
- [48].Siegel S, Castellan NJ., Jr. Nonparametric statistics for the behavioral sciences. 2nd ed. McGraw-Hill Book Company; New York: 1988. [Google Scholar]
- [49].Storey JD. A direct approach to false discovery rates. J R Stat Soc Series B Stat Methodol. 2002;64:479–98. [Google Scholar]
- [50].Espejo EF. Effects of weekly or daily exposure to the elevated plus-maze in male mice. Behav Brain Res. 1997;87:233–8. doi: 10.1016/s0166-4328(97)02286-9. [DOI] [PubMed] [Google Scholar]
- [51].Brown RE, Corey SC, Moore AK. Differences in measures of exploration and fear in MHC-congenic C57/BL6J and B6-H-2K mice. Behav Gen. 1999;29:263–71. [Google Scholar]
- [52].Gubernick DJ, Laskin B. Mechanisms influencing sibling care in the monogamous biparental California mouse, Peromyscus californicus. Anim Behav. 1994;48:1235–7. [Google Scholar]
- [53].Gubernick DJ, Schneider JS, Jeannotte LA. Individual differences in the mechanisms underlying the onset and maintenance of paternal behavior and the inhibition of infanticide in the monogamous biparental California mouse, Peromyscus californicus. Behav Ecol Sociobiol. 1994;34:225–31. [Google Scholar]
- [54].Gubernick DJ. A maternal chemosignal maintains paternal behavior in the biparental California mouse, Peromyscus californicus. Anim Behav. 1990;39:936–42. [Google Scholar]
- [55].Gubernick DJ, Alberts JR. Postpartum maintenance of paternal behaviour in the biparental California mouse, Peromyscus californicus. Anim Behav. 1989;37:656–64. [Google Scholar]
- [56].Lambert KG, Franssen CL, Bardi M, Hampton JE, Hainley L, Karsner S, et al. Characteristic neurobiological patterns differentiate paternal responsiveness in two Peromyscus species. Brain Behav Evol. 2011;77:159–75. doi: 10.1159/000326054. [DOI] [PubMed] [Google Scholar]
- [57].Gubernick DJ, Winslow JT, Jensen P, Jeanotte L, Bowen J. Oxytocin changes in males over the reproductive cycle in the monogamous, biparental California mouse, Peromyscus californicus. Horm Behav. 1995;29:59–73. doi: 10.1006/hbeh.1995.1005. [DOI] [PubMed] [Google Scholar]
- [58].Wynne-Edwards KE, Timonin ME. Paternal care in rodents: Weakening support for hormonal regulation of the transition to behavioral fatherhood in rodent animal models of biparental care. Horm Behav. 2007;52:114–21. doi: 10.1016/j.yhbeh.2007.03.018. [DOI] [PubMed] [Google Scholar]
- [59].Grattan DR. The actions of prolactin in the brain during pregnancy and lactation. Prog Brain Res. 2001;133:153–71. doi: 10.1016/s0079-6123(01)33012-1. [DOI] [PubMed] [Google Scholar]
- [60].Insel TR. Postpartum increases in brain oxytocin binding. Neuroendocrinology. 1986;44:515–8. doi: 10.1159/000124694. [DOI] [PubMed] [Google Scholar]
- [61].Landgraf R, Neumann I, Pittman QJ. Septal and hippocampal release of vasopressin and oxytocin during late pregnancy and parturition in the rat. Neuroendocrinology. 1991;54:378–83. doi: 10.1159/000125917. [DOI] [PubMed] [Google Scholar]
- [62].Neumann I, Landgraf R. Septal and hippocampal release of oxytocin, but not vasopressin, in the conscious lactating rat during suckling. J Neuroendocrinol. 1989;1:305–8. doi: 10.1111/j.1365-2826.1989.tb00120.x. [DOI] [PubMed] [Google Scholar]
- [63].Pi XJ, Grattan DR. Increased prolactin receptor immunoreactivity in the hypothalamus of lactating rats. J Neuroendocrinol. 1999;11:693–705. doi: 10.1046/j.1365-2826.1999.00386.x. [DOI] [PubMed] [Google Scholar]
- [64].Torner L, Toschi N, Nava G, Clapp C, Neumann ID. Increased hypothalamic expression of prolactin in lactation: involvement in behavioural and neuroendocrine stress responses. Eur J Neurosci. 2002;15:1381–9. doi: 10.1046/j.1460-9568.2002.01965.x. [DOI] [PubMed] [Google Scholar]
- [65].van Leengoed E, Kerker E, Swanson HH. Inhibition of post-partum maternal behaviour in the rat by injecting an oxytocin antagonist into the cerebral ventricles. J Endocrinol. 1987;112:275–82. doi: 10.1677/joe.0.1120275. [DOI] [PubMed] [Google Scholar]
- [66].Neumann I, Wigger A, Torner L, Holsboer F, Landgraf R. Brain oxytocin inhibits basal and stress-induced activity of the hypothalamo-pituitary-adrenal axis in male and female rats: partial action within the paraventricular nucleus. J Neuroendocrinol. 2000;12:235–43. doi: 10.1046/j.1365-2826.2000.00442.x. [DOI] [PubMed] [Google Scholar]
- [67].Lister RG. Ethologically-based animal models of anxiety disorders. Pharmac Ther. 1990;46:321–40. doi: 10.1016/0163-7258(90)90021-s. [DOI] [PubMed] [Google Scholar]
- [68].Pellow S, Chopin P, File S, Briley M. Validation of open: closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Meth. 1985;14:149–67. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- [69].Neumann ID, Johnstone HA, Hatzinger M, Liebsch G, Shipston M, Russell JA, et al. Attenuated neuroendocrine responses to emotional and physical stressors in pregnant rats involve adenohypophysial changes. J Physiol. 1998;508:289–300. doi: 10.1111/j.1469-7793.1998.289br.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Neumann ID. Alterations in behavioral and neuroendocrine stress coping strategies in pregnant, parturient and lactating rats. Prog Brain Res. 2001;133:143–52. doi: 10.1016/s0079-6123(01)33011-x. [DOI] [PubMed] [Google Scholar]
- [71].Zuluaga MJ, Agrati D, Pereira M, Uriarte N, Fernandez-Guasti A, Ferreira A. Experimental anxiety in the black and white model in cycling, pregnant and lactating rats. Physiol Behav. 2005;84:279–86. doi: 10.1016/j.physbeh.2004.12.004. [DOI] [PubMed] [Google Scholar]
- [72].Pan Y, Liu Y, Young KA, Zhang Z, Wang Z. Post-weaning social isolation alters anxiety-related behavior and neurochemical gene expression in the brain of male prairie voles. Neurosci Lett. 2009;454:67–71. doi: 10.1016/j.neulet.2009.02.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Rogers RJ, Cole JC. Influence of social isolation, gender, strain, and prior novelty on plus-maze behaviour in mice. Physiol Behav. 1993;54:729–36. doi: 10.1016/0031-9384(93)90084-s. [DOI] [PubMed] [Google Scholar]
- [74].Starkey NJ. Normington G, Bridges NJ. The effects of individual housing on `anxious' behaviour in male and female gerbils. Physiol Behav. 2007;90:545–52. doi: 10.1016/j.physbeh.2006.11.001. [DOI] [PubMed] [Google Scholar]
- [75].Clark FH. Geotropic behavior on a sloping plane of arboreal and non-arboreal races of mice of the genus Peromyscus. J Mammal. 1936;17:44–7. [Google Scholar]
- [76].Day HEW, Masini CV, Campeau S. The pattern of brain c-fos mRNA induced by a component of fox odor, 2,5-dihydro-2,4,5-Trimethylthiazoline (TMT), in rats, suggests both systemic and processive stress characteristics. Brain Res. 2004;1025:139–51. doi: 10.1016/j.brainres.2004.07.079. [DOI] [PubMed] [Google Scholar]
- [77].Figueiredo HF, Bodie BL, Tauchi M, Dolgas CM, Herman JP. Stress integration after acute and chronic predator stress: differential activation of central stress circuitry and sensitization of the hypothalamo-pituitary-adrenocortical axis. Endocrinology. 2003;144:5249–58. doi: 10.1210/en.2003-0713. [DOI] [PubMed] [Google Scholar]
- [78].Rivalland ETA, Clarke LJ, Turner AI, Pompolo S, Tilbrook AJ. Isolation and restraint stress results in differential activation of corticotropin-releasing hormone and arginine vasopressin neurons in sheep. Neuroscience. 2007;145:1048–58. doi: 10.1016/j.neuroscience.2006.12.045. [DOI] [PubMed] [Google Scholar]
- [79].Grippo AJ, Gerena D, Huang J, Kumar N, Sah M, Ughreja R, et al. Social isolation induces behavioral and neuroendocrine disturbances relevant to depression in female and male prairie voles. Psychoneuroendocrinology. 2007;32:966–80. doi: 10.1016/j.psyneuen.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Pournajafi-Nazarloo H, Partoo L, Sanzenbacher L, Esmaeilzadeh M, Paredes J, Hashimoto K, et al. Social isolation modulates corticotropin-releasing factor type 2 receptor, urocortin 1 and urocortin 2 mRNAs expression in the cardiovascular system of prairie voles. Peptides. 2009;30:940–6. doi: 10.1016/j.peptides.2009.01.003. [DOI] [PubMed] [Google Scholar]
- [81].Ruscio MG, Sweeny T, Hazelton J, Suppatkul P, Carter CS. Social environment regulates corticotropin releasing factor, corticosterone and vasopressin in juvenile prairie voles. Horm Behav. 2007;51:54–61. doi: 10.1016/j.yhbeh.2006.08.004. [DOI] [PubMed] [Google Scholar]
- [82].Dallman MF. Stress update: adaptation of the hypothalamic-pituitary-adrenal axis to chronic stress. Trends Endocrinol Metab. 1993;4:62–9. doi: 10.1016/s1043-2760(05)80017-7. [DOI] [PubMed] [Google Scholar]
- [83].Lightman SL. The neuroendocrinology of stress: a never ending story. J Neuroendocrinol. 2008;202:880–4. doi: 10.1111/j.1365-2826.2008.01711.x. [DOI] [PubMed] [Google Scholar]
- [84].Tilders FJH, Schmidt ED, de Goeij DCE. Phenotypic plasticity of CRF neurons during stress. Ann N Y Acad Sci. 1993;697:39–52. doi: 10.1111/j.1749-6632.1993.tb49921.x. [DOI] [PubMed] [Google Scholar]
- [85].Albeck DS, McKittrick CR, Blanchard DC, Blanchard RJ, Nikulina J, McEwen BS, et al. Chronic social stress alters levels of corticotropin-releasing factor and arginine vasopressin mRNA in rat brain. J Neurosci. 1997;17:4895–903. doi: 10.1523/JNEUROSCI.17-12-04895.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Choi DC, Nguyen MMN, Tamashiro KLK, Ma LY, Sakai RR, Herman JP. Chronic social stress in the visible burrow system modulates stress-related gene expression in the bed nucleus of the stria terminalis. Physiol Behav. 2006;89:301–10. doi: 10.1016/j.physbeh.2006.05.046. [DOI] [PubMed] [Google Scholar]
- [87].Ladd CO, Thrivikraman KV, Huot RL, Plotsky PM. Differential neuroendocrine responses to chronic variable stress in adult Long Evans rats exposed to handling-maternal separation as neonates. Psychoneuroendocrinology. 2005;30:520–33. doi: 10.1016/j.psyneuen.2004.12.004. [DOI] [PubMed] [Google Scholar]
- [88].Clinton SM, Vazquez DM, Kabbaj M, Kabbaj M-H, Watson SJ, Akil H. Individual differences in novelty-seeking and emotional reactivity correlate with variation in maternal behavior. Horm Behav. 2007;51:655–64. doi: 10.1016/j.yhbeh.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Byrnes EM, Bridges RS. Reproductive experience alters anxiety-like behavior in the female rat. Horm Behav. 2006;50:70–6. doi: 10.1016/j.yhbeh.2006.01.006. [DOI] [PubMed] [Google Scholar]
- [90].Pereira M, Uriarte N, Agrati D, Zuluaga MJ, Ferreira A. Motivational aspects of maternal anxiolysis in lactating rats. Psychopharmacology. 2005;180:241–8. doi: 10.1007/s00213-005-2157-y. [DOI] [PubMed] [Google Scholar]