Abstract
Oxygen exposure in premature infants is a major risk factor for bronchopulmonary dysplasia and can impair the host response to respiratory viral infections later in life. Similarly, adult mice exposed to hyperoxia as neonates display alveolar simplification associated with a reduced number of alveolar epithelial type II cells and exhibit persistent inflammation, fibrosis, and mortality when infected with influenza A virus. Because type II cells participate in innate immunity and alveolar repair, their loss may contribute to oxygen-mediated sensitivity to viral infection. A genomewide screening of type II cells identified eosinophil-associated RNase 1 (Ear1). Ear1 was also detected in airway epithelium and was reduced in lungs of mice exposed to neonatal hyperoxia. Electroporation-mediated gene delivery of Ear1 to the lung before infection successfully reduced viral replication and leukocyte recruitment during infection. It also diminished the enhanced morbidity and mortality attributed to neonatal hyperoxia. These findings demonstrate that novel epithelial expression of Ear1 functions to limit influenza A virus infection, and its loss contributes to oxygen-associated epithelial injury and fibrosis after infection. People born prematurely may have defects in epithelial innate immunity that increase their risk for respiratory viral infections.
Premature exposure to oxygen is a major risk factor for neonatal lung disease and can promote bronchopulmonary dysplasia (BPD), a chronic form of disease frequently seen in preterm infants with very low birth weight.1 Lungs of infants who died of BPD are less vascularized and have fewer and larger alveoli.2 Although the use of exogenous surfactant, antenatal steroids, and milder ventilation strategies has markedly increased survival of preterm infants, survivors continue to show decreased lung capacity at 5 to 10 years of age and even as young adults.3–5 Moreover, these children are often hospitalized again after respiratory viral infection and are at increased risk for asthma and other cardiopulmonary-related diseases, including hypertension.6–8 Despite a clear appreciation that premature exposure to oxygen contributes to BPD, how early-life oxygen therapy permanently disrupts lung development and modifies the ability to respond to respiratory infections later in life is not known.
Studies in a variety of animal models reveal that neonatal exposure to supplemental oxygen (hyperoxia) affects lung development in several ways, yet the contribution of these effects to long-term health and disease is not fully understood.9 Infants who have died of BPD have pronounced disruptions in vascular development, but it remains uncertain whether alterations in vascular development are the sole cause of morbidity in those who survive. Indeed, long-term changes in alveolar simplification and airway responsiveness observed in animal studies suggest that the oxygen-injured lung may never fully recover.10–12 These studies also suggest that changes in vascular development are not the only modification that affects survival, lung function, and overall health.13,14 Studies of short-term high-oxygen exposure in mice reveal changes in the balance of alveolar epithelial cell types. Along with patent alveolar simplification, lungs of adult mice that were exposed to 100% oxygen during postnatal days 1 to 4 have fewer alveolar epithelial type II cells, as defined by reduced number of cells expressing prosurfactant protein B (proSP-B), prosurfactant protein C (proSP-C), or enhanced green fluorescence protein (EGFP) driven by the human surfactant protein C promoter.11 Lungs of these mice also express more T1α and AQP5, genes expressed by type I epithelial cells. Because type II cells are progenitor cells for type I cells, even short-term neonatal hyperoxia may have depleted or reprogrammed type II cells such that the overall balance is altered, with the simplified lung having more type I cells. The consequences of such an imbalance are not completely understood, but it possibly contributes to the poorer responses to respiratory infection reported in children born prematurely.
We have previously reported that adult mice exposed to hyperoxia as neonates display persistent and enhanced recruitment of leukocytes, pulmonary fibrosis, and mortality when infected with a sublethal dose of influenza A virus.14 Neonatal hyperoxia could alter host responses to influenza virus infection by compromising any of the innate and adaptive host defense pathways involved in controlling infection. Given that alveolar type II cells secrete proteins involved in innate immunity and surfactant homeostasis and participate in alveolar epithelial repair, their reduction in adult mice exposed to hyperoxia as neonates may adversely affect the host response to respiratory viral infections.15–19 We report a study of how the loss of type II cells in adult mice exposed to neonatal hyperoxia led us to identify a novel mechanism of innate host defense in airway and alveolar epithelial cells that is disrupted by neonatal hyperoxia. Understanding how short-term hyperoxia permanently disrupts lung development and the host response to respiratory viral infections is important because it may provide insight into why children born prematurely also display permanent changes in lung function and host defense later in life.
Materials and Methods
Exposure of Mice to Hyperoxia as Neonates and Influenza A Virus as Adults
Newborn C57Bl/6J mice were exposed to room air or 100% oxygen (hyperoxia) between postnatal days 1 and 4 and then returned to room air.14 At 8 weeks of age, female mice were infected by intranasal administration of 120 hemagglutinating units of influenza A virus, strain HKx31 (H3N2). Female mice were used for the study because they consistently exhibit a more robust response to neonatal hyperoxia and infection with influenza A virus than males. Mechanisms by which sex affects the immune response to pathogens is complex and not fully understood.20 Mice were housed in microisolator cages in a specified pathogen-free facility, and the University Committee on Animal Resources approved all protocols using mice.
RNA Isolation and Hybridization to Affymetrix Probes
The generation, characterization, and isolation of type II cells from transgenic human surfactant protein-C (hSP-C)/EGFP mice in the C57Bl/6J inbred background have been described previously.21 Total RNA was isolated from EGFP-positive and EGFP-negative type II cells harvested from uninfected 8- to 10-week-old mice using RNeasy (Qiagen, Valencia, CA). RNA integrity was validated by the presence of strong ribosomal RNA bands with an Agilent Bioanalyzer (Agilent Technologies, Santa Clara, CA). The RNA was amplified during reverse transcription to cDNA and biotinylated with Ovation kits from NuGEN (San Carlos, CA). Biotinylated cDNA was hybridized overnight to the mouse genome 430 2.0 array from Affymetrix. Arrays were stained with streptavidin-phycoerythrin as recommended by Affymetrix. The arrays were scanned for phycoerythrin fluorescence, and the spot intensities were normalized across arrays with the GeneChip Robust Multiarray Averaging method. Quantitative real-time PCR was performed with cDNA that was amplified during reverse transcription with WT-Ovation kits (NuGEN). Taqman primer and probe sets were purchased from Applied Biosystems (Carlsbad, CA), which designed these proprietary assays for use with standard thermal cycling conditions and detector settings that are the same for all assays. These assays were performed with the Applied Biosystems 7900HT real-time PCR instrument and were analyzed with Applied Biosystems SDS software version 2.4, using β-actin as the reference transcript. Real-time PCR of EAR cDNA was performed by amplifying bases 14 to 107 (see Supplemental Figure S1 at http://ajp.amjpathol.org) and detecting the amplicon with locked nucleic acid probe 18 from the Roche Universal Probe Library (Roche Applied Science, Indianapolis, IN).
Electroporation-Mediated Plasmid Delivery
Plasmids were purified using Qiagen Giga-prep kits and resuspended at 1 mg/mL in 10 mmol/L Tris, pH 8.0, 1 mmol/L EDTA, and 140 mmol/L NaCl. Adult mice (8 to 10 weeks old) were lightly anesthetized with isoflurane and placed in the supine position. Pediatric cutaneous pacemaker electrodes (Quik-Combo RTS; Medtronic Physio-Control Corporation, Redmond, WA) cut to 1 × 2 cm were placed on either side of the chest under the forelimbs, using a small amount of petroleum jelly to aid conductance.22 Next, 50 μL of plasmid was administered to the lungs by tracheal instillation during inspiration, and a series of eight consecutive square-wave electric pulses (200 V/cm for 10 milliseconds each) were immediately administered using an ECM830 electroporator (Gentronics, West Hollywood, CA). Mice were monitored as they recovered from the anesthesia and then returned to the vivarium.
RT-PCR
Total RNA was isolated from frozen lung homogenates or isolated type II cells with Trizol (Invitrogen, Grand Island, NY) and reverse transcribed into cDNA with the iScript cDNA Synthesis kit (Bio-Rad, Hercules, CA) using random hexamer primers. For identifying Ear genes expressed in lung and type II cells, Ear primers (forward primer: 5′-GATCGAATTCATGGGTCCGAAGCTGCTTGAGT-3′ and reverse primer: 5′-GATCGGATCCCTAAAATGTCCCATCCAAGTGA-3′) were used to amplify products that were cloned into pBluescript vector (Stratagene, Santa Clara, CA) and sequenced. For semiquantitative RT-PCR, Ear (forward primer: 5′-GATCGAATTCAATACTTTTCTTCATACAA-3′ and reverse primer: 5′-GATCGGATCCGTGAACTGGAACCACTGGATA-3′) and β-actin (forward 5′-GTATGGAATCCTGTGGCATCC-3′ and reverse 5′-TACGCAGCTCAGTAACAGTCC-3′) primers were used to amplify aliquots of cDNA (2 and 1 μL, respectively) by PCR. The thermal cycling profile for Ear and β-actin was 94°C for 4 minutes, followed by 35 (Ear) or 26 (β-actin) cycles of 94°C for 30 seconds, 58°C for 30 seconds, and 72°C for 1 minute, with a final extension of 72°C for 5 minutes. The number of cycles was based on prior studies confirming that amplification was in the linear range.
Immunohistochemistry
Lungs were inflation fixed with 10% neutral-buffered formalin, embedded in paraffin, and sectioned as previously described.11,23 Sections were incubated with rabbit anti-proSP-C,11 rabbit anti-Ear (1:500, James and Nancy Lee, Mayo Clinic, Scottsdale, AZ24), or mouse anti–α-smooth muscle actin14 antibody overnight at 4°C. Slides were washed three times in PBS with 0.05% Triton-X before incubating for 1 hour with appropriate fluorescently labeled secondary antibodies (Jackson Immunoresearch, West Grove, PA). Gomori's trichrome stain was performed according to the manufacturer's instructions (Richard-Allan Scientific, Kalamazoo, MI). All slides were immersed in DAPI and visualized with a Nikon E-800 fluorescence microscope (Nikon Instruments, Melville, NY). Images were captured with a SPOT-RT digital camera (Diagnostic Instruments, Sterling Heights, MI). For quantifying positively stained cells, five random images of the right lung lobes were captured at 20× magnification.11,23 A minimum of 200 cells per image were counted using Metamorph analysis software version 7.7 (Molecular Devices, LLC, Sunnyvale, CA).
In Situ Hybridization
The Ear1 open reading frame was generated by PCR of pVL-mEar1 using the forward 5′-ATGGGTCCGAAGCTGCTTGAGTCT-3′ primer and reverse 5′-CTAAAATGTCCCATCCAAGTGAAC-3′ primer. An EcoRI site was added to the 5′ primer, and a BamHI site was added to the 3′ primer. The EcoRI- and BamHI-digested fragment was ligated into pBluescript II KS plasmid, generating pBS-Ear1. pBS-Ear1 was linearized and antisense and sense probes were synthesized using T7 and T3 promoters in the presence of digoxigenin (DIG)-11-UTP (Roche Diagnostics Corporation, Indianapolis, IA). DIG-labeled probes were hydrolyzed to a length of approximately 200 bp by alkaline hydrolysis and quantified by using an alkaline phosphate–conjugated anti-DIG antibody in a dot blot. Paraffin-embedded sections of lungs fixed in 10% neutral buffered formalin were deparaffinized in xylene and dehydrated followed by proteinase K digestion. Slides were fixed in 4% formaldehyde, equilibrated with 100 mmol/L triethanolamine HCl (pH 8.0), and treated with 0.25% acetic anhydride. The slides were then washed in 2× saline sodium citrate (SSC), and hybridization was performed for 16 hours at 49°C using a hybridization solution containing 50 pg/μL of DIG-labeled probe. The hybridization solution consisted of 45% formamide, 4× SSC, 10 mmol/L DTT 1× Denhardt's reagent, 10% dextran sulfate, and 1 mg/mL of yeast tRNA. Sections were washed, denatured with 1% hydrogen peroxide, digested with RNase A, and washed in RNase buffer. The slides were then washed in 0.1× SSC at 58°C and 0.1× SSC at room temperature, blocked with 3% normal sheep serum, and incubated in horseradish peroxidase–conjugated anti-DIG antibody. Tyramide amplification (NEN Life Science Products, Boston, MA) was performed and slides were incubated in Vectastain ABC (Vector Laboratories, Burlingame, CA) before detection with 3,3′-diaminobenzidine. Tissues were counterstained with hematoxylin, and images were visualized with a Nikon E800 fluorescence microscope (Nikon Instruments) and captured with a SPOT-RT digital camera (Diagnostic Instruments).
Viral Foci Assay
Frozen lungs from individual mice were resuspended in 1 mL of ice-cold PBS, containing 100 U/mL of penicillin and 0.1 mg/mL of streptomycin, and mechanically homogenized (Tissuemiser; Fisher Scientific, Waltham, MA). After centrifugation of tissue homogenates (400 × g for 5 minutes at 4°C), 15 μL of the supernatant was serially diluted (1:100 to 1:107) in zero-serum refeed medium containing penicillin (100 U/mL), streptomycin (100 µg/mL), gentamicin (50 µg/mL), amphotericin B (1.25 µg/mL) (PSGA) containing 4 μg/mL of trypsin. Virus titers were determined by immunocytochemistry on confluent Madin-Darby canine kidney cells in 96-well flat bottom tissue culture plates in duplicate.25 Briefly, cells were incubated with 100 μL of serially diluted supernatant and centrifuged for 1.5 hours at 700 × g, after which wells were aspirated and replaced with fresh zero-serum refeed media. After overnight incubation at 33°C, cells were fixed with 80% acetone/20% water for 30 minutes at −20°C, rinsed with staining wash buffer (1× PBS, 2% fetal bovine serum, 0.1% sodium azide), and then incubated with a biotinylated anti-influenza A monoclonal antibody (1:900 dilution in staining wash buffer; Millipore, Billerica, MA) for 1 hour at 38°C. Cells were then incubated with streptavidin-labeled alkaline phosphatase (1:500 dilution in PBS; Sigma-Aldrich, St Louis, MO) for 1 hour at room temperature, and viral foci, indicative of influenza infection in the cell monolayer, were detected on addition of 5-bromo-4-chloro-3′-indolyphosphate p-toluidine salt and nitro-blue tetrazolium chloride (Sigma-Aldrich). Viral foci were counted and viral foci units per milliliter were determined.
Bronchoalveolar Lavage Fluid Collection and Counting of Cells
Leukocytes were collected by bronchoalveolar lavage (BAL). The trachea was catheterized and the lungs were lavaged with three separate 1-mL aliquots of ice-cold PBS containing 1% bovine serum albumin and 10% HEPES. BAL fluid and cells were separated by centrifugation (200 × g). Supernatant from the first lavage was retained as BAL fluid and was stored at −80°C for quantifying levels of monocyte chemotactic protein 1 (MCP-1) by enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, MN). Cells recovered from all three lavages were pooled and erythrocytes were removed by treatment with ammonium chloride lysing solution (0.15 M NH4Cl, 10 mmol/L NaHCO3, and 1 mmol/L EDTA). The total number of cells was enumerated using a TC10 automated cell counter (Bio-Rad). Cells from individual mice were transferred to microscope slides using a cytologic centrifuge and were stained with Diff-Quik. Monocytes or macrophages, neutrophils, and lymphocytes were enumerated by differential cell counts of at least 200 cells on coded slides by two independent investigators.
Western Blot Analysis
Cells and whole lungs were harvested in lysis buffer, and protein concentrations were determined as previously published.11 Samples were diluted in Laemmli buffer and separated by SDS-PAGE and transferred to polyvinylidene difluoride membranes (Pall Life Sciences, Port Washington, NY). The membranes were then incubated with anti-Ear (1:500), anti-ABCA3 (1:500; Seven Hills, Cinciannati, OH), E-cadherin (1:500; Cell Signaling Technology, Danvers, MA), or anti–Clara cell secretory protein (CCSP)11 antibodies with β-actin (1:2500; Sigma Chemical Company, St. Louis, MO) as a loading control. Membranes were then incubated in horseradish peroxidase–conjugated secondary antibodies (anti-mouse at 1:4000 or anti-rabbit at 1:5000; Southern Biotechnology) and visualized by chemiluminescence (Amersham Biosciences, Sunnyvale, CA). Quantification of Western blots was performed using ImageJ software version 1.46 (NIH, Bethesda, MD).
Statistical Analysis
Statistical analyses of values were assessed by unpaired Student's t-test for single comparisons or an analysis of variance using Fisher's procedure post hoc analysis for group comparisons using Statview software (Abacus Concepts, Piscataway, NJ). Mortality was evaluated with Kaplan-Meier and analyzed for significance with Mantel-Cox test using StatView software version 5.0. Values are expressed as mean ± SEM with P < 0.05 considered significant.
Results
Ear1 Can Be Detected in Some Epithelial Type II Cells
Previous work revealed adult mice exposed to neonatal hyperoxia have fewer type II cells based on counting cells expressing proSP-C or EGFP controlled by the human SP-C promoter.11 This finding led us to hypothesize that neonatal hyperoxia reduced expression of prosurfactant protein A (SP-A) and prosurfactant protein D (SP-D), proteins expressed by type II cells that protect against respiratory viruses. However, levels of SP-A or SP-D in BAL fluid were similar in mice exposed to room air or hyperoxia (data not shown). We therefore decided to use Affymetrix arrays to search for novel innate immune genes expressed by type II cells. Unfortunately, pure populations of mouse type II cells are difficult to obtain. However, we have previously shown how the intrinsic green fluorescence of EGFP can be used to isolate highly pure populations of type II cells from hSP-C/EGFP transgenic mice using fluorescence-activated cell sorting.21 Because EGFP is detected in approximately 5% of type II cells, we first isolated type II cells using Dispase and then separated the cells into EGFP-positive and EGFP-negative populations based on their intrinsic green fluorescence (see Supplemental Figure S2 at http://ajp.amjpathol.org). RNA was isolated, converted to cDNA, and hybridized to the mouse genome 430 2.0 array from Affymetrix. A hierarchal cluster analysis of the 25 most abundantly expressed genes revealed that EGFP-positive and EGFP-negative cells expressed SP-C, as well as SP-A, SP-B, SP-D, lysozyme, forkhead box protein 1A, and thyroid transcription factor 1 (see Supplemental Figure S2 at http://ajp.amjpathol.org). We then looked at the 25 most abundantly expressed genes whose expression was fivefold or greater in EGFP-positive or EGFP-negative type II cells. Changes less than fivefold were not always consistent and were therefore ignored. Approximately 250 (0.6%) of the >45,000 probes on the array were selectively detected in EGFP-positive cells, whereas approximately 15,000 (3.3%) were selectively detected in the EGFP-negative cells. Intriguingly, EGFP-positive cells selectively expressed genes previously identified as playing a role in activating bone morphogenetic protein signaling, asymmetric cell division (Par-3, cdc42-binding protein, Magi1, dock-1, and dock-9), and protection against RNA viruses (eosinophil-associated RNase 2 and 3, also called Ear2 and Ear3) (see Supplemental Table S1 at http://ajp.amjpathol.org). On the other hand, EGFP-negative type II cells expressed claudin 4, matrix metallopeptidase 3, hemoglobin, and many other genes, some of which might be expressed by non–type II cells that co-purified during the initial isolation. Expression of some of these genes was validated by quantitative RT-PCR (see Supplemental Table S2 at http://ajp.amjpathol.org). The complete array data were deposited in the Gene Expression Omnibus at the National Center for Biological Information on December 2, 2010, and may be accessed under accession number GSE25778 (http://www.ncbi.nlm.nih.gov/geo).
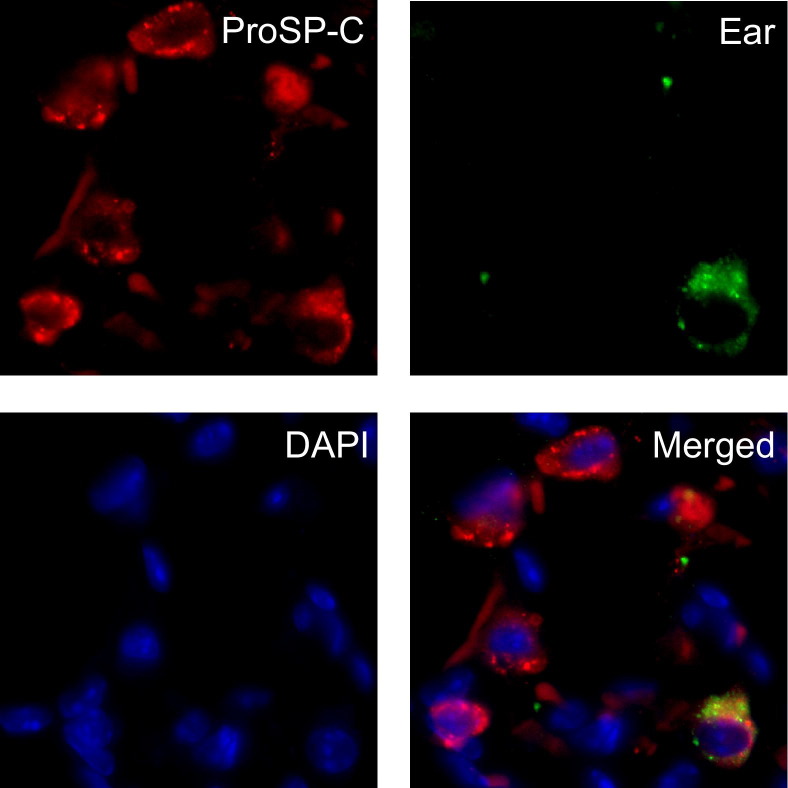
The selective expression of Ear mRNA by EGFP-positive type II cells was of interest because Ear proteins possess potent bactericidal, helminthotoxic, and antiviral properties when expressed in cell lines (for review see Rosenberg26). However, expression of Ear proteins in the respiratory epithelium has not been reported, and function of Ear proteins during in vivo infection with influenza A viruses has not been well characterized. Mus musculus expresses 14 highly homologous members of this family that can only be distinguished by sequence analysis.27,28 Realizing that the array probes were unlikely to distinguish the individual isoforms, Ear cDNAs were amplified from RNA isolated from whole lung of nontransgenic mice or EGFP-positive type II cells isolated from transgenic mice and sequenced (see Supplemental Figure S1 at http://ajp.amjpathol.org). Ear1 and Ear2 were the only isoforms detected in whole lung (four Ear1 and eight Ear2 clones) and EGFP-positive type II cells (10 Ear1, one Ear2, and three mutant Ear1 clones). When the study was repeated, slightly more Ear2 clones were obtained in the EGFP-positive type II cells. To validate that type II cells express Ear genes, lung sections from nontransgenic adult mice were immunostained with an antibody that recognizes all known Ear proteins. Ear-positive staining was restricted to alveolar cells and occasionally overlapped with proSP-C (Figure 1). This finding confirms an earlier report that found that Ear1 and Ear2 are the only forms detected in healthy adult mouse lung and extends it by showing expression by some type II cells.28
Figure 1.
Ear can be detected in some type II cells by immunostaining. Lungs from uninfected adult mice were immunostained for proSP-C (red) and Ear (green) and counterstained with DAPI (blue). EAR-positive staining is clearly evident in the cytoplasm of some but not all proSP-C–positive cells.
Neonatal Hyperoxia Suppresses Epithelial Expression of Ear1 in Nontransgenic Mice
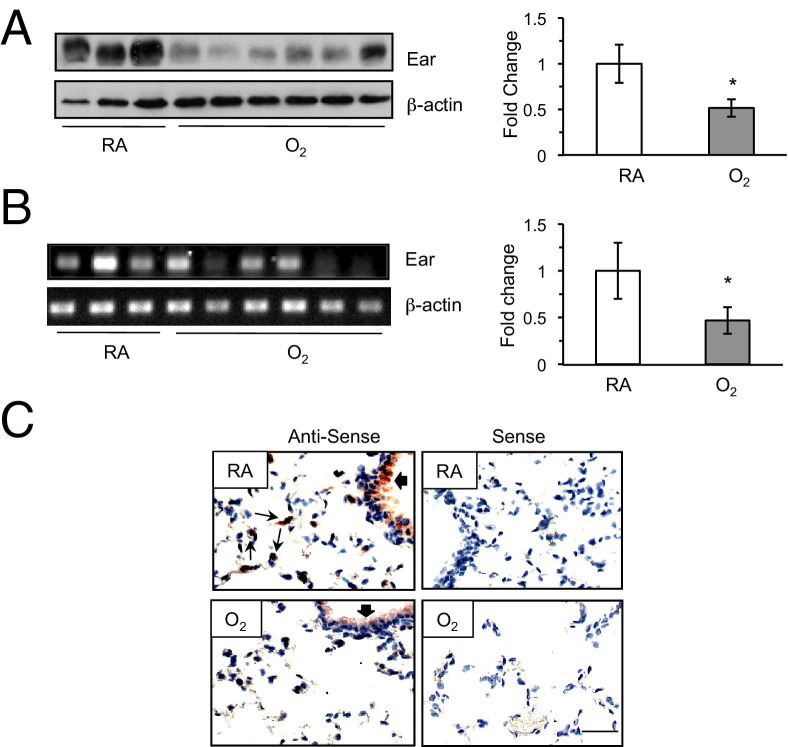
Because adult mice exposed to hyperoxia as neonates have fewer type II cells and some type II cells express Ear1 mRNA, we hypothesized that neonatal hyperoxia reduces expression of Ear1. Ear protein was readily detected in lung homogenates of nontransgenic C57Bl/6J adult mice and was reduced in siblings that had been exposed to hyperoxia as neonates (Figure 2A). Ear mRNA was also reduced in some but not all adult mice exposed to hyperoxia as neonates (Figure 3B). Further, Ear mRNA was detected by in situ hybridization in airway epithelium and some alveolar cells of adult mice exposed to room air (Figure 3C). It was reduced in the airway epithelium and not easily detected in alveoli of sibling mice exposed to hyperoxia as neonates. Hence, adult mice exposed to neonatal hyperoxia express lesser Ear than siblings exposed to room air.
Figure 2.
Neonatal hyperoxia suppresses Ear expression in adult mice. A: Ear protein was detected in lung homogenates of mice exposed to room air (RA) or hyperoxia (O2) as neonates. Expression of Ear in mice exposed to room air (white bars) or hyperoxia (gray bars) was graphed relative to β-actin (n = 3 to 6 mice per group; *P < 0.05). B: Ear mRNA was detected by semiquantitative RT-PCR. Expression of Ear mRNA in mice exposed to RA or O2 were graphed relative to β-actin (n = 3 to 6 mice per group; *P < 0.04). C: Lung tissues from mice exposed to room air or hyperoxia at birth were hybridized with antisense or sense Ear1 riboprobe and counterstained with hematoxylin. Thick arrows indicate positive airway staining, and thin arrows depict positive alveolar staining. Scale bar = 100 μm (B). Mean values from a single experiment reproduced three to five times with similar results.
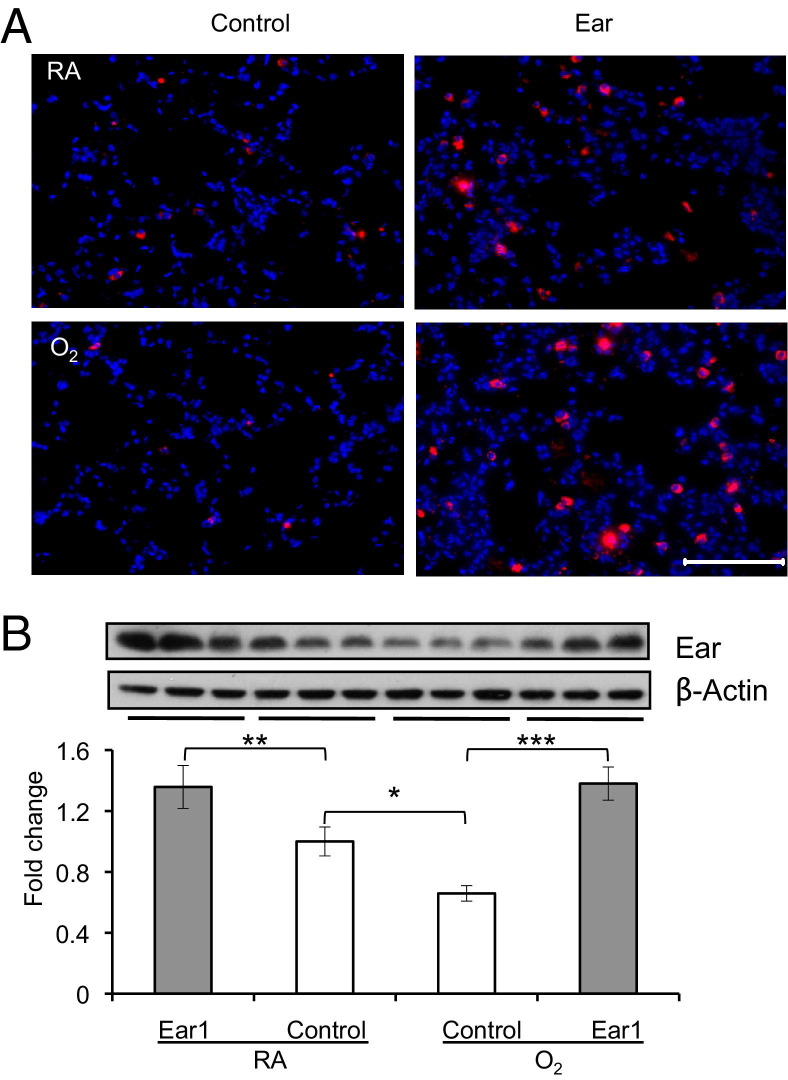
Figure 3.
Electroporation-mediated gene delivery effectively drives high-level expression of Ear1 in the lung. Lungs of adult mice exposed to room air (RA) or hyperoxia (O2) as neonates were electroporated with empty vector (control) or plasmid-expressing Ear1 (Ear1). A: Lungs were immunostained for Ear (red) and counterstained with DAPI (blue). Scale bar = 100 μmol/L. B: Ear protein was detected by immunoblotting lung homogenates, and levels of expression were graphed as fold change relative to β-actin (n = 3 mice per group; *P < 0.002 and **P < 0.05 compared with RA control and ***P < 0.001 compared with O2 control).
Electroporation-Mediated Gene Delivery of Ear1 Protects Against Influenza A Virus Infection
Given that Ear proteins can inhibit replication of respiratory syncytial virus, hepatitis B virus, and HIV virus,29–33 reduced expression of Ear1 in adult mice exposed to hyperoxia could allow for greater influenza virus expansion during infection. However, viral titers were usually not different between the two groups of mice on any given day, and virus was still efficiently cleared by postinfection day 9 (see Supplemental Figure S3 at http://ajp.amjpathol.org). On the other hand, when analyzed across the entire course of infection, viral titers were modestly but significantly higher in infected mice exposed to hyperoxia (P < 0.03 by analysis of variance). To determine whether this could be attributed to lower levels of Ear1, we overexpressed Ear1 using electroporation-mediated delivery of expression plasmids. This method has been used to efficiently drive gene expression to a nonproliferating organ, such as the lung, without inducing an inflammatory response.34 To assess whether restoring Ear1 expression would protect against influenza virus infection, porcine cytomegalovirus (pCMV)–control (empty vector) or pCMV-Ear1 plasmids were first instilled intratracheally into the lungs of naive adult mice exposed to room air or hyperoxia as neonates. The mice were electroporated to facilitate uptake of the plasmid and recovered for 48 hours to allow expression of Ear1. Electroporation-mediated delivery of pCMV-Ear1 plasmid increased expression of Ear1 approximately twofold in both groups of mice (Figure 3A). Even though electroporation with this plasmid facilitates expression in airway epithelium, Ear1 immunostaining was only observed in alveolar cells (Figure 3B).
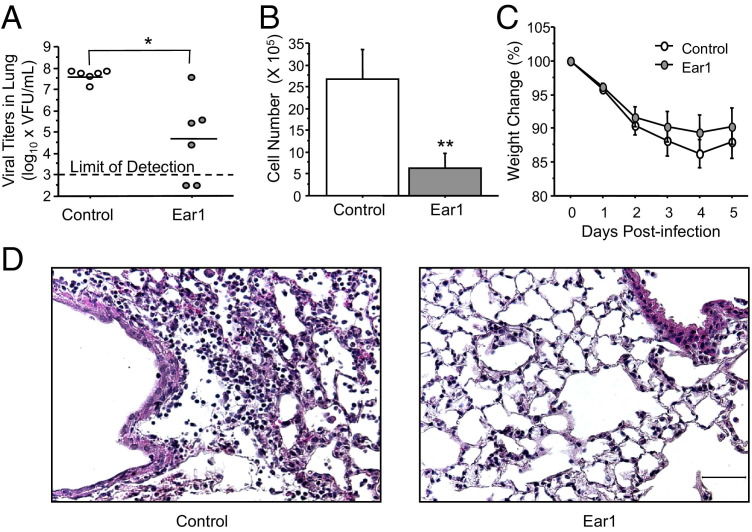
We next determined whether Ear1 gene delivery protects against influenza A virus infection. The pCMV-control or pCMV-Ear1 plasmids were electroporated into the lungs of mice exposed to room air at birth and then infected 48 hours later with a sublethal dose of influenza A virus. Consistent with its antiviral properties, overexpression of Ear1 significantly reduced viral titers and the number of leukocytes recruited to the lung on postinfection day 5 (Figures 4, A and B). Differential counts of macrophages, neutrophils, and lymphocytes were not different, implying overexpression of Ear1 had reduced the recruitment of those leukocytes examined and not just one cell type (data not shown). Overexpression of Ear1 also modestly, but not significantly, attenuated weight loss through postinfection day 5 (Figure 4C). Leukocyte recruitment, alveolar swelling, and airway injury seen in infected mice was also less pronounced in infected mice administered Ear1 before infection (Figure 4D).
Figure 4.
Electroporation-mediated gene delivery of Ear1 protects room air–exposed mice against influenza A virus infection. Lungs of adult mice exposed to room air as neonates were electroporated with empty vector (control) or plasmid-expressing Ear1 (Ear1). Mice were infected intranasally with influenza A virus 48 hours later. A: Overexpression of Ear1 significantly reduced viral titers on postinfection day 5 (*P < 0.005). Each dot represents the virus titer in an individual mouse. B: Overexpression of Ear1 significantly reduced the number of inflammatory cells recruited into the lung on postinfection day 5 (n = 6 control plasmids and 7 Ear1 plasmids; **P < 0.02). C: Expression of Ear 1 did not significantly diminish weight loss during infection (n = 13 mice per group, P = 0.21). Mean values from a single experiment reproduced two to three times with similar results. D: H& E staining of lungs on postinfection day 5 showing overexpression of Ear1 reduced leukocyte recruitment, alveolar edema, and airway injury. Scale bar = 50 μm.
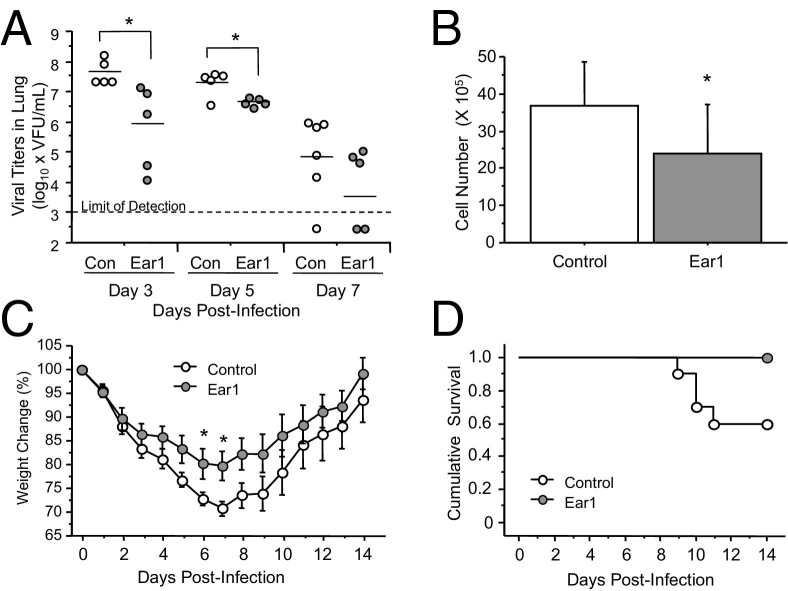
We then investigated in more detail whether restoring Ear1 expression in mice exposed to hyperoxia as neonates would mitigate the morbidity and mortality seen when infected with the same sublethal dose of influenza A virus. Gene delivery of Ear1 significantly reduced viral titers on postinfection days 3 and 5 but not on day 7, and this was associated with reduced number of leukocytes recruited to the lung on day 5 (Figures 5, A and B). As seen with the infected mice born into room air, overexpression of Ear1 reduced recruitment of macrophages, neutrophils, and lymphocytes to the airways (data not shown). Overexpression of Ear1 also significantly reduced levels of MCP-1, a chemokine whose expression during infection is augmented by prior exposure to oxygen,14 from 284.1 ± 29.7 pg/mL to 165.2 ± 12.6 pg/mL (P < 0.005, n = 9 control plasmids and n = 7 Ear1 plasmids). Interestingly, overexpression of Ear1 resulted in a more robust reduction in the levels of MCP-1 in infected siblings exposed to room air, from 203.6 ± 38.2 pg/mL to 12.2 ± 5.5 pg/mL (P < 0.002, n = 6 control plasmids and n = 5 Ear1 plasmids). Overexpression of Ear 1 in infected mice exposed to hyperoxia also significantly attenuated the pronounced weight loss observed on postinfection days 6 and 7 and as a group (P < 0.001) through postinfection day 14 (Figure 5C). Perhaps most striking, none of the mice expressing Ear1 died, whereas 50% of the mice receiving control plasmid died by postinfection day 14 (Figure 5D). The study was repeated two more times, and in both cases, none of the mice receiving pCMV-Ear1 plasmid died (n = 17 mice), whereas 50% of mice receiving the control plasmid died (n = 19 mice). Moreover, none of the oxygen-treated mice died during infection when electroporated with plasmid expressing the related isoform Ear2 (n = 9, data not shown).
Figure 5.
Electroporation-mediated gene delivery of Ear1 protects oxygen-exposed mice against influenza A virus infection. Lungs of adult mice exposed to hyperoxia (O2) as neonates were electroporated with empty vector (control) or plasmid-expressing Ear1 (Ear1). Mice were infected intranasally with influenza A virus 48 hours later. A: Overexpression of Ear1 significantly reduced viral titers on postinfection days 3 and 5 (*P < 0.005). Each dot represents the virus titer in an individual mouse. B: Overexpression of Ear1 significantly reduced the number of inflammatory cells recruited into the lung on postinfection day 5 (n = 9 mice per group; **P < 0.05). C: Overexpression of Ear1 significantly reduced weight loss of infected adult mice exposed to hyperoxia as neonates (n = 10 control plasmids and 8 Ear1 plasmids; *P < 0.0001). D: Overexpression of Ear1 enhanced survival of infected young adult mice exposed to hyperoxia as neonates (n = 10 control plasmids and 8 Ear1 plasmids; P < 0.05). Mean values from a single experiment reproduced three to five times with similar results.
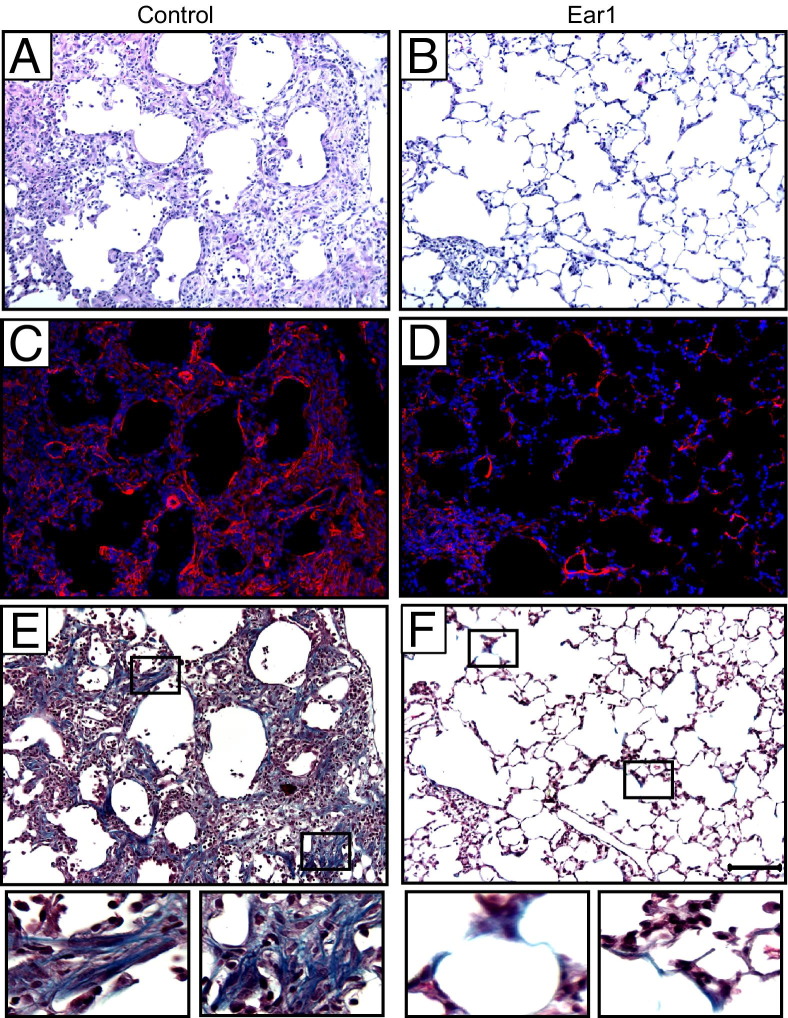
Persistent inflammation and fibrotic scarring were readily evident in the surviving mice that were administered control plasmid (Figure 6A). Fibrotic regions stained positively for α-smooth muscle actin, indicative of activated myofibroblasts, and thickened collagen bundles were observed using trichrome staining (Figures 6, C and E). This severe condition was rarely seen in infected siblings administered plasmid expressing Ear1 (Figures 6, B, D, and F). Although some interstitial thickening was observed, it stained weakly for α-smooth muscle actin or trichrome. In fact, with the exception of alveolar simplification attributed to neonatal oxygen exposure, fairly normal alveolar architecture was evident throughout most of the lung and was routinely observed in all of the mice on postinfection day 14. By sampling lungs on postinfection days 3, 5, 9, and 14 (see Supplemental Figure S4 at http://ajp.amjpathol.org), it was clear that Ear1 markedly reduced infection-associated symptoms throughout the infection.
Figure 6.
Electroporation-mediated gene delivery of Ear1 attenuates fibrotic repair of lungs from infected mice exposed to hyperoxia as neonates. Lungs of adult mice exposed to hyperoxia (O2) as neonates were electroporated with empty vector control (A, C, and E) or plasmid-expressing Ear1 (B, D, and F). Mice were infected intranasally with influenza A virus 2 days later. Lungs were fixed on postinfection day 14 and stained with H&E (A and B), α-smooth muscle actin (red) with DAPI nuclear counterstain (C and D), or Gomori's trichrome (E and F). Boxes in E and F are magnified below each respective image. Scale bar = 100 μm (A–F).
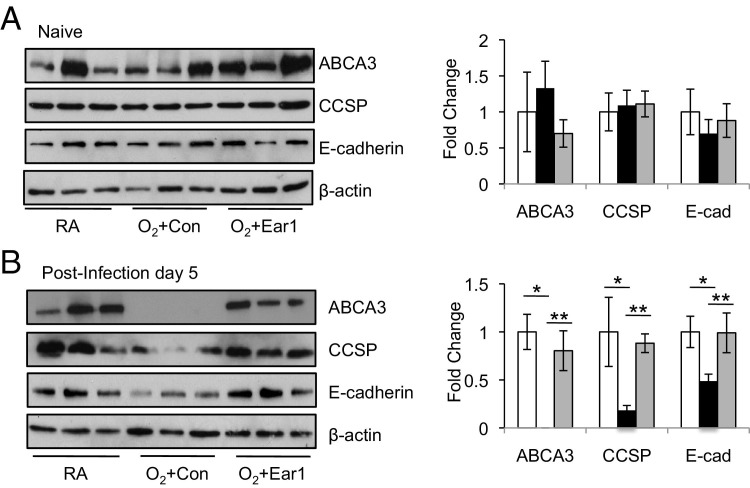
Given that severe epithelial injury in general, and especially to type II cells, often occurs in pulmonary fibrosis,35,36 we investigated whether neonatal oxygen sensitized the respiratory epithelium to virus-induced epithelial injury. However, it was difficult to use proSP-C as a marker of type II cells during infection because levels of proSP-C are low in adult mice exposed to neonatal hyperoxia.11 Expression of ABCA3 (ATP-binding cassette transporter of the A subfamily), a protein highly expressed in type II cells and involved in trafficking of surfactant phospholipids to lamellar bodies, was therefore used as a biomarker of type II cells. In the absence of infection, the 150-kDa mature ABCA3 protein was readily detected in lung homogenates of all mice examined (Figure 7A). Although ABCA3 was present in the lungs of room air-exposed mice on postinfection day 5, it was not detected in infected siblings that had been exposed to hyperoxia as neonates (Figure 7B). Interestingly, the loss of ABCA3 was not seen in infected mice exposed to hyperoxia if they were administered Ear1 expression plasmid before infection. We also evaluated expression of CCSP as an indicator of how Ear affected the airway epithelium and expression of E-cadherin as an indicator of how Ear affected the entire respiratory epithelium. Before infection, levels of CCSP and E-cadherin were comparable among the three groups of mice (Figure 7A). In contrast, infected mice exposed to hyperoxia as neonates expressed less CCSP and E-cadherin than infected siblings that had been exposed to room air; however, this reduction was not observed when Ear1 plasmid was administered (Figure 7B). Collectively, these findings suggest that neonatal oxygen sensitizes the respiratory epithelium to influenza A virus infection and expression of Ear1 attenuates this increased sensitivity.
Figure 7.
Electroporation-mediated gene delivery of Ear1 protects the respiratory epithelium of lungs from infected mice exposed to hyperoxia as neonates. Lungs of adult mice exposed to room air (RA) or hyperoxia (O2) as neonates were electroporated with empty vector (Con) or plasmid-expressing Ear1 (Ear1). Mice were infected with influenza A virus 48 hours later. Lungs were harvested before infection (A) and on postinfection day 5 (B). Lung homogenates were immunoblotted for ABCA3, CCSP, E-cadherin, and β-actin. Band intensities were quantified and graphed relative to β-actin. Neonatal oxygen significantly reduced expression of ABCA3, CCSP, and E-cadherin during infection. (n = 3 mice per group, *P < 0.05) but not when Ear1 was overexpressed (n = 5 mice per group, **P < 0.05).
Discussion
Influenza A viruses are a common cause of illness in the general population, and children born prematurely are at increased risk of complications due to infection with influenza and other respiratory viruses, such as respiratory syncytial virus (RSV). These viruses primarily infect cells of the respiratory tract due to the selective binding of viral coat proteins to specific terminal oligosaccharides on sialic acid residues, which are typically found on airway epithelial cells, alveolar epithelial type II cells, and antigen-presenting cells.37 Analogous to children born prematurely, neonatal hyperoxia increases sensitivity of young adult mice to influenza A virus infection. Increased sensitivity was not attributed to redistribution of sialic acid receptors required for infection because staining with lectin from Maackia amurensis was not qualitatively different between the two groups of mice (data not shown). Instead, increased sensitivity was attributed in part to less epithelial expression of Ear, particularly Ear1, which is a host defense protein secreted by eosinophils, macrophages, and neutrophils.24,38,39 Reduced expression of Ear1 is unlikely to be the only reason why neonatal oxygen increases sensitivity to viral infection because overexpression of Ear1 was more effective at attenuating the host response in room air–exposed mice than in hyperoxia-exposed siblings. Although modulation of Ear proteins may be only one of several targeted pathways, the suppression of innate defense proteins, such as Ear1, in adult mice exposed to neonatal hyperoxia is an exciting discovery that may help explain why children born prematurely respond poorly to respiratory viruses.
Ear1 is a member of the RNase A superfamily consisting of 13 members in human and 14 in Mus musculus (for review see Rosenberg26 and Cho et al40). All members of this family are located on one contiguous region of chromosomes 10 and 14 and are rapidly evolving through gene duplication and accumulation of missense mutations.27,40 In humans, Ears 1 through 8 retain amino acids in their catalytic subunits required for RNase activity, whereas Ears 9 through 13 have additional differences in their amino-terminus and appear to play a role in male reproductive functions.41 Unlike pancreatic RNase A, which has only RNase activity, Ears 1 through 8 are secreted RNases that also possess potent bactericidal, helminthotoxic, and antiviral properties when expressed in cell lines. However, host defense functions for the individual members of this protein family have not been fully elucidated, yet appear to be specific for each member. Moreover, RNase activity is necessary, but not sufficient, for anti-microbial activity.29,31,42 With regard to Ear1 specifically, recombinant rat Ear1 displays antimicrobial activity when expressed in cells, but little is known in general about its function.42 Hence, our finding that Ear1 also functions as an antiviral molecule in vivo provides a new role for this member of the RNase family. Our finding that Ear1 is expressed by the respiratory epithelium suggests it may function to limit viral or bacterial growth during the early stages of an infection. Consistent with this hypothesis, prophylactic delivery of Ear1 to naive mice exposed to room air or hyperoxia at birth reduced viral titers and the associated inflammatory response to influenza A virus infection. Hence, epithelial expression of Ear1 adds to the growing list of innate immune genes, such as SP-A and SP-D, that function as first responders to respiratory pathogens.
Of the 14 known rodent members of the Ear protein family, Ear1 and Ear2 were the only isoforms detected in lungs of naive mice. Although this confirms an earlier report,28 we extended those findings by showing airway epithelium and a limited number of alveolar epithelial type II cells express Ear mRNA. Ear protein staining was only detected in type II cells, perhaps because they, like eosinophils, contain large granules for storing secreted proteins. Consistent with this hypothesis, Ear staining was observed in some airway epithelial cells after viral infection (data not shown). Neonatal hyperoxia suppressed Ear mRNA expression in the airway and depleted the alveolus of type II cells expressing Ear. Prophylactic administration of a plasmid expressing Ear1 reduced influenza A virus infection and, more importantly, alleviated the poorer outcomes from influenza virus infection caused by neonatal hyperoxia. Interestingly, overexpression of Ear1 afforded less protection to infected mice exposed to hyperoxia than to infected siblings exposed to room air. This finding implies that Ear1 is unlikely the sole target of neonatal oxygen. Nonetheless, long-term dosing with anti-RSV antibodies, such as RSV-IGIV or palivizumab, alleviates infection rates or pulmonary complications seen in preterm infants with BPD.43,44 These findings both suggest that antiviral modalities may be efficacious in attenuating sensitivity to respiratory pathogens attributed to prematurity. As such, epithelial expression of Ear should be investigated as a potential biomarker and perhaps a therapeutic target for people at risk for respiratory infections, such as those who were born prematurely or had BPD. However, distinguishing between epithelial and inflammatory sources of these highly homologous members in humans will be challenging, particularly because levels of eosinophil cationic protein in tracheal aspirates, serum, and urine is already used as a biomarker of airway inflammation and bronchial hyperresponsiveness in preterm infants and asthmatic patients.45–48 Fortunately, this was not a concern for the current study in which Ear expression could be assessed in lung tissues harvested from naive mice that did not show a difference in the number of immune cells before infection.14
Another opportunity to learn more about Ear1 in the context of viral infections is suggested by our observation that administration of plasmids encoding Ear1 not only protected against infection-associated morbidity and mortality but also reduced leukocyte recruitment to the lung and ameliorated infection-associated symptoms. Importantly, reduced leukocyte accumulation in the lung was not due to the attenuated recruitment of a single cell type. Macrophages, neutrophils, and lymphocytes were equally affected by overexpression of Ear1. Reduced cellular infiltration to the infected lung could simply be due to early reductions in viral load caused by elevated Ear levels. However, the protective effects of Ear likely extend beyond reducing viral load because viral titers were only modestly elevated in infected mice exposed to neonatal hyperoxia and virus was effectively cleared by postinfection day 9. There is precedence for an interface between Ear proteins and signaling pathways that cue innate cell recruitment or that bridge the innate and adaptive responses to infection. For example, in addition to antiviral activity, Ear2 has been reported to be chemotactic for mouse and human dendritic cells.49 Dendritic cells are critical for the activation of naive, virus-specific CD8+ T cells, which differentiate cytotoxic T lymphocytes that kill virus-infected cells in the lung. There is some evidence that early dampening of viral replication may reduce the magnitude of the CD8+ T-cell response to infection.50 However, other work suggests alterations in early innate responses during an infection can result in greater CD8+ T-cell responses later51 and enhance infection-associated symptoms.52,53 Thus, the “stoichiometric” and kinetic relationship among antigen load, early innate responses, and the magnitude or nature of the adaptive response to infection remains to be fully elucidated. Understanding how Ear modifies recruitment of leukocytes may help clarify these complex interactions.
Altered recruitment of inflammatory cells and the associated cytokine-chemokine surge may contribute to the enhanced epithelial injury and fibrosis observed in infected mice exposed to hyperoxia as neonates. Severe epithelial injury is often observed in fibrosis35,36 and was observed in the current study as defined by a more significant loss of ABCA3, CCSP, and E-cadherin expression. Although pulmonary fibrosis is not typically viewed as a pathological outcome of viral infections, it is possible that primary or secondary defects render a host more susceptible to fibrosis after a second insult. Indeed, genetic disorders controlling surfactant homeostasis have been linked to interstitial lung fibrosis often diagnosed after a severe respiratory viral infection (for review see Whitsett et al54). For example, mutations in exon 4 of proSP-C disrupt normal posttranslational processing, thereby activating endoplasmic reticulum stress and increasing sensitivity to RSV-induced cell death.55 Similarly, mutations in ABCA3 have been associated with childhood interstitial lung disease, including desquamative interstitial pneumonia, nonspecific interstitial pneumonitis, and occasionally usual interstitial pneumonia.56–58 Some mutations in ABCA3 also elevate endoplasmic reticulum stress and promote apoptosis.59 The loss of ABCA3 in infected mice exposed to hyperoxia as neonates found in the current study provides strong evidence that neonatal hyperoxia reprogrammed how type II cells respond to influenza A virus infection. Understanding how this occurs and whether loss of ABCA3 by type II cells contributes to disease progression or merely provides a marker of epithelial cell damage will help clarify how neonatal hyperoxia sensitizes mice to influenza A virus infection.
Our mouse model has several limitations that make it difficult to directly compare our findings to children born prematurely. For example, newborn term mice were exposed to hyperoxia because it is not feasible to expose preterm mice to oxygen in a manner that reflects oxygen exposure in preterm humans. However, oxygen supplementation in term mice is likely to model the effects seen in preterm humans of 24 to 38 weeks' gestation because both have lungs in the saccular stage of development.60 Our study also evaluated how neonatal oxygen reprogrammed the host response to infection of young adult mice. It is not known whether increased sensitivity to respiratory viral infections, such as RSV, seen in children born prematurely is retained when they become adults. However, less Ear protein is observed in lung homogenates of postnatal day 7 mice exposed to hyperoxia between birth and postnatal day 4, and aged mice (>90 weeks old) exposed to hyperoxia as neonates still show increased mortality compared with siblings exposed to room air when infected with a sublethal dose of influenza virus strain HKx31 (data not shown). This finding suggests that children born prematurely may not outgrow their sensitivity to influenza A virus as young or old adults, but that remains to be determined. Our study also began by using transgenic mice expressing EGFP in a subpopulation of epithelial type II cells under control of the human SP-C promoter. Whether these cells truly represent a novel subpopulation of type II cells remains to be proven. Perhaps the biggest limitation is not being able to harvest respiratory epithelial cells from healthy children born prematurely for determining expression of the human ortholog of Ear proteins. However, reduced expression of Ear1 is unlikely to be the only reason neonatal hyperoxia increases sensitivity to influenza A virus in this mouse model because overexpression of Ear1 was more effective at attenuating the host response in infected mice exposed to room air than siblings exposed to hyperoxia. Identifying other innate immune genes that contribute to oxygen-dependent sensitivity to infection may provide better molecular markers for making comparisons between this mouse model and human disease.
In conclusion, the current study identifies a novel mechanism of innate host defense in respiratory epithelial cells that is disrupted in young adult mice exposed to neonatal hyperoxia. To our knowledge, this is the first report of Ear genes being expressed by the respiratory epithelium, and it adds to the growing list of epithelial-derived innate immune genes expressed by type II cells. When expressed in young adult mice, Ear1 served to reduce viral replication, inflammation, and epithelial injury during infection with influenza virus and alleviated some of the consequences of neonatal hyperoxia, including poorer survival. Understanding how neonatal hyperoxia reprograms epithelial expression of Ear and other innate immune defenses may provide new opportunities for identifying and treating children born prematurely and who are at risk for a variety of respiratory infections.
Acknowledgments
We thank Barry Stripp (Duke University, Durham, NC) for providing the anti-CCSP antibody, Helene Rosenberg (NIH) for providing the pVL-mEar1 and mEar2 expression plasmids, Jamie and Nancy Lee (Mayo Clinic, Scottsdale, AZ) for providing the rabbit anti-Ear antibody, David Phelps (Hershey, PA) for analyzing SP-A, Paul Kingma (Cincinnati, OH) for analyzing SP-D in BAL, and Michael Barravecchia, Christopher Kaufman, and Shauna Marr for technical assistance.
Footnotes
Supported in part by NIH grants HL091968 (M.A.O.), HL097141 (M.A.O. and P.L.), ES012409 and ES013863 (P.L.), training grant ES07026 and HL66988 (B.B.), HL81148 and EB9903 (D.D.), March of Dimes grant 6-FY08-264 (M.A.O.), and pilot projects funded by NIH grants ES01247 and 5UL1RR024160 (M.A.O.). The animal inhalation facility and tissue-processing core was supported in part by ES01247.
The authors did not disclose any relevant financial relationships.
Supplemental material for this article can be found at http://ajp.amjpathol.org or at http://dx.doi.org/10.1016/j.ajpath.2012.05.005.
Supplementary data
ClustalW alignment of mouse Ear open reading frames. Mouse Ear1 (GenBank accession AF408692), Ear2 (GenBank accession AF306664.1), and Ear3 (GenBank accession BC153204) open reading frames aligned using MacVector software version 10.0.2. An asterisk defines those nucleotides present in all three isoforms, and lines represent gaps present in Ear1 that are not found in Ear2 or Ear3. Primers 203 and 204 were used to amplify and clone cDNAs from whole lung and isolated EGFP-positive type II cells. Primers 238 and 239 were used for semiquantitative RT-PCR.
Genotypic differences between EGFP-positive and EGFP-negative type II cells. EGFP-positive and EGFP-negative type II cells were isolated in triplicate and RNA hybridized to mouse genome 430 2.0 array from Affymetrix. A: Representative flow cytometry histogram showing separation of EGFP-positive and EGFP-negative type II cells based on intrinsic green fluorescence. B: The hierarchical clustered heat map represents the 25 most abundant genes expressed in both cell populations, most abundant in EGFP-positive type II cells, or most abundant in EGFP-negative type II cells. Names of some genes are listed to the right. Signal intensities range from low (green) to high (red).
Influenza A viral titers in adult mice exposed to neonatal hyperoxia. Adult mice exposed to room air (RA) or hyperoxia (O2) at birth were infected intranasally with 120 hemagglutinating units of influenza A virus strain HKx31 (H3N2). Viral foci assays were performed on dilutions of lung homogenates collected on postinfection days 3, 5, 7, and 9 days. Foci were counted, and influenza viral titers were calculated. The data shown are from a single experiment, which was repeated four times with similar results. Neonatal hyperoxia increased viral titers during infection (n = 5 to 6; P < 0.03 by analysis of variance). Data are representative of five to six separate infections with similar findings.
Electroporation-mediated gene delivery of EAR1 alleviates oxygen-enhanced lung symptoms after influenza A virus infection. Adult mice exposed to neonatal hyperoxia were electroporated with control plasmid (top rows) or EAR1 plasmid (bottom rows) and then infected 2 days later with a nonlethal dose of influenza A virus HKx31 (H3N2). Lungs were harvested on postinfection days 3, 5, 9, and 14 for histologic analysis. Each image represents an individual mouse harvested from a single experiment showing efficacy of Ear protection against influenza A virus–mediated disease.
References
- 1.Eber E., Zach M.S. Long term sequelae of bronchopulmonary dysplasia (chronic lung disease of infancy) Thorax. 2001;56:317–323. doi: 10.1136/thorax.56.4.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhatt A.J., Pryhuber G.S., Huyck H., Watkins R.H., Metlay L.A., Maniscalco W.M. Disrupted pulmonary vasculature and decreased vascular endothelial growth factor, Flt-1, and TIE-2 in human infants dying with bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;164:1971–1980. doi: 10.1164/ajrccm.164.10.2101140. [DOI] [PubMed] [Google Scholar]
- 3.Robin B., Kim Y.J., Huth J., Klocksieben J., Torres M., Tepper R.S., Castile R.G., Solway J., Hershenson M.B., Goldstein-Filbrun A. Pulmonary function in bronchopulmonary dysplasia. Pediatr Pulmonol. 2004;37:236–242. doi: 10.1002/ppul.10424. [DOI] [PubMed] [Google Scholar]
- 4.Doyle L.W. Respiratory function at age 8–9 years in extremely low birthweight/very preterm children born in Victoria in 1991–1992. Pediatr Pulmonol. 2006;41:570–576. doi: 10.1002/ppul.20412. [DOI] [PubMed] [Google Scholar]
- 5.Doyle L.W., Faber B., Callanan C., Freezer N., Ford G.W., Davis N.M. Bronchopulmonary dysplasia in very low birth weight subjects and lung function in late adolescence. Pediatrics. 2006;118:108–113. doi: 10.1542/peds.2005-2522. [DOI] [PubMed] [Google Scholar]
- 6.Smith V.C., Zupancic J.A., McCormick M.C., Croen L.A., Greene J., Escobar G.J., Richardson D.K. Rehospitalization in the first year of life among infants with bronchopulmonary dysplasia. J Pediatr. 2004;144:799–803. doi: 10.1016/j.jpeds.2004.03.026. [DOI] [PubMed] [Google Scholar]
- 7.Doyle L.W., Faber B., Callanan C., Morley R. Blood pressure in late adolescence and very low birth weight. Pediatrics. 2003;111:252–257. doi: 10.1542/peds.111.2.252. [DOI] [PubMed] [Google Scholar]
- 8.Weisman L.E. Populations at risk for developing respiratory syncytial virus and risk factors for respiratory syncytial virus severity: infants with predisposing conditions. Pediatr Infect Dis J. 2003;22:S33–S39. doi: 10.1097/01.inf.0000053883.08663.e5. [DOI] [PubMed] [Google Scholar]
- 9.Jobe A.H., Kallapur S.G. Long term consequences of oxygen therapy in the neonatal period. Semin Fetal Neonatal Med. 2010;15:230–235. doi: 10.1016/j.siny.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schulman S.R., Canada A.T., Fryer A.D., Winsett D.W., Costa D.L. Airway hyperreactivity produced by short-term exposure to hyperoxia in neonatal guinea pigs. Am J Physiol. 1997;272:L1211–L1216. doi: 10.1152/ajplung.1997.272.6.L1211. [DOI] [PubMed] [Google Scholar]
- 11.Yee M., Vitiello P.F., Roper J.M., Staversky R.J., Wright T.W., McGrath-Morrow S.A., Maniscalco W.M., Finkelstein J.N., O'Reilly M.A. Type II epithelial cells are critical target for hyperoxia-mediated impairment of postnatal lung development. Am J Physiol Lung Cell Mol Physiol. 2006;291:L1101–L1111. doi: 10.1152/ajplung.00126.2006. [DOI] [PubMed] [Google Scholar]
- 12.Denis D., Fayon M.J., Berger P., Molimard M., De Lara M.T., Roux E., Marthan R. Prolonged moderate hyperoxia induces hyperresponsiveness and airway inflammation in newborn rats. Pediatr Res. 2001;50:515–519. doi: 10.1203/00006450-200110000-00015. [DOI] [PubMed] [Google Scholar]
- 13.Yee M., White R.J., Awad H.A., Bates W.A., McGrath-Morrow S.A., O'Reilly M.A. Neonatal hyperoxia causes pulmonary vascular disease and shortens life span in aging mice. Am J Pathol. 2011;178:2601–2610. doi: 10.1016/j.ajpath.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O'Reilly M.A., Marr S.H., Yee M., McGrath-Morrow S.A., Lawrence B.P. Neonatal hyperoxia enhances the inflammatory response in adult mice infected with influenza A virus. Am J Respir Crit Care Med. 2008;177:1103–1110. doi: 10.1164/rccm.200712-1839OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCormack F.X., Whitsett J.A. The pulmonary collectins: SP-A and SP-D, orchestrate innate immunity in the lung. J Clin Invest. 2002;109:707–712. doi: 10.1172/JCI15293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fehrenbach H. Alveolar epithelial type II cell: defender of the alveolus revisited. Respir Res. 2001;2:33–46. doi: 10.1186/rr36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang J., Nikrad M.P., Phang T., Gao B., Alford T., Ito Y., Edeen K., Travanty E.A., Kosmider B., Hartshorn K., Mason R.J. Innate immune response to influenza A virus in differentiated human alveolar type II cells. Am J Respir Cell Mol Biol. 2011;45:582–591. doi: 10.1165/rcmb.2010-0108OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang J., Oberley-Deegan R., Wang S., Nikrad M., Funk C.J., Hartshorn K.L., Mason R.J. Differentiated human alveolar type II cells secrete antiviral IL-29 (IFN-lambda 1) in response to influenza A infection. J Immunol. 2009;182:1296–1304. doi: 10.4049/jimmunol.182.3.1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lo B., Hansen S., Evans K., Heath J.K., Wright J.R. Alveolar epithelial type II cells induce T cell tolerance to specific antigen. J Immunol. 2008;180:881–888. doi: 10.4049/jimmunol.180.2.881. [DOI] [PubMed] [Google Scholar]
- 20.Oertelt-Prigione S. The influence of sex and gender on the immune response. Autoimmun Rev. 2011;11:A479–A485. doi: 10.1016/j.autrev.2011.11.022. [DOI] [PubMed] [Google Scholar]
- 21.Roper J.M., Staversky R.J., Finkelstein J.N., Keng P.C., O'Reilly M.A. Identification and isolation of mouse type II cells on the basis of intrinsic expression of enhanced green fluorescent protein. Am J Physiol Lung Cell Mol Physiol. 2003;285:L691–L700. doi: 10.1152/ajplung.00034.2003. [DOI] [PubMed] [Google Scholar]
- 22.Mutlu G.M., Machado-Aranda D., Norton J.E., Bellmeyer A., Urich D., Zhou R., Dean D.A. Electroporation-mediated gene transfer of the Na+,K+ -ATPase rescues endotoxin-induced lung injury. Am J Respir Crit Care Med. 2007;176:582–590. doi: 10.1164/rccm.200608-1246OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yee M., Chess P.R., McGrath-Morrow S.A., Wang Z., Gelein R., Zhou R., Dean D.A., Notter R.H., O'Reilly M.A. Neonatal oxygen adversely affects lung function in adult mice without altering surfactant composition or activity. Am J Physiol Lung Cell Mol Physiol. 2009;297:L641–L649. doi: 10.1152/ajplung.00023.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cormier S.A., Yuan S., Crosby J.R., Protheroe C.A., Dimina D.M., Hines E.M., Lee N.A., Lee J.J. T(H)2-mediated pulmonary inflammation leads to the differential expression of ribonuclease genes by alveolar macrophages. Am J Respir Cell Mol Biol. 2002;27:678–687. doi: 10.1165/rcmb.4882. [DOI] [PubMed] [Google Scholar]
- 25.Bachmann M.F., Ecabert B., Kopf M. Influenza virus: a novel method to assess viral and neutralizing antibody titers in vitro. J Immunol Methods. 1999;225:105–111. doi: 10.1016/s0022-1759(99)00034-4. [DOI] [PubMed] [Google Scholar]
- 26.Rosenberg H.F. RNase A ribonucleases and host defense: an evolving story. J Leukoc Biol. 2008;83:1079–1087. doi: 10.1189/jlb.1107725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang J., Dyer K.D., Rosenberg H.F. Evolution of the rodent eosinophil-associated RNase gene family by rapid gene sorting and positive selection. Proc Natl Acad Sci U S A. 2000;97:4701–4706. doi: 10.1073/pnas.080071397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cormier S.A., Larson K.A., Yuan S., Mitchell T.L., Lindenberger K., Carrigan P., Lee N.A., Lee J.J. Mouse eosinophil-associated ribonucleases: a unique subfamily expressed during hematopoiesis. Mamm Genome. 2001;12:352–361. doi: 10.1007/s003350020007. [DOI] [PubMed] [Google Scholar]
- 29.Domachowske J.B., Dyer K.D., Adams A.G., Leto T.L., Rosenberg H.F. Eosinophil cationic protein/RNase 3 is another RNase A-family ribonuclease with direct antiviral activity. Nucleic Acids Res. 1998;26:3358–3363. doi: 10.1093/nar/26.14.3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bedoya V.I., Boasso A., Hardy A.W., Rybak S., Shearer G.M., Rugeles M.T. Ribonucleases in HIV type 1 inhibition: effect of recombinant RNases on infection of primary T cells and immune activation-induced RNase gene and protein expression. AIDS Res Hum Retroviruses. 2006;22:897–907. doi: 10.1089/aid.2006.22.897. [DOI] [PubMed] [Google Scholar]
- 31.Domachowske J.B., Dyer K.D., Bonville C.A., Rosenberg H.F. Recombinant human eosinophil-derived neurotoxin/RNase 2 functions as an effective antiviral agent against respiratory syncytial virus. J Infect Dis. 1998;177:1458–1464. doi: 10.1086/515322. [DOI] [PubMed] [Google Scholar]
- 32.Domachowske J.B., Bonville C.A., Dyer K.D., Rosenberg H.F. Evolution of antiviral activity in the ribonuclease A gene superfamily: evidence for a specific interaction between eosinophil-derived neurotoxin (EDN/RNase 2) and respiratory syncytial virus. Nucleic Acids Res. 1998;26:5327–5332. doi: 10.1093/nar/26.23.5327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu J., Li Y.H., Xue C.F., Ding J., Gong W.D., Zhao Y., Huang Y.X. Targeted ribonuclease can inhibit replication of hepatitis B virus. World J Gastroenterol. 2003;9:295–299. doi: 10.3748/wjg.v9.i2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou R., Norton J.E., Dean D.A. Electroporation-mediated gene delivery to the lungs. Methods Mol Biol. 2008;423:233–247. doi: 10.1007/978-1-59745-194-9_17. [DOI] [PubMed] [Google Scholar]
- 35.Adamson I.Y., Young L., Bowden D.H. Relationship of alveolar epithelial injury and repair to the induction of pulmonary fibrosis. Am J Pathol. 1988;130:377–383. [PMC free article] [PubMed] [Google Scholar]
- 36.Sisson T.H., Mendez M., Choi K., Subbotina N., Courey A., Cunningham A., Dave A., Engelhardt J.F., Liu X., White E.S., Thannickal V.J., Moore B.B., Christensen P.J., Simon R.H. Targeted injury of type II alveolar epithelial cells induces pulmonary fibrosis. Am J Respir Crit Care Med. 2010;181:254–263. doi: 10.1164/rccm.200810-1615OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ibricevic A., Pekosz A., Walter M.J., Newby C., Battaile J.T., Brown E.G., Holtzman M.J., Brody S.L. Influenza virus receptor specificity and cell tropism in mouse and human airway epithelial cells. J Virol. 2006;80:7469–7480. doi: 10.1128/JVI.02677-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Slifman N.R., Loegering D.A., McKean D.J., Gleich G.J. Ribonuclease activity associated with human eosinophil-derived neurotoxin and eosinophil cationic protein. J Immunol. 1986;137:2913–2917. [PubMed] [Google Scholar]
- 39.Sur S., Glitz D.G., Kita H., Kujawa S.M., Peterson E.A., Weiler D.A., Kephart G.M., Wagner J.M., George T.J., Gleich G.J., Leiferman K.M. Localization of eosinophil-derived neurotoxin and eosinophil cationic protein in neutrophilic leukocytes. J Leukoc Biol. 1998;63:715–722. doi: 10.1002/jlb.63.6.715. [DOI] [PubMed] [Google Scholar]
- 40.Cho S., Beintema J.J., Zhang J. The ribonuclease A superfamily of mammals and birds: identifying new members and tracing evolutionary histories. Genomics. 2005;85:208–220. doi: 10.1016/j.ygeno.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 41.Boix E., Nogues M.V. Mammalian antimicrobial proteins and peptides: overview on the RNase A superfamily members involved in innate host defence. Mol Biosyst. 2007;3:317–335. doi: 10.1039/b617527a. [DOI] [PubMed] [Google Scholar]
- 42.Ishihara K., Asai K., Nakajima M., Mue S., Ohuchi K. Preparation of recombinant rat eosinophil-associated ribonuclease-1 and -2 and analysis of their biological activities. Biochim Biophys Acta. 2003;1638:164–172. doi: 10.1016/s0925-4439(03)00077-2. [DOI] [PubMed] [Google Scholar]
- 43.The PREVENT Study Group Reduction of respiratory syncytial virus hospitalization among premature infants and infants with bronchopulmonary dysplasia using respiratory syncytial virus immune globulin prophylaxis. Pediatrics. 1997;99:93–99. doi: 10.1542/peds.99.1.93. [DOI] [PubMed] [Google Scholar]
- 44.The IMpact RSV Study Group Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. Pediatrics. 1998;102:531–537. [PubMed] [Google Scholar]
- 45.Brostrom E.B., Katz-Salamon M., Lundahl J., Hallden G., Winbladh B. Eosinophil activation in preterm infants with lung disease. Acta Paediatr. 2007;96:23–28. doi: 10.1111/j.1651-2227.2006.00002.x. [DOI] [PubMed] [Google Scholar]
- 46.Remes S., Korppi M., Remes K., Savolainen K., Mononen I., Pekkanen J. Serum eosinophil cationic protein (ECP) and eosinophil protein X (EPX) in childhood asthma: the influence of atopy. Pediatr Pulmonol. 1998;25:167–174. doi: 10.1002/(sici)1099-0496(199803)25:3<167::aid-ppul6>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 47.Nuijsink M., Hop W.C., Sterk P.J., Duiverman E.J., Hiemstra P.S., de Jongste J.C. Urinary eosinophil protein X in children with atopic asthma. Mediators Inflamm. 2007;2007:49240. doi: 10.1155/2007/49240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Raghavender B., Smith J.B. Eosinophil cationic protein in tracheal aspirates of preterm infants with bronchopulmonary dysplasia. J Pediatr. 1997;130:944–947. doi: 10.1016/s0022-3476(97)70281-6. [DOI] [PubMed] [Google Scholar]
- 49.Yang D., Chen Q., Rosenberg H.F., Rybak S.M., Newton D.L., Wang Z.Y., Fu Q., Tchernev V.T., Wang M., Schweitzer B., Kingsmore S.F., Patel D.D., Oppenheim J.J., Howard O.M. Human ribonuclease A superfamily members, eosinophil-derived neurotoxin and pancreatic ribonuclease, induce dendritic cell maturation and activation. J Immunol. 2004;173:6134–6142. doi: 10.4049/jimmunol.173.10.6134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee H.Y., Topham D.J., Park S.Y., Hollenbaugh J., Treanor J., Mosmann T.R., Jin X., Ward B.M., Miao H., Holden-Wiltse J., Perelson A.S., Zand M., Wu H. Simulation and prediction of the adaptive immune response to influenza A virus infection. J Virol. 2009;83:7151–7165. doi: 10.1128/JVI.00098-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tate M.D., Pickett D.L., van Rooijen N., Brooks A.G., Reading P.C. Critical role of airway macrophages in modulating disease severity during influenza virus infection of mice. J Virol. 2010;84:7569–7580. doi: 10.1128/JVI.00291-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tumpey T.M., Garcia-Sastre A., Taubenberger J.K., Palese P., Swayne D.E., Pantin-Jackwood M.J., Schultz-Cherry S., Solorzano A., Van Rooijen N., Katz J.M., Basler C.F. Pathogenicity of influenza viruses with genes from the 1918 pandemic virus: functional roles of alveolar macrophages and neutrophils in limiting virus replication and mortality in mice. J Virol. 2005;79:14933–14944. doi: 10.1128/JVI.79.23.14933-14944.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fujisawa H. Neutrophils play an essential role in cooperation with antibody in both protection against and recovery from pulmonary infection with influenza virus in mice. J Virol. 2008;82:2772–2783. doi: 10.1128/JVI.01210-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Whitsett J.A., Wert S.E., Weaver T.E. Alveolar surfactant homeostasis and the pathogenesis of pulmonary disease. Annu Rev Med. 2010;61:105–119. doi: 10.1146/annurev.med.60.041807.123500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bridges J.P., Xu Y., Na C.L., Wong H.R., Weaver T.E. Adaptation and increased susceptibility to infection associated with constitutive expression of misfolded SP-C. J Cell Biol. 2006;172:395–407. doi: 10.1083/jcb.200508016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bullard J.E., Wert S.E., Whitsett J.A., Dean M., Nogee L.M. ABCA3 mutations associated with pediatric interstitial lung disease. Am J Respir Crit Care Med. 2005;172:1026–1031. doi: 10.1164/rccm.200503-504OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Doan M.L., Guillerman R.P., Dishop M.K., Nogee L.M., Langston C., Mallory G.B., Sockrider M.M., Fan L.L. Clinical, radiological and pathological features of ABCA3 mutations in children. Thorax. 2008;63:366–373. doi: 10.1136/thx.2007.083766. [DOI] [PubMed] [Google Scholar]
- 58.Young L.R., Nogee L.M., Barnett B., Panos R.J., Colby T.V., Deutsch G.H. Usual interstitial pneumonia in an adolescent with ABCA3 mutations. Chest. 2008;134:192–195. doi: 10.1378/chest.07-2652. [DOI] [PubMed] [Google Scholar]
- 59.Weichert N., Kaltenborn E., Hector A., Woischnik M., Schams A., Holzinger A., Kern S., Griese M. Some ABCA3 mutations elevate ER stress and initiate apoptosis of lung epithelial cells. Respir Res. 2011;12:4. doi: 10.1186/1465-9921-12-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Maeda Y., Dave V., Whitsett J.A. Transcriptional control of lung morphogenesis. Physiol Rev. 2007;87:219–244. doi: 10.1152/physrev.00028.2006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ClustalW alignment of mouse Ear open reading frames. Mouse Ear1 (GenBank accession AF408692), Ear2 (GenBank accession AF306664.1), and Ear3 (GenBank accession BC153204) open reading frames aligned using MacVector software version 10.0.2. An asterisk defines those nucleotides present in all three isoforms, and lines represent gaps present in Ear1 that are not found in Ear2 or Ear3. Primers 203 and 204 were used to amplify and clone cDNAs from whole lung and isolated EGFP-positive type II cells. Primers 238 and 239 were used for semiquantitative RT-PCR.
Genotypic differences between EGFP-positive and EGFP-negative type II cells. EGFP-positive and EGFP-negative type II cells were isolated in triplicate and RNA hybridized to mouse genome 430 2.0 array from Affymetrix. A: Representative flow cytometry histogram showing separation of EGFP-positive and EGFP-negative type II cells based on intrinsic green fluorescence. B: The hierarchical clustered heat map represents the 25 most abundant genes expressed in both cell populations, most abundant in EGFP-positive type II cells, or most abundant in EGFP-negative type II cells. Names of some genes are listed to the right. Signal intensities range from low (green) to high (red).
Influenza A viral titers in adult mice exposed to neonatal hyperoxia. Adult mice exposed to room air (RA) or hyperoxia (O2) at birth were infected intranasally with 120 hemagglutinating units of influenza A virus strain HKx31 (H3N2). Viral foci assays were performed on dilutions of lung homogenates collected on postinfection days 3, 5, 7, and 9 days. Foci were counted, and influenza viral titers were calculated. The data shown are from a single experiment, which was repeated four times with similar results. Neonatal hyperoxia increased viral titers during infection (n = 5 to 6; P < 0.03 by analysis of variance). Data are representative of five to six separate infections with similar findings.
Electroporation-mediated gene delivery of EAR1 alleviates oxygen-enhanced lung symptoms after influenza A virus infection. Adult mice exposed to neonatal hyperoxia were electroporated with control plasmid (top rows) or EAR1 plasmid (bottom rows) and then infected 2 days later with a nonlethal dose of influenza A virus HKx31 (H3N2). Lungs were harvested on postinfection days 3, 5, 9, and 14 for histologic analysis. Each image represents an individual mouse harvested from a single experiment showing efficacy of Ear protection against influenza A virus–mediated disease.