Abstract
Purpose
To report spontaneous corneal clearing with improved visual acuity and central endothelial cell repopulation after Descemet’s stripping without endothelial replacement.
Design
Interventional case report.
Methods
A 34 year-old female with bilateral decreased vision secondary to corneal edema from endothelial dysfunction underwent Descemet’s stripping endothelial keratoplasty (DSEK) in the right eye and Descemet’s stripping (DS) only in her contralateral eye. Histopathologic evaluation confirmed a dual diagnosis of Fuchs endothelial dystrophy (Fuchs) and posterior polymorphous membrane dystrophy (PPMD) from Descemet’s membrane specimens removed from each eye. Following primary graft failure with regraft in the right eye, the second posterior corneal lenticule detached, was removed and was not replaced. The cornea cleared and central endothelial cell repopulation was documented by confocal microscopy. Therefore, Descemet’s stripping without endothelial replacement was performed in the left eye. The left cornea also cleared with central endothelial cell repopulation.
Main Outcome Measures
Postoperative visual acuity and central endothelial cell repopulation
Results
Endothelial migration after Descemet’s stripping alone in the second eye, with probably host endothelial cell repopulation in the right eye.
Conclusions
Endothelial cell migration after Descemet’s stripping procedure without insertion of endothelial graft can occur, resulting in host endothelial cell repopulation with corneal clearing and improved visual acuity.
Descemet’s stripping endothelial keratoplasty (DSEK) selectively transplants healthy donor corneal endothelium and Descemet’s membrane while leaving the recipient anterior cornea intact.1 DSEK is becoming the preferred surgical technique for primary endothelial disorders, including Fuchs endothelial dystrohy (Fuchs),2 pseudophakic bullous keratopathy (PBK),3 and less common endothelial disorders such as Iridocorneal Endothelial (ICE) Syndrome and posterior polymorphous dystrophy (PPMD).4, 13 While the usual surgical technique removes host Descemet’s and endothelium, there is debate about the necessity of removing the host tissue in cases with presumably normal Descemet’s (PBK and failed penetrating keratoplasty).5 However, for each DSEK surgical indication, endothelial replacement is deemed absolutely necessary for ultimate corneal clearing, since corneal endothelium is not believed to have the ability to regenerate or repopulate the central cornea.
PPMD is a unique autosomal dominant bilateral corneal endothelial dystrophy characterized by transformation of the endothelium into epithelial-like cells with potentially increased migratory capacity.6 A mis-sense mutation common to both FED and PPMD exists and could explain the occurrence of both these processes simultaneously.7 This case report describes the clinical course of a young patient with both Fuchs and PPMD who had a positive surgical outcome, with corneal clearing and significantly improved visual acuity bilaterally, despite having no functional donor endothelium implanted in either eye. To our knowledge, this is the first definitive report of corneal clearing and spontaneous central endothelial cell repopulation after simple Descemet’s membrane removal.
Case Report
A 34 year-old Caucasian female presented in May 2009 complaining of progressive blurred vision bilaterally. Corrected Distance visual acuity (CDVA) with spectacles was 20/50 bilaterally with a manifest refraction of −2.25 +2.00 × 150 in the right eye and −1.00 +1.75 × 015 in the left eye. Central corneal thickness was 595 and 597 microns in the right and left eyes respectively. Slit lamp examination showed mild corneal edema with multiple pinpoint endothelial irregularities bilaterally (Figure 1). Confocal microscopy demonstrated an irregular endothelial mosaic pattern with patchy alternating hypo and hyper-reflective regions in an inconsistent pattern (Figure 2). The patient had been seen by another corneal specialist 2 years prior and given a diagnosis of Fuchs; however, the clinical appearance was atypical, and based on age and appearance, a presumptive diagnosis of PPMD was made. After discussing treatment options, risks, and benefits, the patient elected to undergo DSEK.
Figure 1.
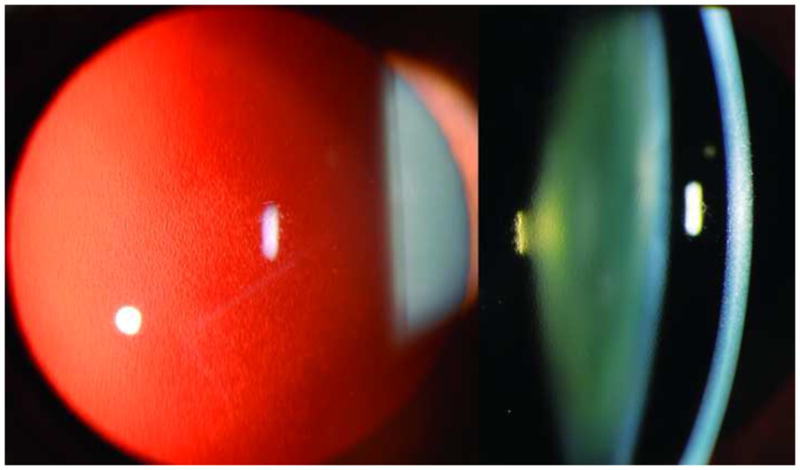
Slit lamp photo of right cornea demonstrating a beaten metal appearance of the endothelium notable in slit view (Left image) and in retroillumination (Right image).
Figure 2.
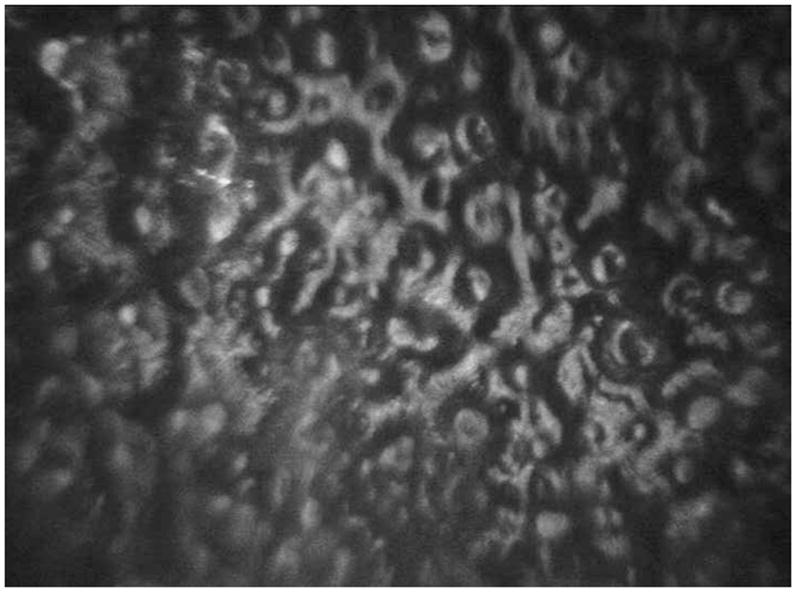
Confocal imaging of the right cornea demonstrating an irregular endothelial mosaic pattern with patchy alternating hypo and hyper-reflective regions in an inconsistent pattern.
An uncomplicated DSEK was performed on the right eye in July 2009 using a standard surgical technique. Briefly, this involved scoring of Descemet’s membrane for 360 degrees at a diameter of 8.0 mm with a reverse Sinsky hook followed by manual tissue stripping and removal of the intact host Descemet’s and endothelium through a 4.0mm incision. The donor tissue was cut with the Moria microkeratome unit (Moria, Inc., Doylestown, PA) with a 350 micron head and then trephined with an 8.0mm trephine. The tissue was folded in a 60/40 fashion, inserted into the anterior chamber, and positioned using the LASIK flap roller (BD Visitec, Franklin Lakes, NJ.) and the reverse Sinsky hook. The corneal lenticule was left with a complete air fill for 8 minutes, after which the anterior chamber was reformed with Basic Salt Solution with a remaining 50% air fill. The patient tolerated the procedure well without complications. Histopathologic analysis revealed thickening of Descemet’s membrane and presence of numerous posterior nodular excrescences (guttata). Endothelial cells were multilayered in areas and immunohistochemical stains were postitive for cytokeratins AE1,3 and MAK 6 in the endothelial cells (Figure 3). The final diagnosis was combined PPMD with Fuchs dystrophy-like changes.
Figure 3.
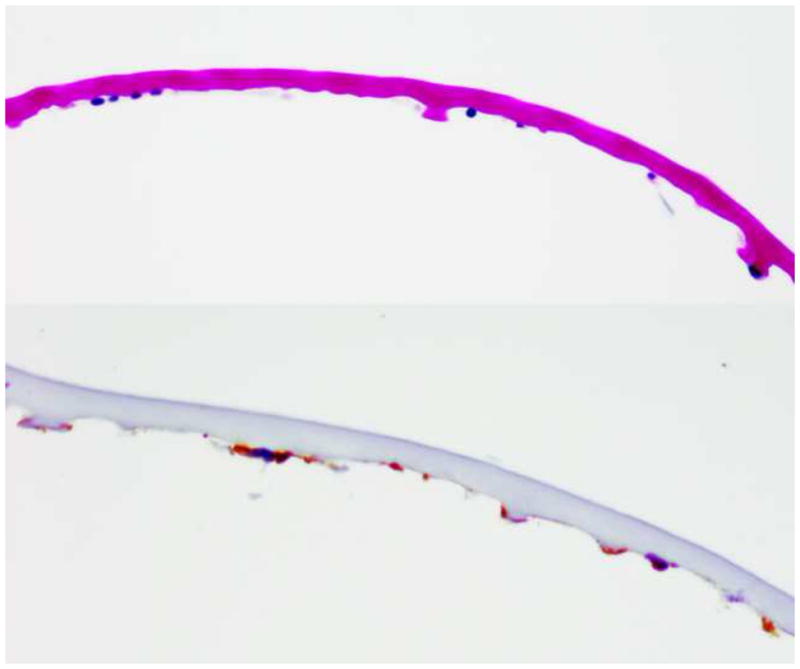
Descemet’s membrane is thickened and contains posterior nodular excrescences with thickened Descemet’s membrane (Top image) with multilayered, scattered endothelial cells positive for cytokeratins AE1,3 and MAK 6 immunohistochemical satins (Bottom image). (Periodic acid schiff, 100X)
At the postoperative day (POD) one visit, the graft was inferiorly displaced and was repositioned without incident. However, one week after repositioning, the lenticule was attached but grossly edematous and primary graft failure was diagnosed. Repeat DSEK was performed in August 2009 using the same basic technique and was again uncomplicated. At POD one, the donor lenticule was appropriately positioned and attached. Nonetheless, over the next two months the donor lenticule began to progressively detach peripherally, with the area of detachment moving centrally at each visit. Interestingly, during this process the patient’s cornea appeared clearest in the peripheral areas with maximal graft detachment (Figure 4) and confocal microscopy demonstrated the presence of endothelial cells in the mid periphery (Figure 5).
Figure 4.
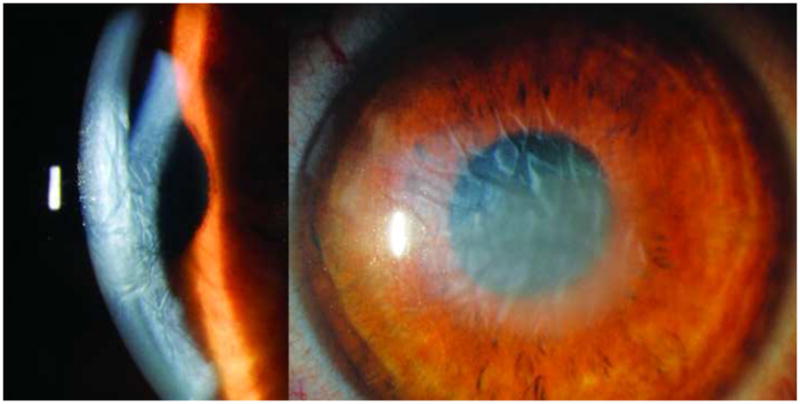
Slit lamp photo of right and left corneas during the healing process after surgery. The right cornea (left image) shows peripheral graft detachment with corneal clearing in the detached section (superiorly in the image) as compared to the persistent central edema in the central regions of graft adherence, while the left cornea (right image) shows similar peripheral corneal clearing with residual central edema after the Descemet’s stripping only procedure.
Figure 5.
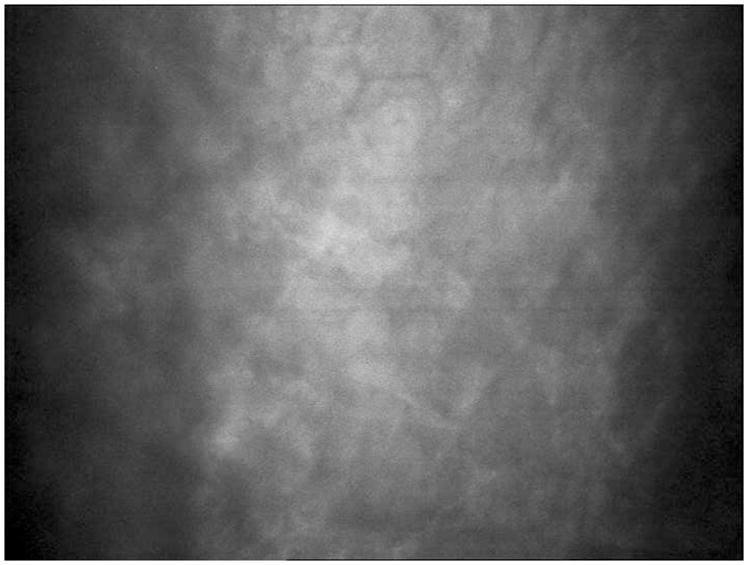
Confocal imaging showing presence of new endothelial cells in the superior cornea of right eye with persistent central edema and haze.
Serial confocal microscopy demonstrated repopulation of endothelial cells specifically in the areas where the donor tissue had detached. By November 2009, the patient’s central cornea had improved clarity. Confocal microscopy revealed near-complete central endothelial cell repopulation. Uncorrected distance visual acuity (UDVA) had improved to 20/80 despite the opaque donor lenticule graft being completely detached and in the anterior chamber. At this juncture, the decision was made to proceed with donor lenticule removal without replacement. Two months after this procedure, the patient’s cornea had cleared and vision had significantly improved to UCVA of 20/25 and CDVA of 20/20 (Figure 6). Confocal analysis demonstrated a complete repopulation of endothelial cells centrally (Figure 7).
Figure 6.
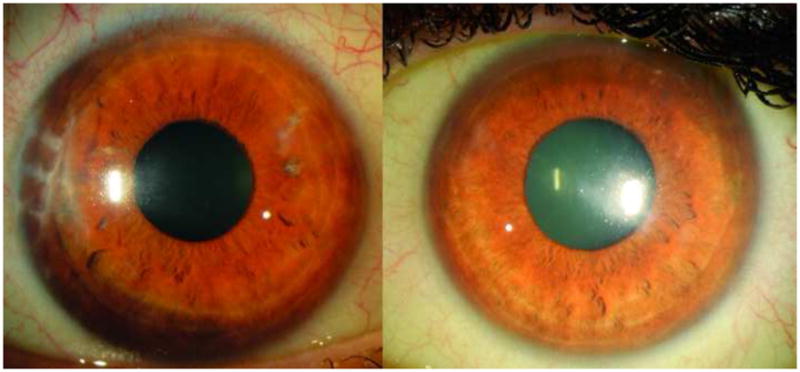
Slit lamp photo of right and left cornea at final evaluation. The right cornea cornea (left image) shows complete resolution of corneal edema with corneal clarity, while the left cornea (right image) shows resolution of central corneal edema with deep paracentral stromal haze in the region corresponding to final endothelial cell repopulation.
Figure 7.
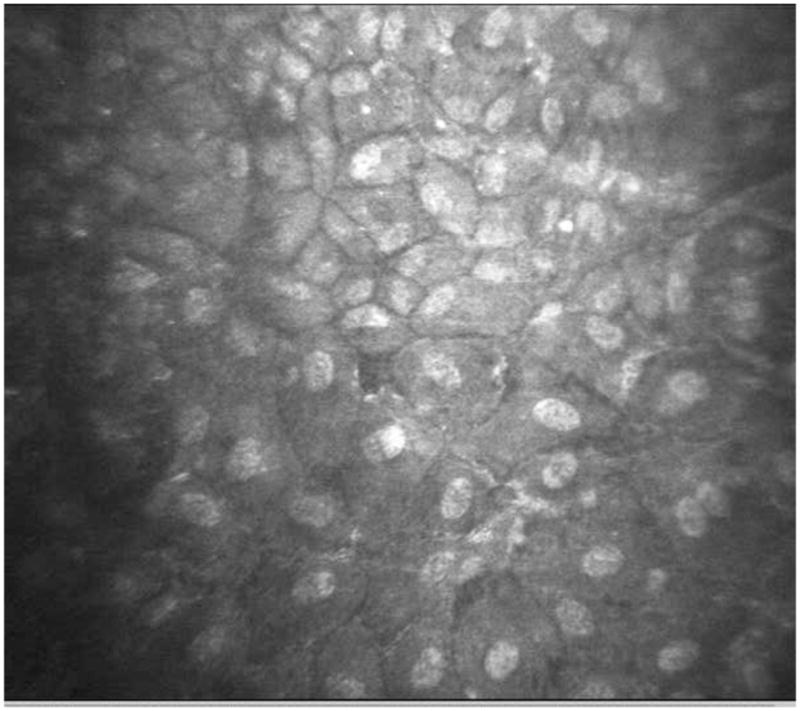
Confocal imaging of right cornea showing repopulation of endothelial cells throughout the central cornea.
After fully discussing the unusual pattern of healing in the right eye and the uncertain outcome or best procedure to utilize for the left eye with the patient, the decision was made to simply strip Descemet’s membrane without donor transplantation. Surgery was performed in February 2010, with scoring of Descemet’s membrane for 360 degrees with the reverse sinsky hook and removal of this tissue using the manual stripper through a slightly enlarged paracentesis. Histopathologic analysis again confirmed PPMD and Fuchs-like changes. At three months postoperatively, the patient’s cornea had cleared significantly and UCVA had improved to 20/60 and peripheral corneal clearing was again noted (Figure 4). By six months postoperatively, the patient’s left cornea cleared with a residual small area of deep stromal haze that appeared to correspond with the last region of endothelial cell repopulation (Figure 6). The patient’s right cornea remained clear and the patient had UCVA of 20/25 bilaterally. Confocal analysis demonstrated the same complete central repopulation of endothelial cells in the left eye (Figure 8).
Figure 8.
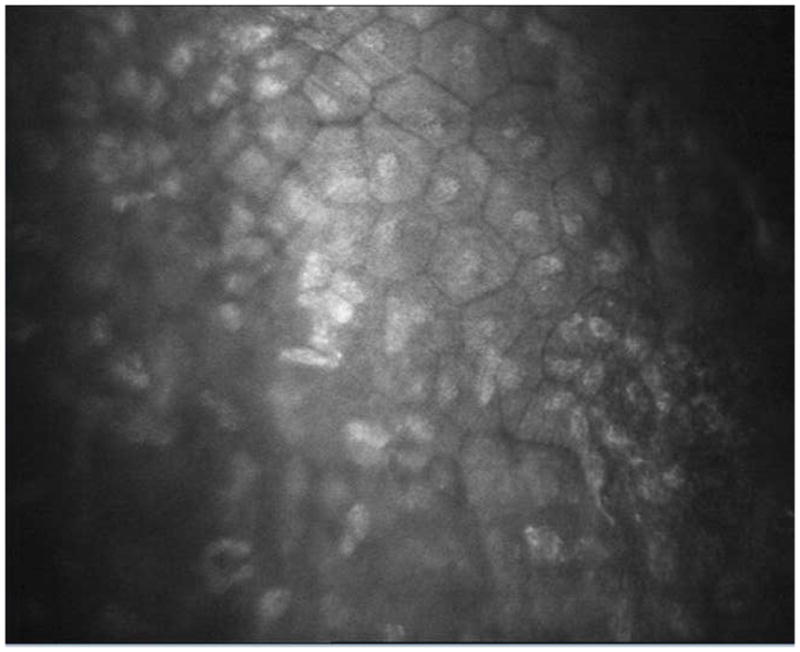
Confocal imaging of left cornea showing repopulation of endothelial cells throughout the central cornea.
Institutional review board (IRB) approval was obtained to review this case. Literature review of the PubMed database (www.pubmed.gov) using the search terms “descemet membrane endothelial keratoplasty” yielded 450 results, which were initially screened by title followed by abstract where available for relevance. Approximately 50 reports were briefly reviewed and we did not find a similar report of endothelial cell repopulation without corneal donor implantation.
Discussion
This report describes, to our knowledge, the first definitive case of spontaneous corneal clearing with endothelial cell repopulation after Descemet’s stripping without donor endothelial cell implantation in a patient with posterior polymorphous corneal dystrophy and Fuchs-like endothelial changes. Our patient had a clinical diagnosis of PPMD and histologic evidence of multi-layered endothelium that expressed cytokeratins, consistent with PPMD.8 There may be overlap in PPMD and Fuchs dystrophy, as a mutation in the TCF8 gene has been associated with both PPMD and Fuchs dystrophy.9 It is possible that our case had overlap of these two dystrophies.
The potential for endothelial cell transfer from donor to host or endothelial cell migration was reported by Balachandran and colleagues.10 They reported corneal clearing with improved visual acuity in 2 eyes of 2 patients with Fuchs after Descemet’s membrane endothelial keratoplasty (DMEK) despite early donor detachment. They proposed that in one case, the recipient endothelium may have migrated from the remaining peripheral rim of recipient Descemet’s membrane onto the bare recipient posterior stroma, from the area of the descemetorhexis. A second proposed mechanism was that an upside-down graft could have facilitated transfer of donor endothelial cells to bare host stroma.10 While our patient had traditional DSEK in the right eye and theoretically could have had endothelial cell migration from the donor to the host, this seems unlikely given the clinical course of peripheral clearing with persistent edema in the areas of graft attachment. However, as no tissue was transplanted in the left eye, the only possible mechanism for central endothelial cell repopulation was by cell migration from the periphery. Because the posterior stromal scar in the left eye corresponded with the last area to be repopulated with host endothelium, the lack of donor tissue may have facilitated the scar formation.
DSEK procedure has been successful for the treatment of PPMD as described in a case report by Bellaveau.11 We hypothesize that in our case, the patient’s underlying PPMD condition contributed to the endothelial cell’s ability to centrally migrate. It is also possible that over time the patient’s corneal clouding may return by this same mechanism. The patient’s young age may have also facilitated endothelial cell migration.
It is unknown whether these results are simply unique to this particular patient or if they are applicable to most patients with PPMD, nor is it known whether patients with PPMD ultimately will do better with standard DSEK or simple Descemet’s removal. Further study and more cases are certainly warranted before any recommendations for DSEK technique changes should be considered.
Acknowledgments
Financial Support: Supported in part by NIH NEI P30EY06360 and an unrestricted departmental grant from Research to Prevent Blindness, Inc.
Footnotes
The Authors have no financial interests in the products or topics in this manuscript
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lee WB, Jacobs DS, Musch DC, et al. Ophthalmic Technology Assessment Committee Cornea and Anterior Segment Disorders Panel. Descemet’s stripping endothelial keratoplasty: safety and outcomes: a report by the American Academy of Ophthalmology. Ophthalmology. 2009;116:1818–30. doi: 10.1016/j.ophtha.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 2.Price MO, Price FW. Descemet’s stripping endothelial keratoplasty. Curr Opin Ophthalmol. 2007;18:290–4. doi: 10.1097/ICU.0b013e3281a4775b. [DOI] [PubMed] [Google Scholar]
- 3.Basak SK. Descemet stripping and endothelial keratoplasty in endothelial dysfunctions: three-month results in 75 eyes. Indian J Ophthalmol. 2008;56:291–6. doi: 10.4103/0301-4738.41412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Price MO, Price FW., Jr Descemet stripping endothelial keratoplasty for treatment of iridocorneal endothelial syndrome. Cornea. 2007;26:493–7. doi: 10.1097/ICO.0b013e318030d274. [DOI] [PubMed] [Google Scholar]
- 5.Caldwell MCM, Afshari NA, Decroos FC, Proia AD. The histology of graft adhesion in Descemet stripping with endothelial keratoplasty. Am J Ophthalmol. 2009;148:277–81. doi: 10.1016/j.ajo.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 6.Krachmer JH. Posterior polymorphous corneal dystrophy: a disease characterized by epithelial-like endothelial cells which influence management and prognosis. Trans Am Ophthalmol Soc. 1985;83:413–75. [PMC free article] [PubMed] [Google Scholar]
- 7.Biswas S, Munier FL, Yardley J, et al. Missense mutations in COL8A2, the gene encoding the alpha2 chain of type VIII collagen, cause two forms of corneal endothelial dystrophy. Hum Mol Genet. 2001;10:2415–23. doi: 10.1093/hmg/10.21.2415. [DOI] [PubMed] [Google Scholar]
- 8.Anderson NJ, Badawi DY, Grossniklaus HE, Stulting RD. Posterior polymorphous membranous dystrophy with overlapping features of iridocorneal endothelial syndrome. Arch Ophthalmol. 2001;119:624–5. doi: 10.1001/archopht.119.4.624. [DOI] [PubMed] [Google Scholar]
- 9.Mehta JS, Vithana EN, Tan DT, et al. Analysis of the posterior polymorphous corneal dystrophy 3 gene, TCF8, in late-onset Fuchs endothelial corneal dystrophy. Invest Ophthalmol Vis Sci. 2008;49:184–8. doi: 10.1167/iovs.07-0847. [DOI] [PubMed] [Google Scholar]
- 10.Balachandran C, Ham L, Verschoor CA, et al. Spontaneous corneal clearance despite graft detachment in Descemet membrane endothelial keratoplasty. Am J Ophthalmol. 2009;148:227–34. doi: 10.1016/j.ajo.2009.02.033. [DOI] [PubMed] [Google Scholar]
- 11.Belliveau MJ, Rocha G, Manchur A, Brownstein S. Bilateral Descemet’s stripping with endothelial keratoplasty for posterior polymorphous corneal dystrophy in a young phakic patient [letter] Can J Ophthalmol. 2010;45:180–1. doi: 10.1139/i09-199. [DOI] [PubMed] [Google Scholar]


