Abstract
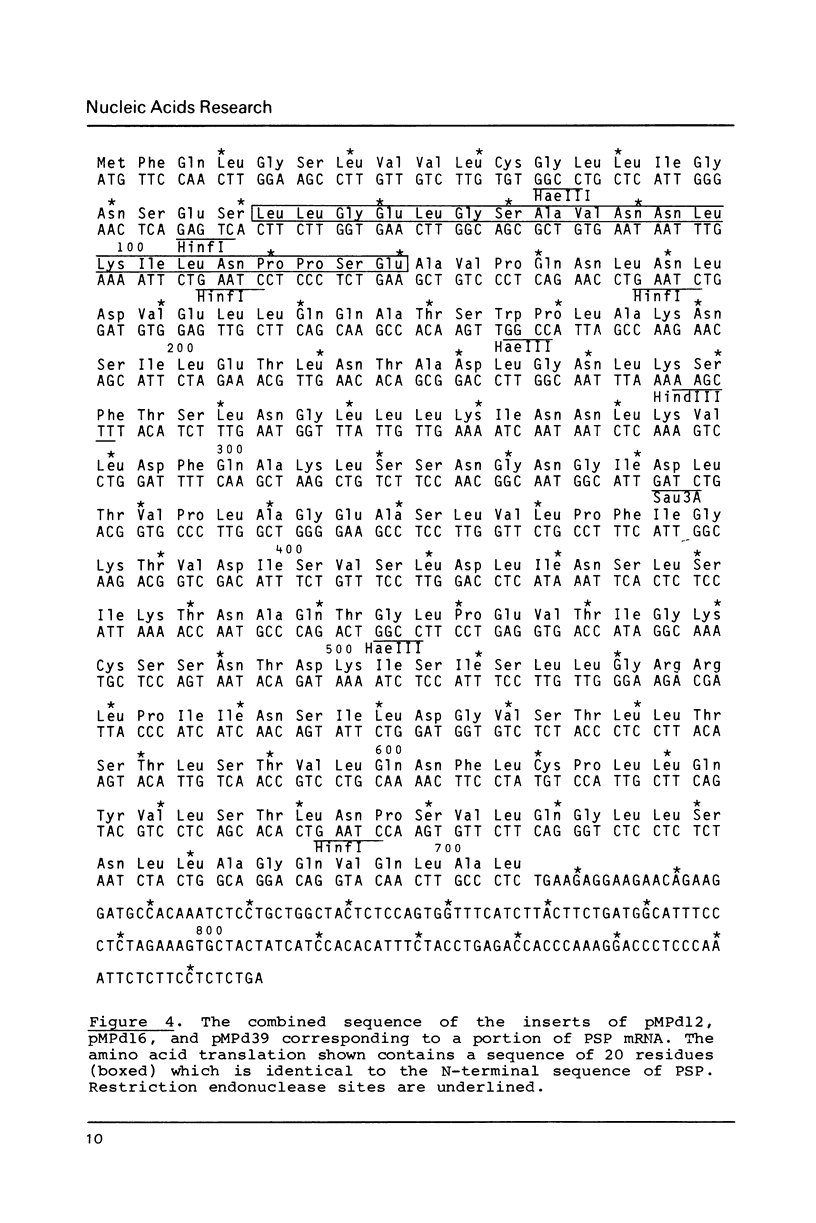
PSP is the most abundant translation product of mouse parotid glands where its production is co-ordinated with that of salivary amylase. The synthesis of these two proteins apparently is restricted to this tissue. In order to enable us to study common regulatory elements in the genes of the two proteins, double stranded cDNA, synthesized for parotid gland poly (A)+ RNA, was cloned. DNA sequencing of three clones complementary to the most abundant messenger indicated overlap and resulted in a total sequence of 867 nucleotides. Translation of this sequence revealed that at one end the amino acid sequence was the same as the N-terminal sequence of PSP. The sequence contains 60 nucleotides coding for part of or the complete signal peptide, 645 nucleotides coding for the PSP protein, and 162 nucleotides that apparently are not translated. Southern blot analysis suggests a simple structure for the PSP gene in mouse and man.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggin M. D., Gibson T. J., Hong G. F. Buffer gradient gels and 35S label as an aid to rapid DNA sequence determination. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3963–3965. doi: 10.1073/pnas.80.13.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blin N., Stafford D. W. A general method for isolation of high molecular weight DNA from eukaryotes. Nucleic Acids Res. 1976 Sep;3(9):2303–2308. doi: 10.1093/nar/3.9.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- CLARKE J. T. SIMPLIFIED "DISC" (POLYACRYLAMIDE GEL) ELECTROPHORESIS. Ann N Y Acad Sci. 1964 Dec 28;121:428–436. doi: 10.1111/j.1749-6632.1964.tb14214.x. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Deininger P. L. Random subcloning of sonicated DNA: application to shotgun DNA sequence analysis. Anal Biochem. 1983 Feb 15;129(1):216–223. doi: 10.1016/0003-2697(83)90072-6. [DOI] [PubMed] [Google Scholar]
- Eicher E. M., Lane P. W. Assignment of LH XVI to chromosome 3 in the mouse. J Hered. 1980 Sep-Oct;71(5):315–318. doi: 10.1093/oxfordjournals.jhered.a109378. [DOI] [PubMed] [Google Scholar]
- Fantoni A., Bozzoni I., Ullu E., Farace M. G. Construction of a recombinant bacterial plasmid containing DNA sequences for a mouse embryonic globin chain. Nucleic Acids Res. 1979 Aug 10;6(11):3505–3517. doi: 10.1093/nar/6.11.3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girvitz S. C., Bacchetti S., Rainbow A. J., Graham F. L. A rapid and efficient procedure for the purification of DNA from agarose gels. Anal Biochem. 1980 Aug;106(2):492–496. doi: 10.1016/0003-2697(80)90553-9. [DOI] [PubMed] [Google Scholar]
- Glisin V., Crkvenjakov R., Byus C. Ribonucleic acid isolated by cesium chloride centrifugation. Biochemistry. 1974 Jun 4;13(12):2633–2637. doi: 10.1021/bi00709a025. [DOI] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagenbüchle O., Bovey R., Young R. A. Tissue-specific expression of mouse-alpha-amylase genes: nucleotide sequence of isoenzyme mRNAs from pancreas and salivary gland. Cell. 1980 Aug;21(1):179–187. doi: 10.1016/0092-8674(80)90125-7. [DOI] [PubMed] [Google Scholar]
- Hjorth J. P. Genetic variation in mouse salivary amylase rate of synthesis. Biochem Genet. 1979 Aug;17(7-8):665–682. doi: 10.1007/BF00502125. [DOI] [PubMed] [Google Scholar]
- Hjorth J. P., Lusis A. J., Nielsen J. T. Multiple structural genes for mouse amylase. Biochem Genet. 1980 Apr;18(3-4):281–302. doi: 10.1007/BF00484242. [DOI] [PubMed] [Google Scholar]
- Karn R. C., Petersen T. E., Hjorth J. P., Nieles J. T., Roepstorff P. Characterization of the amino termini of mouse salivary and pancreatic amylases. FEBS Lett. 1981 Apr 20;126(2):293–296. doi: 10.1016/0014-5793(81)80264-5. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Owerbach D., Hjorth J. P. Inheritance of a parotid secretory protein in mice and its use in determining salivary amylase quantitative variants. Genetics. 1980 May;95(1):129–141. doi: 10.1093/genetics/95.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson B. M., Roberts B. E., Kuff E. L. Structural gene identification and mapping by DNA-mRNA hybrid-arrested cell-free translation. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4370–4374. doi: 10.1073/pnas.74.10.4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roychoudhury R., Jay E., Wu R. Terminal labeling and addition of homopolymer tracts to duplex DNA fragments by terminal deoxynucleotidyl transferase. Nucleic Acids Res. 1976 Jan;3(1):101–116. doi: 10.1093/nar/3.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schibler U., Tosi M., Pittet A. C., Fabiani L., Wellauer P. K. Tissue-specific expression of mouse alpha-amylase genes. J Mol Biol. 1980 Sep 5;142(1):93–116. doi: 10.1016/0022-2836(80)90208-9. [DOI] [PubMed] [Google Scholar]
- Schibler U., Tosi M., Pittet A. C., Fabiani L., Wellauer P. K. Tissue-specific expression of mouse alpha-amylase genes. J Mol Biol. 1980 Sep 5;142(1):93–116. doi: 10.1016/0022-2836(80)90208-9. [DOI] [PubMed] [Google Scholar]
- Skorstengaard K., Thøgersen H. C., Vibe-Pedersen K., Petersen T. E., Magnusson S. Purification of twelve cyanogen bromide fragments from bovine plasma fibronectin and the amino acid sequence of eight of them. Overlap evidence aligning two plasmic fragments, internal homology in gelatin-binding region and phosphorylation site near C terminus. Eur J Biochem. 1982 Nov 15;128(2-3):605–623. doi: 10.1111/j.1432-1033.1982.tb07007.x. [DOI] [PubMed] [Google Scholar]
- Southern E. Gel electrophoresis of restriction fragments. Methods Enzymol. 1979;68:152–176. doi: 10.1016/0076-6879(79)68011-4. [DOI] [PubMed] [Google Scholar]
- Wickens M. P., Buell G. N., Schimke R. T. Synthesis of double-stranded DNA complementary to lysozyme, ovomucoid, and ovalbumin mRNAs. Optimization for full length second strand synthesis by Escherichia coli DNA polymerase I. J Biol Chem. 1978 Apr 10;253(7):2483–2495. [PubMed] [Google Scholar]
- Wood D., Blanchetot A., Jeffreys A. J. Molecular cloning of seal myoglobin mRNA. Nucleic Acids Res. 1982 Nov 25;10(22):7133–7144. doi: 10.1093/nar/10.22.7133. [DOI] [PMC free article] [PubMed] [Google Scholar]