Abstract
Phytochromes are red/far-red photochromic photoreceptors central to regulating plant development. Although they are known to enter the nucleus upon light activation and, once there, regulate transcription, this is not the complete picture. Various phytochrome effects are manifested much too rapidly to derive from changes in gene expression, whereas others seem to occur without phytochrome entering the nucleus. Phytochromes also guide directional responses to light, excluding a genetic signaling route and implying instead plasma membrane association and a direct cytoplasmic signal. However, to date, no such association has been demonstrated. Here we report that a phytochrome subpopulation indeed associates physically with another photoreceptor, phototropin, at the plasma membrane. Yeast two-hybrid methods using functional photoreceptor molecules showed that the phytochrome steering growth direction in Physcomitrella protonemata binds several phototropins specifically in the photoactivated Pfr state. Split-YFP studies in planta showed that the interaction occurs exclusively at the plasma membrane. Coimmunoprecipitation experiments provided independent confirmation of in vivo phy-phot binding. Consistent with this interaction being associated with a cellular signal, we found that phytochrome-mediated tropic responses are impaired in Physcomitrella phot− mutants. Split-YFP revealed a similar interaction between Arabidopsis phytochrome A and phototropin 1 at the plasma membrane. These associations additionally provide a functional explanation for the evolution of neochrome photoreceptors. Our results imply that the elusive phytochrome cytoplasmic signal arises through binding and coaction with phototropin at the plasma membrane.
The breakthrough in understanding plant phytochrome action was the discovery that, upon light activation, the photoreceptor enters the nucleus and there orchestrates major changes in the transcriptional pattern through interactions with transcriptional regulators including PIF family members (1). In the case of phyA, the predominant phytochrome in etiolated cells, the Pr ground state is cytoplasmic, whereas photoconversion to the Pfr state leads to rapid FHY1-mediated nuclear transport. phyB, predominant in light-grown tissue, is thought to act similarly, although the translocation mechanism is much slower and not associated with FHY1 (2–4). Pfr regulation of transcriptional regulators involves phosphorylation, but although the C-terminal domain resembles that of prokaryotic histidine kinases, it seems to be unnecessary for phytochrome signaling (5, 6). Instead, the critical interaction of phyB with PIF3 appears to involve the PAS-GAF cleft in the N-terminal photosensory module (7, 8).
Various phytochrome-mediated effects cannot, however, derive from such a system (4). Several occur within minutes or even seconds, far too fast for gene regulation. In Arabidopsis fhl−/fhy1− mutants, light-dependent phyA nuclear translocation is lost along with most but not all phyA-mediated responses, implying that a cytoplasmic signal also exists (9). In fern and moss protonemal filaments, tip cells show phototropic bending toward red light (R), an effect that is reversible by far-red light (FR). In these groups and also in certain algae, light-induced chloroplast movement too is a R effect reversible by FR. None of these responses can derive from gene regulation, not only because they are too fast but also because they require that directional information from the stimulus is transmitted to the response (which is, of course, impossible for a transcription/translation mechanism). Interestingly, these vectorial responses show strong action dichroism and can even be steered by the polarization of the light stimulus. Wolfgang Haupt evolved an elegant model able to explain all these effects on the basis of phytochromes attached anisotropically to the plasma membrane and showing a rotation of the transition dipole moment between Pr and Pfr (10–12). To date, the only direct evidence for phytochrome association with the plasma membrane derives from a fluorescence correlation microscopy study in Ceratodon that implied that phytochrome assembled with phycoerythrobilin was less mobile at the cell periphery than in the cytoplasm (13). In general, however, phytochromes are not considered to be membrane-associated, Pr at least being freely cytosolic. This paradox was partly solved in cases involving ferns (14) and algae (15) with the discovery that the photoreceptor responsible comprised a phytochrome sensory module fused to a phototropin blue light (B) receptor. This chimeric molecule was named neochrome. As higher plant phototropins are known to be membrane-associated (16, 17), neochromes might show this property too, thereby providing for the predicted action dichroism in accordance with the Haupt model. Neochromes are, however, absent from mosses and higher plants. Indeed, targeted knockout in the moss Physcomitrella patens showed that a canonical phytochrome Pp.phy4 was responsible for vectorial responses including phototropism, polarotropism, and chloroplast relocation (18). We were thus interested in how this and perhaps higher plant phytochromes might provide an appropriate signal within the cytoplasm.
Results
Our initial approach was to try to identify Pp.phy4 holophytochrome interacting partners in yeast. We established both Pp.phy4 and Arabidopsis phytochrome A (At.phyA) as functional, full-length, holoprotein bait molecules fused to the DNA-binding domain (BD) for yeast two-hybrid (Y2H) experiments (Fig. 1A). Both phytochromes showed light-independent homodimerization (Fig. 1B and Fig. S1A) using equivalent prey constructs carrying the activator domain (AD), whereas At.phyA and FHY1 (19) hybrids interacted exclusively in R, the effect being reversed by FR and only seen if the cells were fed with phycocyanobilin (PCB) (Fig. 1 B and C and Fig. S1A). These positive controls indicated that correctly folded, photochromically functional holophytochrome was present in the yeast cells. We then screened a Physcomitrella full-length cDNA prey library with N-terminal ADs using Pp.phy4 bait hybrids with the BD attached either to the C or N terminus (Pp.phy4:BD and BD:Pp.phy4, respectively). Several prey molecules bound the latter R/FR reversibly, indicating that these bait constructs were also functional. Additionally, we found that all four Physcomitrella phototropins (Pp.photA1, -A2, -B1, and -B2) showed phytochrome state-dependent interactions with BD:Pp.phy4 according to both quantitative growth on appropriate dropout media (Fig. 1B) and β-galactosidase assays (Fig. 1C). AD:Pp.photA1 in particular showed moderate interaction even in darkness but in this as in the cases of the other three phototropins, interaction was enhanced three- to fivefold in pulsed R, effects that were fully reversed by subsequent FR pulses. No specific effects of B were apparent. Although the isolated Pp.phy4 sensory module showed no interaction with Pp.phot, constitutive binding was seen for the C terminus (Fig. S1 D and E). These data in yeast pointed to a direct, Pfr-enhanced binding of phytochrome to all four phototropins.
Fig. 1.
Yeast two-hybrid studies reveal Pfr-enhanced phytochrome–phototropin interaction. Various hybrid constructs (A, green and yellow bars indicate HA- and MYC-tags, respectively) were assayed for growth (B) and reporter activity (C) following different light treatments as described in Materials and Methods. Full-length holophytochrome bait constructs with N- or C-terminal BDs were expressed together with different prey constructs in PCB-fed yeast. In B, 2 × 105 AH109 cells were spotted onto QSD medium. In C, β-galactosidase activity of Y187 cultures was measured using the Miller assay with ONPG substrate.
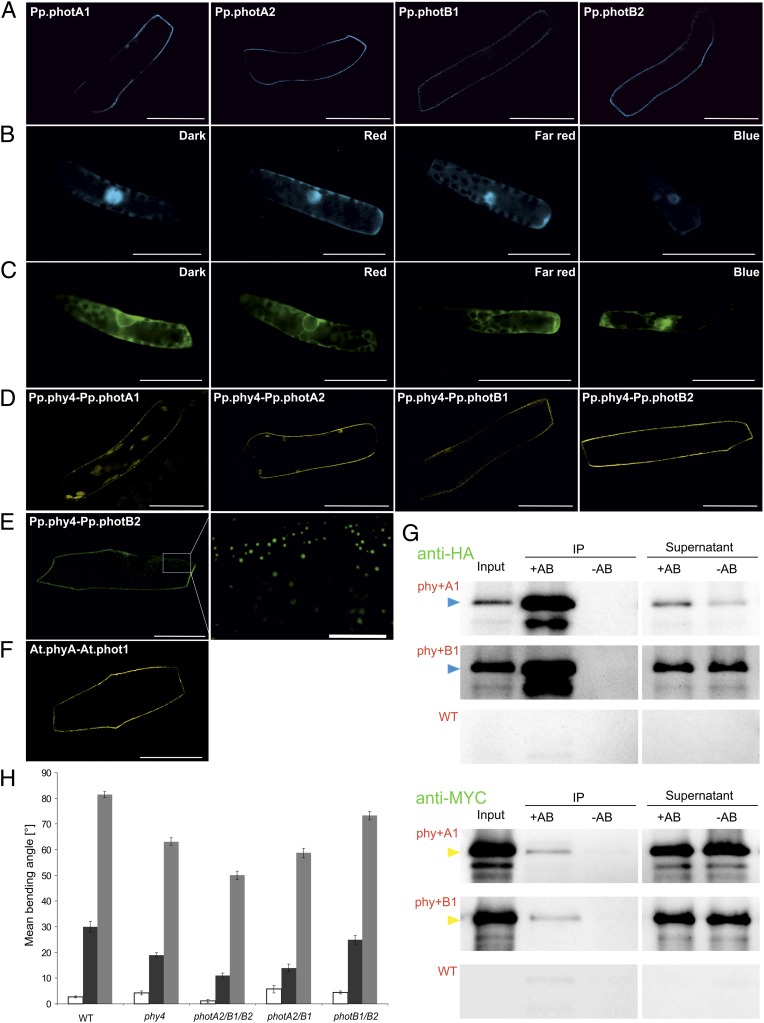
Although phototropins lack obvious features that might lead to a direct association with cell membranes, fluorescent protein (FP)-tagging experiments in Arabidopsis have shown that they are indeed membrane associated (16, 17). Additionally, light-dependent phototropin internalization has been observed; this effect was suppressed by pretreatment with R (20). To date, no studies of phototropin localization in Physcomitrella have been reported. We therefore constructed hybrid proteins in which an FP was attached to the N terminus of each Physcomitrella phototropin and examined the cellular localization in Physcomitrella filaments. This study showed that, as expected, all phototropins are exclusively associated with the plasma membrane (Fig. 2A and Fig. S2; for comparison, Fig. S3 shows plasma membrane, cytoplasmic, and nuclear markers). Similar localization was seen in onion epidermis cells. However, we did not observe dissociation from the plasma membrane upon irradiation as reported for Arabidopsis (20).
Fig. 2.
Physcomitrella phytochrome and phototropin localization and interaction in planta, phytochrome–phototropin coimmunoprecipitation, and genetic evidence for phototropin involvement in phytochrome signaling. (A–C) Localization of phototropins with CFP:Pp.phot constructs (A) and of Pp.phy4 with Pp.phy4:CFP (B) and GFP:Pp.phy4 (C) in Physcomitrella following different light pretreatments. Split-YFP showing interaction of Pp.phy4 with phototropins at the plasma membrane in Physcomitrella filament cells after 15 min R (D) and in onion epidermis cells (E, right at high magnification; scale bar: 10 μm) showing YFPC:Pp.phy4 with YFPN:Pp.photB2 interaction in discrete peripheral areas. Arabidopsis phytochrome A–phototropin 1 interaction at the plasma membrane in onion epidermal cells revealed by split-YPF after 15-min R pretreatment (F, YFPN:At.phot1 with YFPC:At.phyA). (Scale bars: 50 μm.) Anti-HA antibody immunoprecipitation (IP) of HA:Pp.phy4 with MYC:Pp.photA1 or B1 following Agrobacterium-mediated coexpression in Nicotiana benthamiana (G), detected with anti-HA (Upper, HA:Pp.phy4 as blue arrows) and anti-MYC (Lower, MYC:Pp.photA1 or B1 as yellow arrows). Negative controls were simulated IPs without anti-HA (-AB) and extracts from untransformed leaves (WT). All procedures carried out in white light (plant growth and protein extraction) or R (IP). Pp.phy4-mediated steering of directional growth in Physcomitrella wild type and phy4− or multiple phot− knockout filament tip cells (H). R-induced phototropism (dark gray) and polarotropism (light gray). Equivalent phototropism experiments with B induced no significant bending (white). SEs are shown.
Surprisingly, in contrast to phytochromes in higher plants, it was reported earlier that Physcomitrella phytochromes 1–4 were cytoplasmic, showing no tendency to enter the nucleus upon Pfr formation (21). Using the same conventional hybrid configuration (Pp.phy4:FP), we observed a weak fluorescence signal in the nucleus with no particular enhancement following light pretreatments (Fig. 2B and Fig. S4). In the context of the results obtained from the Y2H experiments (Fig. 1), we constructed FP:Pp.phy4 hybrids for in planta studies. Interestingly, with this configuration nuclear localization was never observed, the hybrid being exclusively cytoplasmic (Fig. 2C and Fig. S4). Pp.phy4 with split-YFP moieties attached N-terminally (YFPN:Pp.phy4 and YFPC:Pp.phy4) showed equivalent distributions, additionally indicating homodimerization and thus correct folding (Fig. S5). As in the case of higher plant phytochromes, there was no obvious enhanced fluorescence at the plasma membrane.
We then used split-YFP methods to detect whether a subpopulation of Pp.phy4 nevertheless interacts with phototropin, as seen in Y2H studies (Fig. 1). Indeed, YFPC:Pp.phy4 bound YFPN:Pp.phot hybrids in both Physcomitrella (Fig. 2D and Fig. S6) and onion (Fig. 2E and Fig. S6) exclusively at the plasma membrane. These signals were much weaker than those from FP:Pp.phy4 and split-YFP:Pp.phy4 homodimers in the cytoplasm (Fig. 2C and Figs. S4 and S5). At the highest resolution accessible, the split-YFP signal seemed restricted to distinct tiny areas at or near the plasma membrane (Fig. 2E and Fig. S6E).
We confirmed phytochrome-phototropin interaction independently by coimmunoprecipitation (coIP). HA:Pp.phy4 and MYC:Pp.photA1 or -B1 overproduced in Nicotina benthamiana were liberated from the membrane fraction by mild detergent treatment and pulled down together from the supernatant with anti-HA antibody. Immunoblots probed with anti-MYC antibody clearly showed coIP of both phototropins with Pp.phy4 (Fig. 2G). Experiments carried out in darkness following saturating light pulses given either in vivo 16 h before extraction or in vitro before the pull-down provided no evidence for Pr/Pfr-dependent association (Fig. S7).
We reasoned that if the Pp.phy4-Pp.phot interaction is related to tropic reactions to light, these might be impaired not only in Pp.phy4− (18) but also in phototropin mutants. Indeed, using multiple Pp.phot− knockouts (22), both photo- and polarotropic responses in R were compromised (Fig. 2H). In particular, photA2−/B1−/B2− and photA2−/B1− approximately phenocopied Pp.phy4−, whereas the behavior of photB1−/B2− more resembled that of the wild type, implying that the interaction with photA2 predominates in these tropic responses, but that the other family members capable of interacting with Pp.phy4 can also contribute to the response. Despite the involvement of a B photoreceptor, no phototropic reaction to B was seen, as expected.
We considered that the Physcomitrella phytochrome–phototropin interaction described above and perhaps its cytoplasmic signaling route might be conserved in higher plants. In onion, we observed translocation of At.phyA from the cytoplasm to the nucleus in light using hybrids with the FP conventionally attached to the C terminus (At.phyA:FP; Fig. S8). We estimate that ∼75% of the fluorescence signal was restricted to the nucleus after 1 h of FR irradiation, whereas no significant signal was apparent in the nucleus in the dark controls. Similar behavior was apparent for N-terminal FP hybrids (FP:At.phyA; Fig. S8). In Physcomitrella, At.phyA showed a marked tendency to enter the nucleus in light in the case of At.phyA:FP but not FP:At.phyA (Fig. S9), resembling the pattern seen for Pp.phy4. At.phyB, on the other hand, showed weak light-dependent nuclear localization irrespective of the positioning of the FP tag in both onion and Physcomitrella (Figs. S10 and S11). Equivalent split-YFP experiments with YFPN:At.phyA and YFPC:At.phyA (Fig. S5) showed similar light-dependent distributions and additionally confirmed homodimerization, implying that the hybrids were correctly folded and photochromically functional. Similarly to our findings for Physcomitrella, YFPC:At.phyA with YFPN:At.phot1 yielded a fluorescence signal exclusively at the plasma membrane (Fig. 2F). On the other hand, Y2H investigations analogous to those for Physcomitrella (Fig. 1) gave no evidence for direct binding of At.phyA to At.phot1 or -2, despite the stringent At.phyA-At.FHY1–positive control.
Discussion
Phytochrome Localization.
A major step forward in understanding phytochrome biology came with the discovery that both phyA and phyB are translocated from the cytoplasm to the nucleus upon formation of the physiologically active Pfr state (2, 23–25), providing a phenomenological basis for phytochrome-mediated regulation of the transcriptome (26–29). However, whereas FHL/FHY1-mediated nuclear translocation of phyA in Arabidopsis is rapid, most having been shuttled into the nucleus within an hour of irradiation, the effect is much less pronounced in the case of phyB, most of which remains in the cytoplasm even after 2 h of irradiation (3). Furthermore, the mechanism underlying phyB nuclear localization is unclear, and certainly does not involve the FHL/FHY1 system. phyC, -D, and -E, on the other hand, were reported to be constitutively nuclear localized (30). These studies exploited transgenic phytochromes carrying FP tags at the C terminus. Here, we show that light-dependent translocation of phyA into the nucleus in higher plant cells occurs when the FP tag is attached either to the N or the C terminus (Fig. S8), indicating in planta functionality in both cases.
Remarkably little is known about phytochrome-mediated effects of light on gene expression in lower plants. Surprisingly, earlier studies showed that Physcomitrella phytochromes remained cytoplasmic irrespective of light treatments (21). Our data confirm these findings (Fig. 2C and Fig. S4). It is thus remarkable that At.phyA:FP in Physcomitrella filament cells showed similar Pfr-dependent nuclear translocation to that seen in higher plant cells (Fig. S9), all of the more so because no FHY1 homolog is immediately obvious in the Physcomitrella genome. Detailed searches for FHY1-related functional elements in the Physcomitrella genome (31) revealed XP_001772282.1 as a potential candidate, however.
Physcomitrella Phototropins Are Associated with the Plasma Membrane.
Light-directed growth (phototropism) in lower plants occurs in response to R and is mediated by phytochrome, phototropism in higher plants occurs in response to B and is mediated by phototropin photoreceptors. Although phototropins show no features characteristic of membrane proteins, they are plasma membrane-associated in higher plants and also Chlamydomonas (16, 17, 32, 33). Here we show that all four Physcomitrella phototropins are associated with the plasma membrane (Fig. 2A and Fig. S2). Detailed studies of At.phot1 in Arabidopsis revealed a B-dependent dissociation from the plasma membrane which could be ameliorated by pretreatment with R via phyA (20). However, we failed to observe a similar dissociation effect of B in the case of Physcomitrella phototropins. Phototropin association with the plasma membrane is probably mediated by one or more anchor proteins, either bona fide plasma membrane proteins or partners otherwise fixed at the periphery of the cell. None of the phototropin partners identified to date are likely candidates.
A Subpopulation of Physcomitrella Phytochrome 4 Binds Directly to Phototropin at the Plasma Membrane.
Phytochrome 4 is the photoreceptor predominantly responsible for vectorial light sensing and response in Physcomitrella (18), a paradoxical situation if phytochromes were to act via transcriptional regulation alone (4, 12). Using Y2H methods (Fig. 1) we describe the binding of full-length, photochemically active holophytochrome 4 from Physcomitrella to phototropins. Characteristically, apophytochromes autoassemble with specific bilins to form the functional holoprotein photoreceptor. Full-length phytochromes produced in PCB-fed yeast are obviously able to carry out this process, as only then would they be able to show R/FR reversible binding to prey molecules. In the case of the Pp.phy4 and phototropins, interaction is enhanced in continuous or pulsed R, the latter effect being reversed by subsequent FR pulses, clear evidence of Pfr-enhanced binding. This interaction is likely to be autonomous, as additional factors are probably missing in yeast.
We show bimolecular fluorescence complementation signals at the plasma membrane in both onion epidermis and Physcomitrella filament cells coexpressing Pp.phy4 and Pp.phot constructs carrying N-terminal split-YFP tags (Fig. 2 D and E and Fig. S6). The split-YFP signal was much weaker than that seen from FP:Pp.phy4 constructs, which did not themselves show appreciably enhanced fluorescence at the plasma membrane (Fig. 2 B and C and Fig. S4), implying that only a small proportion of the phytochrome interacts with phototropin. We do not know whether this represents a dynamic equilibrium with the phytochrome population in the cytoplasm or a particular subpopulation of the phytochrome molecules.
Although the phytochrome–phototropin interaction involves a membrane protein complex that would thus not be accessible to coIP methods, we were able to confirm the interaction via coIP by liberating the complex from the plasma membrane by mild detergent treatment before pull-down (Fig. 2G). The success of these experiments implies that phytochrome–phototropin binding is more stable with regard to the detergent treatment than is the association of the complex with the plasma membrane. We found no evidence for light effects on the coIP signal (Fig. S7).
Phytochrome Cytoplasmic Signal Requires Phototropin.
Targeted knockout studies in Physcomitrella implied that chloroplast B avoidance is primarily mediated by photA2 although other family members also contribute to the response (22). However, chloroplast movement is also steered by phytochrome. Interestingly, these workers found that the latter response to R was defective particularly in the photA2− null mutant. Using the same phototropin knockout lines, we show here that phytochrome-mediated R photo- and polarotropism too are dependent particularly upon Pp.photA2 (Fig. 2H).
Kasahara and coworkers (22) formulated an explicit model to explain light-induced chloroplast movement based on signal transfer from phytochrome to phototropin and thence to the actin and myosin cytoskeleton. Phytochrome-mediated phototropism in moss protonemata was shown to involve rearrangements of actin microfilaments (34, 35), consistent with their model and our findings.
Although the coaction implied by these physiological studies does not necessarily require that the photoreceptors interact directly, the fact that they do so at the plasma membrane provides not only a plausible physical basis for the coaction but one entirely consistent with Haupt's proposal to explain both polaro- and phototropic bending of protonemata and chloroplast movements in lower plants (10–12) whereby phytochrome molecules are considered to be anchored anisotropically at the plasma membrane with the transition dipole moment parallel to the cell surface in the Pr ground state. Upon formation of the physiologically active Pfr state in R, the dipole for FR becomes perpendicular to the surface. On this basis, both dichroic effects and fluence rate gradients within the cell can be correlated with local concentrations of Pfr. The weakness of this model was that, up to now, phytochromes were considered to be cytosolic rather than associated with the plasma membrane. The data presented here seem to resolve this problem in the case of mosses. On the other hand, in certain algal and fern groups, neochrome photoreceptors have evolved to fulfill this function (14, 15, 36). Neochromes comprise an N-terminal phytochrome sensory module attached to an almost complete phototropin moiety. We propose that the phytochrome–phototropin binding we describe here has been genetically consolidated in the form of neochromes.
Phytochrome–Phototropin Interaction in Higher Plants.
We show that Arabidopsis phyA and phot1 interact at the plasma membrane in onion epidermis cells (Fig. 2F). At first sight, this observation might seem to contradict the notion of cytosolic Pr and nuclear translocation of Pfr, however, both localization and physiological studies indicate that a proportion of phyA always remains in the cytoplasm irrespective of the light conditions (2–4). Our findings imply that only a small fraction of the phytochrome population is associated with phototropin, explaining also why no signal enrichment at the plasma membrane is apparent from FP:At.phyA constructs (Figs. S8 and S9). Our data are therefore consistent with and provide possible explanations of previous findings. For example, involvement of phytochrome in tropic responses is well established, R enhancing B-induced phototropism in the hypocotyl (37) by suppressing light-induced internalization of phot1 via phyA (20). The recent finding that NPH3 acts as an E3 ubiquitin ligase possibly involved in clathrin-mediated vesicle formation fits well into this scheme (38). Interestingly, R enhancement of B-induced phototropism is retained in the fhl−/fhy1− mutant in which phyA remains in the cytoplasm after Pfr formation (9). However, we see no reason to exclude an additional effect of phyA-mediated changes in gene expression. It was reported recently that nuclear phyA Pfr promotes whereas the fhl−/fhy1− mutation delays phototropism (39), but this does not call the existence of a cytoplasmic signaling route into question. We propose that phyA–phot1 binding at the plasma membrane we report is associated with the enhancement effect of phyA Pfr on phototropism, perhaps representing an evolved role of the analogous interaction in lower plants revealed in Physcomitrella. We do not yet know whether higher plant phytochromes other than phyA associate with phototropin, nor do we know whether phot1 is involved alone or with phot2.
In contrast to our Y2H findings for Physcomitrella (Fig. 1) and the situation in planta (Fig. 2F), interaction between Arabidopsis phyA and phot1 was not apparent in Y2H studies, perhaps because, in higher plants, a larger molecular complex is involved comprising components missing in yeast. One putative candidate is PKS1, which (i) binds phyA, phot1 and its signaling partner NPH3, (ii) is associated with the plasma membrane, (iii) is necessary for both B-induced phototropism in the shoot (40, 41) and R-induced root phototropism (42), and (iv) is absent from lower plants (31, 43).
Phytochrome Cytoplasmic Signaling.
It was recently reported that phytochrome down-regulates protochlorophillide oxidoreductase A (PORA) at the translational level via cytoplasmic Pfr (44). Although the PNT1 putative cap-binding protein involved appears cytosolic, the location of the phy–PNT1 interaction was not studied. We do not yet know if these findings are physiologically related to ours, but it is clear that both are distinct from phytochrome-regulated transcription. The phytochrome–phototropin complex we describe is likely to include a still unknown membrane anchor protein as well as further components associated with signal transfer. At least in the case of protonemata this is likely to connect physically to an actin microfilament organizer to preserve the vectorial information. Various predictions of the Haupt model can be tested on the basis of the phytochrome–phototropin interaction. It will also be interesting to investigate the physiological significance of the apparently related interaction seen in higher plants especially in view of the diverse roles played by phototropins 1 and 2.
Materials and Methods
Cloning.
For Y2H studies, N-terminally fused FHY1, phytochrome, and phototropin bait and prey plasmids were generated in pGBKT7 and pGADT7 (Clontech), respectively. A C-terminal At.PHYA:BD hybrid was created by blunt cloning into pBAC, a derivate of pBRIDGE (Clontech). cDNAs from Physcomitrella PHY4, PHOTA1, PHOTA2, PHOTB1, and PHOTB2 and Arabidopsis PHYA and PHOT1 were cloned into Gateway entry vectors (Invitrogen). Expression clones for N- and C-terminal FP tags were generated by LR-clonase-mediated recombination into appropriate destination vectors (45). N-terminal fusion constructs for split YFP-based in vivo interaction assays were generated in pSAT destination vectors (46). Expression constructs for coIP studies were generated as follows. The Pp.PHY4 entry clone was LR-recombined with pGWB415 (47) yielding HA:PHY4_pGWB415. PHOTA1_pDONR221 and PHOTB1_pDONR221 were obtained by BP-recombination of CFP:PHOTA1 and -B1 constructs with donor vector pDONR221 (Invitrogen), respectively, followed by LR reaction with pGWB421 yielding MYC:PHOTA1_pGWB421 and MYC:PHOTB1_pGWB421.
Y2H Interaction Assays.
PCB was obtained by methanolysis of soluble proteins from Spirulina as described (48). Yeast was transformed as described (49, 50). For quantitative growth assays, 2 × 105 AH109 cells were spotted on -W/-L/-H/-A quadruple synthetic dropout (QSD) selective media containing 30 μM PCB and 3-AT (0.5 mM phyA:BD; 2.5 mM BD:phy4). Plates were incubated for 5 d in 0.7 μmol m−2 s−1 R (660-nm LEDs), 3 μmol m−2 s−1 FR (740-nm LEDs), 0.7 μmol m−2 s−1 B (465-nm LEDs), or darkness. β-galactosidase assays were carried out according to the Yeast Protocols Handbook (Clontech) using yeast strain Y187. Cultures were grown in -W/-L DSD media supplemented with 20 μM PCB in the dark. Overnight cultures were diluted to OD 1, and 1.4 mL was inoculated into 5.6 mL of YPDA expansion cultures with 20 μM PCB. Light pulses were given as follows: R, 5 min at 20 μmol m−2 s−1; FR, 10 min at 20 μmol m−2 s−1; B, 5 min at 12 μmol m−2 s−1. Cultures were incubated for further 3 h in darkness, and Miller assays using 2-nitrophenyl-β-D-galactopyranoside (ONPG) were performed in 540 nm dim green safelight.
Biolistic Transfection and Cytological Studies.
Physcomitrella filaments were grown on solid BCE225 medium for 5 d followed by 5-h equilibration on 0.2 M mannitol to reduce turgor. Biolistic transfection was carried out as described (51), using gold particles (1.6 μm, Inbio Gold) for Physcomitrella cells and tungsten particles (1.1 μm, Bio-Rad) for onion epidermal cells. After transfection, cells were kept in darkness for 2–3 d before preirradiation as appropriate (1 h of R, FR, or B, each at 5 μmol m−2 s−1) immediately before observation/documentation. For split-YFP-based in vivo interaction studies, transfected material was kept in darkness for ∼3 d, then preirradiated in R (660-nm LEDs) for 15 min at 35 μmol m−2 s−1 before observation (see SI Materials and Methodsfor microscopy details).
Coimmunoprecipitation of Pp.phy4 and Pp.photA1 and -B1.
35S-driven HA:Pp.PHY4 and MYC:Pp.PHOTA1 or B1 were coexpressed together with P19 in Nicotiana bethamiana leaves following Agrobacterium infiltration. After 5- to 7-d, 16-h white light (∼100 μmol m−2 s−1)/8-h dark cycle, proteins were extracted in white light by grinding in buffer (100 mM Tris⋅HCl, pH 8.3/5 mM EDTA/0.1% β-mercaptoethanol with Complete protease inhibitor mixture; Roche) on ice; the membrane fraction was pelleted and resuspended in the same buffer containing 0.3% Triton X-100 to solubilize associated proteins. Following clarification, monoclonal anti-HA antibody (Sigma) was added to an aliquot of the supernatant and mixed for 40 min at 4 °C in R. Subsequently, protein G-coupled magnetic beads (NEB) were added and mixed further. The beads were then washed in the same buffer, and the captured proteins analyzed by SDS/PAGE/immunoblotting with anti-HA and anti-MYC monoclonal antibodies according to standard procedures. Similar procedures omitting the pull-down antibody or using untransformed leaf tissue constituted negative controls. Light effects were checked in vivo by pretreating the leaf material with FR followed by 16 h darkness before extraction and coIP in darkness and in vitro by irradiating conventional extract with R and FR before coIP in darkness.
Photo- and Polarotropism in Physcomitrella.
The Pp.phy4 knockout line was as described (18). Double and triple phototropin knockout lines were kindly donated by Masahiro Kasahara (22). Phototropism of negatively gravitropically grown Physcomitrella protonemata was investigated essentially as described (18) using R and B LED arrays (λmax: 660 and 465 nm, respectively; FWHM: 20 nm; Roithner) at 1 μmol m−2 s−1. Polarotropism was assessed using Physcomitrella protonemal filaments grown horizontally under polarized R at 30 nmol m−2 s−1. After 7 d, the plane of polarization was rotated by 45° and growth continued for a further 24 h.
Supplementary Material
Acknowledgments
We thank Masahiro Kasahara (Ritsumeikan University, Kyoto) for the Physcomitrella phototropin knockouts; Tsuyoshi Nakagawa (Shimane University, Matsue, Japan) for the pGWB vectors; John Christie (University of Glasgow, Glasgow, United Kingdom) for the Arabidopsis phototropin clones; David Baulcombe (University of Cambridge, Cambridge, United Kingdom) for the p19 expression plasmid; Hans Sommer (MPI Plant Breeding, Köln, Germany) for the Physcomitrella cDNA library; Milva Mateblowski (University of Giessen, Giessen, Germany) for initial At.phyA studies in Physcomitrella; Anne Holz and Adriaan Dorresteijn (University of Giessen) and Olga Lévai and Manfred Jung (Leica Microsystems, Mannheim, Germany) for providing access to confocal microscopy facilities; and Melanie Bingel, Tanja Gans, and Tina Lang for technical assistance. This work was supported by Deutsche Forschungsgemeinschaft Grants Hu702/5 and Ze485/2.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1120203109/-/DCSupplemental.
References
- 1.Quail PH. Phytochromes. Curr Biol. 2010;20:R504–R507. doi: 10.1016/j.cub.2010.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hisada A, et al. Light-induced nuclear translocation of endogenous pea phytochrome A visualized by immunocytochemical procedures. Plant Cell. 2000;12:1063–1078. doi: 10.1105/tpc.12.7.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nagy F, Schäfer E. Phytochromes control photomorphogenesis by differentially regulated, interacting signaling pathways in higher plants. Annu Rev Plant Biol. 2002;53:329–355. doi: 10.1146/annurev.arplant.53.100301.135302. [DOI] [PubMed] [Google Scholar]
- 4.Rösler J, Jaedicke K, Zeidler M. Cytoplasmic phytochrome action. Plant Cell Physiol. 2010;51:1248–1254. doi: 10.1093/pcp/pcq091. [DOI] [PubMed] [Google Scholar]
- 5.Matsushita T, Mochizuki N, Nagatani A. Dimers of the N-terminal domain of phytochrome B are functional in the nucleus. Nature. 2003;424:571–574. doi: 10.1038/nature01837. [DOI] [PubMed] [Google Scholar]
- 6.Mateos JL, et al. Functional and biochemical analysis of the N-terminal domain of phytochrome A. J Biol Chem. 2006;281:34421–34429. doi: 10.1074/jbc.M603538200. [DOI] [PubMed] [Google Scholar]
- 7.Kikis EA, Oka Y, Hudson ME, Nagatani A, Quail PH. Residues clustered in the light-sensing knot of phytochrome B are necessary for conformer-specific binding to signaling partner PIF3. PLoS Genet. 2009;5:e1000352. doi: 10.1371/journal.pgen.1000352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Essen L-O, Mailliet J, Hughes J. The structure of a complete phytochrome sensory module in the Pr ground state. Proc Natl Acad Sci USA. 2008;105:14709–14714. doi: 10.1073/pnas.0806477105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rösler J, Klein I, Zeidler M. Arabidopsis fhl/fhy1 double mutant reveals a distinct cytoplasmic action of phytochrome A. Proc Natl Acad Sci USA. 2007;104:10737–10742. doi: 10.1073/pnas.0703855104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haupt W, Scheuerlein R. Chloroplast movement. Plant Cell Environ. 1990;13:595–614. [Google Scholar]
- 11.Kraml M. Light direction and polarization. In: Kendrick RE, Kronenberg GHM, editors. Photomorphogenesis in plants. Dortrecht: Kluwer; 1994. pp. 417–446. [Google Scholar]
- 12.Hughes J, Brücker G, Repp A, Zeidler M, Mittmann F. Phytochromes and functions: Studies using gene targeting in Physcomitrella. In: Wada M, Shimazaki Y, Iino M, editors. Light sensing in plants. Tokyo: Springer, Botanical Society of Japan; 2005. pp. 103–110. [Google Scholar]
- 13.Böse G, Schwille P, Lamparter T. The mobility of phytochrome within protonemal tip cells of the moss Ceratodon purpureus, monitored by fluorescence correlation spectroscopy. Biophys J. 2004;87:2013–2021. doi: 10.1529/biophysj.103.038521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nozue K, et al. A phytochrome from the fern Adiantum with features of the putative photoreceptor NPH1. Proc Natl Acad Sci USA. 1998;95:15826–15830. doi: 10.1073/pnas.95.26.15826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suetsugu N, Mittmann F, Wagner G, Hughes J, Wada M. A chimeric photoreceptor gene, NEOCHROME, has arisen twice during plant evolution. Proc Natl Acad Sci USA. 2005;102:13705–13709. doi: 10.1073/pnas.0504734102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sakamoto K, Briggs WR. Cellular and subcellular localization of phototropin 1. Plant Cell. 2002;14:1723–1735. doi: 10.1105/tpc.003293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wan YL, et al. The subcellular localization and blue-light-induced movement of phototropin 1-GFP in etiolated seedlings of Arabidopsis thaliana. Mol Plant. 2008;1:103–117. doi: 10.1093/mp/ssm011. [DOI] [PubMed] [Google Scholar]
- 18.Mittmann F, et al. Targeted knockout in Physcomitrella reveals direct actions of phytochrome in the cytoplasm. Proc Natl Acad Sci USA. 2004;101:13939–13944. doi: 10.1073/pnas.0403140101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hiltbrunner A, et al. FHY1 and FHL act together to mediate nuclear accumulation of the phytochrome A photoreceptor. Plant Cell Physiol. 2006;47:1023–1034. doi: 10.1093/pcp/pcj087. [DOI] [PubMed] [Google Scholar]
- 20.Han IS, Tseng TS, Eisinger W, Briggs WR. Phytochrome A regulates the intracellular distribution of phototropin 1-green fluorescent protein in Arabidopsis thaliana. Plant Cell. 2008;20:2835–2847. doi: 10.1105/tpc.108.059915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uenaka H, Kadota A. Functional analyses of the Physcomitrella patens phytochromes in regulating chloroplast avoidance movement. Plant J. 2007;51:1050–1061. doi: 10.1111/j.1365-313X.2007.03202.x. [DOI] [PubMed] [Google Scholar]
- 22.Kasahara M, Kagawa T, Sato Y, Kiyosue T, Wada M. Phototropins mediate blue and red light-induced chloroplast movements in Physcomitrella patens. Plant Physiol. 2004;135:1388–1397. doi: 10.1104/pp.104.042705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kircher S, et al. Light quality-dependent nuclear import of the plant photoreceptors phytochrome A and B. Plant Cell. 1999;11:1445–1456. doi: 10.1105/tpc.11.8.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamaguchi R, Nakamura M, Mochizuki N, Kay SA, Nagatani A. Light-dependent translocation of a phytochrome B-GFP fusion protein to the nucleus in transgenic Arabidopsis. J Cell Biol. 1999;145:437–445. doi: 10.1083/jcb.145.3.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sakamoto K, Nagatani A. Nuclear localization activity of phytochrome B. Plant J. 1996;10:859–868. doi: 10.1046/j.1365-313x.1996.10050859.x. [DOI] [PubMed] [Google Scholar]
- 26.Tepperman JM, Hwang YS, Quail PH. phyA dominates in transduction of red-light signals to rapidly responding genes at the initiation of Arabidopsis seedling de-etiolation. Plant J. 2006;48:728–742. doi: 10.1111/j.1365-313X.2006.02914.x. [DOI] [PubMed] [Google Scholar]
- 27.Tepperman JM, et al. Expression profiling of phyB mutant demonstrates substantial contribution of other phytochromes to red-light-regulated gene expression during seedling de-etiolation. Plant J. 2004;38:725–739. doi: 10.1111/j.1365-313X.2004.02084.x. [DOI] [PubMed] [Google Scholar]
- 28.Tepperman JM, Zhu T, Chang HS, Wang X, Quail PH. Multiple transcription-factor genes are early targets of phytochrome A signaling. Proc Natl Acad Sci USA. 2001;98:9437–9442. doi: 10.1073/pnas.161300998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ma L, et al. Light control of Arabidopsis development entails coordinated regulation of genome expression and cellular pathways. Plant Cell. 2001;13:2589–2607. doi: 10.1105/tpc.010229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kircher S, et al. Nucleocytoplasmic partitioning of the plant photoreceptors phytochrome A, B, C, D, and E is regulated differentially by light and exhibits a diurnal rhythm. Plant Cell. 2002;14:1541–1555. doi: 10.1105/tpc.001156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rensing SA, et al. The Physcomitrella genome reveals evolutionary insights into the conquest of land by plants. Science. 2008;319:64–69. doi: 10.1126/science.1150646. [DOI] [PubMed] [Google Scholar]
- 32.Zienkiewicz A, Zienkiewicz K, Kopcewicz J. Intracellular distribution of phototropin 1 protein in the short-day plant Ipomoea nil. Protoplasma. 2008;233:141–147. doi: 10.1007/s00709-008-0292-0. [DOI] [PubMed] [Google Scholar]
- 33.Huang K, Kunkel T, Beck CF. Localization of the blue-light receptor phototropin to the flagella of the green alga Chlamydomonas reinhardtii. Mol Biol Cell. 2004;15:3605–3614. doi: 10.1091/mbc.E04-01-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meske V, Rupert V, Hartmann E. Structural basis for the red light induced repolarisation of tip growth in caulonemal cells of Ceratodon purpureus. Protoplasma. 1996;192:189–198. [Google Scholar]
- 35.Meske V, Hartmann E. Reorganization of microfilaments in protonemal tip cells of the moss Ceratodon purpureus during the phototropic response. Protoplasma. 1995;188:59–69. doi: 10.1007/BF01276796. [DOI] [PubMed] [Google Scholar]
- 36.Kawai H, et al. Responses of ferns to red light are mediated by an unconventional photoreceptor. Nature. 2003;421:287–290. doi: 10.1038/nature01310. [DOI] [PubMed] [Google Scholar]
- 37.Briggs WR. The phototropic responses of higher plants. Annu Rev Plant Physiol. 1963;14:311–352. [Google Scholar]
- 38.Roberts D, et al. Modulation of phototropic responsiveness in Arabidopsis through ubiquitination of phototropin 1 by the CUL3-Ring E3 ubiquitin ligase CRL3(NPH3) Plant Cell. 2011;23:3627–3640. doi: 10.1105/tpc.111.087999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kami C, et al. Nuclear phytochrome A signaling promotes phototropism in Arabidopsis. Plant Cell. 2012;24:566–576. doi: 10.1105/tpc.111.095083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fankhauser C. Phytochromes as light-modulated protein kinases. Semin Cell Dev Biol. 2000;11:467–473. doi: 10.1006/scdb.2000.0201. [DOI] [PubMed] [Google Scholar]
- 41.Lariguet P, et al. PHYTOCHROME KINASE SUBSTRATE 1 is a phototropin 1 binding protein required for phototropism. Proc Natl Acad Sci USA. 2006;103:10134–10139. doi: 10.1073/pnas.0603799103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boccalandro HE, et al. PHYTOCHROME KINASE SUBSTRATE1 regulates root phototropism and gravitropism. Plant Physiol. 2008;146:108–115. doi: 10.1104/pp.107.106468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Banks JA, et al. The Selaginella genome identifies genetic changes associated with the evolution of vascular plants. Science. 2011;332:960–963. doi: 10.1126/science.1203810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Paik I, Yang S, Choi G. Phytochrome regulates translation of mRNA in the cytosol. Proc Natl Acad Sci USA. 2012;109:1335–1340. doi: 10.1073/pnas.1109683109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Karimi M, De Meyer B, Hilson P. Modular cloning in plant cells. Trends Plant Sci. 2005;10:103–105. doi: 10.1016/j.tplants.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 46.Citovsky V, et al. Subcellular localization of interacting proteins by bimolecular fluorescence complementation in planta. J Mol Biol. 2006;362:1120–1131. doi: 10.1016/j.jmb.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 47.Nakagawa T, et al. Improved Gateway binary vectors: High-performance vectors for creation of fusion constructs in transgenic analysis of plants. Biosci Biotechnol Biochem. 2007;71:2095–2100. doi: 10.1271/bbb.70216. [DOI] [PubMed] [Google Scholar]
- 48.Song C, et al. Two ground state isoforms and a chromophore D-ring photoflip triggering extensive intramolecular changes in a canonical phytochrome. Proc Natl Acad Sci USA. 2011;108:3842–3847. doi: 10.1073/pnas.1013377108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gietz RD, Woods RA. Transformation of yeast by lithium acetate/single-stranded carrier DNA/polyethylene glycol method. Methods Enzymol. 2002;350:87–96. doi: 10.1016/s0076-6879(02)50957-5. [DOI] [PubMed] [Google Scholar]
- 50.Agatep R, et al. Transformation of Saccharomyces cerevisiae by the lithium acetate/single-stranded carrier DNA/polyethylene glycol protocol. Tech Tips Online. 1998;3:133–137. [Google Scholar]
- 51.Jaedicke K, Rösler J, Gans T, Hughes J. Bellis perennis: A useful tool for protein localization studies. Planta. 2011;234:759–768. doi: 10.1007/s00425-011-1443-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.