Abstract
BACKGROUND
To compare the prognostic value of the American Joint Cancer Committee (AJCC) staging version 6, version 7, and the risk-stratification model of the National Comprehensive Cancer Network (NCCN).
METHODS
Two-thousand four hundred twenty-nine men who received definitive RT with or without ADT (median follow-up: 74 months) were analyzed.
RESULTS
There was a migration of stage II patients to stage I with AJCC v7 (Stage I increased from 1% to 38%, while Stage II decreased from 91% to 55%). One (4%) pair-wise comparison of Kaplan-Meier estimates of biochemical failure, distant metastasis, prostate cancer specific survival, and overall survival between stages were statistically significant for AJCC v6. On the other hand, 16/24 (67%) of comparisons were significant with AJCC v7. For NCCN, 9/12 (75%) of comparisons were significant. Concordance probability estimate (CPE) and standard error (SE) analysis showed uniform and significant improvement in the predictive power of AJCC v7 versus AJCC v6 for all outcomes. CPE±SE values for AJCC v6 versus AJCC v7 was .51±.009 vs .59±.02 for BF, .54±.02 vs. .70±.05 for DM, .57±.009 vs. .76±.007 for PCSS, and .52±.006 vs. .57±.01 for OS. CPE±SE values for NCCN was .59±.02 for BF, 0.72±.05 for DM, .80±.01 for PCSS, and .57±.01 for OS.
CONCLUSIONS
AJCC v7 is a major improvement over AJCC v6 because it better distributes patients among the stages and is more prognostic. The NCCN model is superior to the AJCC v7 and remains the preferred method for risk-based clinical management of prostate cancer with radiotherapy.
Keywords: prostate cancer, staging, radiotherapy, American Joint Committee on Cancer (AJCC), The National Comprehensive Cancer Network (NCCN)
INTRODUCTION
The American Joint Committee on Cancer (AJCC) published the first edition of the Cancer Staging Manual in 1977. It used the T (tumor extent), N (lymph node invasion), and M (presence or absence of metastasis) to stage prostate cancer. Staging is important in order to: (1) categorize the severity of disease, (2) estimate prognosis, (3) recommend treatment, and (4) aid health care providers and researchers exchange information about patients.1 The manual has been revised several times with the advent of new diagnostic tools and treatments.
There are several important changes that have been implemented in AJCC version 7 (AJCC v7).2 For the first time, serum prostate specific antigen,3 a predictor of survival is recognized in prostate cancer staging. Gleason score (GS) is now recognized as the preferred tumor grading system. And perhaps most importantly, PSA and GS are included in the stage grouping (i.e. I, II, III, and IV). Together, T-stage, PSA and GS have been commonly used in various risk group stratification models4-7 that help physicians guide patients in terms of treatment and risks of recurrence, but they have not appeared together in the AJCC staging until now.
It is unclear however, whether these changes have significantly improved the staging system's ability to group patients according to prognosis. The purpose of this investigation is to compare the ability of AJCC v68 and AJCC v72 to predict biochemical failure (BF), distant metastasis (DM), prostate cancer specific survival (PCSS), and overall survival (OS) in men treated with radiotherapy (RT) with or without androgen deprivation therapy.9
MATERIALS AND METHODS
Between 1989 and 2006, 2,469 men with clinical stage T1-4, N0/X-N1, M0 adenocarcinoma of the prostate received definitive RT with or without adjuvant ADT. Three-dimensional-conformal RT (3D-CRT, July 1992 to June 2001) was used in 1,465 men (59%) and intensity modulated RT (IMRT, July 2001 to July 2004) was used in 1,004 men (41%). The mean follow-up time from completion of RT was 74 months (range: 1-213), 70 months (range: 2-108) for the IMRT group, and 86 months (range: 1-213) for the 3D-CRT group. The techniques used for 3D-CRT and IMRT have been previously reported.10, 11
Androgen deprivation therapy
ADT was generally used at the discretion of the treating physician, and consisted oral antiandrogen given for 1 to 4 months and a luteinizing hormone-releasing hormone agonist administered as depot injections.
Patient evaluation and staging
All patients had a history and physical exam including digital rectal exam (DRE), initial serum PSA (PSA), and histologic confirmation of adenocarcinoma with a GS. Thirty-one percent had a CT of the pelvis. Eighty-eight percent of the patients had a bone scan. T-category was established by palpation findings only, without upstaging using pathologic or radiographic information. Patients were staged using the AJCC v68 and v72 guidelines.
Statistical analysis
Univariate analysis was performed using a χ2-test for discrete variables, and the Wilcoxon test was used for continuous variables. Kaplan-Meier estimation method and the Cox proportional hazard model were used to evaluate the ability of each clinical staging system to predict the time to BF, DM, PCSS, and OS. In the multivariate analysis (MVA), treatment (RT versus RT+ADT) and RT dose (< 75.6 Gy versus ≥ 75.6 Gy) effects were accounted for as covariates. The Benjamini & Hochberg adjustment12 was used to produce a series of adjusted p-values. An adjusted p-value < 0.05 was considered significant. The BF was defined as the PSA nadir plus 2ng/mL.13 The concordance probability estimate (CPE)14, 15 and its standard error (SE) were used in assessing the predictive accuracy of the model for various endpoints. The closer the CPE is to 0.5, the lower the predictive accuracy of the model; the closer the CPE is to 1, the better the predictive accuracy.
RESULTS
Patient characteristics
Various patient- and treatment-related characteristics are listed in Table 1. Based on NCCN criteria, the proportion of patients with low-risk disease was 38%, intermediate-risk was 42%, and high-risk was 21%. The majority of patients, 79%, received RT alone (no ADT) to a median RT dose of 76 Gy. Of those men receiving ADT, the median duration was 11.2 months (range: 0.4 – 124).
Table 1.
Various patient and treatment related characteristics (n = 2,469).
| Characteristic | # (%) |
|---|---|
| Age (y) | |
| mean | 68 |
| range | 40 - 89 |
| PSA (ng/mL) | |
| <10 | 1631 (66) |
| 10-20 | 591 (23) |
| >20 | 247 (10) |
| Gleason score | |
| 2-6 | 1608 (65) |
| 7 | 627 (25) |
| 8-10 | 234 (9) |
| ADT | |
| none | 1941 (79) |
| <1 y | 277 (11) |
| 1-2y | 75 (3) |
| > 2 y | 176 (7) |
| Radiation dose (Gy) | |
| Median | 76 |
| Range | 70 - 83 |
| NCCN risk group | |
| low | 938 (38) |
| intermediate | 1012 (41) |
| high | 518 (21) |
Abbreviations: 3D-CRT = three-dimensional conformal radiotherapy; ADT = androgen deprivation therapy; NCCN = National Comprehensive Cancer Network; PSA = prostate-specific antigen
Staging statistics
Patient staging statistics under AJCC v6 and v7 are listed in Table 2. When transitioning from AJCC v6 to v7, the proportion of men in stage I increased (1% to 38%), stage II decreased (91% to 55%), while stages III and IV remained the same (6% and 1%, respectively). Regarding the subdivision of stage II into A and B subcategories with AJCC v7, 35% of all patients had IIA disease and 20% had IIB disease.
Table 2.
Staging statistics under AJCC v6 and v7 guidelines.
| AJCC v6 | # (%) | AJCC v7 | # (%) |
|---|---|---|---|
| Stage I | 28 (1) | Stage I | 936 (38) |
| T1a N0 M0 G1 | T1a-c N0 M0 PSA<10 GS≤6 | 735 | |
| T2a N0 M0 PSA<10 GS≤6 | 201 | ||
| T1-2a N0 M0 PSAX GX | 0 | ||
| Stage II | 2263 (91) | Stage II | 1355 (55) |
| T1a N0 M0 G2-4 | 5 | Stage IIA | 871 |
| T1b N0 M0 Any G | 19 | T1a-c N0 M0 PSA<20 GS7 | 233 |
| T1c N0 M0 Any G | 1316 | T1a-c N0 M0 PSA≥10 <20 GS≤6 | 224 |
| T1 N0 M0 Any G | 1344 | T2a N0 M0 PSA≥10 <20 GS≤6 | 56 |
| T2 N0 M0 Any G | 947 | T2a N0 M0 PSA<20 GS7 | 100 |
| T2b N0 M0 PSA<20 GS≤7 | 258 | ||
| T2b N0 M0 PSAX GX | 0 | ||
| Stage IIB | 484 | ||
| T2c N0 M0 Any PSA Any GS | 134 | ||
| T1-2 N0 M0 PSA≥20 Any GS | 171 | ||
| T1-2 N0 M0 Any PSA GS ≥8 | 179 | ||
| Stage III | 152 (6) | Stage III | 152 (6) |
| T3 N0 M0 Any G | T3a-b N0 M0 Any PSA Any GS | ||
| Stage IV | 26 (1) | Stage IV | 26 (1) |
| T4 N0 M0 Any G | 13 | T4 N0 M0 Any PSA Any GS | 13 |
| Any T N1 M0 Any G | 9 | Any T N1 M0 Any PSA Any GS | 10 |
| Any T4 Any N M1 Any G | 1 | Any T4 Any N M1 Any PSA Any GS | 3 |
Abbreviations: AJCC = American Joint Committee on Cancer; T = tumor; N = node; M = metastasis; G = grade; GS = Gleason score; G1 = well differentiated (GS 2-4), G2-4 = moderately differentiated (GS5-6), G5-6; poorly differentiated (GS7-10); PSA = prostate specific antigen; v6 = 6th edition; v7 = 7th edition
Prognostic Value
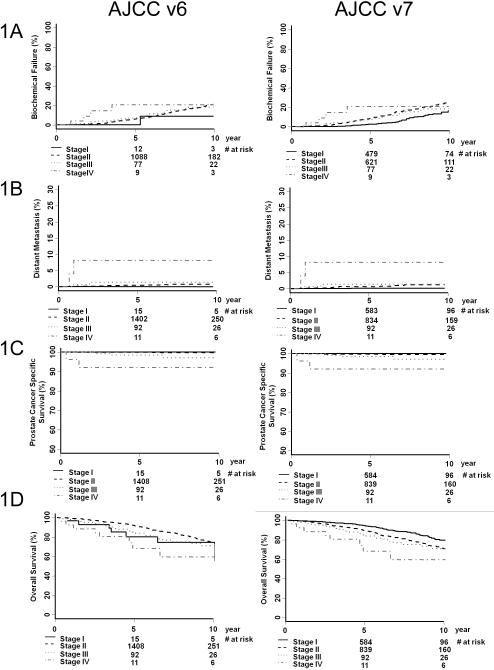
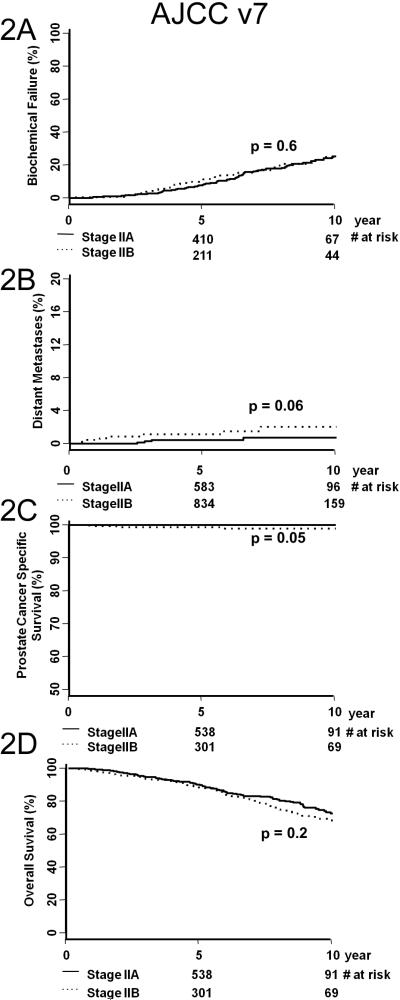
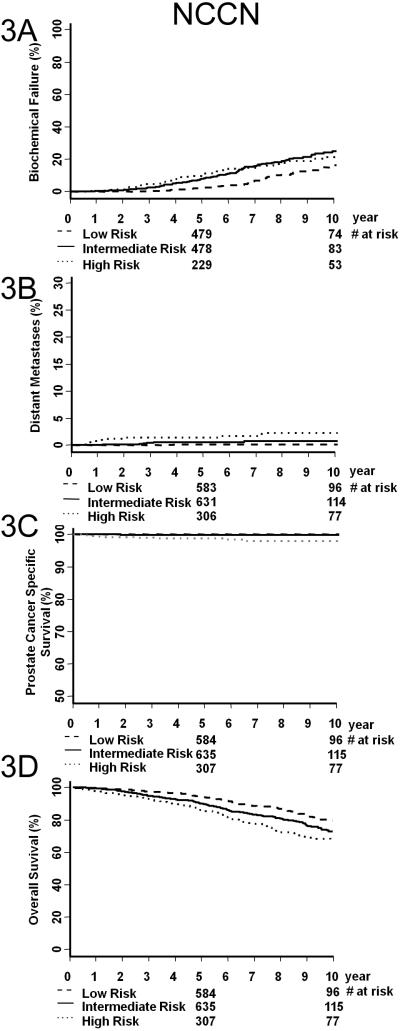
Figure 1 illustrates KM estimates of BF (A), DM (B), PCSS (C), and OS (D) for AJCC v6 (left column) and AJCC v7 (right column). Table 3 shows the paired p-value comparison between any two KM curves from Figure 1. The significant (i.e. <0.05) paired p-values are bold-faced. In the AJCC v6 staging, overall differences in DM, PCSS, and OS were not significant, with the exception being the outcome prediction of PCSS for stage II and III patients. Contrarily, in the AJCC v7 staging, paired p-value comparisons were more likely to be significant. This is a marked improvement compared to AJCC v6. Figure 2 illustrates KM estimates of BF (A), DM (B), PCSS (C), and OS (D) for AJCC v7 stages IIA versus IIB. Substratification between stages IIA and IIB did not provide more prognostic information. Figure 3 illustrates KM estimates of BF (A), DM (B), PCSS (C), and OS (D) based on NCCN risk group. Table 4 shows the paired p-value comparison between any two KM curves from Figure 3. NCCN risk group stratification is significant for most of the KM curves in the outcome measures studies, particularly for OS.
Figure 1.
Biochemical failure (A), overall survival (B), prostate cancer specific survival (C), and distant metastasis (D) by AJCC version 6 grouping (left column) and AJCC version 7 grouping (right column). The number of patients at risk is noted on the horizontal axes.
Table 3.
Paired p-value comparison of stages as assessed by either AJCC v6 or v7.
| Outcome measure | Pair comparison (stage vs. stage) | p-value | ||
|---|---|---|---|---|
| AJCC v6 | AJCC v7 | |||
| Biological failure | I | II | 0.5 | <0.0001 |
| I | III | 0.5 | 0.12 | |
| I | IV | 0.22 | 0.03 | |
| II | III | 0.8 | 0.3 | |
| II | IV | 0.22 | 0.03 | |
| III | IV | 0.37 | 0.22 | |
| Distant metastases | I | II | 0.8 | 0.03 |
| I | III | 0.6 | 0.02 | |
| I | IV | 0.22 | <0.0001 | |
| II | III | 0.37 | 0.5 | |
| II | IV | 0.22 | 0.0003 | |
| III | IV | 0.22 | 0.06 | |
| Prostate cancer specific survival | I | II | 0.8 | 0.1 |
| I | III | 0.5 | 0.0001 | |
| I | IV | 0.2 | <0.0001 | |
| II | III | 0.01 | 0.02 | |
| II | IV | 0.2 | <0.0001 | |
| III | IV | 0.2 | 0.1 | |
| Overall survival | I | II | 0.48 | 0.0001 |
| I | III | 0.8 | 0.0027 | |
| I | IV | 0.5 | 0.0003 | |
| II | III | 0.22 | 0.313 | |
| II | IV | 0.5 | 0.02 | |
| III | IV | 0.2 | 0.12 | |
Abbreviations: AJCC = American Joint Committee on Cancer; v6 = 6th edition; v7 = 7th edition
Bold-faced print denotes p-values <0.05.
Figure 2.
Biochemical failure (A), overall survival (B), prostate cancer specific survival (C), and distant metastasis (D) of AJCC version 7 stage IIA and IIB patients.
Figure 3.
Biochemical failure (A), overall survival (B), prostate cancer specific survival (C), and distant metastasis (D) of patients stratified by NCCN guidelines.
Table 4.
Paired p-value comparison of risk groups as assessed by NCCN guidelines.
| Outcome measure | Pair comparison (risk group vs. risk group) | p-value | |
|---|---|---|---|
| Biological failure | low | intermediate | <0.0001 |
| low | high | 0.0001 | |
| intermediate | high | 0.97 | |
| Distant metastases | low | intermediate | 0.084 |
| low | high | 0.0007 | |
| intermediate | high | 0.04 | |
| Prostate cancer specific survival | low | intermediate | 0.2 |
| low | high | 0.0003 | |
| intermediate | high | 0.0034 | |
| Overall survival | low | intermediate | 0.002 |
| low | high | <0.0001 | |
| intermediate | high | 0.01 | |
Abbreviations: NCCN = National Comprehensive Cancer Network
Bold-faced print denotes p-values <0.05.
Predictive accuracy
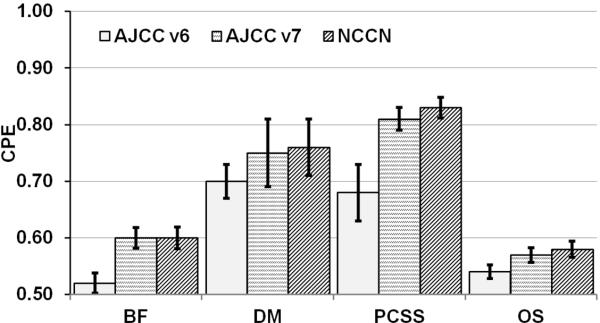
Table 5 lists the CPEs and SEs in evaluating the ability of the AJCC v6, v7, and NCCN risk groups to prognosticate BF, DM, PCSS, and OS after controlling for treatment and dose effects. The results show uniform improvement in the predictive power of the model based on AJCC v7 compared to that on AJCC v6 for all endpoints, in particular for DM and PCSS. NCCN risk group stratification is equally predictive of BF and OS, and it is more predictive of DM and PCSS. Figure 4 illustrates the data presented in Table 5.
Table 5.
The CPEs and their respective SEs in AJCC v6, v7 and NCCN assessment.
| AJCC v6 | AJCC v7 | NCCN | ||||
|---|---|---|---|---|---|---|
| Outcome measure | CPE | SE | CPE | SE | CPE | SE |
| BF | 0.52 | 0.018 | 0.60 | 0.018 | 0.60 | 0.019 |
| DM | 0.70 | 0.030 | 0.75 | 0.060 | 0.76 | 0.060 |
| PCSS | 0.68 | 0.050 | 0.81 | 0.020 | 0.83 | 0.018 |
| OM | 0.54 | 0.012 | 0.58 | 0.014 | 0.58 | 0.014 |
Abbreviations: AJCC = American Joint Committee on Cancer; BF = biochemical failure; CPE = concordance probability estimate; DM = ditant metastasis; NCCN = National Comprehensive Cancer Network, v6 = 6th edition, v7 = 7th edition; OM = overall mortality; PCSS = prostate cancer specific survival; SE = standard error;
Note: The closer the CPE to 1, the better the predictive power of the model; the closer the CPE to 0.5, the worse the predictive power.
Figure 4.
The concordance probability estimates (CPEs) and their respective standard errors (SEs) in assessing the ability of the AJCC v6, v7, and NCCN risk groups to predict biochemical failure, distant metastasis, prostate cancer specific survival, and overall survival. Both treatment (RT versus RT+ADT) and RT dose (< 75.6 Gy versus ≥ 75.6 Gy) effects were included in the model as covariates. The closer the CPE is to 0.5, the lower the predictive accuracy of the model; the closer the CPE is to 1, the better the predictive accuracy. The results show uniform improvement in the predictive power of AJCC v7 and NCCN compared to that of AJCC v6 for all endpoints, in particular for DM and PCSS.
DISCUSSION
Staging is important for radiotherapy because it guides treatment recommendations such the use ADT, radiation dose, and treatment volume (i.e. prostate only versus prostate and pelvis). While several relatively common models that subdivide patients into low-, medium-, and high- risk groups have been proposed,16, 17 there have also been a number of models that are more sophisticated, but less commonly used.5, 18-22 The results from these studies have been integrated in designing AJCC guidelines. We compared the ability of AJCC v68 and v72 to predict BF, DM, PCSS, and OS in men who received RT with or without ADT.
The first major improvement with AJCC v7 is better prognositication between stages I and II. According to AJCC v6, 2,263 (91%) men in our cohort were stage II with the great majority having T1c disease. Nine-hundred-eight (40%) of stage II patients migrated from AJCC v6 Stage II to AJCC v7 Stage I. The remaining 1,355 (60%) AJCC v6 Stage II patients remain Stage II. This migration positively influenced the prognostic value of the AJCC v7. Stage I patients had significantly longer times until BF, DM, and OS, when compared to AJCC v7 stage II patients (Table 3, Figure 1).
The second major advancement associated with AJCC v7 is the improved prognostic value and ability to predict for BF, DM, PCSS, and OS (Tables 3 and 5). The greatest gains were seen for DM and PCSS endpoints. For DM the CPE increased from 0.54 to 0.70 and for PCSS from 0.57 to 0.76. When considering that a CPE 0.5 is associated with purely random ranking, these improvements are a remarkable accomplishment that should be applauded.
These improvements are due to several important changes that distinguish the AJCC v7 from AJCC v6. First, PSA is recognized in TNM staging. Serum PSA is an important prognosticator in patients with a benign prostate exam,23 and lower PSAs have been associated with more favorable pathological findings in clinical stage T1c cancers.24 Second, the AJCC v7 TNM system obligates the GS to be used preferentially in assessing the histopathological tumor grade. The GS is an important determinant of outcome and has been found to be superior to other histopathological reports.7, 25, 26
Problems still exist with AJCC v7 staging. First, there are still too many “intermediate-risk” patients (i.e. stage II, 55%), and there is a lack of sub-stratification of these patients. The clustering of patients in stages I and II are likely secondary to the majority of patients presenting with localized or impalpable disease but an increased PSA.27 Moreover, stage IIA patients do not have a statistically significant difference in rate of BF, DM, PCSS, or OS when compared to stage IIB patients (Figure 2). Second, AJCC v7 does not alter the number of patients in stages III and IV, a finding shown in other patient populations.28 The proportion of patients in stage III was 6% and IV was 1%, which is small compared to stages I and II. Third, AJCC v7 does not have a high predictive accuracy for BF or OS (Table 5, CPEs 0.59 and 0.57, respectively). This is especially true for stratifying and predicting the outcomes of stage II and III patients (Figure 1, right column). Fourth, the NCCN risk groups have a statistically higher predictive accuracy compared to AJCC v7 for PCSS (Table 5, 0.8 vs. 0.76, respectively). Thus, the NCCN model is superior to the AJCC v7 and remains the preferred method for risk-based clinical management of prostate cancer with radiation therapy.
Clinical T-stage, GS, and initial PSA have been shown to be independent predictors of BF and are commonly used in staging systems.16 The TNM staging system is a surgical staging system and does not include many other important pretreatment characteristics. Future models will use more advanced data analysis in predicting outcome. For example, to improve the TNM staging system, one may consider upstaging patients with low or favorable intermediate-risk disease and more than 50% positive core biopsies.28-30 Second, staging systems may include the number of GS 4 or 5 in biopsy specimens, as this value has been shown to be a predictor of BF and DM after RT, independent of GS.31 Third, patients with a PSA velocity >2ng/mL in the year before radical prostatectomy or RT may be upstaged, as these men have been shown to have a lower PCSS.32, 33
Future systems may also integrate patient-specific biomarkers into prognostication. Patient- and disease-specific treatment is demanded by the U.S. government's impetus for expanding comparative effectiveness research (CER).34 CER focuses to develop personalized medicine by examining the racial, ethnic, socioeconomic, and geographic variations in care that affect health outcomes.35, 36 Prostate cancer is a high impact site of CER because of its high prevalence, the many treatment options available, and the emerging biomarkers used in its staging and treatment.37
The future of CER for prostate cancer therapy may integrate specific cancer- and patient-specific biomarkers into staging, including Bcl-2,38-40 Bax,38, 39 the Bcl-2/Bax ratio,39, 41 CD-44,42 e-cadherin,42 p53,42 p21/waf1,41 COX-2,43, 44 MDM2,45 Ki-67,46, 47 P120,46 PCNA,46 and DNA microarrays,48 and p450 polymorphisms.49 Thus, although the TNM staging system may be used to describe almost any patient with prostate cancer, its generalizability precludes its specificity and prognostic capability. Future biomarker-based staging systems, although not universal, may be more specific and have a greater predictive accuracy.
CONCLUSION
AJCC v7 is a major improvement over AJCC v6 because it better distributes patients among the stages and is more prognostic. Further improvements are needed as the majority of men (55%) are stage II and the sub-stratification into IIA and IIB was not prognostic. The NCCN model is superior to the AJCC v7 and remains the preferred method for risk-based clinical management of prostate cancer with radiation therapy.
Acknowledgement
Presented at the Genitourinary Cancer Symposium, February 2-4, 2012, San Francisco, CA. The authors thank Dr. Gerald Hanks for his leadership in the establishment of the Fox Chase Cancer Center database for the treatment of prostate cancer.
Funding Sources: This publication was supported by grant number P30 CA006927 from the National Cancer Institute/NIH and a departmental Varian Grant. Its contents are solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute, the National Institutes of Health, or Varian.
Footnotes
Approval/disclosures: All authors have read and approved the manuscript. We have no financial disclosures. We are not using any copyrighted information, patient photographs, identifiers, or other protected health information in this paper. No text, text boxes, figures, or tables in this article have been previously published or owned by another party.
Conflicts of Interest Notification: We have no conflicts of interests.
REFERENCES
- 1.Yarbro JW, Page DL, Fielding LP, Partridge EE, Murphy GP. American Joint Committee on Cancer prognostic factors consensus conference. Cancer. 1999;86(11):2436–46. doi: 10.1002/(sici)1097-0142(19991201)86:11<2436::aid-cncr35>3.0.co;2-#. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=10590388. [DOI] [PubMed] [Google Scholar]
- 2.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. AJCC Cancer Staging Manual. 7th edition Springer; New York, NY: 2010. Prostate. pp. 457–68. [Google Scholar]
- 3.Karadeniz T, Topsakal M, Aydogmus A, Beksan M. Role of RigiScan in the etiologic differential diagnosis of erectile dysfunction. Urol Int. 1997;59(1):41–5. doi: 10.1159/000283015. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=9313323. [DOI] [PubMed] [Google Scholar]
- 4.Pisansky TM, Kahn MJ, Bostwick DG. An enhanced prognostic system for clinically localized carcinoma of the prostate. Cancer. 1997;79(11):2154–61. doi: 10.1002/(sici)1097-0142(19970601)79:11<2154::aid-cncr13>3.0.co;2-v. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=9179062. [DOI] [PubMed] [Google Scholar]
- 5.Zagars GK, Pollack A, von Eschenbach AC. Prognostic factors for clinically localized prostate carcinoma: analysis of 938 patients irradiated in the prostate specific antigen era. Cancer. 1997;79(7):1370–80. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=9083160. [PubMed] [Google Scholar]
- 6.Hanks GE, Hanlon AL, Pinover WH, Horwitz EM, Price RA, Schultheiss T. Dose selection for prostate cancer patients based on dose comparison and dose response studies. Int J Radiat Oncol Biol Phys. 2000;46(4):823–32. doi: 10.1016/s0360-3016(99)00498-8. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=10705002. [DOI] [PubMed] [Google Scholar]
- 7.Roach M, Lu J, Pilepich MV, Asbell SO, Mohiuddin M, Terry R, et al. Four prognostic groups predict long-term survival from prostate cancer following radiotherapy alone on Radiation Therapy Oncology Group clinical trials. Int J Radiat Oncol Biol Phys. 2000;47(3):609–15. doi: 10.1016/s0360-3016(00)00578-2. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=10837943. [DOI] [PubMed] [Google Scholar]
- 8.Greene FL, Page DL, Fleming ID, et al., editors. AJCC Cancer Staging Manual. 6th edition Lippincott Raven; Philadelphia: 2002. Prostate. pp. 309–16. [Google Scholar]
- 9.Zeliadt SB, Potosky AL, Etzioni R, Ramsey SD, Penson DF. Racial disparity in primary and adjuvant treatment for nonmetastatic prostate cancer: SEER-Medicare trends 1991 to 1999. Urology. 2004;64:1171–76. doi: 10.1016/j.urology.2004.07.037. [DOI] [PubMed] [Google Scholar]
- 10.Horwitz EM, Hanlon AL, Pinover WH, Anderson PR, Hanks GE. Defining the optimal radiation dose with three-dimensional conformal radiation therapy for patients with nonmetastatic prostate carcinoma by using recursive partitioning techniques. Cancer. 2001;92:1281–7. doi: 10.1002/1097-0142(20010901)92:5<1281::aid-cncr1449>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 11.Price RA, Murphy S, McNeeley SW, Ma CM, Horwitz E, Movsas B, et al. A method for increased dose conformity and segment reduction for SMLC delivered IMRT treatment of the prostate. Int J Radiat Oncol Biol Phys. 2003;57(3):843–52. doi: 10.1016/s0360-3016(03)00711-9. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=14529792. [DOI] [PubMed] [Google Scholar]
- 12.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Royal Stat Soc Series. 1995;57:289–300. [Google Scholar]
- 13.Roach M, 3rd, Hanks G, Thames H, Jr., Schellhammer P, Shipley WU, Sokol GH, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys. 2006;65(4):965–74. doi: 10.1016/j.ijrobp.2006.04.029. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16798415. [DOI] [PubMed] [Google Scholar]
- 14.Gönen M, Heller G. Concordance probability and discriminatory power in proportional hazards regression. Biometrika. 2011;92(4):965–70. [Google Scholar]
- 15.R Development Core Team . A language and environment for statistical computing Vienna. R Foundation for Statistical Computing; Austria: 2011. [Google Scholar]
- 16.D'Amico AV, Whittington R, Malkowicz SB, Schultz D, Blank K, Broderick GA, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280(11):969–74. doi: 10.1001/jama.280.11.969. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=9749478. [DOI] [PubMed] [Google Scholar]
- 17.Zelefsky MJ, Fuks Z, Hunt M, Lee HJ, Lombardi D, Ling CC, et al. High dose radiation delivered by intensity modulated conformal radiotherapy improves the outcome of localized prostate cancer. J Urol. 2001;166(3):876–81. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=11490237. [PubMed] [Google Scholar]
- 18.Kattan MW, Zelefsky MJ, Kupelian PA, Cho D, Scardino PT, Fuks Z, et al. Pretreatment nomogram that predicts 5-year probability of metastasis following three-dimensional conformal radiation therapy for localized prostate cancer. J Clin Oncol. 2003;21(24):4568–71. doi: 10.1200/JCO.2003.05.046. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=14673043. [DOI] [PubMed] [Google Scholar]
- 19.Movsas B, Hanlon AL, Teshima T, Hanks GE. Analyzing predictive models following definitive radiotherapy for prostate carcinoma. Cancer. 1997;80(6):1093–102. doi: 10.1002/(sici)1097-0142(19970915)80:6<1093::aid-cncr12>3.0.co;2-3. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=9305710. [DOI] [PubMed] [Google Scholar]
- 20.Partin AW, Kattan MW, Subong EN, Walsh PC, Wojno KJ, Oesterling JE, et al. Combination of prostate-specific antigen, clinical stage, and Gleason score to predict pathological stage of localized prostate cancer. A multi-institutional update. JAMA. 1997;277(18):1445–51. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=9145716. [PubMed] [Google Scholar]
- 21.Pisansky TM, Kahn MJ, Rasp GM, Cha SS, Haddock MG, Bostwick DG. A multiple prognostic index predictive of disease outcome after irradiation for clinically localized prostate carcinoma. Cancer. 1997;79(2):337–44. doi: 10.1002/(sici)1097-0142(19970115)79:2<337::aid-cncr17>3.0.co;2-1. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=9010107. [DOI] [PubMed] [Google Scholar]
- 22.Roach M, 3rd, Marquez C, Yuo HS, Narayan P, Coleman L, Nseyo UO, et al. Predicting the risk of lymph node involvement using the pre-treatment prostate specific antigen and Gleason score in men with clinically localized prostate cancer. Int J Radiat Oncol Biol Phys. 1994;28(1):33–7. doi: 10.1016/0360-3016(94)90138-4. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=7505775. [DOI] [PubMed] [Google Scholar]
- 23.Catalona WJ, Smith DS, Ornstein DK. Prostate cancer detection in men with serum PSA concentrations of 2.6 to 4.0 ng/mL and benign prostate examination. Enhancement of specificity with free PSA measurements. JAMA. 1997;277(18):1452–5. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=9145717. [PubMed] [Google Scholar]
- 24.Makarov DV, Humphreys EB, Mangold LA, Walsh PC, Partin AW, Epstein JI, et al. Pathological outcomes and biochemical progression in men with T1c prostate cancer undergoing radical prostatectomy with prostate specific antigen 2.6 to 4.0 vs 4.1 to 6.0 ng/ml. J Urol. 2006;176(2):554–8. doi: 10.1016/j.juro.2006.03.058. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16813888. [DOI] [PubMed] [Google Scholar]
- 25.Tollefson MK, Leibovich BC, Slezak JM, Zincke H, Blute ML. Long-term prognostic significance of primary Gleason pattern in patients with Gleason score 7 prostate cancer: impact on prostate cancer specific survival. J Urol. 2006;175(2):547–51. doi: 10.1016/S0022-5347(05)00152-7. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16406993. [DOI] [PubMed] [Google Scholar]
- 26.Epstein JI. An update of the Gleason grading system. J Urol. 2010;183(2):433–40. doi: 10.1016/j.juro.2009.10.046. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=20006878. [DOI] [PubMed] [Google Scholar]
- 27.Jani AB, Vaida F, Hanks G, Asbell S, Sartor O, Moul JW, et al. Changing face and different countenances of prostate cancer: racial and geographic differences in prostate-specific antigen (PSA), stage, and grade trends in the PSA era. Int J Cancer. 2001;96(6):363–71. doi: 10.1002/ijc.1035. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=11745507. [DOI] [PubMed] [Google Scholar]
- 28.Huang J, Vicini FA, Williams SG, Ye H, McGrath S, Ghilezan M, et al. Percentage of Positive Biopsy Cores: A Better Risk Stratification Model for Prostate Cancer? Int J Radiat Oncol Biol Phys. 2011 doi: 10.1016/j.ijrobp.2011.09.043. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=22099043. [DOI] [PubMed]
- 29.San Francisco IF, Regan MM, Olumi AF, DeWolf WC. Percent of cores positive for cancer is a better preoperative predictor of cancer recurrence after radical prostatectomy than prostate specific antigen. J Urol. 2004;171(4):1492–9. doi: 10.1097/01.ju.0000118690.05943.c0. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15017206. [DOI] [PubMed] [Google Scholar]
- 30.D'Amico AV, Renshaw AA, Cote K, Hurwitz M, Beard C, Loffredo M, et al. Impact of the percentage of positive prostate cores on prostate cancer-specific mortality for patients with low or favorable intermediate-risk disease. J Clin Oncol. 2004;22(18):3726–32. doi: 10.1200/JCO.2004.01.164. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15365069. [DOI] [PubMed] [Google Scholar]
- 31.D'Ambrosio DJ, Hanlon AL, Al-Saleem T, Feigenberg SJ, Horwitz EM, Uzzo RG, et al. The proportion of prostate biopsy tissue with Gleason pattern 4 or 5 predicts for biochemical and clinical outcome after radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2007;67(4):1082–7. doi: 10.1016/j.ijrobp.2006.09.050. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17241749. [DOI] [PubMed] [Google Scholar]
- 32.D'Amico AV, Chen MH, Roehl KA, Catalona WJ. Preoperative PSA velocity and the risk of death from prostate cancer after radical prostatectomy. N Engl J Med. 2004;351(2):125–35. doi: 10.1056/NEJMoa032975. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15247353. [DOI] [PubMed] [Google Scholar]
- 33.D'Amico AV, Renshaw AA, Sussman B, Chen MH. Pretreatment PSA velocity and risk of death from prostate cancer following external beam radiation therapy. Jama. 2005;294(4):440–7. doi: 10.1001/jama.294.4.440. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16046650. [DOI] [PubMed] [Google Scholar]
- 34.Vogel L. New US centre to boost comparative health research. CMAJ. 2010;182(10):E457–E58. doi: 10.1503/cmaj.109-3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Concato J, Lawler EV, Lew RA, Gaziano JM, Aslan M, Huang GD. Observational methods in comparative effectiveness research. The American Journal of Medicine. 2010;123(12A):e16–e23. doi: 10.1016/j.amjmed.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 36.Epstein RS, Teagarden JR. Comparative effectiveness research and personalized medicine: Catalyzing or colliding? Pharmacoeconomics. 2010;28(10):905–13. doi: 10.2165/11535830-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 37.Shen X, Zaorsky NG, Mishra MV, Foley KA, Hyslop T, Hegarty S, et al. Comparative effectiveness research for prostate cancer radiation therapy: current status and future directions. Future Oncol. 2012;8(1):37–54. doi: 10.2217/fon.11.131. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=22149034. [DOI] [PubMed] [Google Scholar]
- 38.Khor LY, Moughan J, Al-Saleem T, Hammond EH, Venkatesan V, Rosenthal SA, et al. Bcl-2 and Bax expression predict prostate cancer outcome in men treated with androgen deprivation and radiotherapy on radiation therapy oncology group protocol 92-02. Clin Cancer Res. 2007;13(12):3585–90. doi: 10.1158/1078-0432.CCR-06-2972. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17575222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pollack A, Cowen D, Troncoso P, Zagars GK, von Eschenbach AC, Meistrich ML, et al. Molecular markers of outcome after radiotherapy in patients with prostate carcinoma: Ki-67, bcl-2, bax, and bcl-x. Cancer. 2003;97(7):1630–8. doi: 10.1002/cncr.11230. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12655519. [DOI] [PubMed] [Google Scholar]
- 40.Pollack A, DeSilvio M, Khor L, Li R, Al-Saleem T, Hammond M, et al. Ki-67 staining is a strong predictor of patient outcome for prostate cancer patients treated with androgen deprivation plus radiotherapy: an analysis of RTOG 92-02. Int J Radiat Oncol Biol Phys. 2003;57(2 Suppl):S200–1. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12965456. [Google Scholar]
- 41.Zhou JR, Yu L, Zerbini LF, Libermann TA, Blackburn GL. Progression to androgen-independent LNCaP human prostate tumors: cellular and molecular alterations. Int J Cancer. 2004;110(6):800–6. doi: 10.1002/ijc.20206. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15170660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brewster SF, Oxley JD, Trivella M, Abbott CD, Gillatt DA. Preoperative p53, bcl-2, CD44 and E-cadherin immunohistochemistry as predictors of biochemical relapse after radical prostatectomy. J Urol. 1999;161(4):1238–43. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=10081877. [PubMed] [Google Scholar]
- 43.Pan Y, Zhang JS, Gazi MH, Young CY. The cyclooxygenase 2-specific nonsteroidal anti-inflammatory drugs celecoxib and nimesulide inhibit androgen receptor activity via induction of c-Jun in prostate cancer cells. Cancer Epidemiol Biomarkers Prev. 2003;12(8):769–74. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12917209. [PubMed] [Google Scholar]
- 44.Lorenzo GD, Placido SD, Autorino R, Laurentiis MD, Mignogna C, D'Armiento M, et al. Expression of biomarkers modulating prostate cancer progression: implications in the treatment of the disease. Prostate Cancer Prostatic Dis 2005. doi: 10.1038/sj.pcan.4500768. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15655565. [DOI] [PubMed]
- 45.Khor LY, Desilvio M, Al-Saleem T, Hammond ME, Grignon DJ, Sause W, et al. MDM2 as a predictor of prostate carcinoma outcome: an analysis of Radiation Therapy Oncology Group Protocol 8610. Cancer. 2005;104(5):962–7. doi: 10.1002/cncr.21261. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16007688. [DOI] [PubMed] [Google Scholar]
- 46.Bantis A, Giannopoulos A, Gonidi M, Liossi A, Aggelonidou E, Petrakakou E, et al. Expression of p120, Ki-67 and PCNA as proliferation biomarkers in imprint smears of prostate carcinoma and their prognostic value. Cytopathology. 2004;15(1):25–31. doi: 10.1046/j.0956-5507.2003.00090.x. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=14748788. [DOI] [PubMed] [Google Scholar]
- 47.Rubin MA, Dunn R, Strawderman M, Pienta KJ. Tissue microarray sampling strategy for prostate cancer biomarker analysis. Am J Surg Pathol. 2002;26(3):312–9. doi: 10.1097/00000478-200203000-00004. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=11859202. [DOI] [PubMed] [Google Scholar]
- 48.Sunami E, Shinozaki M, Higano CS, Wollman R, Dorff TB, Tucker SJ, et al. Multimarker circulating DNA assay for assessing blood of prostate cancer patients. Clin Chem. 2009;55(3):559–67. doi: 10.1373/clinchem.2008.108498. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19131636. [DOI] [PubMed] [Google Scholar]
- 49.Li X, Strauss L, Makela S, Streng T, Huhtaniemi I, Santti R, et al. Multiple structural and functional abnormalities in the p450 aromatase expressing transgenic male mice are ameliorated by a p450 aromatase inhibitor. Am J Pathol. 2004;164(3):1039–48. doi: 10.1016/S0002-9440(10)63191-4. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=14982857. [DOI] [PMC free article] [PubMed] [Google Scholar]