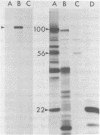
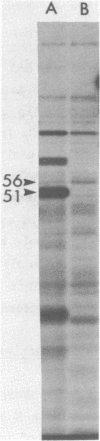
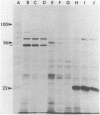
Abstract
The three products of the early region of polyoma virus have been cloned for expression in E. coli using the Tac promoter. Although the identical promoter and ribosome binding site are used in each final construction, the observed level of protein expression is different for each protein. While plasmids expressing wild type T antigens as well as a plasmid expressing the truncated Py-1387T middle T antigen lacking the membrane-anchoring sequence give rise to synthesis of proteins readily detectible by 35S-methionine labeling and immunoprecipitation, only small T and the middle T of Py-1387T are made in amounts sufficient for ready detection in total cell protein. Unlike middle T expressed in animal cells, middle T produced in E. coli is not detectibly phosphorylated. Further, the E. coli protein lacks tyrosine kinase activity.
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asselin C., Gelinas C., Bastin M. Role of the three polyoma virus early proteins in tumorigenesis. Mol Cell Biol. 1983 Aug;3(8):1451–1459. doi: 10.1128/mcb.3.8.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backman K., Ptashne M., Gilbert W. Construction of plasmids carrying the cI gene of bacteriophage lambda. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4174–4178. doi: 10.1073/pnas.73.11.4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
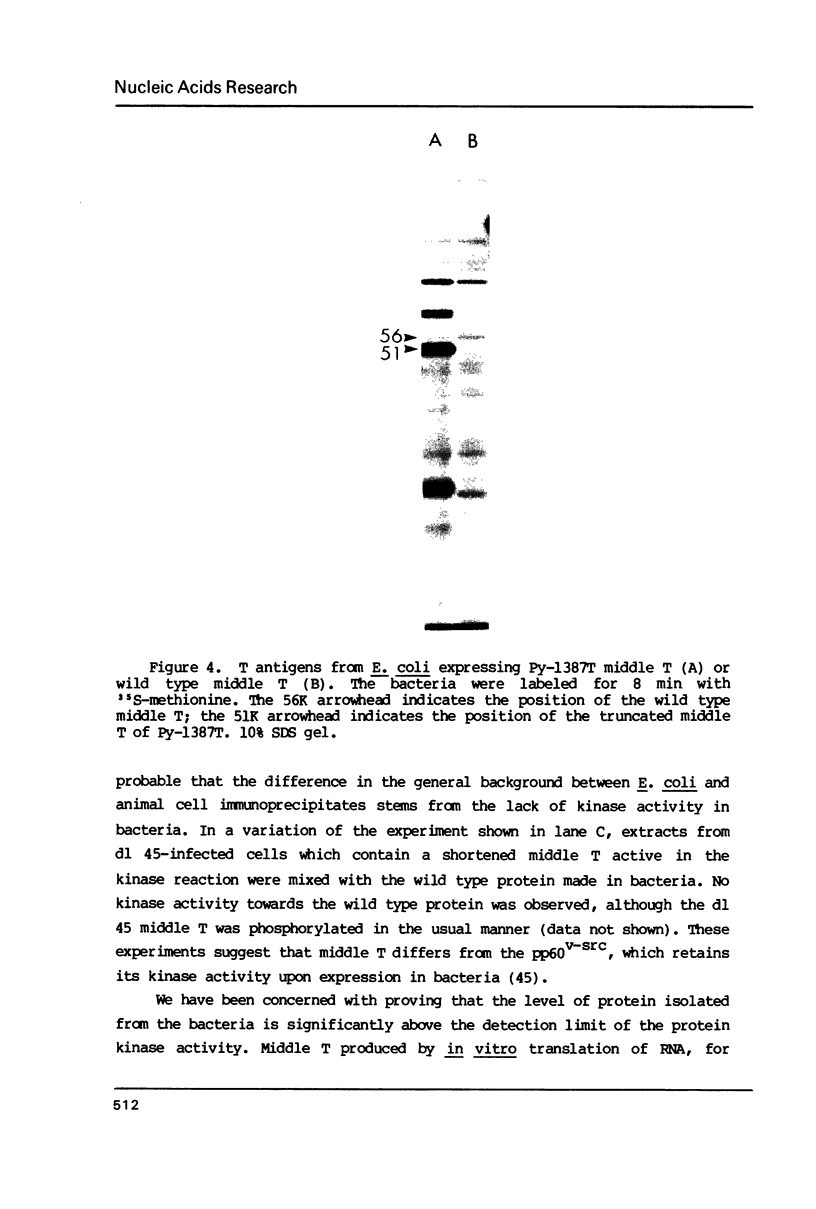
- Bendig M. M., Thomas T., Folk W. R. Viable deletion mutant in the medium and large T-antigen-coding sequences of the polyoma virus genome. J Virol. 1980 Mar;33(3):1215–1220. doi: 10.1128/jvi.33.3.1215-1220.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikel I., Roberts T. M., Bladon M. T., Green R., Amann E., Livingston D. M. Purification of biologically active simian virus 40 small tumor antigen. Proc Natl Acad Sci U S A. 1983 Feb;80(4):906–910. doi: 10.1073/pnas.80.4.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukhari A. I., Zipser D. Mutants of Escherichia coli with a defect in the degradation of nonsense fragments. Nat New Biol. 1973 Jun 20;243(129):238–241. doi: 10.1038/newbio243238a0. [DOI] [PubMed] [Google Scholar]
- Carmichael G. G., Schaffhausen B. S., Dorsky D. I., Oliver D. B., Benjamin T. L. Carboxy terminus of polyoma middle-sized tumor antigen is required for attachment to membranes, associated protein kinase activities, and cell transformation. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3579–3583. doi: 10.1073/pnas.79.11.3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael G., Schaffhausen B. S., Mandel G., Liang T. J., Benjamin T. L. Transformation by polyoma virus is drastically reduced by substitution of phenylalanine for tyrosine at residue 315 of middle-sized tumor antigen. Proc Natl Acad Sci U S A. 1984 Feb;81(3):679–683. doi: 10.1073/pnas.81.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A. C., Nunberg J. H., Kaufman R. J., Erlich H. A., Schimke R. T., Cohen S. N. Phenotypic expression in E. coli of a DNA sequence coding for mouse dihydrofolate reductase. Nature. 1978 Oct 19;275(5681):617–624. doi: 10.1038/275617a0. [DOI] [PubMed] [Google Scholar]
- Chung C. H., Goldberg A. L. The product of the lon (capR) gene in Escherichia coli is the ATP-dependent protease, protease La. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4931–4935. doi: 10.1073/pnas.78.8.4931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogen B. Virus-specific early RNA in 3T6 cells infected by a tsA mutant of polyoma virus. Virology. 1978 Mar;85(1):222–230. doi: 10.1016/0042-6822(78)90426-9. [DOI] [PubMed] [Google Scholar]
- Colbere-Garapin F., Chousterman S., Horodniceanu F., Kourilsky P., Garapin A. C. Cloning of the active thymidine kinase gene of herpes simplex virus type 1 in Escherichia coli K-12. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3755–3759. doi: 10.1073/pnas.76.8.3755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtneidge S. A., Smith A. E. Polyoma virus transforming protein associates with the product of the c-src cellular gene. Nature. 1983 Jun 2;303(5916):435–439. doi: 10.1038/303435a0. [DOI] [PubMed] [Google Scholar]
- Della Valle G., Fenton R. G., Basilico C. Polyoma large T antigen regulates the integration of viral DNA sequences into the genome of transformed cells. Cell. 1981 Feb;23(2):347–355. doi: 10.1016/0092-8674(81)90130-6. [DOI] [PubMed] [Google Scholar]
- Devare S. G., Shatzman A., Robbins K. C., Rosenberg M., Aaronson S. A. Expression of the PDGF-related transforming protein of simian sarcoma virus in E. coli. Cell. 1984 Jan;36(1):43–49. doi: 10.1016/0092-8674(84)90072-2. [DOI] [PubMed] [Google Scholar]
- FRIED M. CELL-TRANSFORMING ABILITY OF A TEMPERATURE-SENSITIVE MUTANT OF POLYOMA VIRUS. Proc Natl Acad Sci U S A. 1965 Mar;53:486–491. doi: 10.1073/pnas.53.3.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feunteun J., Sompayrac L., Fluck M., Benjamin T. Localization of gene functions in polyoma virus DNA. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4169–4173. doi: 10.1073/pnas.73.11.4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francke B., Eckhart W. Polyoma gene function required for viral DNA synthesis. Virology. 1973 Sep;55(1):127–135. doi: 10.1016/s0042-6822(73)81014-1. [DOI] [PubMed] [Google Scholar]
- Garcea R. L., Benjamin T. L. Host range transforming gene of polyoma virus plays a role in virus assembly. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3613–3617. doi: 10.1073/pnas.80.12.3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmer T. M., Erikson R. L. Rous sarcoma virus transforming protein, p60src, expressed in E. coli, functions as a protein kinase. Nature. 1981 Dec 24;294(5843):771–773. doi: 10.1038/294771a0. [DOI] [PubMed] [Google Scholar]
- Guarente L., Lauer G., Roberts T. M., Ptashne M. Improved methods for maximizing expression of a cloned gene: a bacterium that synthesizes rabbit beta-globin. Cell. 1980 Jun;20(2):543–553. doi: 10.1016/0092-8674(80)90640-6. [DOI] [PubMed] [Google Scholar]
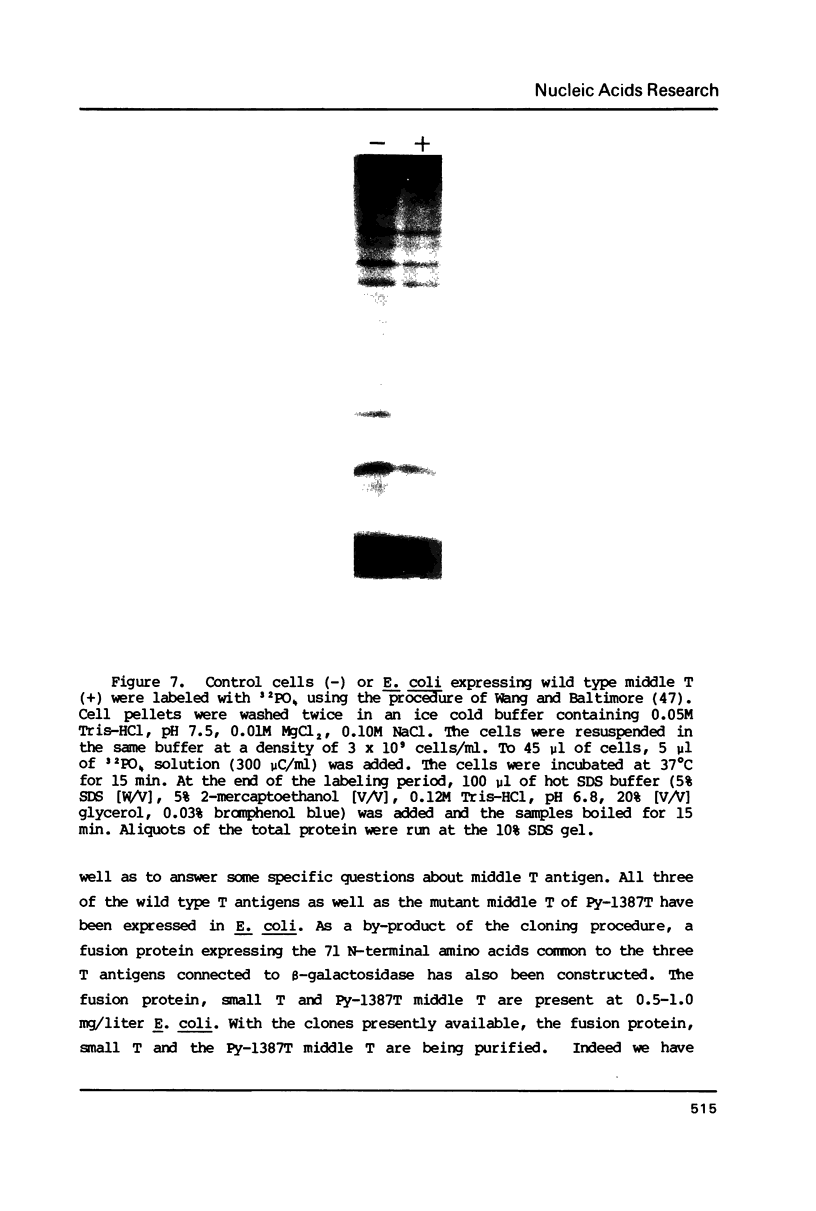
- Harvey R., Oostra B. A., Belsham G. J., Gillett P., Smith A. E. An antibody to a synthetic peptide recognizes polyomavirus middle-T antigen and reveals multiple in vitro tyrosine phosphorylation sites. Mol Cell Biol. 1984 Jul;4(7):1334–1342. doi: 10.1128/mcb.4.7.1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwich A., Koop A. H., Eckhart W. Expression of the gene for the polyoma small T antigen in Escherichia coli. J Virol. 1980 Oct;36(1):125–132. doi: 10.1128/jvi.36.1.125-132.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwich A., Koop A. H., Eckhart W. Syntheses and stabilities of proteins related to the polyoma small T antigen in Escherichia coli. Mol Cell Biol. 1982 Jan;2(1):88–92. doi: 10.1128/mcb.2.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T., Hutchinson M. A., Eckhart W. Polyoma middle-sized T antigen can be phosphorylated on tyrosine at multiple sites in vitro. EMBO J. 1984 Jan;3(1):73–79. doi: 10.1002/j.1460-2075.1984.tb01763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y. Polyoma virus-specific 55K protein isolated from plasma membrane of productively infected cells is virus-coded and important for cell transformation. Virology. 1979 Oct 15;98(1):261–266. doi: 10.1016/0042-6822(79)90545-2. [DOI] [PubMed] [Google Scholar]
- Ito Y., Spurr N., Griffin B. E. Middle T antigen as primary inducer of full expression of the phenotype of transformation by polyoma virus. J Virol. 1980 Jul;35(1):219–232. doi: 10.1128/jvi.35.1.219-232.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lauer G., Pastrana R., Sherley J., Ptashne M. Construction of overproducers of the bacteriophage 434 repressor and cro proteins. J Mol Appl Genet. 1981;1(2):139–147. [PubMed] [Google Scholar]
- Lautenberger J. A., Kan N. C., Court D., Pry T., Showalter S., Papas T. S. High-level expression of oncogenes in Escherichia coli. Gene Amplif Anal. 1983;3:147–174. [PubMed] [Google Scholar]
- Magnusson G., Berg P. Construction and analysis of viable deletion mutants of polyoma virus. J Virol. 1979 Nov;32(2):523–529. doi: 10.1128/jvi.32.2.523-529.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oostra B. A., Harvey R., Ely B. K., Markham A. F., Smith A. E. Transforming activity of polyoma virus middle-T antigen probed by site-directed mutagenesis. Nature. 1983 Aug 4;304(5925):456–459. doi: 10.1038/304456a0. [DOI] [PubMed] [Google Scholar]
- Rassoulzadegan M., Cowie A., Carr A., Glaichenhaus N., Kamen R., Cuzin F. The roles of individual polyoma virus early proteins in oncogenic transformation. Nature. 1982 Dec 23;300(5894):713–718. doi: 10.1038/300713a0. [DOI] [PubMed] [Google Scholar]
- Rassoulzadegan M., Naghashfar Z., Cowie A., Carr A., Grisoni M., Kamen R., Cuzin F. Expression of the large T protein of polyoma virus promotes the establishment in culture of "normal" rodent fibroblast cell lines. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4354–4358. doi: 10.1073/pnas.80.14.4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts T. M., Bikel I., Yocum R. R., Livingston D. M., Ptashne M. Synthesis of simian virus 40 t antigen in Escherichia coli. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5596–5600. doi: 10.1073/pnas.76.11.5596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts T. M., Kacich R., Ptashne M. A general method for maximizing the expression of a cloned gene. Proc Natl Acad Sci U S A. 1979 Feb;76(2):760–764. doi: 10.1073/pnas.76.2.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts T. M., Lauer G. D. Maximizing gene expression on a plasmid using recombination in vitro. Methods Enzymol. 1979;68:473–482. doi: 10.1016/0076-6879(79)68036-9. [DOI] [PubMed] [Google Scholar]
- Rundell K., Hearing P., Yang Y. C. SV40 17K protein is associated with two cellular proteins. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):211–214. doi: 10.1101/sqb.1980.044.01.024. [DOI] [PubMed] [Google Scholar]
- Schaffhausen B. S., Benjamin T. L. Phosphorylation of polyoma T antigens. Cell. 1979 Dec;18(4):935–946. doi: 10.1016/0092-8674(79)90206-x. [DOI] [PubMed] [Google Scholar]
- Schaffhausen B. S., Dorai H., Arakere G., Benjamin T. L. Polyoma virus middle T antigen: relationship to cell membranes and apparent lack of ATP-binding activity. Mol Cell Biol. 1982 Oct;2(10):1187–1198. doi: 10.1128/mcb.2.10.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffhausen B. S., Silver J. E., Benjamin T. L. Tumor antigen(s) in cell productively infected by wild-type polyoma virus and mutant NG-18. Proc Natl Acad Sci U S A. 1978 Jan;75(1):79–83. doi: 10.1073/pnas.75.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffhausen B., Benjamin T. L. Comparison of phosphorylation of two polyoma virus middle T antigens in vivo and in vitro. J Virol. 1981 Oct;40(1):184–196. doi: 10.1128/jvi.40.1.184-196.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe J. G. Complete nucleotide sequence of the Escherichia coli plasmid pBR322. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):77–90. doi: 10.1101/sqb.1979.043.01.013. [DOI] [PubMed] [Google Scholar]
- Taniguchi T., Guarente L., Roberts T. M., Kimelman D., Douhan J., 3rd, Ptashne M. Expression of the human fibroblast interferon gene in Escherichia coli. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5230–5233. doi: 10.1073/pnas.77.9.5230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Templeton D., Eckhart W. Mutation causing premature termination of the polyoma virus medium T antigen blocks cell transformation. J Virol. 1982 Mar;41(3):1014–1024. doi: 10.1128/jvi.41.3.1014-1024.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenen D. G., Garewal H., Haines L. L., Hudson J., Woodard V., Light S., Livingston D. M. Purification of simian virus 40 tumor antigen from a line of simian virus 40-transformed human cells. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3745–3749. doi: 10.1073/pnas.74.9.3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjian R. The binding site on SV40 DNA for a T antigen-related protein. Cell. 1978 Jan;13(1):165–179. doi: 10.1016/0092-8674(78)90147-2. [DOI] [PubMed] [Google Scholar]
- Treisman R., Cowie A., Favaloro J., Jat P., Kamen R. The structures of the spliced mRNAs encoding polyoma virus early region proteins. J Mol Appl Genet. 1981;1(2):83–92. [PubMed] [Google Scholar]
- Treisman R., Novak U., Favaloro J., Kamen R. Transformation of rat cells by an altered polyoma virus genome expressing only the middle-T protein. Nature. 1981 Aug 13;292(5824):595–600. doi: 10.1038/292595a0. [DOI] [PubMed] [Google Scholar]
- Türler H. The tumor antigens and the early functions of polyoma virus. Mol Cell Biochem. 1980 Sep 15;32(2):63–93. doi: 10.1007/BF00227801. [DOI] [PubMed] [Google Scholar]
- WINOCOUR E. Purification of polyoma virus. Virology. 1963 Feb;19:158–168. doi: 10.1016/0042-6822(63)90005-9. [DOI] [PubMed] [Google Scholar]
- Wang J. Y., Queen C., Baltimore D. Expression of an Abelson murine leukemia virus-encoded protein in Escherichia coli causes extensive phosphorylation of tyrosine residues. J Biol Chem. 1982 Nov 25;257(22):13181–13184. [PubMed] [Google Scholar]