Abstract
Context:
We have previously reported that premenopausal women with idiopathic osteoporosis based on fractures (IOP) or idiopathic low bone mineral density (ILBMD) exhibit markedly reduced bone mass, profoundly abnormal trabecular microstructure, and significant deficits in trabecular bone stiffness. Bone remodeling was heterogeneous. Those with low bone turnover had evidence of osteoblast dysfunction and the most marked deficits in microstructure and stiffness.
Objective:
Because osteoblasts and marrow adipocytes derive from a common mesenchymal precursor and excess marrow fat has been implicated in the pathogenesis of bone fragility in anorexia nervosa, glucocorticoid excess, and thiazolidinedione exposure, we hypothesized that marrow adiposity would be higher in affected women and inversely related to bone mass, microarchitecture, bone formation rate, and osteoblast number.
Design:
We analyzed tetracycline-labeled transiliac biopsy specimens in 64 premenopausal women with IOP or ILBMD and 40 controls by three-dimensional micro-computed tomography and two-dimensional quantitative histomorphometry to assess marrow adipocyte number, perimeter, and area.
Results:
IOP and ILBMD subjects did not differ with regard to any adipocyte parameter, and thus results were combined. Subjects had substantially higher adipocyte number (by 22%), size (by 24%), and volume (by 26%) than controls (P < 0.0001 for all). Results remained significant after adjusting for age, body mass index, and bone volume. Controls demonstrated expected direct associations between marrow adiposity and age and inverse relationships between marrow adiposity and bone formation, volume, and microstructure measures. No such relationships were observed in the subjects.
Conclusions:
Higher marrow adiposity and the absence of expected relationships between marrow adiposity and bone microstructure and remodeling in women with IOP or ILBMD suggest that the relationships between fat and bone are abnormal; excess marrow fat may not arise from a switch from the osteoblast to the adipocyte lineage in this disorder. Whether excess marrow fat contributes to the pathogenesis of this disorder remains unclear.
Osteoporosis is uncommon in premenopausal women, and most affected young women have a secondary cause of bone loss or fragility (1–3). However, some young, otherwise healthy women with intact gonadal function and no known secondary cause of bone loss present with unexplained low trauma fractures, whereas others have very low areal bone mineral density (aBMD) but no history of low trauma fractures (4). To investigate the etiology of idiopathic osteoporosis (IOP), we compared iliac crest bone biopsies from 64 affected premenopausal women to 40 premenopausal controls with normal aBMD and no history of adult low trauma fractures. In the affected group, we included women with a history of one or more low trauma fractures, regardless of whether they had frankly low aBMD measurements (the IOP group). We also included women with low aBMD (defined as a Z score ≤−2.0) but no history of adult low trauma fracture [the idiopathic low bone mineral density (BMD) or ILBMD group]. We initially hypothesized that those with ILBMD would have low aBMD due to small bone size but would have more normal volumetric BMD (vBMD), microarchitecture, and strength than those with a history of low trauma fracture.
We recently reported that women with IOP had severe microarchitectural abnormalities, with thinner cortices; fewer, thinner, more widely separated, and heterogeneously distributed trabeculae; and reduced estimated stiffness of bone biopsy specimens compared with controls (5). They also had lower cortical and trabecular vBMD and reduced stiffness by two noninvasive three-dimensional imaging modalities—high resolution peripheral quantitative computed tomography at the radius and tibia (6), and central quantitative computed tomography at the spine and hip (7). Bone remodeling, whether based upon serum bone turnover markers or tetracycline-based quantitative dynamic histomorphometry, was heterogeneous and, on average, did not differ between either group of affected subjects and controls. However, those with low bone formation rates on bone biopsy had higher serum IGF-I concentrations and appeared to synthesize less bone matrix per remodeling site, suggesting that osteoblast dysfunction and resistance to IGF-I may be involved in the pathogenesis (5). Contrary to our hypotheses, we found no differences in any parameter according to whether the women had fractures or only had low aBMD.
In the bone marrow, osteoblasts and adipocytes derive from a common mesenchymal precursor cell (8). Recent work suggests that control of the differentiation of this precursor cell into either an adipocyte or an osteoblast is an important determinant of bone structural integrity (9–18). A reciprocal relationship between marrow adipocytes and osteoblasts has been implicated in osteoporosis related to aging, menopause, anorexia nervosa, glucocorticoids, and thiazolidinediones (9–16). Some human imaging studies suggest that alterations in marrow fat may contribute to bone fragility and fracture risk independent of bone mineral density (BMD) (17, 18). Studies in animal models suggest that bone marrow adipocytes and osteoblasts are regulated independently (19–21).
To investigate relationships between marrow adiposity and bone microstructure and remodeling in subjects with IOP and ILBMD, we measured adipocyte parameters on the transiliac crest bone biopsy specimens from our affected subjects and in controls. We hypothesized that marrow fat would be higher in affected than normal premenopausal women and would be inversely related to bone mass, microarchitecture, and bone formation, assessed by serum bone turnover markers and quantitative histomorphometry.
Patients and Methods
Patient population
Premenopausal women, aged 18–48 yr, were recruited at Columbia University Medical Center (CUMC; New York, NY) and Creighton University (Omaha, NE) by advertisement, self-referral, or physician referral as previously described in detail (4). Included were women with a documented low-trauma fracture after age 18 (regardless of whether aBMD was low; IOP group) as well as women with low aBMD by dual-energy x-ray absorptiometry (DXA; T score ≤−2.5 or Z score ≤−2.0) at the spine or hip without history of adult low trauma fracture (ILBMD group). Fractures were ascertained by review of radiographs or reports and categorized as low trauma (equivalent to a fall from a standing height or less) after review by a physician panel (A.C., E.M.S., R.R.R., and E.S.). Controls had normal aBMD by DXA (T score ≥−1.0 or Z score ≥−1.0) and no history of adult low trauma fractures.
Inclusion and exclusion criteria were previously reported (4). We defined premenopausal status as regular menses off hormonal contraception and early follicular phase FSH levels below 20 mIU/ml. Secondary causes of osteoporosis were excluded in subjects and controls by detailed history and physical and biochemical evaluation (4). Normal PTH level on screening laboratories was required for study entry. Women with serum 25-hydroxyvitamin D (25-OHD) levels below 10 ng/ml were excluded; those with screening 25-OHD levels between 10 and 20 ng/ml were eligible if their serum PTH was normal (10–65 pg/ml). Serum 25-OHD levels below 20 ng/ml were found in 11 subjects (17%) and 11 controls (28%). Among subjects and controls with serum 25-OHD levels below 20 ng/ml, mean PTH level was normal (24.3 ± 11.3 pg/ml). All subjects provided written informed consent. The Institutional Review Boards of both institutions approved these studies.
Laboratory assessments
To assess for secondary causes of osteoporosis, fasting morning blood was drawn, and a 24-h urine was collected during the early follicular phase of the menstrual cycle on the participant's usual diet and supplement regimen and analyzed in a clinical laboratory (Quest Diagnostics, Madison, NJ). Additional serum was archived and stored at −80 C for batch analyses in the Bone Marker Laboratory of the Metabolic Bone Diseases Program and the CTSA Biomarkers Core Laboratory at CUMC. Serum leptin was assessed by RIA (Linco Research, St. Charles, MO). Details regarding other laboratory assessments have been previously published (4).
Areal BMD
aBMD was measured by DXA (QDR-4500; Hologic Inc., Walton, MA) at Columbia and Creighton University Medical Centers as previously described (4).
Transiliac bone biopsy
After double-labeling with tetracycline, transiliac biopsy was performed using a Bordier-type trephine with an inner diameter of 7.5 mm (22). The intact biopsy specimens were fixed and dehydrated in ethanol, subjected to micro-computed tomography (μCT 40; Scanco Medical AG, Brüttisellen, Switzerland) with the long axis oriented along the rotation axis of the scanner at an isotropic, nominal resolution of 8 μm, as previously described in detail (23, 24). Trabecular indices, including bone volume/total volume (BV/TV), trabecular thickness (Tb.Th), trabecular separation (Tb.Sp), and trabecular number (Tb.N), were determined using a direct three-dimensional approach as previously described (25).
Apparent Young's modulus of the biopsy specimens was calculated by micro-finite element analysis (μFE) of μCT trabecular images as previously described (24). A rectangular volume of interest (640*640*300 voxels) was isolated in the center of the biopsy image; each voxel was converted to an eight-node brick element. A single load case was applied, representing uniaxial compression in the medial-lateral direction. A custom in-house parallel conjugate gradient finite element solver with multilevel preconditioning (26) was used. Detailed μCT and μFE results of this study have previously been reported (5).
After μCT, biopsy specimens were embedded in polymethylmethacrylate for quantitative histomorphometry, sectioned (7 μm), and stained (Goldner trichrome) according to established procedures (24). Histomorphometry was performed with a digitizing image-analysis system (OsteoMeasure, version 4.00C; OsteoMetrics, Inc., Atlanta, GA). All structural and remodeling variables were calculated according to American Society for Bone and Mineral Research (ASBMR) recommendations (27). Detailed histomorphometry results of this study have previously been reported (5).
Adipocyte, osteoblast, and osteoclast analyses
Biopsy specimens were assessed in a blinded manner. An intact section with an intact marrow/cancellous bone compartment and two cortices was required to qualify for the analysis; 103 of 104 original biopsy samples qualified for analysis. Adipocytes were analyzed according to the method of Syed et al. (15) on sections stained with Goldner's trichrome. We analyzed a uniform number of fields in all sections. Each biopsy was oriented with the thinner of the two cortices at the top of the microscopic field. Starting at the farthest left-hand margin of the endocortical surface of the uppermost cortex, the observer moved three fields to the right and three fields down. This randomly selected field was the first of four fields (two consecutive fields on top, followed by the two consecutive fields immediately below) that were analyzed with a 20× objective using the Osteomeasure analysis system (Osteometrics Inc., Decatur, IL) When a field was observed to have a significant artifact (e.g. a fold in the section), that field was excluded and the immediately adjacent field was analyzed. The number, perimeter, and area of adipocytes, and the field area in those sampled fields were measured and expressed as total adipocyte area (Ad.Ar; square millimeters), total adipocyte perimeter (Ad.Pm; in millimeters, measured by tracing each individual adipocyte in all of the fields analyzed and summing the individual values), adipocyte number (Ad.N) per square millimeter in the analyzed fields including marrow tissue area and bone area, percentage adipocyte volume per marrow volume (Ad.V/Ma.V), and adipocyte density (Ad.D; adipocyte number per square millimeter of marrow tissue area in the analyzed fields; no./mm2).
To assess representativeness of adipocyte parameters, we compared two different areas of the biopsy sample (the top, as previously described, and center) in 10 subjects. Correlation coefficients between adipocyte parameters between the two regions ranged from 0.65–0.90 (P = 0.0003–0.04).
Osteoblasts and osteoclasts were identified according to the criteria established by the ASBMR nomenclature committee (27) and expressed as number of osteoblasts (N.Ob) and number of osteoclasts (N.Oc) per millimeter of bone surface (no./mm).
Statistical analysis
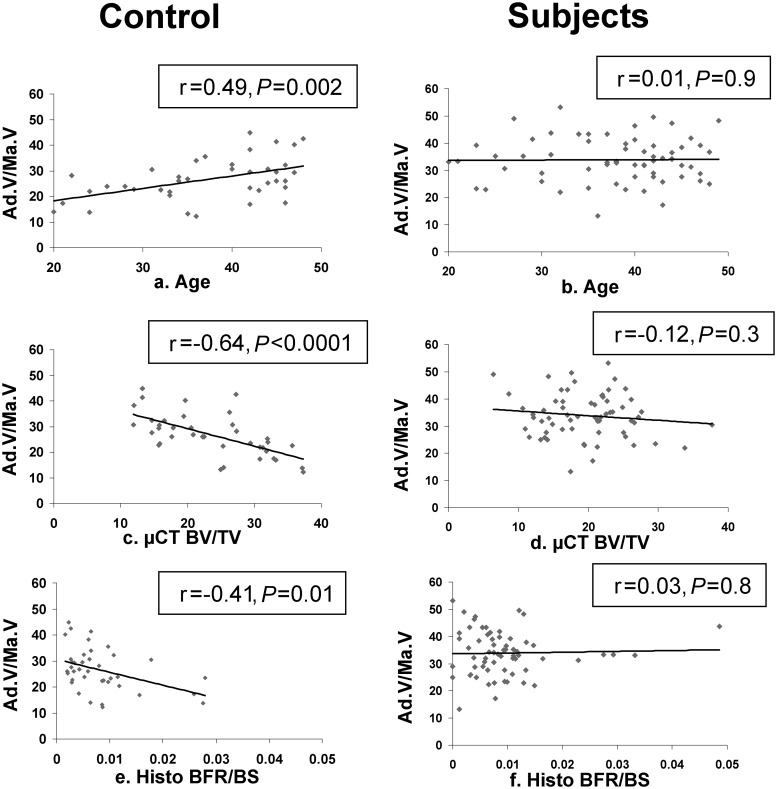
Statistical analyses were performed using SAS software (SAS Institute, Cary, NC). Between-group comparisons were conducted using Student's t tests. Multivariate logistic regression analyses were used to control for covariates [age, body mass index (BMI), BV/TV] that have previously described relationships with marrow adiposity (12, 28–30). Because many variables were not normally distributed (Kolmogorov-Smirnov test), Spearman correlation coefficients were calculated to test associations between variables. Because there were few differences between the Spearman and Pearson correlation coefficients, the latter are presented when regression lines are shown (see Fig. 3). All data are expressed as mean ± sd. Results were considered significant at P < 0.05.
Fig. 3.
Correlations (Pearson) between marrow adiposity (Ad.V/Ma.V) and age, BV/TV measured on biopsy specimens via μCT, and BFR/BS measured on biopsy specimens by histomorphometry.
Results
Clinical, densitometric, and histomorphometric characteristics
As in our previous reports, this study compares data from iliac crest bone biopsies performed in 64 affected premenopausal women to 40 premenopausal controls with normal aBMD and no history of adult low trauma fractures. Among the 64 affected subjects, 45 women had low trauma adult fractures (IOP), and 19 had low aBMD but no low trauma adult fractures (ILBMD). The number of fractures per IOP subject ranged from 1 to 12; 25 subjects had multiple fractures, ranging from 2 to 12. Eleven subjects had vertebral, 11 had rib, seven had hip, five had pelvic, 12 had forearm, six had humerus, six had lower leg, six had ankle, and nine had metatarsal fractures. The mean age at first adult fracture was 30 ± 9 yr, and the mean time between the evaluation and the most recent fracture was 4 ± 4 yr. Clinical, densitometric, and biochemical characteristics were recently reported in detail (4) and are presented in abbreviated form in Table 1. Because there were no differences between IOP and ILBMD groups in any histomorphometric or adipocyte parameters, the two groups were combined in a single group designated “affected subjects.”
Table 1.
Characteristics of the subjects and controls
| Controls | All affected subjects | IOP subjects | ILBMD subjects | |
|---|---|---|---|---|
| n | 40 | 64 | 45 | 19 |
| Anthropometric characteristics | ||||
| Age (yr) | 37.3 ± 8.2 | 37.8 ± 7.4 | 37.0 ± 7.7 | 39.6 ± 6.3 |
| Height (cm) | 165.4 ± 7.3 | 163.3 ± 6.8 | 163.8 ± 7.2 | 162.2 ± 5.7 |
| Weight (kg) | 70.7 ± 14.6 | 61.2 ± 13.9** | 63.0 ± 14.9* | 57.1 ± 10.2*** |
| BMI (kg/m2) | 25.8 ± 4.7 | 22.9 ± 4.6** | 23.4 ± 4.9* | 21.6 ± 3.5** |
| aBMD (g/cm2) | ||||
| Lumbar spine (L1–L4) | 1.099 ± 0.093 | 0.850 ± 0.129*** | 0.875 ± 0.139*** | 0.793 ± 0.078***,## |
| Total hip | 0.984 ± 0.075 | 0.776 ± 0.127*** | 0.792 ± 0.138*** | 0.736 ± 0.090*** |
| Femoral neck | 0.878 ± 0.075 | 0.659 ± 0.120*** | 0.677 ± 0.129*** | 0.615 ± 0.089*** |
| Distal radius (1/3) | 0.721 ± 0.051 | 0.686 ± 0.051*** | 0.692 ± 0.047** | 0.679 ± 0.045** |
| BMD Z score | ||||
| Lumbar spine (L1–L4) | 0.74 ± 0.88 | −1.55 ± 1.19*** | −1.33 ± 1.28*** | −2.06 ± 0.73***,## |
| Total hip | 0.48 ± 0.65 | −1.21 ± 1.02*** | −1.09 ± 1.12*** | −1.48 ± 0.69*** |
| Femoral neck | 0.39 ± 0.74 | −1.45 ± 1.06*** | −1.31 ± 1.15*** | −1.77 ± 0.76*** |
| Distal radius (1/3) | 0.68 ± 0.89 | 0.19 ± 0.83** | 0.26 ± 0.82* | 0.01 ± 0.85** |
| Body composition by DXA and serum leptin | ||||
| % Lean mass | 64.5 ± 7.2 | 66.3 ± 7.3 | 66.1 ± 7.8 | 66.7 ± 6.0 |
| % Body fat mass | 35.5 ± 7.2 | 33.7 ± 7.3 | 33.9 ± 7.8 | 33.3 ± 6.0 |
| % Trunk fat mass | 32.5 ± 8.9 | 30.0 ± 8.7 | 30.5 ± 9.3 | 28.9 ± 7.4 |
| % Trunk fat/% body fat | 90.5 ± 9.2 | 87.7 ± 9.3 | 88.3 ± 9.9 | 86.3 ± 7.7 |
| Leptin (ng/ml) | 17.7 ± 11.7 | 14.2 ± 12.1 | 15.5 ± 13.3 | 11.0 ± 7.6* |
| Bone turnover markers | ||||
| P1NP (μg/liter) | 47 ± 18 | 48 ± 18 | 47 ± 18 | 52 ± 18 |
| Osteocalcin (ng/ml) | 15 ± 8 | 16 ± 7 | 16 ± 8 | 16 ± 6 |
| CTx (ng/ml) | 0.287 ± 0.186 | 0.348 ± 0.175 | 0.354 ± 0.19 | 0.333 ± 0.13 |
| TRAP5b (U/liter) | 1.6 ± 1.0 | 2.3 ± 1.1** | 2.2 ± 1.2** | 2.3 ± 1.0** |
| Trabecular structure/stiffness: 3-dimensional μCT | ||||
| BV/TV (%) | 23.7 ± 7.7 | 19.7 ± 5.9** | 19.8 ± 6.5* | 19.3 ± 4.4** |
| Tb.N (n/mm) | 1.8 ± 0.4 | 1.5 ± 0.2*** | 1.6 ± 0.2*** | 1.5 ± 0.2*** |
| Tb.Th (μm) | 161 ± 38 | 165 ± 37 | 162 ± 39 | 171 ± 30 |
| Tb.Sp (μm) | 625 ± 61 | 710 ± 77*** | 697 ± 72*** | 741 ± 81***,# |
| Stiffness by μFE (E; MPa) | 545 ± 350 | 352 ± 203*** | 374 ± 222* | 302 ± 140** |
| Cancellous N.Ob and N.Oc (no. of cells/mm bone surface) | ||||
| N.Ob | 1.03 ± 0.57 | 1.18 ± 0.76 | 1.14 ± 0.74 | 1.27 ± 0.83 |
| N.Oc | 0.03 ± 0.02 | 0.04 ± 0.03 | 0.04 ± 0.03 | 0.04 ± 0.03 |
| Static and dynamic cancellous bone surface remodeling | ||||
| O.Wi (no. of lamellae) | 4.1 ± 1.0 | 3.6 ± 1.1* | 3.6 ± 1.0 | 3.7 ± 1.1 |
| W.Wi (μm) | 35.0 ± 3.6 | 33.8 ± 3.9 | 33.7 ± 3.8 | 33.7 ± 4.0 |
| Md.Pm (%) | 3.3 ± 2.6 | 4.0 ± 3.1 | 4.1 ± 3.5 | 3.7 ± 1.7 |
| MAR (μm/d) | 0.642 ± 0.090 | 0.628 ± 0.096 | 0.64 ± 0.09 | 0.59 ± 0.10 |
| BFR/BS (mm2/mm/yr) | 0.008 ± 0.007 | 0.009 ± 0.008 | 0.010 ± 0.009 | 0.008 ± 0.004 |
| Act.F (cycle/yr) | 0.291 ± 0.237 | 0.349 ± 0.269 | 0.365 ± 0.303 | 0.312 ± 0.164 |
P1NP, N-terminal propeptides of procollagen type 1; CTx, C-telopeptide; E, Young's modulus; MPa, megapascals.
P < 0.05 vs. controls;
P < 0.01 vs. controls;
P < 0.001 vs. controls.
P < 0.05, and
P < 0.01, IOP vs. ILBMD.
Affected subjects had lower BMI and serum leptin levels than controls, but there were no differences in body fat or lean mass measured by DXA. As expected, subjects had significantly lower aBMD than controls, and differences remained significant (P < 0.03) after controlling for BMI. As previously reported, most bone turnover markers were normal and did not differ between subjects and controls, or between IOP and ILBMD subjects (previously reported in Ref. 4). Serum tartrate-resistant acid phosphatase isoform 5b (TRAP5b) was normal, although significantly higher in subjects than controls. There were no differences between subjects and controls in terms of white blood cell count, hemoglobin, or hematocrit (data not shown).
As previously reported, subjects had substantially lower BV/TV by μCT and estimated stiffness by μFE than controls (Table 1) (5). Cancellous osteoid width (O.Wi) was 12% lower (P = 0.02) in subjects than controls. Mean wall width (W.Wi), which represents the average thickness of completed osteons, did not differ at the cancellous surface but was significantly lower in subjects at the endocortical and intracortical surfaces (5). Dynamic parameters of remodeling, such as bone formation rate/bone surface (BFR/BS) and mineral apposition rate (MAR), were heterogeneous and comparable between subjects and controls, as were N.Ob and N.Oc. Histomorphometric parameters were also indistinguishable between subjects with and without fractures (IOP vs. ILBMD; data not shown) (5).
Adipocyte measurements
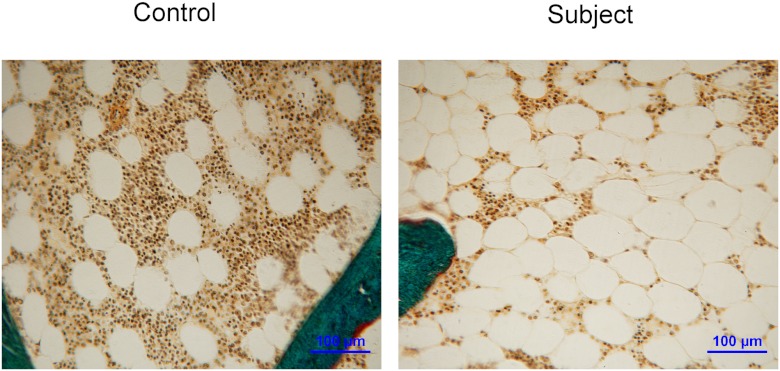
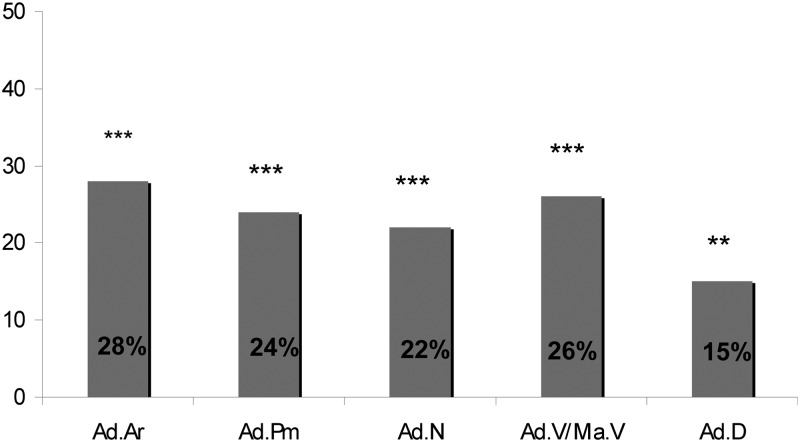
Biopsy specimens from all 64 subjects and 39 of 40 controls were suitable for adipocyte analyses. Adipocytes appear as distinct, translucent, yellow elliptical cells in the marrow cavity (Fig. 1). Ad.Ar, Ad.N, Ad.V/Ma.V, Ad.D, and Ad.Pm, a measure of adipocyte size, were substantially and significantly higher (by 15 to 28%; Fig. 2) in subjects than controls, both in unadjusted analyses and after adjustment for age and BMI (Table 2). Moreover, even after additional adjustment for BV/TV, between-group differences remained significant for all parameters except Ad.D. IOP and ILBMD subjects did not differ from each other in terms of any adipocyte parameter (data not shown).
Fig. 1.
Representative images of marrow adipocytes performed on bone biopsy samples from a control and an affected subject. Magnification, ×200.
Fig. 2.
Adipocyte parameters in the subjects: percentage difference from controls. Ad.Ar, Ad.Pm, Ad.N, Ad.V/Ma.V, and Ad.D are shown. **, P < 0.01; ***, P < 0.0001.
Table 2.
Marrow adiposity
| Controls (n = 39) | Subjects (n = 64) | P (unadjusted) | P (adjusted for age and BMI) | P (adjusted for age, BMI, BV/TV) | |
|---|---|---|---|---|---|
| Ad.Ar (mm2) | 0.25 ± 0.07 | 0.32 ± 0.08 | <0.0001 | <0.0001 | 0.001 |
| Ad.Pm (mm) | 25 ± 7 | 31 ± 7 | <0.0001 | 0.0004 | 0.007 |
| Ad.N (no./mm2) | 170 ± 43 | 207 ± 45 | <0.0001 | 0.003 | 0.04 |
| Ad.V/Ma.V (%) | 27 ± 8 | 34 ± 8 | <0.0001 | 0.0004 | 0.006 |
| Ad.D (no./mm2) | 193 ± 48 | 223 ± 46 | 0.003 | 0.04 | 0.2 |
Ad.N, Adipocyte number per square millimeter tissue area; Ad.D, adipocyte density (adipocyte number per square millimeter marrow tissue area).
In controls, Ad.N and Ad.V/Ma.V demonstrated the expected direct associations with age (Table 3 and Fig. 3A) and were not significantly associated with BMI or serum leptin. We found no significant relationships between serum IGF-I and measures of marrow adiposity. Although there were no significant relationships between marrow adipocyte parameters and aBMD at the spine and hip, there were highly significant, inverse relationships with BV/TV (Fig. 3C), trabecular microarchitecture, and estimated stiffness (Table 3), which remained significant after controlling for age (data not shown). In terms of remodeling, adipocyte parameters were inversely correlated with serum bone turnover markers and certain dynamic indices of bone formation, including mineralized perimeter (Md.Pm), BFR/BS (Fig. 3E), and activation frequency (Act.F), but not with static remodeling parameters (O.Wi, W.Wi, N.Ob and N.Oc; Table 3).
Table 3.
Spearman correlations (r) between adipocyte parameters and subject characteristics in subjects and controls
| Controls |
Subjects |
|||||
|---|---|---|---|---|---|---|
| Ad.N | Ad.V/Ma.V | Ad.D | Ad.N | Ad.V/Ma.V | Ad.D | |
| Clinical characteristics | ||||||
| Age | 0.35a | 0.46b | 0.22 | 0.08 | −0.002 | 0.09 |
| BMI | −0.03 | 0.27 | −0.08 | −0.24 | −0.09 | −0.28a |
| Body composition by DXA | ||||||
| % Body fat | 0.12 | 0.40a | 0.12 | −0.24 | 0.05 | −0.25a |
| % Trunk fat | 0.10 | 0.37a | 0.09 | −0.20 | 0.10 | −0.24a |
| % Trunk fat/% body fat | −0.01 | 0.25 | −0.04 | −0.09 | 0.14 | −0.16 |
| aBMD | ||||||
| Lumbar spine | 0.06 | −0.06 | 0.01 | −0.20 | −0.02 | −0.20 |
| Total hip | 0.09 | 0.01 | 0.04 | −0.23 | −0.27a | −0.25a |
| Femoral neck | 0.17 | 0.03 | 0.09 | −0.23 | −0.16 | −0.21 |
| Bone turnover markers, IGF-I, and leptin | ||||||
| P1NP | −0.30 | −0.40a | −0.24 | −0.07 | −0.12 | −0.07 |
| Osteocalcin | −0.23 | −0.42b | −0.13 | 0.08 | 0.04 | 0.09 |
| CTx | −0.32a | −0.42b | −0.22 | −0.10 | −0.19 | −0.07 |
| TRAP5b | −0.25 | −0.35a | −0.15 | 0.03 | −0.07 | 0.09 |
| IGF-I | −0.19 | −0.19 | −0.06 | 0.10 | 0.08 | 0.07 |
| Leptin | 0.10 | 0.29 | 0.12 | −0.15 | 0.13 | −0.21 |
| Microstructure and stiffness by μCT and μFE of biopsy specimens | ||||||
| BV/TV | −0.57c | −0.66c | −0.49b | −0.04 | −0.03 | 0.05 |
| Tb.N | −0.59c | −0.63c | −0.50b | −0.13 | −0.08 | −0.03 |
| Tb.Th | −0.50b | −0.59c | −0.45b | −0.06 | −0.08 | −0.03 |
| Tb.Sp | 0.42b | 0.40a | 0.32 | 0.14 | 0.09 | 0.05 |
| Stiffness (E) | −0.60c | −0.70c | −0.49b | −0.08 | −0.11 | 0.02 |
| Cancellous N.Ob and N.Oc | ||||||
| N.Ob | −0.29 | −0.21 | −0.20 | 0.06 | 0.14 | 0.12 |
| N.Oc | −0.14 | −0.06 | −0.10 | −0.10 | −0.10 | −0.05 |
| Bone remodeling by histomorphometry at the cancellous surface | ||||||
| O.Wi | −0.14 | −0.07 | −0.07 | 0.04 | 0.07 | 0.15 |
| W.Wi | −0.18 | −0.17 | −0.14 | −0.17 | −0.16 | −0.12 |
| Md.Pm | −0.33a | −0.45b | −0.28 | 0.03 | −0.05 | 0.12 |
| MAR | −0.16 | −0.12 | −0.10 | −0.06 | −0.07 | −0.08 |
| BFR/BS | −0.33a | −0.42b | −0.28 | 0.00 | −0.07 | 0.08 |
| Act.F | −0.31 | −0.40a | −0.26 | 0.03 | −0.04 | 0.11 |
P1NP, N-terminal propeptides of procollagen type 1; CTx, C-telopeptide.
P < 0.05;
P < 0.01;
P < 0.001.
In contrast, none of the expected relationships observed in controls were detected in affected subjects (Table 3 and Fig. 3). Adipocyte parameters did not correlate with age (Fig. 3B), BV/TV (Fig. 3D), estimated stiffness, bone turnover markers, or static or dynamic indices of remodeling, including BFR/BS (Fig. 3F). Ad.D was inversely associated with BMI, body fat, and total hip aBMD in subjects and not in controls (Table 3). Serum and urinary calcium, PTH, vitamin D metabolites, free estradiol, and age-adjusted IGF-I did not correlate with adipocyte parameters in either subjects or controls (data not shown).
N.Ob correlated directly with BFR/BS in subjects (r = 0.53; P = 0.0005) and controls (r = 0.49; P < 0.0001) and tended to be inversely associated with Ad.N in controls (r = −0.29; P = 0.07). No relationships were found between adipocyte indices and N.Ob or N.Oc in subjects (Table 3).
Adipocyte parameters in subgroups based on bone formation rate
We next compared marrow adipocyte parameters between those subjects in the lowest and highest tertiles of BFR/BS. As expected, compared with the high turnover group, those in the low turnover group had significantly lower N.Ob (0.71 ± 0.28 vs. 1.53 ± 1.07 no./mm; P < 0.01) and N.Oc (0.02 ± 0.02 vs. 0.05 ± 0.04 no./mm; P < 0.01). Although the low turnover group had significantly lower bone area (0.137 ± 0.049 vs. 0.173 ± 0.039 mm2; P = 0.01) and reciprocally higher marrow space area (0.956 ± 0.049 vs. 0.920 ± 0.039 mm2; P = 0.01) than the high turnover group, no marrow adiposity parameter differed between the groups.
Discussion
To our knowledge, this is the only study of adipocyte parameters in transiliac bone biopsy specimens from premenopausal women with IOP and ILBMD. Because higher marrow adiposity has been found in other populations with osteoporosis, we hypothesized that marrow fat would be higher in women with IOP and ILBMD than normal women. Consistent with this hypothesis, we found substantially higher Ad.Ar, Ad.Pm, Ad.N, Ad.V/Ma.V, and Ad.D in affected subjects compared with healthy premenopausal controls, indicating that both the number and the size of adipocytes were increased. Also, consistent with our previous work (5, 6), adipocyte parameters were similarly elevated, whether or not the subjects had low aBMD (ILBMD) or had low trauma fractures (IOP). Additionally, we hypothesized that adipocyte parameters would be inversely related to bone volume fraction (BV/TV), and histomorphometric indices of microarchitecture and bone formation. However, whereas these expected inverse relationships were observed in normal premenopausal women, we found no relationships between marrow adipocytes and indices of bone volume and microarchitecture and remodeling in affected subjects. Moreover, whereas N.Ob and N.Oc were directly and significantly related to BFR/BS in the subjects, adipocyte parameters bore no relationship to BFR/BS or to N.Ob or N.Oc and did not differ between subjects with low turnover and high turnover. This lack of expected relationships between marrow adiposity and bone structural and remodeling parameters in the subjects suggests that the relationships between osteoblasts and adipocytes are abnormal in premenopausal women with IOP and ILBMD. Moreover, the fact that differences in adipocyte parameters between subjects and controls remained significant after controlling for the lower BV/TV in the subjects suggests that the increased marrow fat is not simply because there is less bone but rather is independent of the lower trabecular BV/TV.
Within the bone marrow microenvironment, adipocytes and osteoblasts descend from the same bipotential mesenchymal stem cell. The mechanisms by which the mesenchymal progenitor cell develops into adipocytes or osteoblasts are complex and have been extensively reviewed elsewhere (31–33). However, two major transcription factors play a central role in this process—peroxisome proliferator-activated receptor-γ2 (PPAR-γ2) for adipogenesis, and Runx2 (runt-related transcription factor 2) for osteoblastogenesis. With aging, expression of PPAR-γ2 increases in the bone marrow (34). Histological studies in humans document that vertebral bone volume decreases and vertebral marrow fat increases with aging (12, 30). In humans, marrow fat begins to increase before the third decade of life, before age-related decreases in bone mass (29). Conversely, multiple studies have shown that aging is also associated with a decrease in bone formation (35). Whether the age-related declines in bone formation are due to decreased Runx2 expression or shifts from the osteoblast to the adipocyte cell lineage is uncertain. However, such shifts have been postulated to play a role in the pathogenesis of other forms of bone loss. Thiazolidinediones (PPAR-γ agonists) are associated with bone loss and increased fracture risk by a mechanism that may be related to increased marrow adiposity and suppression of osteoblastogenesis (10). The pathogenesis of glucocorticoid-induced osteoporosis may also be related to shifts from osteoblastic toward adipocyte pathways because glucocorticoids induce bone loss via favoring osteoblast apoptosis and also increase marrow adiposity (11).
Our finding that marrow fat was higher in premenopausal women with IOP and ILBMD than controls with normal BMD and no fractures was not unexpected. Previous bone biopsy studies have reported that patients with osteoporosis have increased adipocyte parameters (12–14). In a histomorphometric study of male and female, pediatric and adult subjects with varying forms of osteoporosis, Verma et al. (14) reported increased adipocyte parameters that were inversely associated with parameters of bone formation, supporting the hypothesis of a switch from the osteoblast to the adipocyte lineage in osteoporosis. Similarly, women with anorexia nervosa, a disorder characterized by suppressed bone formation, have greater lumbar and femoral marrow fat as measured by magnetic resonance spectroscopy than controls, and marrow fat is inversely associated with BMD (16). An inverse relationship between bone mass and marrow fat has also been demonstrated both in Chinese men with osteoporosis (36) and in healthy Caucasian women by magnetic resonance spectroscopy (37). In postmenopausal women with osteoporosis and excess marrow fat accumulation, a study examining bone biopsy specimens from estrogen- and placebo-treated postmenopausal women found that treatment with estrogen decreased adipocyte volume and prevented increases in adipocyte number and size (15). Our results in premenopausal women are consistent with the literature reporting increased marrow adiposity in patients with osteoporosis.
Our affected subjects differed in several interesting ways from normal premenopausal women. First, consistent with the known direct relationship between marrow fat and age, adipocyte parameters were positively associated with age in controls. In contrast, marrow fat was not associated with age in the subjects, although mean age and the range of ages were the same in subjects and controls. Second, consistent with the known inverse relationship between marrow fat and bone volume, adipocyte parameters were negatively associated with BV/TV, trabecular number, separation and thickness, and bone stiffness in normal women. In contrast, no such relationships were detected in women with IOP and ILBMD, despite the fact that there was considerable variability in these parameters within the subject group. Moreover, the difference in most of the adipocyte parameters between subjects and controls persisted after controlling for age, BMI, and BV/TV. Third, the normal women exhibited inverse relationships between adipocyte parameters and dynamic histomorphometric parameters of bone formation and serum bone turnover markers, as would be expected given the reciprocal relationship between marrow adipogenesis and osteoblastogenesis. Yet, in the affected subjects, there was no relationship, direct or inverse, between adipocyte parameters and remodeling, despite considerable heterogeneity in bone remodeling activity. Thus, whatever the specific pathogenesis of IOP/ILBMD may be, the normal relationships between marrow fat and bone appear to be disrupted.
Prior studies have shown that both obesity (28) and undernutrition/anorexia nervosa (16) are associated with higher marrow adiposity in premenopausal women. Consistent with these studies, we found that marrow adiposity was associated with lower body fat in the (lower weight) subjects whereas the reciprocal relationship was present in the (higher weight) controls. Because weight and BMI varied significantly between subjects and controls, this may have allowed us to observe different ends of the previously described (16, 28) and apparently U-shaped relationship between body fat and marrow adiposity.
Taken together, the differing relationships between normal premenopausal women and women with IOP/ILBMD with respect to adipocyte parameters and age, BMI, bone volume, and remodeling suggest that the affected subjects have a distinct bone physiology. The lack of relationships between bone formation parameters and adipocytes in the subjects, however, suggests that, in at least this form of osteoporosis, excess marrow adiposity may not arise from a switch from the osteoblast to the adipocyte cell line. Such a possibility is supported by animal studies that also suggest that bone marrow adipogenesis and osteoblastogenesis can be regulated independently (19–21). At present, it is unclear whether increased marrow fat in premenopausal women with osteoporosis alters bone metabolism in a manner that promotes bone loss or osteoporosis, develops in response to an alteration in bone metabolism, or is simply an epiphenomenon that reflects a decrease in bone volume, but not bone formation.
This study has several limitations. We included women in their twenties who may not have reached peak bone mass. The findings of lower vBMD, disrupted microarchitecture, lower estimated stiffness, and higher marrow fat in our subjects with ILBMD may not be generalizable to the larger population of women with low aBMD. The slightly lower osteoid width in the subjects may have affected μFE results because μFE assumptions include uniform mineralization of osteoid. Analyses comparing subgroups based upon BFR/BS involved small sample sizes; this may have limited our ability to detect between-group differences. Additionally, these studies examined marrow adiposity only at the iliac crest, and it is not known whether these results are generalizable to other skeletal locations. We are currently conducting studies to investigate this issue.
In summary, compared with normal premenopausal controls, premenopausal women with IOP and ILBMD have evidence of excess marrow adiposity independent of age, BMI, and bone mass and unrelated to bone formation and remodeling, whether assessed by serum bone turnover markers or dynamic histomorphometry. It is unclear whether the excess fat is an innocent bystander or is in some way related to the pathogenesis of IOP and/or ILBMD. Further studies will be necessary to elucidate whether and how osteoblast and adipocyte differentiation pathways may contribute to the pathogenesis of this condition.
Acknowledgments
This work was supported by the following National Institutes of Health funding sources: R01 AR049896 (to E.S.), K24 AR 05266 (to E.S.), and K23 AR054127 (to A.C.).
Disclosure Summary: All authors state that they have nothing to disclose.
Footnotes
- aBMD
- Areal BMD
- Act.F
- activation frequency
- Ad.Ar
- adipocyte area
- Ad.D
- adipocyte density
- Ad.Pm
- adipocyte perimeter
- Ad.V/Ma.V
- adipocyte volume/marrow volume
- BFR/BS
- bone formation rate/bone surface
- BMD
- bone mineral density
- BMI
- body mass index
- BV/TV
- bone volume/total volume fraction
- μCT
- micro-computed tomography
- DXA
- dual-energy x-ray absorptiometry
- μFE
- micro-finite element analysis
- ILBMD
- idiopathic low BMD
- IOP
- idiopathic osteoporosis
- MAR
- mineral apposition rate
- Md.Pm
- mineralized perimeter
- N.Ob
- number of osteoblasts
- N.Oc
- number of osteoclasts
- 25-OHD
- 25-hydroxyvitamin D
- O.Wi.
- osteoid width
- PPAR-γ2
- peroxisome proliferator-activated receptor-γ2
- Tb.N
- trabecular number
- Tb.Sp
- trabecular separation
- Tb.Th
- trabecular thickness
- TRAP5b
- tartrate-resistant acid phosphatase isoform 5b
- vBMD
- volumetric BMD
- W.Wi.
- wall width.
References
- 1. Cohen A, Fleischer J, Freeby MJ, McMahon DJ, Irani D, Shane E. 2009. Clinical characteristics and medication use among premenopausal women with osteoporosis and low BMD: the experience of an osteoporosis referral center. J Womens Health (Larchmt) 18:79–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cohen A, Shane E. 2008. Premenopausal osteoporosis. In: Rosen CJ, ed. Primer on the metabolic bone diseases and other disorders of bone and mineral metabolism. Washington, DC: American Society for Bone and Mineral Research; 289–293 [Google Scholar]
- 3. Heshmati HM, Khosla S. 1998. Idiopathic osteoporosis: a heterogeneous entity. Ann Med Interne (Paris) 149:77–81 [PubMed] [Google Scholar]
- 4. Cohen A, Recker RR, Lappe J, Dempster DW, Cremers S, McMahon DJ, Stein EM, Fleischer J, Rosen CJ, Rogers H, Staron RB, Lemaster J, Shane E. 2012. Premenopausal women with idiopathic low-trauma fractures and/or low bone mineral density. Osteoporos Int 23:171–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cohen A, Dempster DW, Recker RR, Stein EM, Lappe JM, Zhou H, Wirth AJ, van Lenthe GH, Kohler T, Zwahlen A, Müller R, Rosen CJ, Cremers S, Nickolas TL, McMahon DJ, Rogers H, Staron RB, LeMaster J, Shane E. 2011. Abnormal bone microarchitecture and evidence of osteoblast dysfunction in premenopausal women with idiopathic osteoporosis. J Clin Endocrinol Metab 96:3095–3105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cohen A, Liu XS, Stein EM, McMahon DJ, Rogers HF, Lemaster J, Recker RR, Lappe JM, Guo XE, Shane E. 2009. Bone microarchitecture and stiffness in premenopausal women with idiopathic osteoporosis. J Clin Endocrinol Metab 94:4351–4360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cohen A, Lang T, Rogers H, Stein E, Guo XE, Liu XS, Dempster D, McMahon DJ, Lappe JM, Zhang C, Recker R, Shane E. 2010. Central QCT reveals cortical and trabecular structural deficits in premenopausal women with idiopathic osteoporosis whether diagnosis is based on fragility fracture or low areal bone mineral density. Proc 32nd Annual Meeting of American Society for Bone and Mineral Research, Toronto, 2010, p 1128 (Abstract) [Google Scholar]
- 8. Rosen CJ, Bouxsein ML. 2006. Mechanisms of disease: is osteoporosis the obesity of bone? Nat Clin Pract Rheumatol 2:35–43 [DOI] [PubMed] [Google Scholar]
- 9. Lecka-Czernik B, Moerman EJ, Grant DF, Lehmann JM, Manolagas SC, Jilka RL. 2002. Divergent effects of selective peroxisome proliferator-activated receptor-γ2 ligands on adipocyte versus osteoblast differentiation. Endocrinology 143:2376–2384 [DOI] [PubMed] [Google Scholar]
- 10. Grey A. 2009. Thiazolidinedione-induced skeletal fragility—mechanisms and implications. Diabetes Obes Metab 11:275–284 [DOI] [PubMed] [Google Scholar]
- 11. Canalis E, Mazziotti G, Giustina A, Bilezikian JP. 2007. Glucocorticoid-induced osteoporosis: pathophysiology and therapy. Osteoporos Int 18:1319–1328 [DOI] [PubMed] [Google Scholar]
- 12. Meunier P, Aaron J, Edouard C, Vignon G. 1971. Osteoporosis and the replacement of cell populations of the marrow by adipose tissue. A quantitative study of 84 iliac bone biopsies. Clin Orthop Relat Res 80:147–154 [DOI] [PubMed] [Google Scholar]
- 13. Justesen J, Stenderup K, Ebbesen EN, Mosekilde L, Steiniche T, Kassem M. 2001. Adipocyte tissue volume in bone marrow is increased with aging and in patients with osteoporosis. Biogerontology 2:165–171 [DOI] [PubMed] [Google Scholar]
- 14. Verma S, Rajaratnam JH, Denton J, Hoyland JA, Byers RJ. 2002. Adipocytic proportion of bone marrow is inversely related to bone formation in osteoporosis. J Clin Pathol 55:693–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Syed FA, Oursler MJ, Hefferanm TE, Peterson JM, Riggs BL, Khosla S. 2008. Effects of estrogen therapy on bone marrow adipocytes in postmenopausal osteoporotic women. Osteoporos Int 19:1323–1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bredella MA, Fazeli PK, Miller KK, Misra M, Torriani M, Thomas BJ, Ghomi RH, Rosen CJ, Klibanski A. 2009. Increased bone marrow fat in anorexia nervosa. J Clin Endocrinol Metab 94:2129–2136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schellinger D, Lin CS, Hatipoglu HG, Fertikh D. 2001. Potential value of vertebral proton MR spectroscopy in determining bone weakness. AJNR Am J Neuroradiol 22:1620–1627 [PMC free article] [PubMed] [Google Scholar]
- 18. Schellinger D, Lin CS, Lim J, Hatipoglu HG, Pezzullo JC, Singer AJ. 2004. Bone marrow fat and bone mineral density on proton MR spectroscopy and dual-energy x-ray absorptiometry: their ratio as a new indicator of bone weakening. AJR Am J Roentgenol 183:1761–1765 [DOI] [PubMed] [Google Scholar]
- 19. Justesen J, Mosekilde L, Holmes M, Stenderup K, Gasser J, Mullins JJ, Seckl JR, Kassem M. 2004. Mice deficient in 11β-hydroxysteroid dehydrogenase type 1 lack bone marrow adipocytes, but maintain normal bone formation. Endocrinology 145:1916–1925 [DOI] [PubMed] [Google Scholar]
- 20. Martin RB, Zissimos SL. 1991. Relationships between marrow fat and bone turnover in ovariectomized and intact rats. Bone 12:123–131 [DOI] [PubMed] [Google Scholar]
- 21. Tornvig L, Mosekilde LI, Justesen J, Falk E, Kassem M. 2001. Troglitazone treatment increases bone marrow adipose tissue volume but does not affect trabecular bone volume in mice. Calcif Tissue Int 69:46–50 [DOI] [PubMed] [Google Scholar]
- 22. Dempster DW, Shane E. 2002. Bone quantification and dynamics of bone turnover. In: Becker KL, ed. Principles and practice of endocrinology and metabolism. Philadelphia: J. B. Lippincott Co.; 475–479 [Google Scholar]
- 23. Rüegsegger P, Koller B, Müller R. 1996. A microtomographic system for the nondestructive evaluation of bone architecture. Calcif Tissue Int 58:24–29 [DOI] [PubMed] [Google Scholar]
- 24. Cohen A, Dempster DW, Müller R, Guo XE, Nickolas TL, Liu XS, Zhang XH, Wirth AJ, van Lenthe GH, Kohler T, McMahon DJ, Zhou H, Rubin MR, Bilezikian JP, Lappe JM, Recker RR, Shane E. 2010. Assessment of trabecular and cortical architecture and mechanical competence of bone by high-resolution peripheral computed tomography: comparison with transiliac bone biopsy. Osteoporos Int 21:263–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hildebrand T, Laib A, Müller R, Dequeker J, Rüegsegger P. 1999. Direct three-dimensional morphometric analysis of human cancellous bone: microstructural data from spine, femur, iliac crest, and calcaneus. J Bone Miner Res 14:1167–1174 [DOI] [PubMed] [Google Scholar]
- 26. Arbenz P, van Lenthe GH, Mennel U, Müller R, Sala M. 2008. A scalable multi-level preconditioner for matrix-free micro-finite element analysis of human bone structures. Int J Numer Meth Engrg 73:927–947 [Google Scholar]
- 27. Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR. 1987. Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res 2:595–610 [DOI] [PubMed] [Google Scholar]
- 28. Bredella MA, Torriani M, Ghomi RH, Thomas BJ, Brick DJ, Gerweck AV, Rosen CJ, Klibanski A, Miller KK. 2011. Vertebral bone marrow fat is positively associated with visceral fat and inversely associated with IGF-1 in obese women. Obesity (Silver Spring) 19:49–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Di Iorgi N, Rosol M, Mittelman SD, Gilsanz V. 2008. Reciprocal relation between marrow adiposity and the amount of bone in the axial and appendicular skeleton of young adults. J Clin Endocrinol Metab 93:2281–2286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dunnill MS, Anderson JA, Whitehead R. 1967. Quantitative histological studies on age changes in bone. J Pathol Bacteriol 94:275–291 [DOI] [PubMed] [Google Scholar]
- 31. Kawai M, Rosen CJ. 2010. PPARγ: a circadian transcription factor in adipogenesis and osteogenesis. Nat Rev Endocrinol 6:629–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rosen CJ, Ackert-Bicknell C, Rodriguez JP, Pino AM. 2009. Marrow fat and the bone microenvironment: developmental, functional, and pathological implications. Crit Rev Eukaryot Gene Expr 19:109–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ng A, Duque G. 2010. Osteoporosis as a lipotoxic disease. IBMS BoneKEy 7:103–107 [Google Scholar]
- 34. Moerman EJ, Teng K, Lipschitz DA, Lecka-Czernik B. 2004. Aging activates adipogenic and suppresses osteogenic programs in mesenchymal marrow stroma/stem cells: the role of PPAR-γ2 transcription factor and TGF-β/BMP signaling pathways. Aging Cell 3:379–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kassem M, Marie PJ. 2011. Senescence-associated intrinsic mechanisms of osteoblast dysfunctions. Aging Cell 10:191–197 [DOI] [PubMed] [Google Scholar]
- 36. Griffith JF, Yeung DK, Antonio GE, Lee FK, Hong AW, Wong SY, Lau EM, Leung PC. 2005. Vertebral bone mineral density, marrow perfusion, and fat content in healthy men and men with osteoporosis: dynamic contrast-enhanced MR imaging and MR spectroscopy. Radiology 236:945–951 [DOI] [PubMed] [Google Scholar]
- 37. Shen W, Chen J, Punyanitya M, Shapses S, Heshka S, Heymsfield SB. 2007. MRI-measured bone marrow adipose tissue is inversely related to DXA-measured bone mineral in Caucasian women. Osteoporos Int 18:641–647 [DOI] [PMC free article] [PubMed] [Google Scholar]