Abstract
Context:
Male hormonal contraception (MHC) combines hypothalamic-pituitary-gonadal axis blockade with exogenous androgen delivery to maintain extragonadal androgen end-organ effects. Concern exists that MHC may adversely impact prostate health.
Objective:
The objective of the study was to determine the molecular impact of MHC on intraprostatic androgen concentrations and androgen action.
Design:
This was a single-blind, randomized, placebo-controlled study.
Setting:
The study was conducted at an academic medical center.
Participants:
32 healthy men aged 25–55 yr participated in the study.
Intervention:
Interventions included placebo, daily transdermal testosterone (T) (T-gel), T-gel + depomedroxyprogesterone acetate (T+DMPA), or T-gel + dutasteride daily (T+D) for 12 wk, and prostate biopsy during treatment wk 10.
Main Outcome Measures:
Serum and prostate androgen concentrations and prostate epithelial-cell gene expression were measured.
Results:
Thirty men completed the study. Serum T levels were significantly increased in T-gel and T+D groups compared with baseline (P < 0.05) but were decreased with the addition of DMPA. Intraprostatic androgens were no different from placebo with T-gel treatment. Addition of DMPA to T resulted in 40% lower intraprostatic dihydrotestosterone (DHT) concentration (P = 0.0273 vs. placebo), whereas combining dutasteride with T resulted in a 90% decrease in intraprostatic DHT (P = 0.0012), 11-fold increased intraprostatic T (P = 0.0011), and 7-fold increased intraprostatic androstenedione (P = 0.0011). Significant differences in global or androgen-regulated prostate epithelial-cell gene expression were not observed. Androgen-regulated gene expression correlated with epithelial-cell androgen receptor and prostatic DHT in placebo, T-gel, and T+DMPA arms and with T and androstenedione levels in the T+D arm.
Conclusions:
MHC regimens do not markedly alter gene expression in benign prostate epithelium, suggesting they may not alter risk of prostate disease. Longer-term studies examining the impact of MHC on prostate health are needed.
Unplanned pregnancies remain a critical public health and economic problems worldwide. Male contraceptive options are extremely limited, with condoms the only truly reversible option. Male hormonal contraception (MHC) relies on hypothalamic-pituitary-gonadal axis blockade combined with exogenous androgen delivery to maintain extragonadal androgen end-organ effects. MHC regimens generally include an exogenous androgen plus a progestin, the latter to maximize gonadotropin suppression (1). MHC are currently in phase II trials (1, 2), achieving efficacy rates of 90–95%. In a proof-of-principle study, long-acting testosterone (T) pellets plus the potent progestin depomedoxyprogesterone acetate (DMPA) demonstrated contraceptive efficacy in 55 couples (3). Consistent with these results, our group has demonstrated that daily transdermal T-gel (1%, 10 g/d) combined with DMPA effectively inhibits spermatogenesis to levels consistent with contraceptive efficacy (≤1 million/ml) in 80–90% of men (4). Moreover, ongoing work from our group suggests the combination of T plus dutasteride, a 5α-reductase inhibitor, may be an effective combination for prostate-sparing androgen delivery in a MHC regimen (5–7). This is of particular interest because long-term use of 5α-reductase inhibitors has been associated with reductions in prostate cancer incidence, albeit among concerns of unclear significance regarding a possible increased detection of high-grade cancers (8).
Although short-term side effects from MHC regimens are minimal, it is possible that long-term hormonal manipulation might impact risk for hormonally sensitive diseases. For example, long-term estrogen plus progestin contraceptive use in women decreases the risk of ovarian and endometrial cancer yet increases the risk of cervical malignancy (9). In this respect, there is a paucity of data regarding absolute risks of androgen manipulation on the prostate, which is androgen dependent for both development and maintenance (10). Importantly, emerging data suggest that alterations in serum androgens do not necessarily result in parallel changes in prostate androgen levels or tissue androgen activity. In older men with prostate disease or hypogonadism, recent studies demonstrate that serum and tissue hormone levels are not equivalent (11–17). Furthermore, in healthy young and middle-aged men, such as those who might use a MHC, serum androgen manipulation via either castration or administration of exogenous dihydrotestosterone (DHT) does not directly correlate with equivalent changes in prostate androgen concentrations (16, 18). Similarly, short-term manipulation of serum androgen in these studies was not associated with changes in apoptosis, cell proliferation, or gene expression in prostate epithelial cells (from which prostate cancers arise).
Because MHC might be used over many years, even small changes in prostate epithelial cell biology could have profound implications for prostate health over a lifetime. The objective of this study was to determine the potential molecular impact of androgen and progestin regimens used in MHC on the prostate androgen axis by examining the in vivo response to hormonal manipulation directly in the tissue of interest. We conducted a 3-month, four-arm, placebo-controlled, single-blind, intervention trial examining the impact of transdermal T alone, or in combination with dutasteride or DMPA, on prostate tissue androgen levels and androgen-regulated gene expression. An improved understanding of the molecular consequences of androgen manipulation within the human prostate will help guide the design, safety monitoring, and selection of MHC agents and provide valuable insights into human prostate biology.
Materials and Methods
Subjects
Healthy male volunteers, 25–55 yr old, were recruited via advertisement and gave written informed consent before screening. All study procedures took place at the University of Washington Medical Center (Seattle WA) and were approved by the institutional review board. Inclusion criteria included general good health, normal serum total T level (3–11 ng/ml) and gonadotropins, serum prostate-specific antigen (PSA) less than 2.0 ng/ml, International Prostate Symptom Score less than 10, normal seminal fluid analysis (≥15 million sperm/ml, >50% normal motility, >15% normal forms), digital rectal examination, and transrectal prostate ultrasound. Exclusion criteria included a history of prostate or breast cancer, therapy for benign prostatic hyperplasia, history of urinary retention, current or past treatment with a 5α-reductase inhibitor, or a history of anti/androgenic drug administration that might interfere with steroid metabolism within the past 3 months.
Study design and randomization
After screening, subjects were assigned by the investigational drug pharmacist using a random block allocation (block size = 8) to the following treatment groups: 1) PLACEBO: daily placebo gel + daily placebo pill + placebo injection (once); 2) TESTOSTERONE (T): 1% transdermal T gel 10 g daily (Testim; Auxilium Pharmaceuticals, Malvern, PA) + daily placebo pill + placebo injection; 3) T+DUTASTERIDE (T+D): 1% transdermal T gel 10 g daily + daily dutasteride (GlaxoSmithKline, Research Triangle Park, NC) 0.5 mg pill + placebo injection; or 4) T+DMPA: 1% transdermal T gel 10 g daily + daily placebo pill + im DMPA 300 mg (once) (Upjohn Pharmaceuticals, Kalamazoo, MI). Subjects received 12 wk of the assigned treatment. Subjects were asked to apply their treatment gel at the same time every day, after showering.
International Prostate Symptom Score questionnaires (19) and blood for serum hormone measurements and chemistries were collected monthly. Of note, subjects were instructed not to apply their treatment gel on the morning before their study visits. A transrectal prostate ultrasound was performed at baseline, and at wk 10 subjects underwent an ultrasound-guided prostate biopsy using local anesthetic (1% lidocaine). Using an18-gauge needle, 10 prostate core biopsies were procured, snap frozen in liquid nitrogen, and stored at −70 C or formalin fixed. A third prostate ultrasound for size quantification, as well as a blood collection and examination, were performed 6–8 wk after the cessation of the drug treatment (recovery).
Serum hormone assays and safety laboratory tests
Serum androgens were measured by liquid chromatography-tandem mass spectrometry as described previously (20). The lower limit of quantification for each androgen using this assay was 0.01 ng/ml using 100 μl of serum. The intraassay coefficients of variation were: T, 4.9%; DHT, 4.4%; dehydroepiandrosterone (DHEA), 7.6%; and androstenedione, 3.5%. Serum LH, FSH, and SHBG were quantified by immunofluorometric assay and estradiol by RIA (20). For estradiol, the intraassay coefficient of variation was 9%. Samples for all subjects were measured in one assay in duplicate. Safety laboratories including PSA, serum chemistries, and complete blood counts were measured in the clinical laboratory at the University of Washington Medical Center (Seattle, WA).
Tissue hormone assays
Prostate tissue androgen concentrations were measured using liquid chromatography-tandem mass spectrometry as described previously (21, 22). For each subject, two separate prostate core biopsies were thawed, homogenized, and individually extracted. Individual core concentrations for each androgen were averaged for each subject. Cores weighed an average of 5.2 ± 1.3 mg.
Microarray hybridization and quantitative RT-PCR (qRT-PCR)
Flash-frozen prostate biopsy cores were used for laser capture microdissection of prostate epithelial glands, RNA isolation and amplification, and microarray hybridization as previously described (23). For global expression profiling, 44,000 whole human genome expression oligomicroarray slides were used (Agilent Technologies, Inc., Santa Clara, CA) (18). Fluorescence array images were collected (Agilent DNA oligo-array scanner G2565BA, Agilent Technologies), and Agilent Feature Extraction software used to normalize data. Spots of poor quality or low average intensity levels (<300) were removed from further analysis. Microarray data can be accessed via the Gene Expression Omnibus database (accession number pending). cDNA was generated in a random-primed reverse transcription reaction, and qRT-PCR was performed using primers and reaction conditions previously described (18).
Statistical analyses
Statistical analyses of hormone data were performed using STATA version 10 (State Corp., College Station, TX). Because hormone results were nonnormally distributed, nonparametric statistics were used. Comparison of results between groups at a given time point were made using a Kruskal-Wallis ANOVA with a Wilcoxon rank-sum post hoc test, without corrections for multiplicity. Comparisons of results from baseline within a group were made using a Wilcoxon sign rank test. For all comparisons, a P < 0.05 was considered significant.
For microarray data, unsupervised hierarchical average linkage clustering was performed using Cluster 3.0 software (http://bonsai.ims.u-tokyo.ac.jp/∼mdehoon/software/cluster/software.htm) and plotted using TreeView version 1.6 (http://rana.lbl.gov/EisenSoftware.htm), using the top 1000 genes whose expression demonstrated the highest interquartile range across all groups. Differential expression between each treatment group and placebo was evaluated using two sample t tests (Statistical Analysis of Microarray (http://www-stat.stanford.edu/∼tibs/SAM/). False discovery rate (FDR) less than 5% was considered significant (24). For analysis of qRT-PCR data, the mean cycle threshold (Ct) for each gene was normalized to expression of the housekeeping gene RPL13A in the same sample (δCt). One-way ANOVA was used to compare the mean δCt for each gene across the treatment groups. Correlations were performed using Pearson's technique. P < 0.05 was considered significant.
Results
Study population and adverse events
Of 45 subjects screened, 32 met study criteria, were randomized, and completed all study procedures through wk 10 (the prostate biopsy). Two subjects were lost to follow-up after the wk 11 visit. Two subjects in the T+D group were noncompliant with drug administration and were excluded from analysis. No significant adverse events occurred during the study. In the placebo group, one subject experienced fatigue, and in the T-only group, one subject had a mild rash and one a mild headache. In the T+DMPA group, one subject had occasional mild headaches. All of these adverse events resolved without intervention. In the T+D group, one participant developed a leg abscess over a site of unrelated trauma that resolved with oral antibiotics.
There were no significant differences at baseline between subjects in the four treatment groups (mean age 38.4 ± 8.6 yr), except for a slightly lower serum PSA level in volunteers randomized to T+DMPA (Table 1). However, for all active treatment groups, serum PSA concentrations and prostate volume remained unchanged over the course of the study compared with baseline (Table 1).
Table 1.
Baseline and change in study end points from baseline expressed as medians (25th, 75th percentile)
| Placebo (n = 8) |
Testosterone gel (n = 7) |
T gel + Dutasteride (n = 7) |
T gel + DMPA (n = 7) |
|||||
|---|---|---|---|---|---|---|---|---|
| Wk 0 Baseline | Wk 10 End of treatment | Wk 0 Baseline | Wk 10 End of treatment | Wk 0 Baseline | Wk 10 End of treatment | Wk 0 Baseline | Wk 10 End of treatment | |
| LH (IU/liter) | 4.0 (3.5, 5.6) | 5.1 (2.7, 5.9) | 3.9 (2.7, 5.5) | 1.3 (0.4, 1.8)a | 5.3 (4.2, 6, 6) | 3.6 (2.7, 6.7) | 3.2 (2.7, 4.0) | 0.5 (0.0, 2.6)a |
| FSH (IU/liter) | 3.6 (2.6, 4.4) | 3.5 (2.7, 4.7) | 3.3 (2.6, 3.7) | 1.3 (0.5, 1.8)a | 4.0 (2.4, 5.5) | 2.9 (1.1, 3.6)a | 2.8 (2.6, 4.2) | 1.3 (0.1, 2.1)a |
| SHBG (nmol/liter) | 33 (24, 45) | 34 (25, 48) | 36 (13, 50) | 34 (16, 55) | 38 (26, 52) | 43 (26, 58) | 33 (17, 38) | 31 (13, 52) |
| DHEA (ng/ml) | 3.8 (3.0, 4.7) | 4.3 (2.7, 5.5) | 6 (2.9, 9.4) | 3.5 (2.0, 7.5)a | 4.2 (2.5, 6.0) | 3.8 (3.5, 6.3) | 4.2 (2.4, 5.2) | 3.2 (2.1, 4.0) |
| AED (ng/ml) | 0.9 (0.7, 1.2) | 0.9 (0.8, 1.1) | 1.1 (0.9, 1.2) | 0.9 (0.8, 1.3) | 0.9 (0.7, 0.9) | 1.8 (1.3, 3.7)a,b | 1.0 (0.6, 1.3) | 0.7 (0.6, 1.0) |
| DHT (ng/ml) | 0.5 (0.4, 0.5) | 0.5 (0.4, 0.6) | 0.6 (0.4, 0.9) | 1.8 (1.2, 2.5)a,b | 0.7 (0.6, 1.0) | 0.5 (0.3, 0.5)a | 0.6 (0.4, 0.8) | 0.6 (0.2, 1.8) |
| T (ng/ml) | 3.9 (3.8, 5.0) | 4.0 (2.9, 5.6) | 3.9 (2.9, 5.9) | 4.4 (3.2, 6.2) | 4.8 (3.8, 6.3) | 7.0 (4.4, 12.4)a,b | 4.0 (3.0, 5.8) | 1.8 (1.3, 2.9)a,b |
| Free T (pg/ml) | 63 (59, 77) | 57 (45, 83) | 73 (58, 89) | 65 (56, 106) | 73 (64, 92) | 104 (79, 122)a,b | 73 (62, 103) | 58 (37, 80) |
| E2 (pg/ml) | 63 (59, 77) | 57 (45, 83) | 73 (58, 89) | 65 (56, 106) | 73 (64, 92) | 104 (79, 122)a,b | 73 (62, 103) | 58 (37, 80) |
| PSA (ng/ml) | 0.7 (0.6, 1.0) | 0.8 (0.7, 1.2) | 0.7 (0.4, 1.1) | 0.9 (0.3, 1.2) | 0.9 (0.7, 1.1) | 0.7 (0.7, 1.1) | 0.5 (0.4, 0.6)c | 0.4 (0.4, 0.6) |
| Prostate volume (ml) | 25 (16, 29) | 22 (18, 24)a | 15 (14, 19) | 16 (14, 17) | 15 (13, 21) | 15 (13, 19) | 19 (14, 21) | 17 (14, 19) |
E2, Estradiol.
P < 0.05 vs. baseline.
P < 0.05 compared with placebo and other groups at wk 10.
P < 0.05 compared with placebo at baseline.
Serum hormones
Because most study visits (>90%) occurred in the morning and subjects were instructed not to apply their transdermal gel before their clinic visits, serum androgen levels were generally trough levels, measured 20–30 h after the previous gel application.
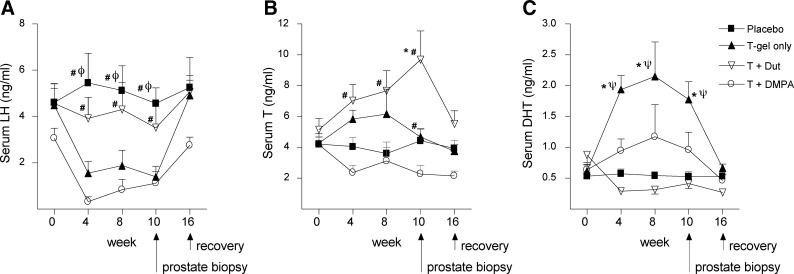
Compared with placebo treatment (Fig. 1, A-C, black squares), T gel administration suppressed LH and resulted in higher serum T and particularly serum DHT levels over the course of the study (Fig. 1, A–C, black triangles). The combination of DMPA with T gel strongly suppressed LH (and therefore endogenous T production), preventing the increase in serum T and DHT observed with T gel treatment alone and thereby resulting in serum T levels below baseline and only nonsignificant increases in serum DHT (Fig. 1, A–C). Inhibiting the conversion of T to DHT by adding dutasteride to T gel resulted in an increase in serum T and marked suppression of serum DHT (Fig. 1, B and C, white triangles) as well as increased serum levels of androstenedione (AED; a precursor of T) and estradiol (derived by aromatization of T) (Table 1). Despite these changes, the addition of dutasteride to T attenuated the magnitude of LH suppression achieved by T gel alone (Fig. 1A). Changes in FSH levels paralleled changes in LH, and SHBG was not significantly affected in any group (Table 1).
Fig. 1.
Serum LH and androgen concentrations in healthy men treated with placebo (black squares), T gel (black triangles), T gel + DMPA (white circles), or T gel + dutasteride (T + Dut; white triangles). Values shown at each time point are medians ± se. *, P < 0.05 vs. placebo; φ, P < 0.05 vs. T gel; ψ, P < 0.05 vs. T gel + dutasteride; #, P < 0.05 vs. T+DMPA. Serum LH (A) was measured by immunofluorometric assay, and serum T (B) and DHT (C) were measured by mass spectrometry in samples obtained during 10 wk of treatment and a 6-wk washout period.
Intraprostatic androgen concentrations
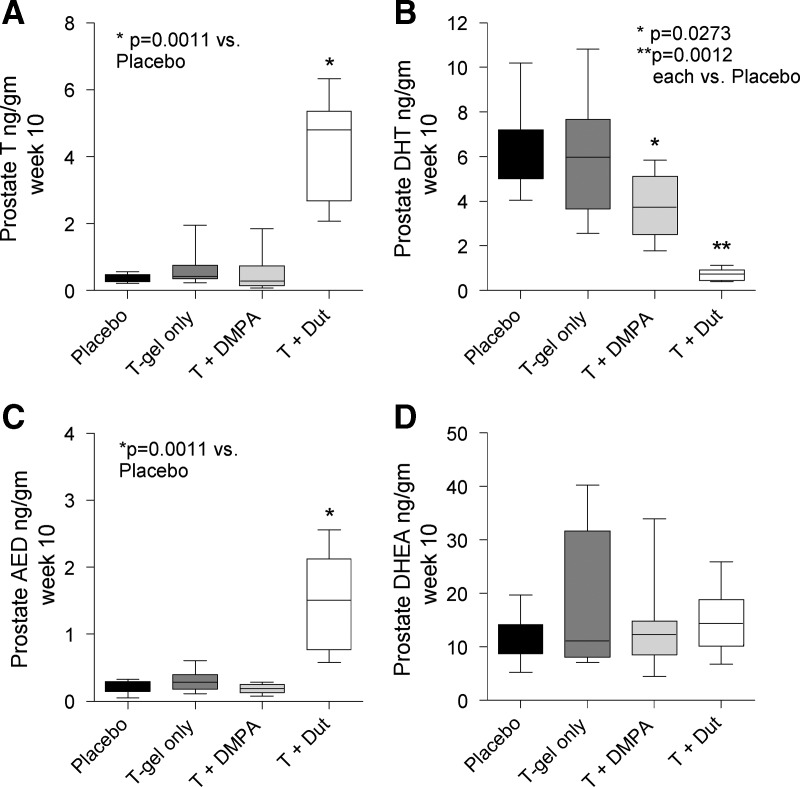
To determine whether MHC regimens may influence prostatic androgen activity, we evaluated androgen levels by mass spectroscopy in prostate biopsies obtained after 10 wk of treatment (Fig. 2; numeric data in Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org). Notably, despite an increase in serum T, and especially serum DHT, over the course of the study, administration of T-gel did not alter intraprostatic levels of T, DHT, or their precursors AED and DHEA at the 10-wk biopsy (Fig. 2, A–D, dark gray bars). Interestingly, the combination of T+DMPA (which resulted in the lowest serum T levels) did not alter prostate T concentrations (Fig. 2A) but was associated with a nearly 40% reduction in prostate DHT concentrations (Table 2, P = 0.027) compared with placebo (Fig. 2B, light gray bar). As anticipated, the combination of T+D led to nearly 90% reduction of intraprostatic DHT levels (P = 0.0012) and significant increases in intraprostatic T (11-fold, P = 0.0011) as well as AED (7-fold, P = 0.0011) compared with placebo (Fig. 2, A–C, white bars). No correlations between prostate and serum T or DHT levels were observed within the treatment groups (data not shown), which may reflect the limited number of observations per group.
Fig. 2.
Box and whiskers plots of prostate androgen concentrations in healthy men treated with placebo (black columns), T gel (dark gray columns), T gel + DMPA (light gray columns), or T gel + dutasteride (white columns). Intraprostatic T (A), DHT (B), AED (C), and DHEA (D) were measured by mass spectrometry in prostate biopsies obtained at the 10-wk time point of treatment.
Table 2.
Correlation of androgen-regulated genes with AR and prostate androgen levels
| Gene | Non-dutasteride arms P, T gel, T+DMPA |
T+dutasteride |
||||||
|---|---|---|---|---|---|---|---|---|
| AR | ip AED | ip T | ip DHT | AR | ip AED | ip T | ip DHT | |
| PSA | 0.83a | 0.2 | 0.0 | 0.51b | 0.1 | 0.92b | 0.99a | −0.80 |
| TMPRSS2 | 0.73a | 0.3 | −0.1 | 0.41 | −0.2 | 0.90b | 0.90b | −0.69 |
| NKX3.1 | 0.75a | 0.2 | −0.2 | 0.37b | 0.5 | 0.87b | 0.90b | −0.98a |
| FOLH1 | 0.57b | 0.3 | 0.1 | 0.43 | 0.0 | 0.98a | 0.92b | −0.78 |
| FKBP5 | 0.72a | 0.2 | −0.1 | 0.31 | 0.2 | 0.62 | 0.71 | −0.87 |
P, Placebo. P values are from Pearson correlations.
P < 0.0005.
P < 0.05.
Prostatic expression of androgen-regulated genes
To more carefully assess the impact of MHC regimens on prostate cellular constituents, we used microarray profiling to quantify gene expression in prostate epithelium microdissected from on-treatment biopsy cores. Notably, unsupervised clustering of the samples based on overall similarity in gene expression did not segregate samples by treatment group (Fig. 3A). Consistent with this observation, gene expression differences meeting a FDR less than 5% were not identified between any of the treatment groups compared with placebo. Specific interrogation of the microarray data for a panel of androgen-regulated genes we have previously described (18) (including genes with high and low signal intensities) demonstrated similar expression of these genes across the placebo and treated samples, with no consistent differences in any of the treatment groups (Fig. 3B). qRT-PCR for the androgen receptor (AR) and several canonical androgen-regulated genes (e.g. PSA, NKX3.1, TMPRSS2, FKBP5) confirmed the microarray data, demonstrating no statistically significant differences in expression of these genes among the treatment groups (Fig. 3C).
Fig. 3.
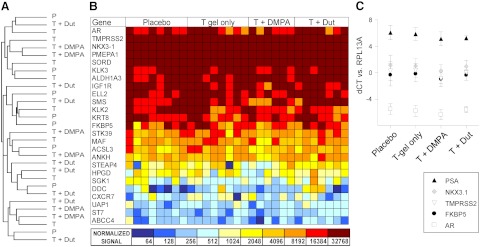
Comparison of prostate gene expression in laser-capture microdissected prostate epithelium from placebo-, T gel only-, T+DMPA-, and T + dutasteride-treated subjects. A, Unsupervised hierarchical clustering of samples based on the 1000 genes demonstrating the highest variability in expression across the four treatment groups (as determined by the interquartile range). This method clusters the samples based on how similarly or differently they express a set of genes. B, Heat map of the normalized absolute signal intensity for 25 genes commonly identified as androgen regulated, spanning a range of abundant to lowly expressed transcripts (lower expression is blue; higher expression is red brown). Gene expression was evaluated using two sample t tests, comparing all genes on the microarray for each treatment group vs. placebo. No q values were significant (FDR <5%). C, Measurement of transcript levels for the AR and androgen-regulated genes by qRT-PCR (normalized to expression of the housekeeping gene RPL13A). The mean expression level (with sd) for the indicated gene is shown in each treatment group. No differences among the treatment groups were identified (one way ANOVA, P < 0.05 considered significant).
Because treatment with T+D resulted in highly significant changes in intraprostatic androgen levels compared with no or less significant changes in the other groups, we further interrogated the microarray data by comparing T+D samples to the combined group of non-dutasteride-treated samples. Again, gene expression differences meeting an FDR less than 5% were not identified; however, evaluation of genes based on uncorrected P values demonstrates that subtle changes in androgen-regulated gene expression in the T+D group can be identified (Supplemental Fig. 1).
Correlation of androgen-regulated genes with prostatic AR and androgen levels
Expression of androgen-regulated genes reflects the net contribution of cellular AR numbers and androgen concentrations, and both AR and androgen levels have been correlated with expression of androgen regulated genes after hormonal manipulation of benign and neoplastic prostate tissues (23, 25). Therefore, although absolute changes in the expression of androgen-regulated genes were not observed in the MHC treatment groups, we sought to determine whether treatment was associated with changes in the correlation of AR or androgen levels with gene expression. Because treatment with T+D resulted in highly significant changes in intraprostatic androgen levels compared with no or less significant changes in the other groups, we determined the correlation of androgen-regulated gene expression with AR levels and androgen ligand concentrations in the T+D treatment group compared with the three nondutasteride treatment groups combined. Notably, expression of androgen-regulated genes in the nondutasteride treated samples correlated strongly with levels of transcripts encoding AR and, albeit to a lesser degree, with prostate DHT levels (Table 2). In contrast, expression of these genes correlated primarily with prostatic levels of T and AED in the T+D group, consistent with the suppression of DHT and accompanying rise in prostate T and AED observed in these samples. [Evaluation of the entire data set (i.e. all treatment groups together) demonstrated strong correlations between expression of AR and androgen regulated genes such as PSA, FKBP5, NKX3.1, and TMPRSS2 (P < 0.0001 for all), but no significant correlations between gene expression and androgen levels (data not shown).]
Discussion
This is the first placebo-controlled trial to examine the impact of MHC regimens on intraprostatic androgens and prostate epithelial gene expression. Overall, our data demonstrate that several promising MHC regimens, which alter circulating gonadotropin and androgen levels, do not substantially alter global or androgen-regulated gene expression in prostate epithelial cells after 10 wk of therapy.
Administration of exogenous, transdermal T, which significantly increased serum DHT and to a less consistent extent, serum T, had no impact on prostate T or DHT levels or on the expression of androgen-regulated genes within the prostate. The combination of T with DMPA, which resulted in the lowest serum T levels and attenuated the rise in serum DHT caused by T gel alone, did not alter prostate T concentrations but actually decreased prostate DHT by 40%. However, this magnitude of difference in prostate androgen concentration was not associated with measurable differences in prostate gene expression.
Because the prostate is an androgen-sensitive organ, concerns have been raised regarding prostate health in the context of long-term androgen administration in healthy men. Studies of the human prostatic microenvironment to date have been predominantly limited to either older, hypogonadal men (26) or men with prostate cancer (17, 23). Here we extend these studies to demonstrate that exogenous androgens at physiologic doses as might be used in MHC do not significantly alter the intraprostatic androgen environment in healthy men. Moreover, we show that the addition of a progestin, a requirement for more potent gonadotropin suppression during MHC, does not appear to further impact the prostate. Previous studies of MHC have used PSA and, in some cases, prostate volume as a surrogate for changes in prostate health (27); our data provide more direct evidence that MHC does not have substantial impacts on the prostate.
The failure of exogenous T gel to alter intraprostatic hormones despite significant increases in serum DHT is consistent with our prior data specifically evaluating the impact of exogenous DHT administration on prostatic androgen activity. In that study, we found that markers of prostate androgen response, including serum PSA, prostate volume, intraprostatic androgens, and prostate epithelial cell gene expression were not increased compared with placebo, despite 7-fold increases in serum DHT (18). Similarly, in a study of older, hypogonadal men, exogenous T replacement to achieve normal circulating levels did not impact intraprostatic T or DHT concentrations nor androgen-regulated gene expression (26).
In contrast, despite overall relatively modest changes in circulating androgens, T+DMPA administration resulted in a significantly lower intraprostatic DHT, compared with placebo. It is possible that this reflects the decrease of serum T to nearly hypogonadal levels (∼2 ng/ml) in this treatment arm, leading to decreased prostatic uptake of circulating T and intraprostatic conversion to DHT. Alternatively, the decrease in prostatic DHT might reflect a modest ability of DMPA to inhibit 5α-reductase (28). That this decrement in prostatic DHT did not impact androgen-regulated gene expression is consistent with our prior study demonstrating that medical castration in healthy men, resulting in more significant prostate androgen suppression, was also associated with very modest changes in expression of androgen-regulated genes (16, 17). That treatment with T+DMPA did not alter prostatic androgen-regulated gene expression is also notable in that MPA has been demonstrated to bind the AR and exhibit partial agonist properties in vitro (29).
The combination of T with dutasteride achieved the expected decreases in serum and prostate DHT levels, accompanied by increases in serum T, prostate T, and prostate AED. Remarkably, despite the higher potency of DHT compared with T for activating AR (30), the nearly 90% reduction of prostatic DHT in this treatment group was also without statistically significant impact on global or androgen regulated gene expression in the prostate (although when compared with the combined non-dutasteride treated groups, nonsignificant changes could be observed). Importantly, whereas expression of canonical androgen regulated genes was correlated with prostate epithelial AR and DHT levels in the placebo, T gel, and T+DMPA treatment groups, expression of androgen regulated genes in the T+D treatment group was strongly correlated with T and AED levels, consistent with the suppression of DHT and accompanying rise in prostate T and AED observed in this group. Because the absolute magnitude of gene expression was not altered relative to placebo, these data suggest that the significant increase in prostatic T and its precursor, AED, in the T+D samples may sufficiently compensate for the decrease in prostatic DHT levels, thereby maintaining homeostatic gene expression.
In a previous study of older men with prostate cancer undergoing neoadjuvant treatment with dutasteride for 4 months before prostatectomy, dutasteride treatment was associated with statistically significant inhibition of androgen-regulated gene expression compared with surgery alone (23). However, despite being nearly 90% lower than in placebo samples, the prostatic DHT levels in the T+D arm of our study were still 2- to 3-fold higher than those observed in the neoadjuvant study of dutasteride. This finding is potentially related to the younger age of participants, the lower dose of dutasteride used here, the inclusion of exogenous T gel, and the shorter duration of therapy. Moreover, the presence of prostate cancer might impact the degree of androgen sensitivity within the epithelium.
Because inhibition of intraprostatic DHT may contribute to lowering the risk for prostate cancer (8, 31), there has been concern that exogenous androgens might increase prostate cancer risk. However, a meta-analysis of small trials of androgen replacement did not find a cumulative increase in prostate cancer risk (32), and higher levels of endogenous androgens are not associated with an increased risk of developing prostate cancer (33). Our study adds to the growing body of evidence demonstrating that interventions significantly altering serum androgen levels do not markedly affect the prostate microenvironment. Although serum changes of the magnitude achieved by castration clearly impact prostate androgen levels and gene expression (15, 16), the present data nonetheless demonstrate that the prostate is able to maintain intraprostatic androgen concentrations and action despite fairly significant alterations in serum levels, perhaps by regulating intraprostatic androgen transport, synthesis, and metabolism (26, 34).
This study is limited by small numbers of participants and a relatively short duration of drug exposure because interpatient heterogeneity due to relatively small numbers in each treatment arm is a limitation in demonstrating significant expression changes. Thus, we cannot exclude the possibility that small, and perhaps clinically significant, changes in intraprostatic hormones, gene expression, or epithelial cell proliferation might be detected with larger cohort sizes or occur during longer-term therapy. Although no studies have been powered to assess the true impact, if any, of androgen-based therapies on prostate cancer risk, our data suggest that significant changes in serum androgen levels may not be associated with marked disturbances within the prostate and perhaps may not alter prostate disease risk. Longer-term studies adequately powered to address this question directly are clearly needed.
Supplementary Material
Acknowledgments
We thank Karl Thoreson, Roger Coleman, and Qi Tian for technical assistance and Ilsa Coleman for assistance with data analysis. We are grateful to Marilyn Busher, R.N., and Kathryn Torrez Duncan, research study coordinator, for their assistance conducting the study and to our research study volunteers, without whom this work would not be possible. All study procedures took place at the University of Washington Medical Center (Seattle WA) and were approved by the University of Washington Institutional Review Board. Informed consent was obtained by the investigator in all cases before any study procedures.
This work was supported by the National Institutes of Health through National Institute of Aging Grants K23-AG027238 and RO1-AG037603A, the Eunice Kennedy Shriver National Institute of Child Health and Human Development Grant U54-HD-42454, the National Cancer Institute Grant K23 CA122820-01, and the Pacific Northwest Prostate Cancer SPORE Grant P50-CA097186 E.A.M. was also supported by a Damon Runyon-Genentech Clinical Investigator Award CI-40-08 and the Prostate Cancer Foundation.
Disclosure Summary: Testosterone and placebo gel were provided by Auxilium Pharmaceuticals (Malvern, PA). S.T.P., J.K.A., P.S.N., and A.M.M. have received grant support from GlaxoSmithKline. P.S.N. has been a consultant for GlaxoSmithKline and Tokai. In addition, A.M.M. has received grant support from Abbott Pharmaceuticals and has been a consultant for Abbott Pharmaceuticals, Endo Pharmaceuticals, and Ligand. E.A.M., D.W.L., B.T.M., and J.L.W. have no potential conflicts of interest.
Footnotes
- AED
- Androstenedione
- AR
- androgen receptor
- Ct
- cycle threshold
- DHEA
- dehydroepiandrosterone
- DHT
- dihydrotestosterone
- DMPA
- depomedoxyprogesterone acetate
- FDR
- false discovery rate
- MHC
- male hormonal contraception
- PSA
- prostate-specific antigen
- qRT-PCR
- quantitative RT-PCR
- T
- testosterone.
References
- 1. Amory JK, Page ST, Bremner WJ. 2006. Recent advances in male hormonal contraception. Nat Clin Pract Endocrinol Metab 2:32–41 [DOI] [PubMed] [Google Scholar]
- 2. Brady BM, Amory JK, Perheentupa A, Zitzmann M, Hay CJ, Apter D, Anderson RA, Bremner WJ, Pollanen P, Nieschlag E, Wu FC, Kersemaekers WM. 2006. A multicentre study investigating subcutaneous etonogestrel implants with injectable testosterone decanoate as a potential long-acting male contraceptive. Hum Reprod 21:285–294 [DOI] [PubMed] [Google Scholar]
- 3. Turner L, Conway AJ, Jimenez M, Liu PY, Forbes E, McLachlan RI, Handelsman DJ. 2003. Contraceptive efficacy of a depot progestin and androgen combination in men. J Clin Endocrinol Metab 88:4659–4667 [DOI] [PubMed] [Google Scholar]
- 4. Page ST, Amory JK, Anawalt BD, Irwig MS, Brockenbrough AT, Matsumoto AM, Bremner WJ. 2006. Testosterone gel combined with depomedroxyprogesterone acetate is an effective male hormonal contraceptive regimen and is not enhanced by the addition of a GnRH antagonist. J Clin Endocrinol Metab 91:4374–4380 [DOI] [PubMed] [Google Scholar]
- 5. Amory JK, Bremner WJ. 2005. Oral testosterone in oil plus dutasteride in men: a pharmacokinetic study. J Clin Endocrinol Metab 90:2610–2617 [DOI] [PubMed] [Google Scholar]
- 6. Amory JK, Page ST, Bremner WJ. 2006. Oral testosterone in oil: pharmacokinetic effects of 5α reduction by finasteride or dutasteride and food intake in men. J Androl 27:72–78 [DOI] [PubMed] [Google Scholar]
- 7. Amory JK, Watts NB, Easley KA, Sutton PR, Anawalt BD, Matsumoto AM, Bremner WJ, Tenover JL. 2004. Exogenous testosterone or testosterone with finasteride increases bone mineral density in older men with low serum testosterone. J Clin Endocrinol Metab 89:503–510 [DOI] [PubMed] [Google Scholar]
- 8. Thompson IM, Goodman PJ, Tangen CM, Lucia MS, Miller GJ, Ford LG, Lieber MM, Cespedes RD, Atkins JN, Lippman SM, Carlin SM, Ryan A, Szczepanek CM, Crowley JJ, Coltman CA., Jr 2003. The influence of finasteride on the development of prostate cancer. N Engl J Med 349:215–224 [DOI] [PubMed] [Google Scholar]
- 9. Burkman R, Schlesselman JJ, Zieman M. 2004. Safety concerns and health benefits associated with oral contraception. Am J Obstet Gynecol 190:S5–S22 [DOI] [PubMed] [Google Scholar]
- 10. George FW, Wilson JD. 1994. The physiology of reproduction. New York: Raven Press, Ltd [Google Scholar]
- 11. Geller J. 1990. Effect of finasteride, a 5α-reductase inhibitor on prostate tissue androgens and prostate-specific antigen. J Clin Endocrinol Metab 71:1552–1555 [DOI] [PubMed] [Google Scholar]
- 12. Geller J, Albert J. 1987. Effects of castration compared with total androgen blockade on tissue dihydrotestosterone (DHT) concentration in benign prostatic hyperplasia (BPH). Urol Res 15:151–153 [DOI] [PubMed] [Google Scholar]
- 13. Mohler JL, Gregory CW, Ford OH, 3rd, Kim D, Weaver CM, Petrusz P, Wilson EM, French FS. 2004. The androgen axis in recurrent prostate cancer. Clin Cancer Res 10:440–448 [DOI] [PubMed] [Google Scholar]
- 14. Forti G, Salerno R, Moneti G, Zoppi S, Fiorelli G, Marinoni T, Natali A, Costantini A, Serio M, Martini L, et al. 1989. Three-month treatment with a long-acting gonadotropin-releasing hormone agonist of patients with benign prostatic hyperplasia: effects on tissue androgen concentration, 5 alpha-reductase activity and androgen receptor content. J Clin Endocrinol Metab 68:461–468 [DOI] [PubMed] [Google Scholar]
- 15. Habib FK, Ross M, Tate R, Chisholm GD. 1997. Differential effect of finasteride on the tissue androgen concentrations in benign prostatic hyperplasia. Clin Endocrinol (Oxf) 46:137–144 [DOI] [PubMed] [Google Scholar]
- 16. Page ST, Lin DW, Mostaghel EA, Hess DL, True LD, Amory JK, Nelson PS, Matsumoto AM, Bremner WJ. 2006. Persistent intraprostatic androgen concentrations after medical castration in healthy men. J Clin Endocrinol Metab 91:3850–3856 [DOI] [PubMed] [Google Scholar]
- 17. Mostaghel EA, Page ST, Lin DW, Fazli L, Coleman IM, True LD, Knudsen B, Hess DL, Nelson CC, Matsumoto AM, Bremner WJ, Gleave ME, Nelson PS. 2007. Intraprostatic androgens and androgen-regulated gene expression persist after testosterone suppression: therapeutic implications for castration-resistant prostate cancer. Cancer Res 67:5033–5041 [DOI] [PubMed] [Google Scholar]
- 18. Page ST, Lin DW, Mostaghel EA, Marck BT, Wright JL, Wu J, Amory JK, Nelson PS, Matsumoto AM. 2011. Dihydrotestosterone administration does not increase intraprostatic androgen concentrations or alter prostate androgen action in healthy men: a randomized-controlled trial. J Clin Endocrinol Metab 96:430–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Barry MJ, Fowler FJ, Jr, O'Leary MP, Bruskewitz RC, Holtgrewe HL, Mebust WK, Cockett AT. 1992. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol 148:1549–1557; discussion 1564 [DOI] [PubMed] [Google Scholar]
- 20. Roth MY, Page ST, Lin K, Anawalt BD, Matsumoto AM, Snyder CN, Marck BT, Bremner WJ, Amory JK. 2010. Dose-dependent increase in intratesticular testosterone by very low-dose human chorionic gonadotropin in normal men with experimental gonadotropin deficiency. J Clin Endocrinol Metab 95:3806–3813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kalhorn TF, Page ST, Howald WN, Mostaghel EA, Nelson PS. 2007. Analysis of testosterone and dihydrotestosterone from biological fluids as the oxime derivatives using high-performance liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrum 21:3200–3206 [DOI] [PubMed] [Google Scholar]
- 22. Montgomery RB, Mostaghel EA, Vessella R, Hess DL, Kalhorn TF, Higano CS, True LD, Nelson PS. 2008. Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer Res 68:4447–4454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mostaghel EA, Geng L, Holcomb I, Coleman IM, Lucas J, True LD, Nelson PS. 2010. Variability in the androgen response of prostate epithelium to 5α-reductase inhibition: implications for prostate cancer chemoprevention. Cancer Res 70:1286–1295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tusher VG, Tibshirani R, Chu G. 2001. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci USA 98:5116–5121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mostaghel EA, Marck BT, Plymate SR, Vessella RL, Balk S, Matsumoto AM, Nelson PS, Montgomery RB. 2011. Resistance to CYP17A1 inhibition with abiraterone in castration-resistant prostate cancer: induction of steroidogenesis and androgen receptor splice variant. Clin Cancer Res 17:5913–5925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Marks LS, Mazer NA, Mostaghel E, Hess DL, Dorey FJ, Epstein JI, Veltri RW, Makarov DV, Partin AW, Bostwick DG, Macairan ML, Nelson PS. 2006. Effect of testosterone replacement therapy on prostate tissue in men with late-onset hypogonadism: a randomized controlled trial. JAMA 296:2351–2361 [DOI] [PubMed] [Google Scholar]
- 27. Matthiesson KL, McLachlan RI. 2006. Male hormonal contraception: concept proven, product in sight? Hum Reprod Update 12:463–482 [DOI] [PubMed] [Google Scholar]
- 28. Lax ER, Baumann P, Schriefers H. 1984. Changes in the activities of microsomal enzymes involved in hepatic steroid metabolism in the rat after administration of androgenic, estrogenic, progestational, anabolic and catatoxic steroids. Biochem Pharmacol 33:1235–1241 [DOI] [PubMed] [Google Scholar]
- 29. Africander D, Verhoog N, Hapgood JP. 2011. Molecular mechanisms of steroid receptor-mediated actions by synthetic progestins used in HRT and contraception. Steroids 76:636–652 [DOI] [PubMed] [Google Scholar]
- 30. Wilson JD. 2001. The role of 5α-reduction in steroid hormone physiology. Reprod Fertil Dev 13:673–678 [DOI] [PubMed] [Google Scholar]
- 31. Andriole GL, Bostwick DG, Brawley OW, Gomella LG, Marberger M, Montorsi F, Pettaway CA, Tammela TL, Teloken C, Tindall DJ, Somerville MC, Wilson TH, Fowler IL, Rittmaster RS, Group RS. 2010. Effect of dutasteride on the risk of prostate cancer. N Engl J Med 362:1192–1202 [DOI] [PubMed] [Google Scholar]
- 32. Calof OM, Singh AB, Lee ML, Kenny AM, Urban RJ, Tenover JL, Bhasin S. 2005. Adverse events associated with testosterone replacement in middle-aged and older men: a meta-analysis of randomized, placebo-controlled trials. J Gerontol A Biol Sci Med Sci 60:1451–1457 [DOI] [PubMed] [Google Scholar]
- 33. Roddam AW, Allen NE, Appleby P, Key TJ. 2008. Endogenous sex hormones and prostate cancer: a collaborative analysis of 18 prospective studies. J Natl Cancer Inst 100:170–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Luu-The V, Bélanger A, Labrie F. 2008. Androgen biosynthetic pathways in the human prostate. Best Pract Res Clin Endocrinol Metab 22:207–221 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.