Abstract
Context:
Epidemiological studies reported an inverse or U-shaped relationship between sleep duration and weight. The relationship between sleep and resting energy expenditure (REE) has not been well characterized.
Objective:
The aim of the study was to determine the relationship between sleep, REE, and stress hormones.
Design and Setting:
We conducted a cross-sectional evaluation of a prospective cohort study at a tertiary referral research clinical center.
Subjects:
Subjects included 126 obese individuals (30 males, 96 females; age, 40.5 ± 6.9 yr; body mass index, 38.6 ± 6.5 kg/m2; sleep duration, 360 ± 50 min/night; and sleep efficiency, 79.5 ± 7.5%).
Main Outcome Measure(s):
REE and respiratory quotient (RQ) were assessed by indirect calorimetry. Sleep duration and sleep efficiency were assessed by actigraphy. Sleep quality was estimated by questionnaires, and sleep apnea was evaluated by respiratory disturbance index (RDI). Morning plasma ACTH, serum cortisol, and 24-h urinary free cortisol and catecholamines were also measured.
Results:
RDI was positively correlated with REE adjusted by fat-free mass (r = 0.307; P = 0.003) and RQ (r = 0.377; P < 0.001). Sleep efficiency was inversely correlated with RQ (r = −0.200; P = 0.033). The relationship of RDI score and REE was stronger in men than women (P = 0.03). In women, serum cortisol was positively correlated (r = 0.407; P < 0.001), and Epworth sleepiness score tended to be inversely (r = −0.190; P = 0.086) correlated with adjusted REE. The RQ was positively related to RDI in women, whereas subjective sleep time was related to RQ in men. In a multiple regression model, RDI, serum cortisol, and urinary norepinephrine were directly related to REE, whereas serum cortisol also directly related to adjusted REE.
Conclusion:
Poor sleep quality was associated with increased REE, a higher RQ indicating a shift from fat toward carbohydrate oxidation, and activation of the stress system.
The amount of sleep needed varies among individuals, and no objective method is available for its determination. Nonetheless, an average of 7.5 h/d is deemed optimal in adults (1, 2). Sleep is considered adequate when no sleepiness or suboptimal functioning is experienced during the daytime. Insufficient sleep impairs memory, mood, and behavior (3, 4). Increasing occupational and social demands, the advent of artificial lighting at the beginning of the last century and, more recently, the widespread use of computers and other electronic media (5) have curtailed sleep duration from 9 h per night in 1910 to 7.5 h by 1975, and less than 7 h today (2). One fourth of adults and a larger proportion of children and adolescents are sleep-deprived (2). Sleep deprivation due to “social jet lag” is increasing (6); sleeping 5–6 h per night during the week is becoming the norm (2).
The trend toward decreased sleep duration has been paralleled by a worldwide increase in prevalence of obesity and insulin resistance/type 2 diabetes (7, 8). Mainly cross-sectional and some prospective epidemiological studies have reported an association between short habitual sleep and obesity or diabetes. This association has been mostly observed in children and younger adults and attenuates at an older age (9–11). Furthermore, sleep deprivation is more common in individuals of lower socioeconomic status (12).
Depending on the study, either an inverse or U-shaped relationship has been described between sleep and weight (9, 13). Epidemiological studies rarely address causation, and experimental studies, although informative of causal links and physiological mechanisms, are often not representative of real-life situations because of the artificial setting in which they are conducted. Thus, despite a growing body of evidence, it is still unclear whether chronic sleep deprivation and impaired sleep quality are causally related to obesity or whether they simply represent coincidental secular trends (5). According to a survey of 25-to 45-yr-old individuals, short sleepers are a heterogeneous group, including both insomniacs, representing approximately 16%, and those with a sleep debt, about 45% (14). As noted recently (15), thus far the association between short sleep and obesity has been explained mostly with increased appetite and food intake (16–19), whereas the repercussions of chronic sleep deprivation on resting energy expenditure (REE), and basal metabolic rate have not been explored yet.
Sleep deprivation causes interconnected systemic endocrine and immune system dysregulation (20–22). Sleep curtailment also affects the endocrine system in its entirety, alters the sympathovagal balance, and leads to the activation of both efferent limbs of the stress system, the hypothalamic-pituitary-adrenal (HPA) axis and the sympathetic nervous system (23, 24). Concomitantly, the GH, gonadal, and thyroid axes are inhibited (25). Sleep deprivation is associated with alterations in body temperature regulation (26), heart rate (4), and metabolic rate (27). Given the known stimulatory effects of cortisol and catecholamines on energy expenditure, sleep deprivation may in theory lead to increased energy expenditure (28). Acute activation of the stress system is adaptive because it augments the energy that is readily available for “fight or flight,” whereas protracted activation, whether secondary to sleep deprivation or other stressor, is associated with stress-induced pathologies (25).
Our aim was to investigate, in a real-life situation, the relationships between sleep and REE, and the role of the HPA axis and sympathetic activity on these relationships in a prospectively assembled cohort of obese individuals reporting sleeping less than 6.5 h per night.
Subjects and Methods
Study cohort
We report here on selected baseline characteristics of subjects of the Sleep Extension Study. The design of this randomized, prospective, long-term, intervention trial of obese adult men and premenopausal women conducted at the National Institutes of Health (NIH) Clinical Center (NIDDK protocol 06-DK-0036; www.ClinicalTrials.gov identifier NCT00261898) has been described in detail earlier (29). The study protocol was approved by the NIDDK Institutional Review Board, and appropriate written consent was obtained from each participant.
Recruitment occurred between January 22, 2007, and June 28, 2011, for obese individuals [body mass index (BMI), 30–55 kg/m2] who reported sleeping on average less than 6.5 h per night. In addition, self-reported body weight had to have remained within 5% over the previous 6 months. A recruitment center screened out by phone those who were less than 18 yr or more than 50 yr old; who reported not being obese; who reported uncontrolled hypertension or diabetes, or stated sleeping more than 6.5 h; or who were postmenopausal. Those remaining after a second, semistructured telephone interview came for a clinic visit, during which informed written consent was obtained. A medical history, physical examination, electrocardiogram, clinical blood work, and sleep assessment were completed.
Study procedures
Individuals who met study criteria were invited for another visit at the NIH Clinical Center. Whenever feasible, subjects arrived the evening before the visit; if this was not possible, they were instructed to arrive after an overnight fast at 0700 h. Upon arrival, subjects were asked to void, and a 24-h urine collection was started. If the subjects were unable to spend the night, they were given supplies and instructions regarding urine collection at home. Anthropometric measurements were taken, and sleep questionnaires were filled out. Fasting blood was drawn at 0800 h. An iv line was started approximately 30 min before sample collection. Indirect calorimetry was then performed as detailed.
Subjects then received instruction for wearing the Actiwatch (Mini Mitter/Respironics/Philips, Bend, OR) and maintaining sleep diaries for the following 2 wk. Those participants who performed an overnight visit were outfitted with the Unicorder (Advanced Brain Monitoring Inc., Carlsbad, CA) for sleep apnea recordings. Subjects who elected to do their sleep apnea recordings at home were instructed on the correct use of the Unicorder, which they wore during the subsequent night.
Body composition
Body composition was measured by dual-energy x-ray absorptiometry (Lunar iDXA; General Electric, Chicago, IL; software version enCORE 11.10.053). Height was measured to the nearest centimeter using a wall-mounted stadiometer (SECA 242; SECA North America East, Hanover, MD), and weight was measured to the nearest 0.1 kg in a hospital gown using a stand-on scale (SR555 SR Scale; SR Instruments, Inc., Tonawanda, NY). Waist circumference was measured by placing the measuring tape in a horizontal plane around the abdomen above the uppermost lateral border of the right iliac crest at the end of a normal expiration. If this site could not be determined, the maximum circumference was measured at or near the level of the umbilicus.
Indirect calorimetry
Indirect calorimetry for the determination of REE and respiratory quotient (RQ) was performed the following morning after an overnight fast. After resting in bed in a quiet room for 30 min, a transparent plastic hood was placed over the head of the subject for another 30 min. Individuals were instructed to remain motionless but awake during the test. To allow for acclimation to this procedure, only the measurements taken during the last 20 min were used. Oxygen consumption and CO2 production were computed from continuous measurements of O2 and CO2 concentrations in inspired and expired air diluted in a constant air flow generated by the metabolic cart (TrueOne 2400 metabolic cart; ParvoMedics, Sandy, UT). REE was calculated from VO2 and VCO2 using the Weir equation (30). RQ was calculated as the ratio of VCO2/VO2. REE was corrected for fat-free mass, age, and sex.
Sleep measures
Total sleep time and sleep efficiency were determined by a wrist actigraphy monitor (Actiwatch-64), similar in size to a wristwatch. This device records gross motor activity using a highly sensitive piezoelectric accelerometer with a sensitivity of 0.05 g, sampling frequency of 32 Hz, and filters set to 3–11 Hz. One-minute epochs were used in this study. Data were analyzed by Actiware-Sleep software versions 5.04 and 5.57 (Mini Mitter/Respironics/Philips Bend, OR). Sleep start and end times were set according to sleep diaries, if available, or by using best clinical judgment. The Actiwatch has been validated against polysomnography previously (31). Sleep start time was calculated as the first 10-min period in which no more than one epoch was scored as mobile, whereas sleep end time was calculated as the last 10-min period during which no more than one epoch scored as mobile. Two measures were included from the actigraphy data: sleep duration, the amount of actual sleep obtained during the 24-h period; and sleep efficiency, the percentage of time in bed spent still and, therefore, presumably sleeping.
Sleep disordered breathing was assessed overnight, either in the clinic or at the subject's home using a portable screening device (Unirecorder-Apnea Risk Evaluation System; Advanced Brain Monitoring, Inc., Carlsbad, CA) that has been approved and verified for ambulatory use. This device consists of a nasal cannula, adjustable head strap, forehead reflectance pulse oximeter, an accelerometer to measure head movement, and a calibrated acoustic microphone. Bedtimes were at the subject's discretion. Data were automatically scored, then reviewed. The device provides an estimate of the respiratory disturbance index (RDI), the number of apneas and hypopneas per hour of sleep. Apnea was defined as a cessation of airflow for at least 10 sec. Hypopneas included events identified as airflow less than 50%, with a 3.5% or greater desaturation and 1% resaturation. Hypopneas were also determined as a minimum 1% desaturation and resaturation plus at least one surrogate arousal indicator (head movement, changes in snoring, or changes in pulse rate). When validated against polysomnography, this device has demonstrated high sensitivity and specificity (32). Seventy-eight participants completed the recording at home, and 26 completed the recording in the clinic. Participants were asked to complete their testing in the clinic instead of at home as a cost-saving measure to minimize loss of equipment. Gender breakdown of home vs. lab monitoring was similar: 17 of the 78 (21%) home participants were males, and six of the 26 (23%) clinic participants were males.
Subjects completed the Pittsburgh Sleep Quality Index (PSQI), a validated 21-item questionnaire with inquiries about sleep, including perceived quality over the past month. PSQI scores range from 0 to 21, higher scores indicating worse quality. Scores were dichotomized at 5 or less and greater than 5, the threshold conventionally used for poor sleep quality (33). Subjective sleep duration was estimated prospectively from the sleep diaries that were kept over the period of actigraphy and retrospectively by question C4 of the PSQI. Daytime sleepiness was assessed by the Epworth Sleepiness Scale (ESS), a validated eight-item questionnaire (34). ESS scores range from 0 to 24. Higher scores represent increased daytime sleepiness, scores greater than 10 indicating an excessive level of sleepiness.
Clinical laboratory analysis
Plasma fasting samples were consistently obtained at 0800 h. Plasma ACTH and serum cortisol were measured with chemiluminescent immunoassays (Immulite 2500; Siemens Health Diagnostics, Deerfield, IL). The 24-h urinary free cortisol (UFC) was measured by liquid chromatography-tandem mass spectrometry, and 24-h urinary catecholamines were measured by HPLC.
Statistical analysis
We calculated descriptive statistics for each variable for the cohort as a whole and separately by gender. We compared men and women by t test for difference in means of continuous variables, Wilcoxon rank test for skewed variables, Fisher exact test, and Pearson χ2 test for difference in counts and frequency.
We related REE, RQ, and stress hormones to sleep parameters in exploratory linear regression analyses, predefined on the basis of physiology. The hormone measurements were related to the sleep measurements obtained immediately preceding the blood or urine sampling. Before each regression, normality of the dependent variables was examined by Q-Q normality plots. The regression analyses were stratified by gender. Those variables that were found to be related using a threshold for P value of 0.1 were used in the regression models. Using backward stepwise regression models, we then determined which of the sleep and hormone variables predict REE. Data are reported as mean ± sd, median, and range as deemed appropriate, and a P value ≤0.05 was considered statistically significant. Analyses were performed using SAS version 9.1.3 (SAS Institute Inc., Cary, NC), JMP version 8.0 (SAS Institute Inc.), and SPSS version 19 (IBM SPSS North America, Chicago, IL).
Results
Demographic and anthropometric characteristics
The average age of the 126 subjects (30 males and 96 females) was 40.5 ± 6.9 yr, and two thirds were minorities (Table 1). Eight percent were current smokers; a small portion of individuals were on various medications, including statins and antihypertensive agents. Average BMI was 38.6 ± 6.5 kg/m2; as expected, men had a larger neck circumference than women, and women had less fat-free mass and a higher percentage of body fat than men.
Table 1.
Demographic and anthropometric characteristics of the study population
| All | Men | Women | P value | |
|---|---|---|---|---|
| n | 126 | 30 | 96 | |
| Age (yr) | 40.5 ± 6.9 | 40.7 ± 7.3 | 40.5 ± 6.8 | 0.890 |
| Range | 23–50 | 23–50 | 23–50 | |
| Race/ethnicity (n)a | 0.012 | |||
| Non-Hispanic black | 75 (58.9) | 11 (36.7) | 64 (66.7) | |
| Non-Hispanic white | 45 (35.7) | 17 (56.7) | 27 (28.1) | |
| Other | 6 (4.8) | 2 (6.7) | 5 (5.2) | |
| Smoking status (n)a | 10 (7.9) | 3 (10) | 7 (7.3) | 0.256 |
| Medication (n)a | 0.178 | |||
| Psychiatric | 9 (7.1) | 2 (6.6) | 7 (7.2) | |
| Antidiabetic (oral) | 4 (3.2) | 1 (0.3) | 3 (3.1) | |
| Antiinflammatory | 10 (7.9) | 3 (10.0) | 7 (7.3) | |
| Statins | 7 (5.6) | 3 (10.0) | 4 (4.2) | |
| Antihypertensive | 18 (14.2) | 4 (13.3) | 14 (14.6) | |
| Corticosteroids | 8 (6.3) | 4 (13.3) | 4 (4.2) | |
| Oral contraceptives | 8 (6.3) | 8 (8.3) | ||
| Height (cm) | 167.4 ± 9.0 | 176.8 ± 8.5 | 164.4 ± 6.9 | <0.001 |
| Weight (kg) | 108.3 ± 20.7 | 115.2 ± 23.3 | 106.2 ± 19.5 | 0.036 |
| BMI (kg/m2) | 38.6 ± 6.5 | 36.7 ± 6.1 | 39.2 ± 6.5 | 0.067 |
| Waist circumference (cm) | 113.8 ± 13.0 | 118.1 ± 14.1 | 112.5 ± 12.4 | 0.038 |
| Neck circumference (cm) | 39.2 ± 4.0 | 44.0 ± 3.6 | 37.7 ± 2.6 | <0.001 |
| Fat-free mass (kg) | 60.9 ± 11.9 | 74.5 ± 12.2 | 56.1 ± 7.1 | <0.001 |
| % Body fat | 41.3 ± 7.0 | 33.4 ± 5.7 | 44.1 ± 5.0 | <0.001 |
Data are expressed as number (percentage) or mean ± sd.
χ2.
Sleep characteristics
Sleep duration and sleep efficiency were approximately 6 h and 80%, respectively (Table 2). Subjects wore the Actiwatch for 12.9 ± 2.0 d (mean ± sd; 5–14 d, range). Global PSQI scores indicated poor sleep quality, and sleepiness scores tended to be abnormal. Half of the subjects had sleep apnea. There were significant differences in RDI scores between those subjects who did the recordings at home vs. those who did the recordings in the laboratory (10.49 ± 14.44 vs. 18.20 ± 19.07 events per hour for home vs. lab recordings, respectively; P = 0.013). A likely explanation of the difference may be related to the fact that the hospital is an unfamiliar environment and is likely to interfere with sleep. This location may have influenced the results but it remains unclear to what extent. There is also a possibility that the difference was due merely to chance.
Table 2.
Sleep characteristics of the subjects
| All | Men | Women | P value | |
|---|---|---|---|---|
| n | 126 | 30 | 96 | |
| Actigraphy sleep duration (min/night) | 360 ± 50 | 341 ± 55 | 366 ± 47 | 0.021 |
| Actigraphy sleep efficiency (%) | 79.5 ± 7.5 | 78.0 ± 7.9 | 80.1 ± 7.3 | 0.190 |
| Subjective sleep duration (min/night) | 386 ± 48 | 372 ± 45 | 390 ± 49 | 0.131 |
| Question C4 of PSQI (hours/24 h) | 5.6 ± 0.9 | 5.8 ± 1.0 | 5.5 ± 0.9 | 0.153 |
| PSQI global score (optimal; 0–5) | 8.2 ± 2.9 | 8.0 ± 3.2 | 8.2 ± 2.8 | 0.210 |
| ESS (optimal; 0–10) | 8.2 ± 4.5 | 8.9 ± 4.6 | 8.0 ± 4.5 | 0.362 |
| RDI score (no. of events/h) | 12.6 ± 15.6 | 20.9 ± 24.0 | 9.5 ± 11.1 | 0.099a |
| Normal | 42 (42.9) | 5 (22.7) | 37 (48.7) | |
| Mild | 35 (35.7) | 9 (40.9) | 25 (34.1) | |
| Moderate | 12 (12.2) | 4 (18.2) | 8 (10.5) | |
| Severe | 9 (9.2) | 4 (18.2) | 5 (6.6) |
Data are expressed as number (percentage) or mean ± sd.
Wilcoxon rank-sum test.
The following gender differences were noted: sleep duration by actigraphy was approximately 25 min shorter in men than in women (P = 0.021). Only one of five men, compared with 50% of women, was free of sleep apnea. When present, sleep apnea tended to be more severe in men (P ≤ 0.09).
Gender differences in REE and stress hormones
ACTH, cortisol, UFC, and urinary catecholamines were borderline to significantly higher in men than women (Table 3).
Table 3.
REE and hormone stress parameters of the study population
| All | Men | Women | P value | |
|---|---|---|---|---|
| n | 116 | 27 | 89 | |
| REE (kcal/24 h) | 1700 ± 300 | 1993 ± 342 | 1608 ± 218 | <0.001 |
| Adjusted REE (kcal/24 h)a | 1697 ± 154 | 1692 ± 200 | 1699 ± 137 | 0.822 |
| Serum ACTH (pg/ml)b | 24.5 ± 24.90 | 30.9 ± 37.0 | 21.6 ± 19.4 | 0.111 |
| Plasma cortisol (μg/dl)b | 9.6 ± 4.3 | 10.6 ± 3.7 | 9.3 ± 4.4 | 0.123 |
| UFC (μg/24 h)b | 21.3 ± 14.0 | 29.8 ± 17.8 | 18.6 ± 11.5 | 0.001 |
| Urinary NE (μg/24 h)b | 44.8 ± 19.1 | 53.6 ± 23.6 | 42.0 ± 16.7 | 0.004 |
| Urinary epinephrine (μg/24 h)b | 4.4 ± 2.9 | 5.7 ± 3.6 | 3.9 ± 2.5 | 0.004 |
| Urinary dopamine (μg/24 h)b | 264 ± 93 | 295 ± 96 | 254 ± 91 | 0.037 |
Data are expressed as mean ± sd.
REE was adjusted for fat-free mass, age, and gender.
To convert gravimetric units for hormones to SI units, use the following conversion factors: cortisol, μg/dl × 27.59 = nmol/liter; epinephrine, μg/24 h × 5.46 = nmol/24 h; NE, μg/24 h × 5.91 = nmol/24 h; corticotropin (ACTH), pg/ml × 0.22 = pmol/liter; and dopamine, μg/24 h × 6.58 = nmol/24 h.
Relationship of severity of sleep apnea with energy metabolism and hormone measurements
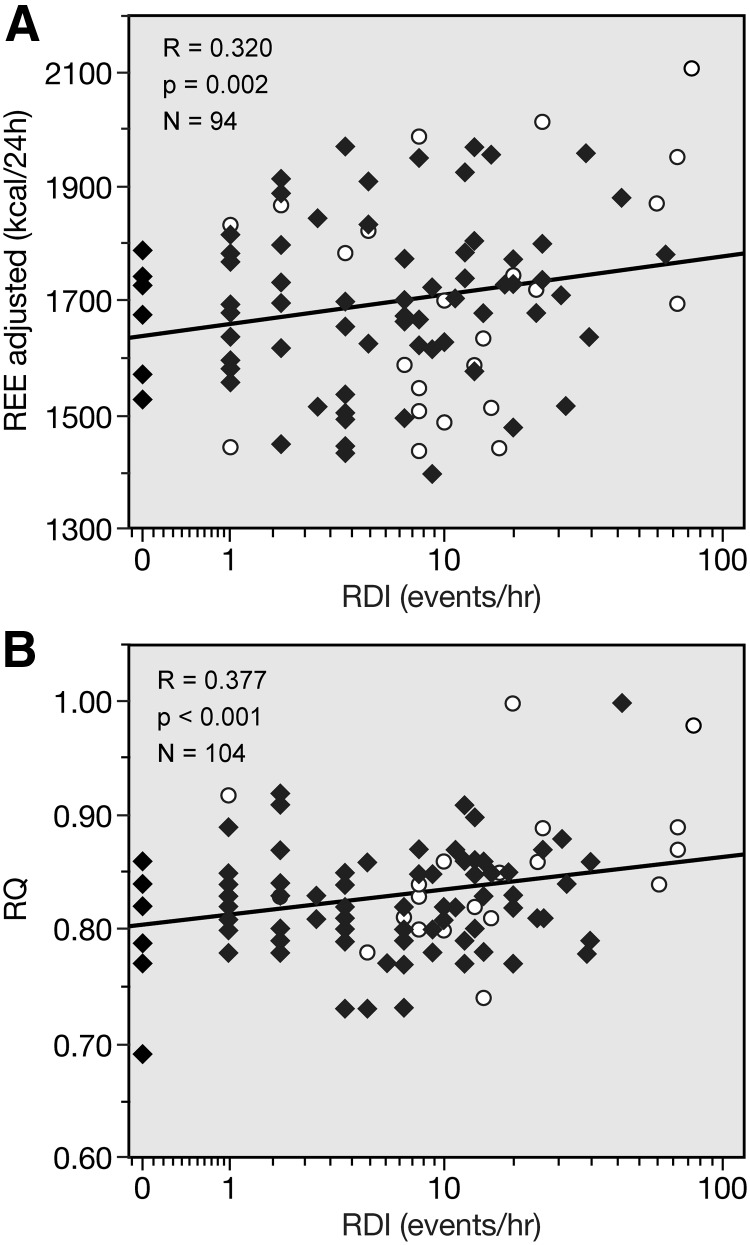
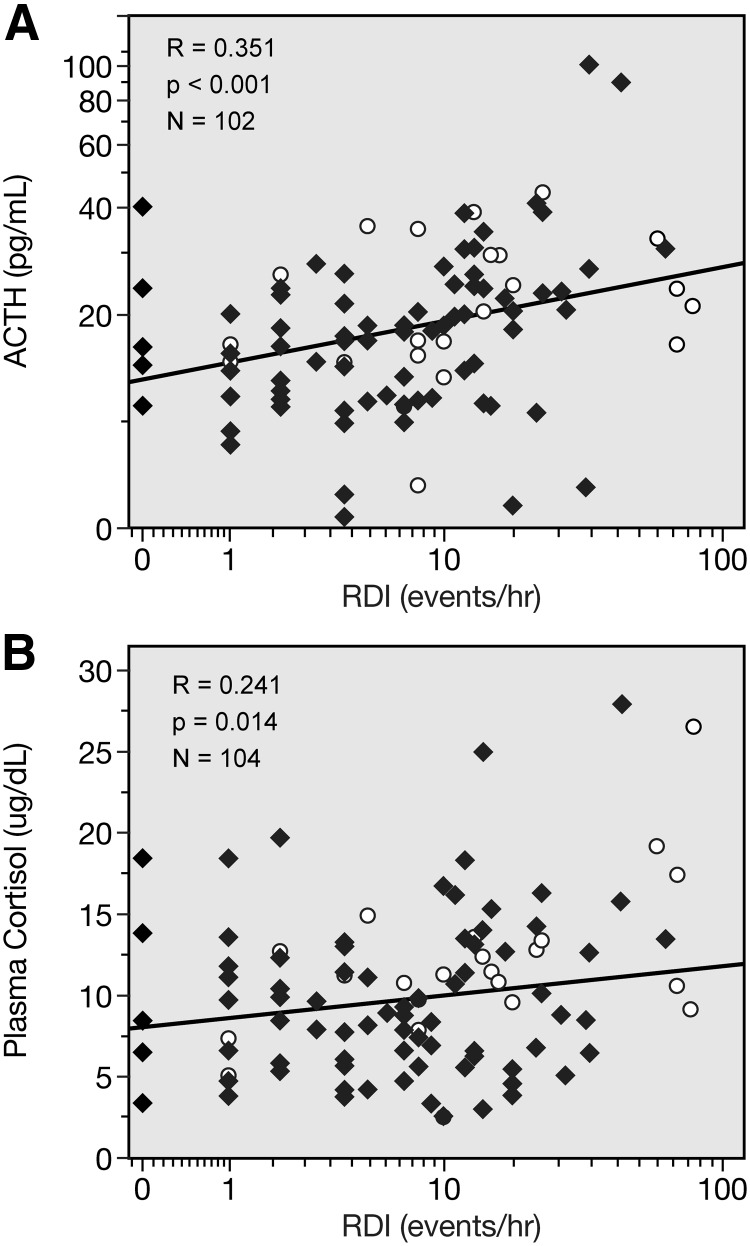
RDI, the number of events of apnea, was directly correlated with REE and RQ (Fig. 1). RDI was also directly related to plasma ACTH and serum cortisol (Fig. 2). Because of the observed difference in RDI scores between those subjects who did the recordings at home and those who did the recordings in the laboratory, we analyzed the relationship of RDI vs. REE, RQ, ACTH, and cortisol after controlling for measurement location. RDI remained significantly related to these parameters: RDI vs. REE, r = 0.584, P = 0.000; vs. RQ, r = 0.535, P = 0.001; vs. ACTH, r = 0.199, P = 0.043; and vs. cortisol, r = 0.263, P = 0.027.
Fig. 1.
Correlation of RDI with REE adjusted (A) and RQ (B). Closed diamonds, women; open circles, men.
Fig. 2.
Correlation of RDI with ACTH (A) and plasma cortisol (B).
In addition, the shorter the total sleep time by actigraphy, the higher the REE (r = −0.218; P = 0.017; n = 120) and the higher the UFC (r = −0.236; P = 0.011; n = 117) and the urinary dopamine (r = −0.248; P = 0.007; n = 116). The poorer the sleep efficiency, the higher the RQ (r = −0.200; P = 0.033; n = 114); and poor sleep quality, as assessed by the PSQI global score, is inversely related to serum cortisol (r = −0.196; P = 0.031; n = 123).
Associations between energy expenditure, sleep, and metabolic variables
Table 4 reports a backwise multiple regression model of energy expenditure vs. hormones and sleep parameters. RDI and REE were related in a positive fashion: 10 additional events of apnea would increase REE by 84 kcal/d and increase RQ by 0.01, roughly a 5% shift in calories from fat to carbohydrate oxidation. Serum cortisol was positively related to REE: a 10 μg/dl increase in cortisol would lead to 132 and 200 kcal/d increases in REE and adjusted REE, respectively. Urinary norepinephrine (NE) was also positively related to REE. Of note, the relationship between RDI and REE was 3-fold stronger in men than women (P = 0.035), and this was still present when corrected for plasma cortisol levels (data not shown).
Table 4.
Backward stepwise linear regression models relating sleep and hormone parameters with energy expenditure variables
| REE (kcal/d) |
Adjusted REE (kcal/d)a |
RQ |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameter estimate | se | P value | Parameter estimate | se | P value | Parameter estimate | se | P value | |
| Total group | |||||||||
| RDI (events/h) | 8.40 | 1.55 | <0.001 | 0.0010 | 0.00030 | <0.001 | |||
| Serum cortisol (μg/dl) | 13.18 | 5.97 | 0.032 | 20.20 | 7.38 | 0.007 | |||
| NE (μg/24 h) | 3.60 | 1.35 | 0.009 | ||||||
| Women | |||||||||
| RDI (events/h) | 3.84 | 2.02 | 0.063 | 0.0014 | 0.00049 | 0.004 | |||
| Serum cortisol (μg/dl) | 12.32 | 5.21 | 0.030 | 13.41 | 3.60 | <0.001 | |||
| ESS | −5.14 | 2.14 | 0.014 | ||||||
| Men | |||||||||
| RDI (events/h) | 10.16 | 2.43 | <0.001 | 4.44 | 1.47 | 0.006 | |||
| Subjective sleep (h/night) | 0.026 | 0.009 | 0.008 | ||||||
Adjusted by age, gender, and fat-free mass.
Discussion
We determined the presence and severity of sleep apnea and assessed sleep duration and quality in a large prospectively assembled cohort of obese individuals who regularly slept less than 6.5 h per night. In cross-sectional analyses, sleep apnea, short sleep, and sleep disturbances were associated with activation of the HPA axis and the sympathoadrenal system, the two major components of the stress system. These findings are clinically important for weight control, body composition, and stress-related pathologies (23).
Because sleep apnea and short sleep are both associated with obesity, their individual contribution cannot be easily extricated. It is known that subjects with sleep apnea report gaining weight before being diagnosed with sleep apnea (35, 36). It is unclear whether this gain is due to increased energy intake, decreased energy expenditure, or a combination of these two factors. Severity of sleep apnea was directly related to REE. We hypothesize that in this sample, sleep apnea acted as a stressor that activated the catecholaminergic system, thus increasing REE. It remains to be determined whether over time there may be a desensitization to this β-adrenergic stimulation. It is known that sleep apnea causes hypoxia; the greater the oxygen desaturation, the lower the REE (37). Prospective assessment of subjects with sleep apnea may determine the net effects on weight of these different contributing factors.
Sleep apnea and poor sleep efficiency were associated with increased fasting RQ. RQ is an index of substrate oxidation: higher values herald preference for carbohydrate vs. fat oxidation. High RQ strongly predicts fat accumulation over time (38). Two studies showed an increase in RQ with decreased sleep time (16, 39), whereas two other studies reported no effect (27, 40). These conflicting results were likely due to differences in participants and study design. We suggest that the relationship between sleep and energy balance is more complex than initially thought and may change over time with development of adaptive mechanisms to hypoxia.
Insufficient sleep impairs recovery from fatigue and causes long-term negative consequences (23). Based on our results, a 10-μg/dl increase in serum cortisol would increase the basal metabolic rate by approximately 10%. In healthy lean subjects, both experimentally induced total sleep deprivation and sleep fragmentation increase energy expenditure by approximately 7% (27, 39). It remains to be determined whether a similar effect is observed in obese subjects in naturalistic conditions.
The effects of sleep apnea on ACTH and cortisol have been extensively studied with varying results. Alterations in ACTH and cortisol secretion dynamics, as well as low cortisol levels, have been reported (41, 42). As recently summarized in a meta-analysis, the collective evidence supporting cortisol alterations in individuals with sleep apnea is inconclusive (43). Withdrawal from continuous positive airway pressure therapy is associated with an increase in urinary NE and epinephrine, suggesting an involvement of the catecholaminergic system as well (44).
We observed several gender differences. Morning levels of plasma ACTH and serum cortisol and 24-h UFC, NE, epinephrine, and dopamine were higher in men than women. Interestingly, women slept 30 min longer than men, as measured by actigraphy, but the perceived sleep duration was considerably shorter than the one assessed by actigraphy. The relationship between sleep apnea and REE was stronger in men than women, so that a 10 U increase in RDI would translate to a 100-calorie per day increase in men, but only 30 calories per day in women. These findings indicate that sleep apnea and chronic sleep deprivation have qualitatively and quantitatively different effects in men and women. Because our study was not designed to assess gender differences, these associations should be considered to be of exploratory nature and hypothesis generating.
In this naturalistic study, women were examined regardless of their menstrual cycle. This represents a limitation of the study. Menstrual cycle influences many parameters, including energy expenditure and sleep. REE is increased, via a β-adrenergic mechanism, by approximately 2% in the midluteal phase vs. the early follicular phase (45). Sleep time and quality and sleepiness are different in the different phases of the menstrual cycle (46). Some of the sex steroid actions on sleep are direct, whereas others are secondary to changes in HPA axis functioning across the menstrual cycle. As an example, the cortisol awakening response, a useful marker of HPA axis activity, varies across the menstrual phases, with higher values around the time of ovulation (47).
Study merits include a large, prospectively assembled, and well-characterized sample with a good representation of minorities, which reflects the epidemiology of sleep deprivation. Sleep was characterized by an array of objective measures and standardized questionnaires. Our analyses were, however, cross-sectional, and, as per study design, we lacked a control group of obese, non-sleep-deprived individuals.
Overall, our data suggest that sleep apnea, sleep time, and sleep efficiency are important determinants of REE and RQ. According to the National Health and Nutrition Examination Survey, 12% of obese men and 7% of obese women have sleep apnea (48). A recent CDC survey indicated that 35% of the adult population sleeps less than 7 h per night (2). Our findings have bearing on weight control for the increasing portion of the U.S. population who are obese, chronically sleep-deprived, and suffer from sleep apnea, because sleep apnea and sleep deprivation are modifiable factors and both can be corrected by continuous positive airway pressure devices and sleeping longer, respectively. Clinical approaches to this large segment of the U.S. population should be informed by clinical assessment of sleep apnea. Future studies should assess the long-term negative consequences of chronic activation of the stress system due to sleep deprivation and sleep apnea.
In conclusion, poor sleep quality was associated with increased REE, a higher RQ indicating a shift from fat toward carbohydrate oxidation, and activation of the stress system.
Acknowledgments
We thank the following colleagues for their scientific advice and critical suggestions in the development and conduct of the study protocol: Karim Calis, Janet Gershengorn, Gregor Hasler, Emmanuel Mignot, Kristina I. Rother, Monica Skarulis, Duncan Wallace, Robert Wesley, Elizabeth Wright. We also thank the past and present members of the study team: Peter Bailey, Meredith Coyle, Paula Marincola, Patrick Michaels, Svetlana Primma, Angela Ramer, Rebecca Romero, Megan Sabo, Tanner Slayden, Sara Torvik, Elizabeth Widen, and Lyda Williams. The bioinformatics support of Mr. Frank Pierce (Esprit Health) is gratefully acknowledged. Finally, we are grateful to all of our enthusiastic study subjects.
This study was supported by the Intramural Programs of the National Institute of Diabetes, Digestive, and Kidney Diseases (NIDDK), and the Clinical Center, National Institutes of Health (NIH). This study was conducted under the NIDDK protocol 06-DK-0036 and is listed in www.ClinicalTrials.gov (identifier: NCT00261898). Statistical expertise and a central sample-handling and assays facility are provided by the NIDDK Intramural Obesity Initiative of the NIH Clinical Center.
Disclosure Summary: None of the authors had a conflict of interest.
Footnotes
- BMI
- Body mass index
- HPA
- hypothalamic-pituitary-adrenal
- NE
- norepinephrine
- RDI
- respiratory disturbance index
- REE
- resting energy expenditure
- RQ
- respiratory quotient
- UFC
- urinary free cortisol.
References
- 1. Grandner MA, Hale L, Moore M, Patel NP. 2010. Mortality associated with short sleep duration: the evidence, the possible mechanisms, and the future. Sleep Med Rev 14:191–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Centers for Disease Control and Prevention (CDC) 2011. Effect of short sleep duration on daily activities—United States, 2005–2008. MMWR Morb Mortal Wkly Rep 60:239–242 [PubMed] [Google Scholar]
- 3. Johnson EO. 2000. Sleep in America: 2000. Results from The National Sleep Foundation's 2000 Omnibus Sleep Poll. Washington, DC: National Sleep Foundation [Google Scholar]
- 4. Monroe LJ. 1967. Psychological and physiological differences between good and poor sleepers. J Abnorm Psychol 72:255–264 [DOI] [PubMed] [Google Scholar]
- 5. Siervo M, Wells JC, Cizza G. 2009. The contribution of psychosocial stress to the obesity epidemic: an evolutionary approach. Horm Metab Res 41:261–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wittmann M, Dinich J, Merrow M, Roenneberg T. 2006. Social jet lag: misalignment of biological and social time. Chronobiol Int 23:497–509 [DOI] [PubMed] [Google Scholar]
- 7. Flegal KM, Carroll MD, Ogden CL, Johnson CL. 2002. Prevalence and trends in obesity among US adults, 1999–2000. JAMA 288:1723–1727 [DOI] [PubMed] [Google Scholar]
- 8. Ayas NT, White DP, Al-Delaimy WK, Manson JE, Stampfer MJ, Speizer FE, Patel S, Hu FB. 2003. A prospective study of self-reported sleep duration and incident diabetes in women. Diabetes Care 26:380–384 [DOI] [PubMed] [Google Scholar]
- 9. Chaput JP, Brunet M, Tremblay A. 2006. Relationship between short sleeping hours and childhood overweight/obesity: results from the “Québec en Forme” Project. Int J Obes (Lond) 30:1080–1085 [DOI] [PubMed] [Google Scholar]
- 10. Nilsson PM, Rööst M, Engström G, Hedblad B, Berglund G. 2004. Incidence of diabetes in middle-aged men is related to sleep disturbances. Diabetes Care 27:2464–2469 [DOI] [PubMed] [Google Scholar]
- 11. von Kries R, Toschke AM, Wurmser H, Sauerwald T, Koletzko B. 2002. Reduced risk for overweight and obesity in 5- and 6-y-children by duration of sleep—a cross-sectional study. Int J Obes Relat Metab Disord 26:710–716 [DOI] [PubMed] [Google Scholar]
- 12. Van Cauter E, Spiegel K. 1999. Sleep as a mediator of the relationship between socioeconomic status and health: a hypothesis. Ann NY Acad Sci 896:254–261 [DOI] [PubMed] [Google Scholar]
- 13. Taheri S, Lin L, Austin D, Young T, Mignot E. 2004. Short sleep is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med 1:e62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Léger D, Roscoat E, Bayon V, Guignard R, Pâquereau J, Beck F. 2011. Short sleep in young adults: insomnia or sleep debt? Prevalence and clinical description of short sleep in a representative sample of 1004 young adults from France. Sleep Med 12:454–462 [DOI] [PubMed] [Google Scholar]
- 15. Cizza G, Requena M, Galli G, de Jonge L. 2011. Chronic sleep deprivation and seasonality: implications for the obesity epidemic. J Endocrinol Invest 34:793–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nedeltcheva AV, Kilkus JM, Imperial J, Schoeller DA, Penev PD. 2010. Insufficient sleep undermines dietary efforts to reduce adiposity. Ann Intern Med 153:435–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Penev PD. 2007. Sleep deprivation and energy metabolism: to sleep, perchance to eat? Curr Opin Endocrinol Diabetes Obes 14:374–381 [DOI] [PubMed] [Google Scholar]
- 18. Spiegel K, Tasali E, Penev P, Van Cauter E. 2004. Brief communication: sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med 141:846–850 [DOI] [PubMed] [Google Scholar]
- 19. St-Onge MP, Roberts AL, Chen J, Kelleman M, O'Keeffe M, RoyChoudhury A, Jones PJ. 2011. Short sleep duration increases energy intakes but does not change energy expenditure in normal-weight individuals. Am J Clin Nutr 94:410–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stamatakis KA, Punjabi NM. 2010. Effects of sleep fragmentation on glucose metabolism in normal subjects. Chest 137:95–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schmid SM, Hallschmid M, Jauch-Chara K, Wilms B, Lehnert H, Born J, Schultes B. 2011. Disturbed glucoregulatory response to food intake after moderate sleep restriction. Sleep 34:371–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Spiegel K, Leproult R, Van Cauter E. 1999. Impact of sleep dept on metabolic and endocrine function. Lancet 354:1435–1439 [DOI] [PubMed] [Google Scholar]
- 23. McEwen BS, Seeman T. 1999. Protective and damaging effects of mediators of stress. Elaborating and testing the concepts of allostasis and allostatic load. Ann NY Acad Sci 896:30–47 [DOI] [PubMed] [Google Scholar]
- 24. Vgontzas AN, Mastorakos G, Bixler EO, Kales A, Gold PW, Chrousos GP. 1999. Sleep deprivation effects on the activity of the hypothalamic-pituitary-adrenal and growth axes: potential clinical implications. Clin Endocrinol (Oxf) 51:205–215 [DOI] [PubMed] [Google Scholar]
- 25. Chrousos GP, Gold PW. 1992. The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. JAMA 267:1244–1252 [PubMed] [Google Scholar]
- 26. Lack LC, Gradisar M, Van Someren EJ, Wright HR, Lushington K. 2008. The relationship between insomnia and body temperatures. Sleep Med Rev 12:307–317 [DOI] [PubMed] [Google Scholar]
- 27. Jung CM, Melanson EL, Frydendall EJ, Perreault L, Eckel RH, Wright KP. 2011. Energy expenditure during sleep, sleep deprivation and sleep following sleep deprivation in adult humans. J Physiol 589:235–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tataranni PA, Larson DE, Snitker S, Young JB, Flatt JP, Ravussin E. 1996. Effects of glucocorticoids on energy metabolism and food intake in humans. Am J Physiol 271:E317–E325 [DOI] [PubMed] [Google Scholar]
- 29. Cizza G, Marincola P, Mattingly M, Williams L, Mitler M, Skarulis M, Csako G. 2010. Treatment of obesity with extension of sleep duration: a randomized, prospective, controlled trial. Clin Trials 7:274–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cunningham JJ. 1990. Calculation of energy expenditure from indirect calorimetry: assessment of the Weir equation. Nutrition 6:222–223 [PubMed] [Google Scholar]
- 31. Lichstein KL, Stone KC, Donaldson J, Nau SD, Soeffing JP, Murray D, Lester KW, Aguillard RN. 2006. Actigraphy validation with insomnia. Sleep 29:232–239 [PubMed] [Google Scholar]
- 32. Blackman A, McGregor C, Dales R, Driver HS, Dumov I, Fleming J, Fraser K, George C, Khullar A, Mink J, Moffat M, Sullivan GE, Fleetham JA, Ayas N, Bradley TD, Fitzpatrick M, Kimoff J, Morrison D, Ryan F, Skomro R, Series F. 2010. Canadian Sleep Society/Canadian Thoracic Society position paper on the use of portable monitoring for the diagnosis of obstructive sleep apnea/hypopnea in adults. Can Respir J 17:229–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Backhaus J, Junghanns K, Broocks A, Riemann D, Hohagen F. 2002. Test-retest reliability and validity of the Pittsburgh Sleep Quality Index in primary insomnia. J Psychosom Res 53:737–740 [DOI] [PubMed] [Google Scholar]
- 34. Nguyen AT, Baltzan MA, Small D, Wolkove N, Guillon S, Palayew M. 2006. Clinical reproducibility of the Epworth Sleepiness Scale. J Clin Sleep Med 2:170–174 [PubMed] [Google Scholar]
- 35. Phillips BG, Hisel TM, Kato M, Pesek CA, Dyken ME, Narkiewicz K, Somers VK. 1999. Recent weight gain in patients with newly diagnosed obstructive sleep apnea. J Hypertens 17:1297–1300 [DOI] [PubMed] [Google Scholar]
- 36. Traviss KA, Barr SI, Fleming JA, Ryan CF. 2002. Lifestyle-related weight gain in obese men with newly diagnosed obstructive sleep apnea. J Am Diet Assoc 102:703–706 [DOI] [PubMed] [Google Scholar]
- 37. Hins J, Sériès F, Alméras N, Tremblay A. 2006. Relationship between severity of nocturnal desaturation and adaptive thermogenesis: preliminary data of apneic patients tested in a whole-body indirect calorimetry chamber. Int J Obes (Lond) 30:574–577 [DOI] [PubMed] [Google Scholar]
- 38. Ravussin E, Swinburn BA. 1993. Metabolic predictors of obesity: cross-sectional versus longitudinal data. Int J Obes Relat Metab Disord 17:S28–S31 [PubMed] [Google Scholar]
- 39. Hursel R, Rutters F, Gonnissen HK, Martens EA, Westerterp-Plantenga MS. 2011. Effects of sleep fragmentation in healthy men on energy expenditure, substrate oxidation, physical activity, and exhaustion measured over 48 h in a respiratory chamber. Am J Clin Nutr 94:804–808 [DOI] [PubMed] [Google Scholar]
- 40. Bosy-Westphal A, Hinrichs S, Jauch-Chara K, Hitze B, Later W, Wilms B, Settler U, Peters A, Kiosz D, Muller MJ. 2008. Influence of partial sleep deprivation on energy balance and insulin sensitivity in healthy women. Obes Facts 1:266–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Henley DE, Russell GM, Douthwaite JA, Wood SA, Buchanan F, Gibson R, Woltersdorf WW, Catterall JR, Lightman SL. 2009. Hypothalamic-pituitary-adrenal axis activation in obstructive sleep apnea: the effect of continuous positive airway pressure therapy. J Clin Endocrinol Metab 94:4234–4242 [DOI] [PubMed] [Google Scholar]
- 42. Vgontzas AN, Pejovic S, Zoumakis E, Lin HM, Bentley CM, Bixler EO, Sarrigiannidis A, Basta M, Chrousos GP. 2007. Hypothalamic-pituitary-adrenal axis activity in obese men with and without sleep apnea: effects of continuous positive airway pressure therapy. J Clin Endocrinol Metab 92:4199–4207 [DOI] [PubMed] [Google Scholar]
- 43. Tomfohr LM, Edwards KM, Dimsdale JE. 2012. Is obstructive sleep apnea associated with cortisol levels? A systematic review of the research evidence. Sleep Med Rev 16:243–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kohler M, Stoewhas AC, Ayers L, Senn O, Bloch KE, Russi EW, Stradling JR. 2011. Effects of continuous positive airway pressure therapy withdrawal in patients with obstructive sleep apnea: a randomized controlled trial. Am J Respir Crit Care Med 184:1192–1129 [DOI] [PubMed] [Google Scholar]
- 45. Day DS, Gozansky WS, Van Pelt RE, Schwartz RS, Kohrt WM. 2005. Sex hormone suppression reduces resting energy expenditure and β-adrenergic support of resting energy expenditure. J Clin Endocrinol Metab 90:3312–3317 [DOI] [PubMed] [Google Scholar]
- 46. Driver HS, Baker FC. 1998. Menstrual factors in sleep. Sleep Med Rev 2:213–229 [DOI] [PubMed] [Google Scholar]
- 47. Wolfram M, Bellingrath S, Kudielka BM. 2011. The cortisol awakening response (CAR) across the female menstrual cycle. Psychoneuroendocrinology 36:905–912 [DOI] [PubMed] [Google Scholar]
- 48. Gurubhagavatula I, Maislin G, Pack AI. 2001. An algorithm to stratify sleep apnea risk in a sleep disorders clinic population. Am J Respir Crit Care Med 164:1904–1909 [DOI] [PubMed] [Google Scholar]