Abstract
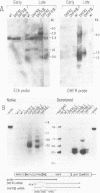
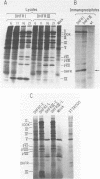
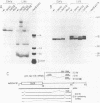
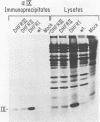
The EIa region of an Adenovirus 5 recombinant has been substituted by a modular gene encoding dihydrofolate reductase (DHFR). In this recombinant, the mouse DHFR cDNA was positioned behind sequences of the major late promoter and the complete tripartite leader. The leader sequences end in the normal 5' splice site (SS) of the third leader, so that RNA splicing joins the tripartite leader to a 3' splice site immediately upstream of the DHFR cDNA. At late stages of infection, high levels of DHFR mRNAs were synthesized. At early times in the late stage, this mRNA was efficiently translated; however, at later times translation of DHFR decreased probably due to poor competition with other late mRNAs. Synthesis of DHFR protein from an analogous Adenovirus 5 recombinant containing only the first late leader was studied in parallel. Equivalent levels of DHFR mRNA were expressed after infection with this recombinant virus; however, the efficiency of DHFR translation was at least 20 fold lower than that of the DHFR mRNA containing the tripartite leader. This suggests that the tripartite leader sequence is important for translation in the late stage of infection. As reported previously, the Ad5 recombinant containing only the first leader vastly overexpresses polypeptide IX from a novel mRNA, formed by the splicing of the first leader in the modular DHFR gene to the 3' splice site in the EIb region. Cells infected with this recombinant synthesize very little normal mRNA from the EIb region. Here, we demonstrated that coinfection of 293 cells with this recombinant and wild type Adenovirus 5 also results in decreased EIb mRNA synthesis. We propose that the overproduction of polypeptide IX suppresses mRNA expression from the EIb and IX promoter sites, probably by an autoregulation loop active during lytic growth.
Full text
PDF


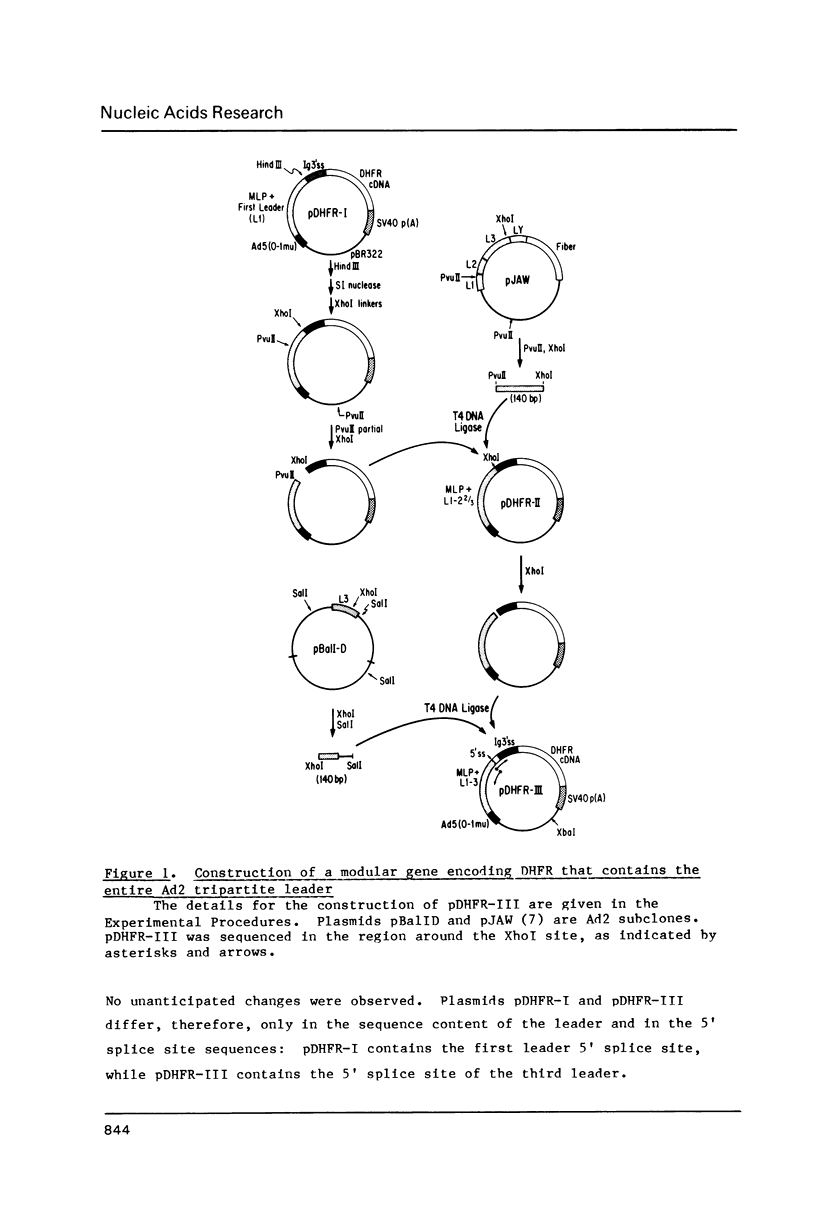

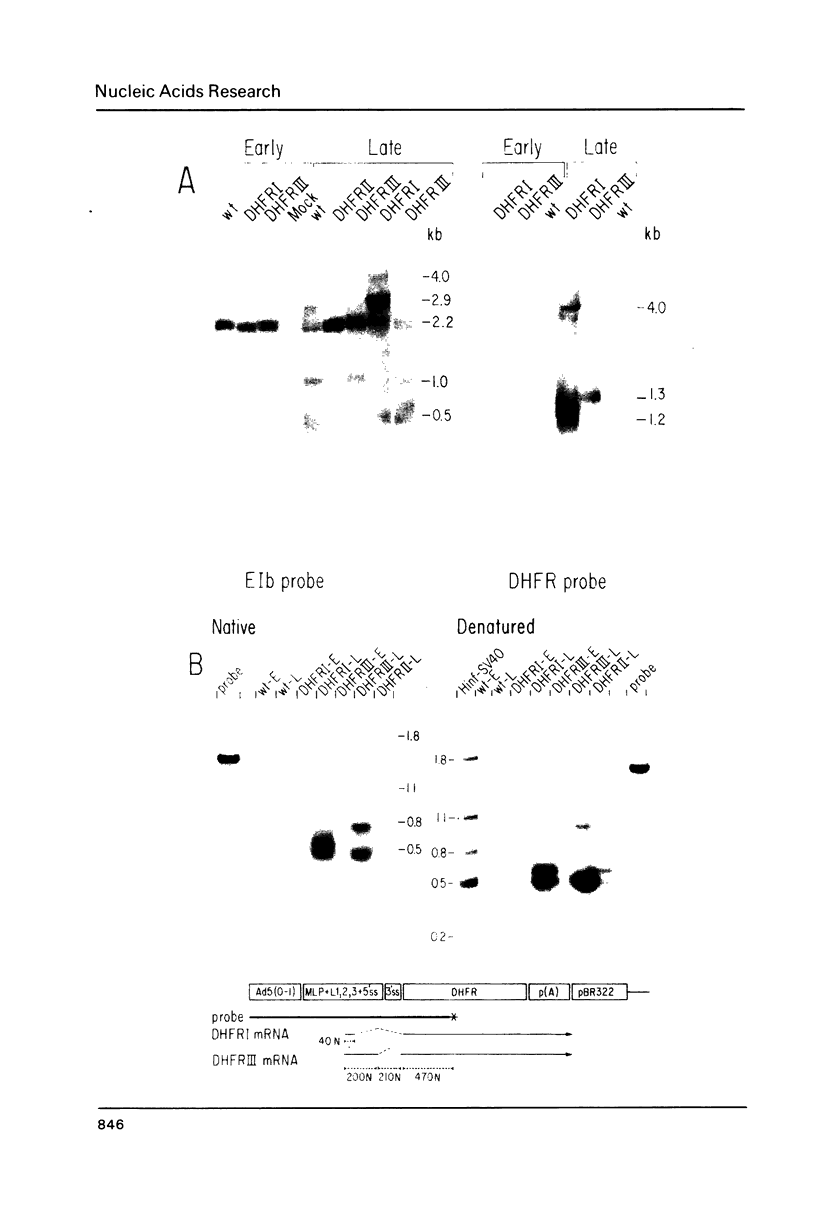

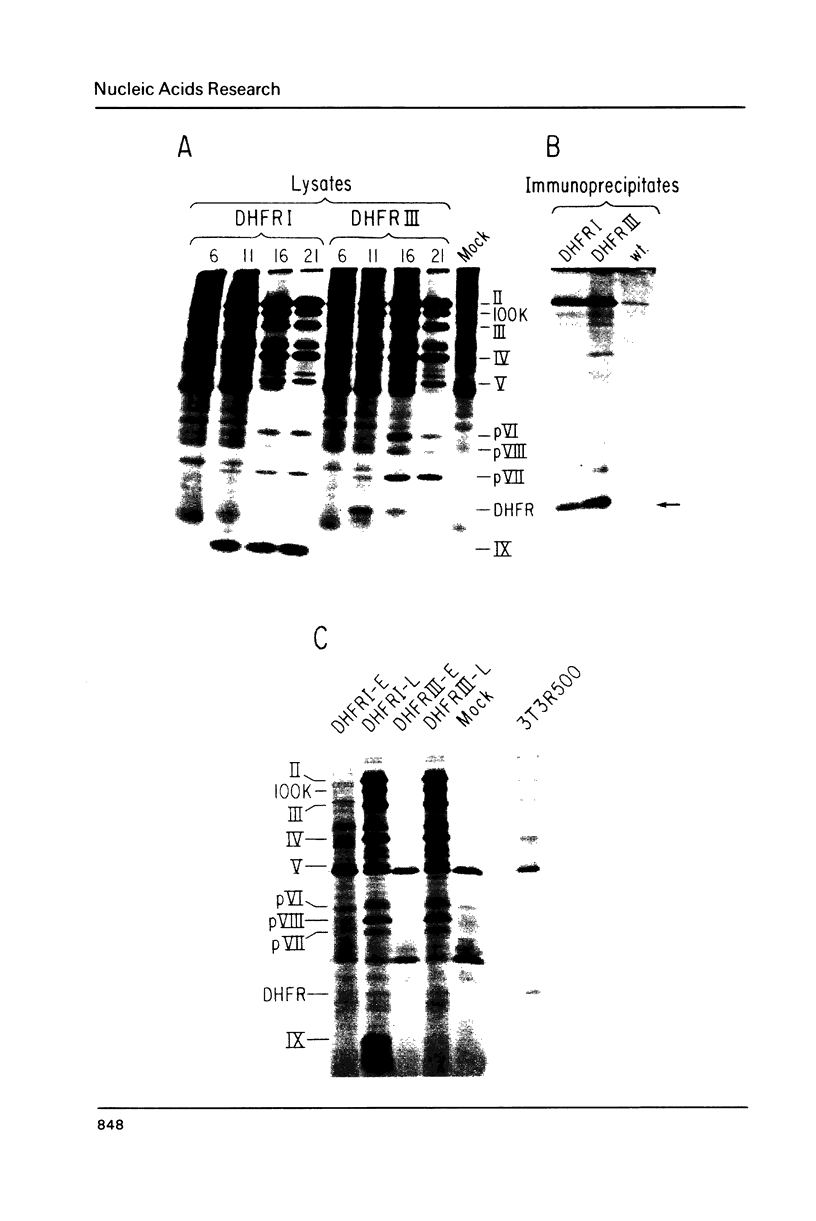

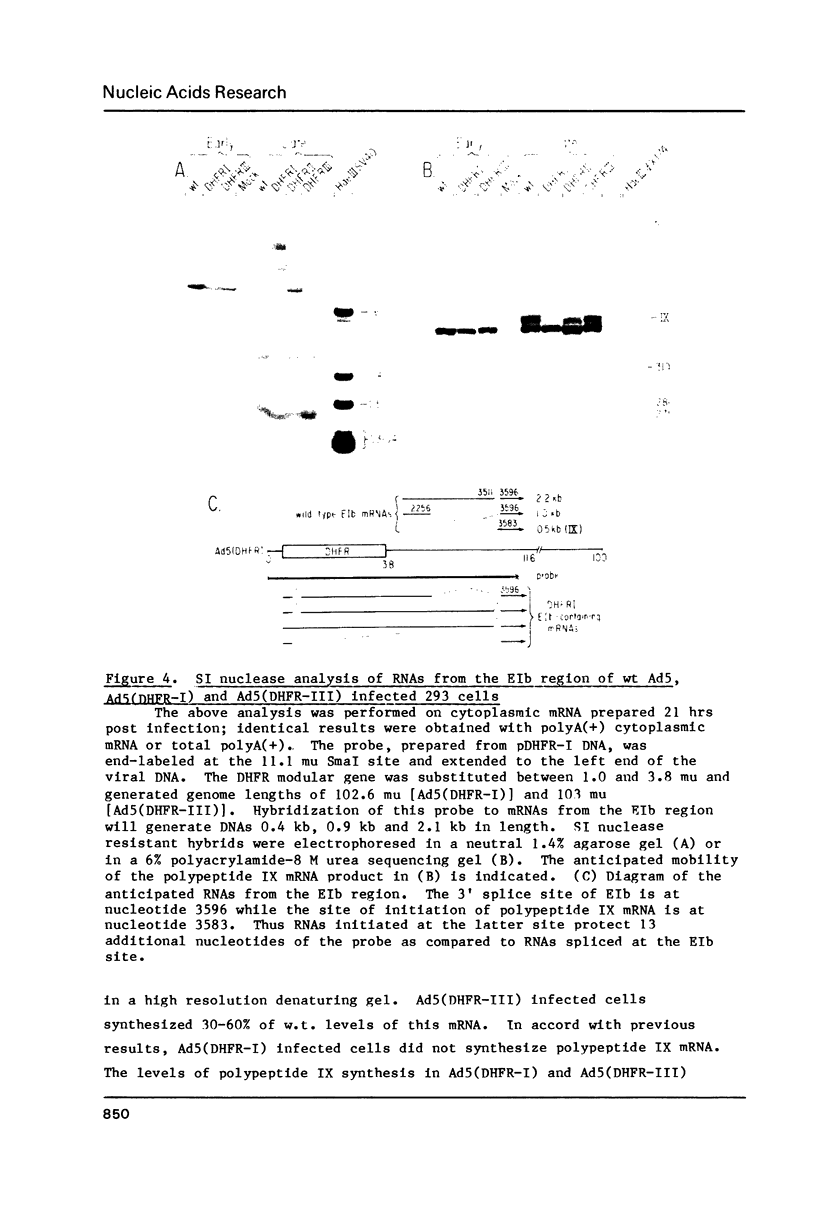

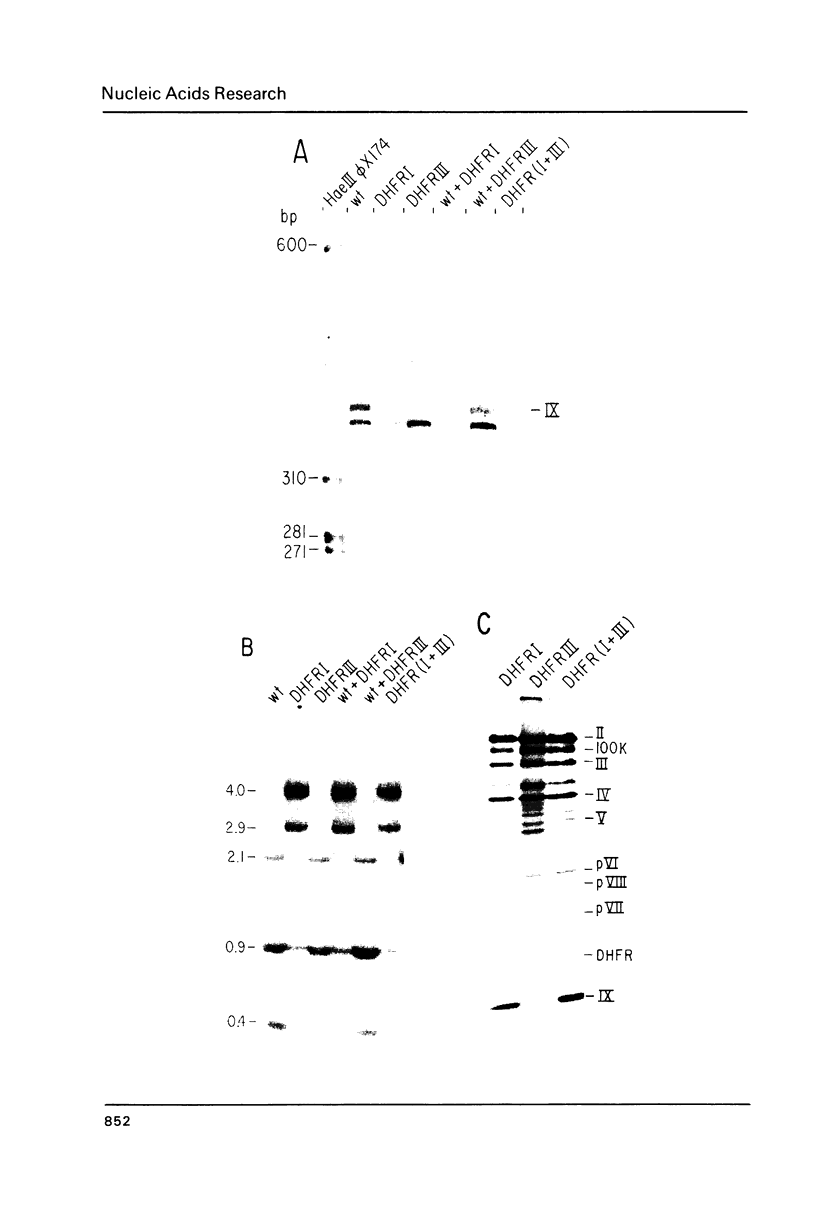





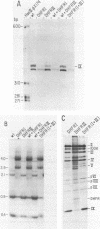
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berget S. M., Moore C., Sharp P. A. Spliced segments at the 5' terminus of adenovirus 2 late mRNA. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3171–3175. doi: 10.1073/pnas.74.8.3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkner K. L., Sharp P. A. Expression of dihydrofolate reductase, and of the adjacent EIb region, in an Ad5-dihydrofolate reductase recombinant virus. Nucleic Acids Res. 1984 Feb 24;12(4):1925–1941. doi: 10.1093/nar/12.4.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepko C. L., Changelian P. S., Sharp P. A. Immunoprecipitation with two-dimensional pools as a hybridoma screening technique: production and characterization of monoclonal antibodies against adenovirus 2 proteins. Virology. 1981 Apr 30;110(2):385–401. doi: 10.1016/0042-6822(81)90069-6. [DOI] [PubMed] [Google Scholar]
- Chow L. T., Roberts J. M., Lewis J. B., Broker T. R. A map of cytoplasmic RNA transcripts from lytic adenovirus type 2, determined by electron microscopy of RNA:DNA hybrids. Cell. 1977 Aug;11(4):819–836. doi: 10.1016/0092-8674(77)90294-x. [DOI] [PubMed] [Google Scholar]
- Graham F. L., Smiley J., Russell W. C., Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977 Jul;36(1):59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Kellems R. E., Morhenn V. B., Pfendt E. A., Alt F. W., Schimke R. T. Polyoma virus and cyclic AMP-mediated control of dihydrofolate reductase mRNA abundance in methotrexate-resistant mouse fibroblasts. J Biol Chem. 1979 Jan 25;254(2):309–318. [PubMed] [Google Scholar]
- Logan J., Shenk T. Adenovirus tripartite leader sequence enhances translation of mRNAs late after infection. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3655–3659. doi: 10.1073/pnas.81.12.3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montell C., Fisher E. F., Caruthers M. H., Berk A. J. Inhibition of RNA cleavage but not polyadenylation by a point mutation in mRNA 3' consensus sequence AAUAAA. Nature. 1983 Oct 13;305(5935):600–605. doi: 10.1038/305600a0. [DOI] [PubMed] [Google Scholar]
- Solnick D. Shuffling adenovirus promoters: a viral recombinant with early region 1A under late transcriptional control. EMBO J. 1983;2(6):845–851. doi: 10.1002/j.1460-2075.1983.tb01512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stow N. D. Cloning of a DNA fragment from the left-hand terminus of the adenovirus type 2 genome and its use in site-directed mutagenesis. J Virol. 1981 Jan;37(1):171–180. doi: 10.1128/jvi.37.1.171-180.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson C., Akusjärvi G. Adenovirus VA RNAI: a positive regulator of mRNA translation. Mol Cell Biol. 1984 Apr;4(4):736–742. doi: 10.1128/mcb.4.4.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thimmappaya B., Weinberger C., Schneider R. J., Shenk T. Adenovirus VAI RNA is required for efficient translation of viral mRNAs at late times after infection. Cell. 1982 Dec;31(3 Pt 2):543–551. doi: 10.1016/0092-8674(82)90310-5. [DOI] [PubMed] [Google Scholar]
- Thummel C., Tjian R., Grodzicker T. Expression of SV40 T antigen under control of adenovirus promoters. Cell. 1981 Mar;23(3):825–836. doi: 10.1016/0092-8674(81)90447-5. [DOI] [PubMed] [Google Scholar]
- Thummel C., Tjian R., Hu S. L., Grodzicker T. Translational control of SV40 T antigen expressed from the adenovirus late promoter. Cell. 1983 Jun;33(2):455–464. doi: 10.1016/0092-8674(83)90427-0. [DOI] [PubMed] [Google Scholar]
- Van Ormondt H., Maat J., De Waard A., Van der Eb A. J. The nucleotide sequence of the transforming HpaI-E fragment of adenovirus type 5 DNA. Gene. 1978 Dec;4(4):309–328. doi: 10.1016/0378-1119(78)90048-3. [DOI] [PubMed] [Google Scholar]
- Zain S., Sambrook J., Roberts R. J., Keller W., Fried M., Dunn A. R. Nucleotide sequence analysis of the leader segments in a cloned copy of adenovirus 2 fiber mRNA. Cell. 1979 Apr;16(4):851–861. doi: 10.1016/0092-8674(79)90100-4. [DOI] [PubMed] [Google Scholar]