The mammalian meiosis-specific KASH protein KASH5 connects the telomere-associated SUN1 protein to the cytoplasmic force–generating mechanism involved in meiotic chromosome movement.
Abstract
In yeasts and worms, KASH (Klarsicht/ANC-1/Syne/homology) domain and SUN (Sad-1/UNC-84) domain nuclear envelope (NE) proteins play a crucial role in meiotic chromosome movement and homologue pairing. However, although the vertebrate SUN domain protein SUN1 is involved in these processes, its partner has remained identified. Based on subcellular localization screening in mouse spermatocytes, we identified a novel germ cell–specific protein, KASH5, that localized exclusively at telomeres from the leptotene to diplotene stages in both spermatocytes and oocytes. KASH5 possesses hitherto unknown KASH-related sequences that directly interacted with SUN1 and mediated telomere localization. Thus, KASH5 is a mammalian meiosis-specific KASH domain protein. We show that meiotic chromosome movement depended on microtubules and that KASH5 interacted with the microtubule-associated dynein–dynactin complex. These results suggest that KASH5 connects the telomere-associated SUN1 protein to the cytoplasmic force–generating mechanism involved in meiotic chromosome movement. Our study strongly suggests that the meiotic homologue-pairing mechanism mediated by the SUN–KASH NE bridge is highly conserved among eukaryotes.
Introduction
Many cellular and developmental events, such as cell migration, cell division, and fertilization, occur depending on proper nuclear localization and movement. These processes are controlled by cytoplasmic microtubule and actin-based networks. The SUN (Sad-1/UNC-84) domain family of inner nuclear membrane (INM) proteins interacts with KASH (Klarsicht/ANC-1/Syne/homology) domain proteins, which are localized to the outer nuclear membrane (ONM). Thus, the SUN–KASH protein complexes bridge across the INM and ONM. Because cytoplasmic extensions of the KASH domain proteins tether the nucleus to the cytoskeleton, the SUN–KASH protein complexes play a crucial role in transferring the driving force generated by the cytoskeleton to the nuclear envelope (NE; Fridkin et al., 2009; Razafsky and Hodzic, 2009; Starr and Fridolfsson, 2010).
The pairing of homologous chromosomes during meiosis is a vital event for proper meiotic recombination and chromosome segregation, and this process largely depends on the dynamic chromosome movements specifically observed during meiotic prophase (Scherthan, 2001; Bhalla and Dernburg, 2008). In yeasts and worms, SUN domain proteins are tethered to telomeres and specific chromosomal loci (pairing centers), respectively, and SUN–KASH protein complexes connect the chromosomes to cytoskeleton, promoting chromosome movements and homologue pairing during meiosis (Hiraoka and Dernburg, 2009).
In mammalian spermatocytes, nuclear movements (nuclear rotation and chromosome movement) are observed from late leptotene toward zygotene, slowing down in early pachytene (Scherthan et al., 1996). In mice, SUN domain protein SUN1 localizes at the NE in somatic cells but concentrates at telomeres in meiotic prophase I to promote telomere movement and homologue pairing (Ding et al., 2007). However, because a putative KASH domain protein acting with SUN1 for homologue pairing remains to be identified, it is unknown whether the mechanism discovered in yeasts and worms is indeed conserved in mammals. Based on subcellular localization screening in mouse germ cells, we now identified a meiosis-specific KASH domain protein, KASH5, which localizes at telomeres and interacts with SUN1, thus implicated in meiotic chromosome dynamics and homologue pairing.
Results and discussion
With the aim of identifying an interacting protein for the mouse cohesin protector shugoshin 2 during meiosis (Lee et al., 2008), we performed yeast two-hybrid screening using a testis cDNA library. The expression profiles of the obtained candidate genes were examined by RT-PCR, and meiosis-specific genes were selected. We produced the full-length cDNAs of the genes using mRNA, fused them to GFP, and expressed them in spermatocytes under control of an ectopic promoter. This enabled us to screen for meiotic factors showing characteristic localization in mouse germ cells even though they might not be relevant to shugoshin 2. During this screening, we identified an uncharacterized protein named coiled-coil domain–containing protein 155 (Ccdc155), which localized at several punctate dots in the spermatocytes (not depicted; see Full column in Fig. 4 B). Database searches for proteins homologous to Ccdc155 revealed that Ccdc155 was highly conserved in vertebrate species (Fig. S1). To detect endogenous Ccdc155 expression, we raised antibodies against Ccdc155 (Fig. 1 A) and used these to immunostain spermatocytes. Although some of the Ccdc155 dots colocalized with centromere protein C (CENP-C), additional Ccdc155 dots devoid of CENP-C were detected (Fig. 1 B). As centromeres of mouse chromosomes are all telocentric, this result suggests that Ccdc155 dots might localize to telomeres locating at both ends of the chromosome rather than to the centromere located near one end. To examine this possibility, we immunostained spermatocytes with antibodies against the telomere-binding protein TRF2 together with antibodies against synaptonemal complex protein 3 (SCP3). Ccdc155 colocalized with TRF2 at both ends of synapsed chromosomal axes in the pachytene stage (Fig. 1 C). These results indicate that Ccdc155 localizes at or close to telomeres in spermatocytes and that two-hybrid interaction between Ccdc155 and the centromeric protein shugoshin 2 might be insignificant.
Figure 4.
The KASH domain of KASH5 and inner NE protein SUN1 are required for telomere localization of KASH5. (A) Schematic representations of GFP-tagged KASH5 mutants. TM, transmembrane. (B) Spermatocytes from the testis transfected with GFP-tagged KASH5 mutants were stained with GFP and SCP3 antibodies. (C) Pachytene spermatocytes of wild-type (WT) and SUN1 KO mice were stained by the indicated antibodies. The top images show the localization of KASH5 signals (anti-KASH5 antibody) in the areas boxed in the bottom images. Note that KASH5 localization to telomeres was abolished in SUN1 KO mice. Bars, 5 µm.
Figure 1.
Identification of a novel mammalian KASH protein. (A) Total testis extracts were loaded, and Western blotting was performed with KASH5 serum. A single band was detected corresponding to the predicted size (72 kD). (B) Chromosome spreads from spermatocytes were stained with KASH5 and CENP-C antibodies. (C) Chromosome spreads from spermatocytes were stained with KASH5 and TRF2 antibodies. Magnified images of the boxed area are shown on the right. (D) Amino acid sequence alignment of KASH proteins. Identical amino acids are shaded in black, and similar amino acids are shaded in gray. Dm, Drosophila melanogaster; Mm, Mus musculus; Ce, C. elegans; Sp, S. pombe. (E and F) mRNA expression in various tissues was analyzed by RT-PCR. E, embryonic day; GAPDH, glyceraldehyde 3-phosphate dehydrogenase. Bars, 3 µm.
Although four KASH domain proteins, Nesprin/Syne1–4, are present in the database of mouse proteins (Grady et al., 2005), all of these proteins show clear similarity to the canonical KASH domain protein ANC-1 of Caenorhabditis elegans. Notably, worms also have a divergent KASH domain protein, ZYG-12, carrying an exceptionally short KASH domain, and this protein acts with a SUN domain protein for homologue pairing during meiosis (Fig. 1 D; Penkner et al., 2007; Fridkin et al., 2009). Furthermore, fission yeast Kms1 is required for meiotic homologue pairing and also carries a short KASH domain (Fig. 1 D; Miki et al., 2004; Starr and Fischer, 2005). Therefore, proteins carrying a divergent KASH domain might remain unidentified in mammals. In this regard, we found a leucine-rich region and a subsequent short proline-rich sequence at the C-terminal end of Ccdc155, representing putative transmembrane and luminal regions (LRs) of the KASH domain, respectively (Fig. 1 D). To explore the expression profile of mouse KASH domain proteins, including Ccdc155, we performed RT-PCR assays using mRNAs prepared from various tissues. The results indicate that Nesprins 1–3 are expressed ubiquitously, whereas Nesprin 4 is expressed in a relatively restricted manner in the liver and kidney. In contrast, Ccdc155 expression is limited to the testis and the early ootidogenesis ovary (Fig. 1, E and F). Collectively, these results suggest that Ccdc155 is a meiosis-specific KASH domain protein. We named this protein as KASH5 (a fifth member of the KASH domain protein in mouse).
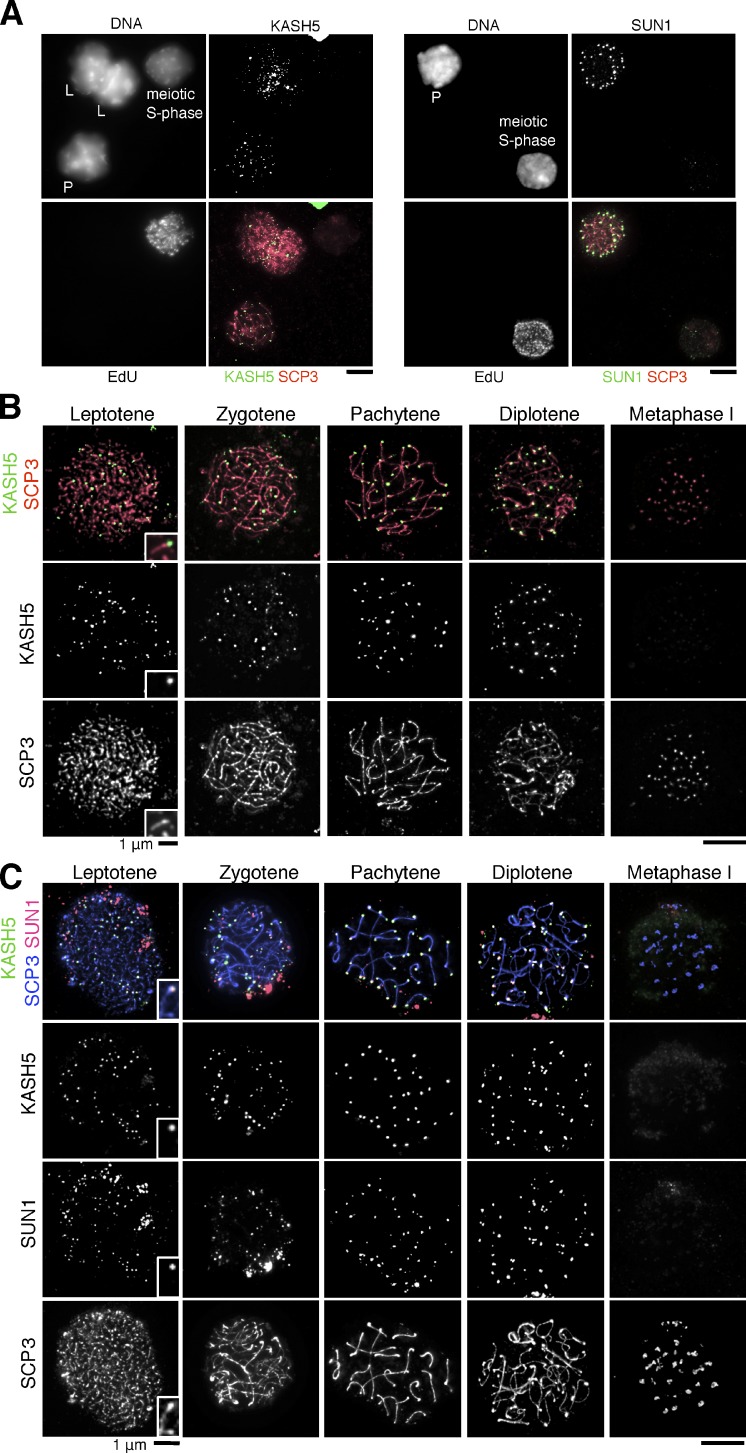
To delineate the localization of KASH5 in germ cells, we first immunostained KASH5 in mouse spermatocytes from meiotic S phase to meiosis I. KASH5 signals were not detected in meiotic S-phase spermatocytes but became visible at the ends of chromosomes in leptotene just before the bouquet stage (Fig. 2, A and B), during which telomeres are attached to the NE to promote chromosome pairing and synapsis. KASH5 signals are visible throughout pachytene until the diplotene stage and then become undetectable in metaphase I when telomeres are completely detached from the NE (Fig. 2 B). The dynamic localization of KASH5 during meiotic prophase was confirmed by immunostaining testis sections (Fig. S2 A). Immunostaining KASH5 in fetal oocytes at several stages revealed that the spatiotemporal distribution of KASH5 was very similar between spermatocytes and oocytes (Fig. S2 B). These results indicate that KASH5 is a telomere-associated protein at early meiosis during both male and female meiosis.
Figure 2.
KASH5 localizes at telomeres with SUN1 during meiotic prophase. (A) Spermatocytes were cultured with EdU and stained with Alexa Flour 488 azide and antibodies against SCP3 and KASH5 (left) or SUN1 (right). L, leptotene; P, pachytene. (B) Chromosome spreads from spermatocytes were stained with KASH5 and SCP3 antibodies. (C) Chromosome spreads from spermatocytes were stained with KASH5, SUN1, and SCP3 antibodies. Bars, 5 µm (unless otherwise indicated).
KASH domain proteins interact directly with SUN domain proteins that connect the nucleus to the cytoskeleton network (Fridkin et al., 2009). Although five SUN proteins have been identified in mammals (Hiraoka and Dernburg, 2009), only SUN1 has been shown to localize at the telomere sites on the NE during meiotic prophase (Ding et al., 2007; Lei et al., 2009). Furthermore, SUN1-deficient mice show defects in homologue pairing and synapsis (Ding et al., 2007). To explore the relationship between KASH5 and SUN1 localization, we examined the immunostaining patterns of these proteins in spermatocytes. Like KASH5, SUN1 appears at telomeres in leptotene (but not before) and disappears after diplotene (Fig. 2, A and C). Importantly, telomeric SUN1 and KASH5 signals were mostly colocalized in the prophase spermatocytes (Fig. 2 C), suggesting a potential causal link between their localization.
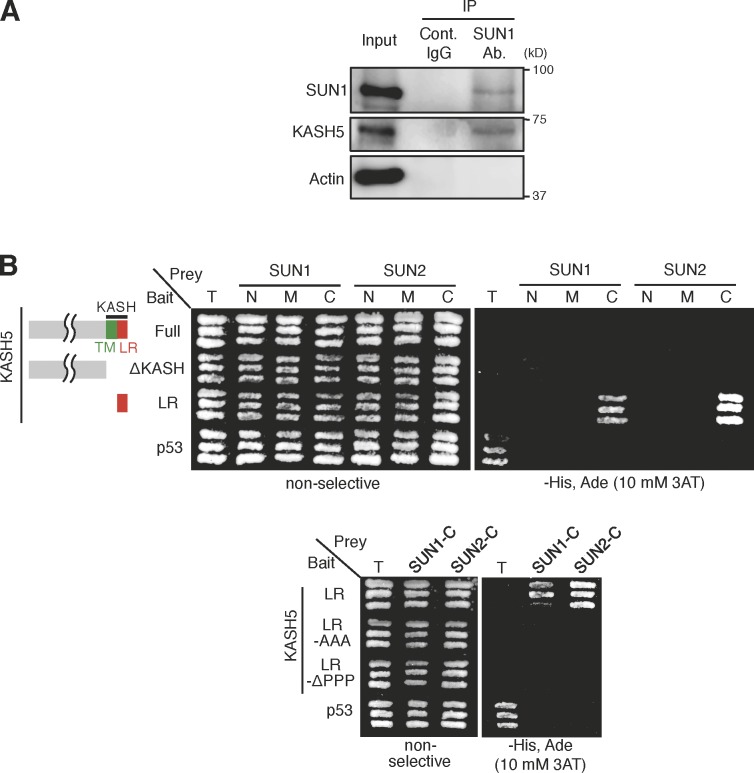
If the putative KASH domain protein KASH5 is the partner of the SUN domain protein SUN1, they should directly interact. We first prepared cytoplasmic extracts of mouse testis and performed immunoprecipitation using antibodies against SUN1. The immunoblot assays detected KASH5 in the SUN1 precipitates, suggesting that they form a complex in vivo (Fig. 3 A). Next, we used a yeast two-hybrid system to examine the direct interaction between KASH5 and SUN1 (Fig. 3 B). The full-length KASH5 did not show an interaction with SUN1/2 in this assay, presumably because of the deleterious effect of the transmembrane domain. Crucially, however, the last 22 amino acids of the divergent KASH domain (LR) of KASH5 interacted with the SUN domain of SUN1 (Fig. 3 B). When the stretch of proline residues in the LR was deleted or substituted with alanine, the interaction with SUN1 was eliminated (Fig. 3 B). Collectively, these results suggest that KASH5 interacts directly and forms a complex with SUN1 at the NE attachment sites of meiotic telomeres.
Figure 3.
KASH5 interacts with SUN1/2 via the KASH domain. (A) Immunoprecipitates (IP) from mouse testis using a SUN1 antibody (Ab.) or control (Cont.) IgG were analyzed by immunoblot using the indicated antibodies. Total soluble extract (0.1%) was loaded as input. (B) Yeast two-hybrid assay using KASH5 fragments. T-antigen (T) and p53 act as a positive control. C, C-terminal region; M, middle region; N, N-terminal region; TM, transmembrane.
To delineate domains in KASH5 important for targeting to telomeres in vivo, we constructed plasmids encoding GFP-tagged KASH5 and its mutant versions and injected them into mouse spermatocytes (Fig. 4 A). 2 d after injection, the spermatocytes were fixed and stained for GFP and SCP3 (Fig. 4 B). GFP-tagged full-length KASH5 showed telomere localization similar to endogenous KASH5, whereas GFP-tagged KASH5 lacking the LR lost the specific localization. GFP-tagged KASH5 containing only the KASH domain showed intact localization, indicating that the KASH domain was required and sufficient for targeting to telomere sites in the NE. We then examined the importance of the conserved triple proline residues (PPP) in the LR of the KASH domain, which were essential for binding with SUN1 (Fig. 3 B). Strikingly, the mutant versions of the KASH domain fragment tagged with GFP (KASH-AAA and KASH-ΔPPP) failed to localize at telomere sites on the NE in spermatocytes (Fig. 4, A and B). These results indicate that the C-terminal short peptide of KASH5 is required and sufficient for localization to telomeres at the NE and, thus, acts as a canonical KASH domain (Padmakumar et al., 2005; Ketema et al., 2007; Minn et al., 2009; Roux et al., 2009). To examine whether SUN1 is required for the localization of KASH5 at telomeres, we immunostained KASH5 in spermatocytes of SUN1 knockout (KO) mice. We found that localization of meiotic KASH5 to telomeres was abolished in the SUN1 KO mice (Fig. 4 C). Ectopic expression of KASH5 in mitotic HeLa cells and depletion of endogenous SUN domain proteins recapitulated the requirement of SUN domain proteins for KASH5 localization to the NE, although SUN domain proteins and KASH5 localize uniformly on the NE in mitotic cells (Fig. S3). Collectively, these analyses indicate that KASH5 is localized to the NE near telomeres through the direct interaction with SUN1 in early meiosis.
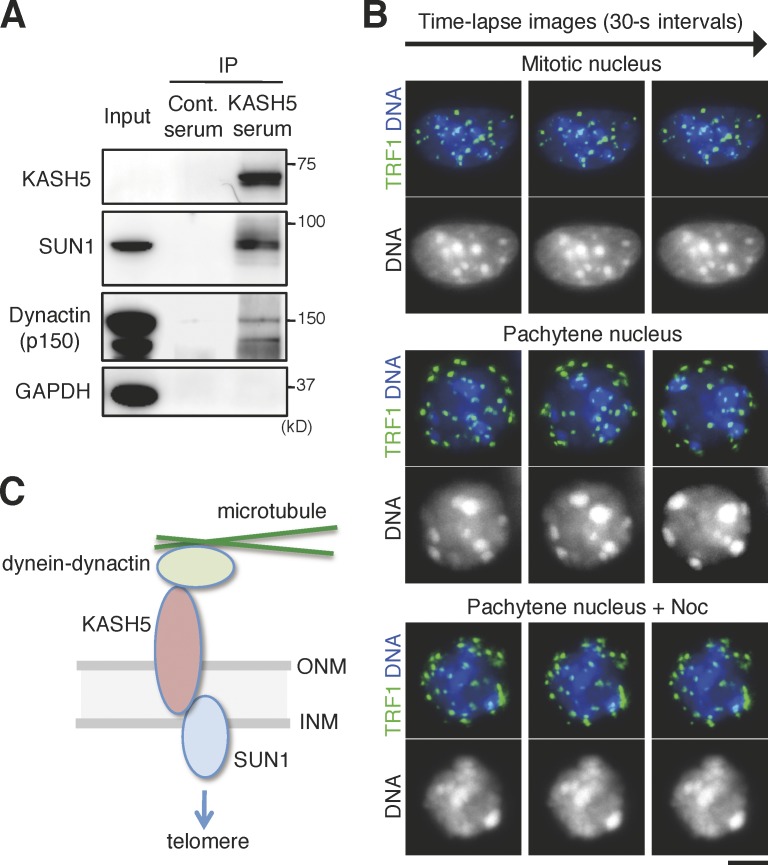
It has been reported that mouse KASH proteins Syne1 and Syne2 act for nuclear movement during neurogenesis by interacting with the dynein–dynactin microtubule motor (Zhang et al., 2009). Therefore, we examined the possibility that the SUN1–KASH5 complex might also interact with cytoplasmic dynein–dynactin complexes for driving telomere movement. Indeed, antibodies against KASH5 precipitated the dynactin p150 subunit as well as SUN1 from cytoplasmic extracts of mouse testis (Fig. 5 A). If cytoplasmic dynein–dynactin complexes act for telomere movement, inhibition of microtubule activity will prevent the movement. To examine this possibility, we cultured spermatocytes and performed time-lapse live-imaging analysis of pachytene nuclei. We prepared spermatocytes transiently expressing GFP-fused telomere protein TRF1, added Hoechst 33342 to the medium, and collected time-lapse images of GFP-TRF1 as well as DNA. Although GFP-TRF1 and DNA were stably positioned in mitotic interphase nuclei throughout filming, they moved dramatically in pachytene nuclei (Fig. 5 B), supporting the notion that dynamic telomere movement is meiosis specific and that this movement promotes chromosome pairing (Scherthan, 2001). Crucially, GFP-TRF1 did not show any dynamics in the presence of nocodazole (Fig. 5 B). These results suggest that meiosis-specific chromosome dynamics is driven by microtubule-induced force, presumably through the telomere–SUN1–KASH5–dynactin interaction (Fig. 5 C).
Figure 5.
Microtubule-dependent meiotic nuclear movement and the interaction between KASH5 and dynein. (A) Immunoprecipitates (IP) from mouse testis using a KASH5 antibody or control (Cont.) IgG were analyzed by immunoblot using the indicated antibodies. Total soluble extract (0.05%) was loaded as input. GAPDH, glyceraldehyde 3-phosphate dehydrogenase. (B) Time-lapse images of a mitotic cell or pachytene spermatocytes treated with or without nocodazole (Noc) were collected for 5 min at 30-s intervals (Video 1–3). Note that TRF1 signals distribute randomly in the nucleus of mitotic cells, whereas TRF1 signals localize near the NE of pachytene spermatocytes. Bar, 5 µm. (C) A model for mechanical bridging of the NE by SUN1 and KASH5 proteins during mammalian meiotic prophase. SUN1 and KASH5 locate at the INM and ONM, respectively, of the NE and interact across the lumen between the membranes. KASH5 associates with the dynein–dynactin complexes, whereas SUN1 may associate with telomeres on the opposite outsides of the NE, thus transferring the driving force generated by cytoplasmic microtubules to telomeres in the nucleus.
In the fission yeast Schizosaccharomyces pombe, the SUN–KASH complex Sad-1–Kms1/2 is a key element for dynein-mediated telomere clustering in the horse tail stage, and the absence of Kms1 causes defects in telomere tethering to the spindle pole body (Shimanuki et al., 1997; Miki et al., 2004; Chikashige et al., 2007). In the nematode C. elegans, SUN1 and ZYG-12 accumulate at the pairing center, and mutations in SUN1 or ZYG-12 result in reduced homologue pairing and aberrant synapsis (Penkner et al., 2007; Sato et al., 2009). In mammals, the SUN domain protein SUN1 might be engaged in this process (Ding et al., 2007). However, because the associated KASH protein has remained elusive (Hiraoka and Dernburg, 2009), it is uncertain whether the SUN–KASH complex indeed acts in homologue pairing in mammalian meiosis. In the current study, we have identified a novel meiosis-specific mammalian KASH protein, KASH5, that forms a complex and colocalizes with SUN1 at the NE attachment sites of telomeres in prophase I. Crucially, KASH5 localization to the NE is largely dependent on SUN1 in spermatocytes. It was previously shown that treatment with a microtubule polymerization inhibitor, colchicine, induces defects in homologous chromosome synapsis similar to that of SUN1-deficient mice (Tepperberg et al., 1999; Ding et al., 2007). Our study extended this observation by monitoring telomere movement in live imaging and by demonstrating the movement dependent on microtubules. Given that KASH5 associates with dynactin, the SUN1–KASH5 complex may drive telomere movement by linking microtubule-induced forces to chromosomes similar to the SUN–KASH complex in S. pombe and C. elegans (Hiraoka and Dernburg, 2009; Razafsky and Hodzic, 2009; Starr and Fridolfsson, 2010). Furthermore, the fact that SUN1–KASH5 localizes at telomeres in both spermatocytes and oocytes implies that the mechanism driving dynamic telomere movement and chromosome pairing is shared between male and female gametocytes in mammals. Future studies, including the generation of KASH5 KO mice, are required to validate this conclusion. Moreover, as proteins connecting SUN1 and telomeres still remain to be discovered, the identification of these factors will be the next major target of study in this field.
Materials and methods
Cell culture, expression vectors, and RNAi experiments
HeLa cells were cultured as previously described (Tanno et al., 2010). For the ectopic expression of KASH5 mutants in HeLa cells, cDNAs were inserted into the vector pEGFP-C2 (Takara Bio Inc.). Following the manufacturer’s protocol (Promega), HeLa cells were transfected with pEGFP-C2 constructs using FuGENE HD (Promega). RNAi experiments were performed based on a previous study (Kitajima et al., 2005). In brief, cells in Opti-MEM (Invitrogen) were transfected with siRNA duplexes at a final concentration of 200 nM using Oligofectamine (Invitrogen). Then, the Opti-MEM was replaced with complete medium containing 10% FBS after 6 h. Synthetic oligonucleotides for RNAi of hSUN1 (5′-GGACGUGCAAGUCAGAGAATT-3′) and hSUN2 (5′-GCAGAAAGAAGGUGUGAUUTT-3′) were obtained from JBioS (Japan Bio Services Co., LTD.). All control samples were similarly treated, except with the addition of H2O instead of siRNA.
Antibody production
A cDNA fragment encoding mouse KASH5 (amino acid residues 242–648) was inserted into pET28c (EMD Millipore). The His-tagged recombinant proteins were extracted from BL21-CodonPlus (DE3), solubilized in a denaturing buffer (6 M HCl-guanidine and 30 mM Tris-HCl, pH 7.5), and purified by Ni–nitrilotriacetic acid (QIAGEN) under denaturing conditions. The recombinant proteins were dialyzed in PBS and used to immunize imprinting control region mice. The serum recovered from mouse blood was used without purification. Antiserum against mouse SCP3 was prepared similarly by using a cDNA fragment encoding mouse SCP3 (residues 1–254) and by immunizing rats.
Antibodies and reagents
The following antibodies were used: rabbit polyclonal antibodies against SUN1 (Abcam), SUN1 (Sigma-Aldrich), SUN2 (Sigma-Aldrich), GFP (Invitrogen), SCP3 (Kawashima et al., 2010), TRF2 (Novus Biologicals), and CENP-C (Ishiguro et al., 2011); mouse polyclonal antibodies against SCP3 (Kawashima et al., 2010) and KASH5; mouse monoclonal antibodies against GFP (Roche) and glyceraldehyde 3-phosphate dehydrogenase (EMD Millipore); goat polyclonal antibodies against actin (Santa Cruz Biotechnology, Inc.), p150 (Abcam), and Lamin B (Santa Cruz Biotechnology, Inc.); and rat polyclonal antibodies against SCP3.
Preparation of testis extracts and immunoprecipitation
To prepare testis extracts, testes were removed from male C57BL/6 mice, detuncated, and then resuspended in extraction buffer (20 mM Tris-HCl, pH 7.4, 200 mM KCl, 0.4 mM EDTA, 5 mM MgCl2, 10% glycerol, 0.1% Triton X-100, and 1 mM β-mercaptoethanol) supplemented with Complete Protease Inhibitor (Roche) and Phosphatase Inhibitor (Roche). After homogenization, the cell extracts were incubated on ice for 30 min, and the cytoplasmic fractions were then obtained by centrifugation at 10,000 g for 10 min at 4°C. To purify the soluble fractions further, the extracts were centrifuged at 50,000 g for 20 min at 4°C and then filtered. For immunoprecipitation, 2 µg of anti-SUN1 and control rabbit IgG antibodies or 10 µl of KASH5 antiserum and control mouse serum were added to the cytoplasmic extracts, and the extracts were incubated for 1 h at 4°C followed by incubation with protein A Dynabeads (Invitrogen) for 1 h at 4°C.
RT-PCR
Total RNA was isolated from tissues using TRIZOL (Invitrogen). cDNA generation and PCR amplification were performed using Platinum Taq DNA Polymerase (Invitrogen). The primers used in this assay were as follows: mKASH5-forward, 5′-CGGGCAGAGGAGACCGCCTA-3′; mKASH5-reverse, 5′-GCTCCTTCTCTGTGTGCCGGG-3′; mNesprin1-forward, 5′-TTTCCTCAGGAGCTGGCGCA-3′; mNesprin1-reverse, 5′-CAATGTCCTGGTTAACAGCCGCCTC-3′; mNesprin2-forward, 5′-AGCAGCCAGGTGCCTTCGAC-3′; mNesprin2-reverse, 5′-GCTTCGGGGCTCTGGCTTGG-3′; mNesprin3a-forward, 5′-CGAGAGACAGAGAAAATCTGCCAG-3′; mNesprin3a-reverse, 5′-CTCAGCTGCCATTGCTTCTCC-3′; mNesprin3b-forward, 5′-TCGAGAGACAGAGTCGCATCGAG-3′; mNesprin3b-reverse, 5′-GGCTCTGGCCTTCACGGCAT-3′; mNesprin4-forward, 5′-CAAGACGGGGGCAAGCCCTG-3′; and mNesprin4-reverse, 5′-TGGCCTTCTCAAGCACCAGGC-3′.
Exogenous expression in testis
Testes were injected based on previous studies (Ogawa et al., 1997; Peters et al., 1997; Shoji et al., 2005). In brief, male mice at 16–20 dpp were anesthetized with pentobarbital. Testes were pulled from the abdominal cavity, and ∼50 µg of plasmid DNA was injected into each testis using glass capillaries under a stereo microscope (M165C; Leica). Testes were held between a pair of tweezer-type electrodes (CUY21; BEX Co., Ltd.), and electric pulses were applied four times and again four times in the reverse direction at 30 V for 50 min for each pulse. The testis was then returned to the abdominal cavity, and the abdominal wall and skin were closed with sutures.
Immunostaining of spermatocytes and oocytes
Immunostaining of chromosome spreads from spermatocytes and fetal oocytes were performed based on a previous study (Peters et al., 1997). In brief, ovaries and testes were incubated in trypsin-EDTA solution at 37°C for 15 min and washed briefly in PBS. Trypsinized ovaries and testes were pipetted repeatedly and centrifuged followed by resuspension in PBS. Cell suspensions were placed on slides containing 75 mM KCl solution and 0.1% Triton X-100. The slides were fixed in 1% PFA for 3 h at room temperature and air dried. For immunostaining, slides were incubated with the primary antibodies in PBS containing 3% BSA for 1 h at room temperature, washed with PBS, and incubated with Alexa Fluor 488, 568, and 647 (Invitrogen) secondary antibodies (1:1,000 dilution) for 1 h at room temperature. After washing with PBS, slides were mounted using VECTASHIELD medium with DAPI (Vector Laboratories).
Immunostaining of HeLa cells
Cells on coverslips were fixed with 4% PFA in PBS for 10 min and then incubated with 0.2% Triton X-100 in PBS for 10 min. Fixed cells were incubated with antibodies in PBS containing 3% BSA. DNA was stained with DAPI.
EdU staining of meiotic S-phase spermatocytes
Spermatocytes were incubated in DME/10% FBS with 10 µM EdU at 34°C for 30 min. Chromosome spreads from spermatocytes labeled with EdU were detected using Alexa Fluor 488 azide and a Click-iT EdU imaging kit (Invitrogen).
Microscopy
Images were taken on a microscope (Olympus IL-X71 DeltaVision; Applied Precision) equipped with 100× NA 1.40 and 60× NA 1.42 objectives, a camera (CoolSNAP HQ; Photometrics), and softWoRx 5.5.5 acquisition software (DeltaVision). Acquired images were processed with Photoshop (Adobe).
Live imaging
For analyzing telomere dynamics in spermatocytes, pCAG-GFP plasmid (Matsuda and Cepko, 2004) harboring mouse TRF1 cDNA sequences were electroporated into testes. GFP-positive cells from the testicular cells were imaged in phenol red–free Leibovitz’s L-15 medium (Gibco) supplemented with 400 ng/ml Hoechst 33342 (Wako Chemicals USA) at 33°C with or without 5 nM nocodazole (Sigma-Aldrich). Exposures of 0.15 s (for GFP) and 0.025 s (for Hoechst 33324) were acquired every 30 s for 5 min using a 100× NA 1.40 objective on a microscope (Olympus IL-X71 DeltaVision). Cells showing a round shape of DNA signals with its telomeres (GFP-TRF1 signals) attached to the NE were regarded as pachytene spermatocytes. Cells showing an ellipse shape of DNA signals with its telomeres scattered in the inner region of the nucleus were regarded as mitotic cells.
Yeast two-hybrid screening and assay
For yeast two-hybrid screening, full-length (residues 1–1,165), N-terminal (residues 1–407), middle (residues 408–773), and C-terminal regions of the mouse shugoshin 2 cDNA were subcloned into the vector pGBKT7 and transformed into the yeast strain AH109, resulting in bait strains. A mouse testis cDNA library (Takara Bio Inc.) was transformed into the bait strains; positive transformants were selected on nutrition-restricted plates. Prey plasmids were extracted from candidate clones and sequenced. To examine the interaction between KASH5 and SUN proteins, full-length, ΔKASH (residues 1–600), and LR (residues 627–648) portions of KASH5 were subcloned into the vector pGBKT7 and used as bait. N-terminal (residues 1–210), middle (residues 188–478), and C-terminal (residues 458–913) regions of mouse SUN1 and N-terminal (residues 1–193), middle (residues 173–400), and C-terminal (residues 383–731) regions of mouse SUN2 were subcloned into the vector pGADT7 and cotransformed into the yeast strain AH109 with the bait.
Online supplemental material
Fig. S1 shows alignment of the KASH5 homologues in vertebrates. Fig. S2 shows the immunostaining of KASH5 in testis slices and in fetal oocytes. Fig. S3 reports the requirement of SUN domain proteins for the NE localization of KASH5 ectopically expressed in HeLa cells. Videos 1–3 show live imaging of a mitotic cell recovered from testes, of a pachytene spermatocyte cell, and of a pachytene spermatocyte cell in the presence of nocodazole, respectively. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.201204085/DC1.
Supplementary Material
Acknowledgments
We thank Y. Kishi and the Gotoh Laboratory for the technical advice and kind instructions about the testis injection experiments. We also thank all the members of our laboratory for their valuable support and discussion.
This work was supported in part by a National Hi-Tech Research and Development program of China (863; to X. Zhu) and in part by a Japan Society for the Promotion of Science Research Fellowship (to J. Kim), a Grant-in-Aid for Scientific Research on Priority Areas, a Grant-in-Aid for Young Scientists (to K. Ishiguro), the Global Centers of Excellence Program (Integrative Life Science Based on the Study of Biosignaling Mechanisms; to H. Shibuya and Y. Watanabe), and a Grant-in-Aid for Specially Promoted Research (to Y. Watanabe) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Footnotes
Abbreviations used in this paper:
- INM
- inner nuclear membrane
- KO
- knockout
- LR
- luminal region
- NE
- nuclear envelope
- ONM
- outer nuclear membrane
References
- Bhalla N., Dernburg A.F. 2008. Prelude to a division. Annu. Rev. Cell Dev. Biol. 24:397–424 10.1146/annurev.cellbio.23.090506.123245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikashige Y., Haraguchi T., Hiraoka Y. 2007. Another way to move chromosomes. Chromosoma. 116:497–505 10.1007/s00412-007-0114-8 [DOI] [PubMed] [Google Scholar]
- Ding X., Xu R., Yu J., Xu T., Zhuang Y., Han M. 2007. SUN1 is required for telomere attachment to nuclear envelope and gametogenesis in mice. Dev. Cell. 12:863–872 10.1016/j.devcel.2007.03.018 [DOI] [PubMed] [Google Scholar]
- Fridkin A., Penkner A., Jantsch V., Gruenbaum Y. 2009. SUN-domain and KASH-domain proteins during development, meiosis and disease. Cell. Mol. Life Sci. 66:1518–1533 10.1007/s00018-008-8713-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady R.M., Starr D.A., Ackerman G.L., Sanes J.R., Han M. 2005. Syne proteins anchor muscle nuclei at the neuromuscular junction. Proc. Natl. Acad. Sci. USA. 102:4359–4364 10.1073/pnas.0500711102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraoka Y., Dernburg A.F. 2009. The SUN rises on meiotic chromosome dynamics. Dev. Cell. 17:598–605 10.1016/j.devcel.2009.10.014 [DOI] [PubMed] [Google Scholar]
- Ishiguro K., Kim J., Fujiyama-Nakamura S., Kato S., Watanabe Y. 2011. A new meiosis-specific cohesin complex implicated in the cohesin code for homologous pairing. EMBO Rep. 12:267–275 10.1038/embor.2011.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima S.A., Yamagishi Y., Honda T., Ishiguro K., Watanabe Y. 2010. Phosphorylation of H2A by Bub1 prevents chromosomal instability through localizing shugoshin. Science. 327:172–177 10.1126/science.1180189 [DOI] [PubMed] [Google Scholar]
- Ketema M., Wilhelmsen K., Kuikman I., Janssen H., Hodzic D., Sonnenberg A. 2007. Requirements for the localization of nesprin-3 at the nuclear envelope and its interaction with plectin. J. Cell Sci. 120:3384–3394 10.1242/jcs.014191 [DOI] [PubMed] [Google Scholar]
- Kitajima T.S., Hauf S., Ohsugi M., Yamamoto T., Watanabe Y. 2005. Human Bub1 defines the persistent cohesion site along the mitotic chromosome by affecting Shugoshin localization. Curr. Biol. 15:353–359 10.1016/j.cub.2004.12.044 [DOI] [PubMed] [Google Scholar]
- Lee J., Kitajima T.S., Tanno Y., Yoshida K., Morita T., Miyano T., Miyake M., Watanabe Y. 2008. Unified mode of centromeric protection by shugoshin in mammalian oocytes and somatic cells. Nat. Cell Biol. 10:42–52 10.1038/ncb1667 [DOI] [PubMed] [Google Scholar]
- Lei K., Zhang X., Ding X., Guo X., Chen M., Zhu B., Xu T., Zhuang Y., Xu R., Han M. 2009. SUN1 and SUN2 play critical but partially redundant roles in anchoring nuclei in skeletal muscle cells in mice. Proc. Natl. Acad. Sci. USA. 106:10207–10212 10.1073/pnas.0812037106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda T., Cepko C.L. 2004. Electroporation and RNA interference in the rodent retina in vivo and in vitro. Proc. Natl. Acad. Sci. USA. 101:16–22 10.1073/pnas.2235688100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki F., Kurabayashi A., Tange Y., Okazaki K., Shimanuki M., Niwa O. 2004. Two-hybrid search for proteins that interact with Sad1 and Kms1, two membrane-bound components of the spindle pole body in fission yeast. Mol. Genet. Genomics. 270:449–461 10.1007/s00438-003-0938-8 [DOI] [PubMed] [Google Scholar]
- Minn I.L., Rolls M.M., Hanna-Rose W., Malone C.J. 2009. SUN-1 and ZYG-12, mediators of centrosome-nucleus attachment, are a functional SUN/KASH pair in Caenorhabditis elegans. Mol. Biol. Cell. 20:4586–4595 10.1091/mbc.E08-10-1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa T., Aréchaga J.M., Avarbock M.R., Brinster R.L. 1997. Transplantation of testis germinal cells into mouse seminiferous tubules. Int. J. Dev. Biol. 41:111–122 [PubMed] [Google Scholar]
- Padmakumar V.C., Libotte T., Lu W., Zaim H., Abraham S., Noegel A.A., Gotzmann J., Foisner R., Karakesisoglou I. 2005. The inner nuclear membrane protein Sun1 mediates the anchorage of Nesprin-2 to the nuclear envelope. J. Cell Sci. 118:3419–3430 10.1242/jcs.02471 [DOI] [PubMed] [Google Scholar]
- Penkner A., Tang L., Novatchkova M., Ladurner M., Fridkin A., Gruenbaum Y., Schweizer D., Loidl J., Jantsch V. 2007. The nuclear envelope protein Matefin/SUN-1 is required for homologous pairing in C. elegans meiosis. Dev. Cell. 12:873–885 10.1016/j.devcel.2007.05.004 [DOI] [PubMed] [Google Scholar]
- Peters A.H., Plug A.W., van Vugt M.J., de Boer P. 1997. A drying-down technique for the spreading of mammalian meiocytes from the male and female germline. Chromosome Res. 5:66–68 10.1023/A:1018445520117 [DOI] [PubMed] [Google Scholar]
- Razafsky D., Hodzic D. 2009. Bringing KASH under the SUN: The many faces of nucleo-cytoskeletal connections. J. Cell Biol. 186:461–472 10.1083/jcb.200906068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux K.J., Crisp M.L., Liu Q., Kim D., Kozlov S., Stewart C.L., Burke B. 2009. Nesprin 4 is an outer nuclear membrane protein that can induce kinesin-mediated cell polarization. Proc. Natl. Acad. Sci. USA. 106:2194–2199 10.1073/pnas.0808602106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato A., Isaac B., Phillips C.M., Rillo R., Carlton P.M., Wynne D.J., Kasad R.A., Dernburg A.F. 2009. Cytoskeletal forces span the nuclear envelope to coordinate meiotic chromosome pairing and synapsis. Cell. 139:907–919 10.1016/j.cell.2009.10.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherthan H. 2001. A bouquet makes ends meet. Nat. Rev. Mol. Cell Biol. 2:621–627 10.1038/35085086 [DOI] [PubMed] [Google Scholar]
- Scherthan H., Weich S., Schwegler H., Heyting C., Härle M., Cremer T. 1996. Centromere and telomere movements during early meiotic prophase of mouse and man are associated with the onset of chromosome pairing. J. Cell Biol. 134:1109–1125 10.1083/jcb.134.5.1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimanuki M., Miki F., Ding D.Q., Chikashige Y., Hiraoka Y., Horio T., Niwa O. 1997. A novel fission yeast gene, kms1+, is required for the formation of meiotic prophase-specific nuclear architecture. Mol. Gen. Genet. 254:238–249 10.1007/s004380050412 [DOI] [PubMed] [Google Scholar]
- Shoji M., Chuma S., Yoshida K., Morita T., Nakatsuji N. 2005. RNA interference during spermatogenesis in mice. Dev. Biol. 282:524–534 10.1016/j.ydbio.2005.03.030 [DOI] [PubMed] [Google Scholar]
- Starr D.A., Fischer J.A. 2005. KASH ’n Karry: The KASH domain family of cargo-specific cytoskeletal adaptor proteins. Bioessays. 27:1136–1146 10.1002/bies.20312 [DOI] [PubMed] [Google Scholar]
- Starr D.A., Fridolfsson H.N. 2010. Interactions between nuclei and the cytoskeleton are mediated by SUN-KASH nuclear-envelope bridges. Annu. Rev. Cell Dev. Biol. 26:421–444 10.1146/annurev-cellbio-100109-104037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanno Y., Kitajima T.S., Honda T., Ando Y., Ishiguro K., Watanabe Y. 2010. Phosphorylation of mammalian Sgo2 by Aurora B recruits PP2A and MCAK to centromeres. Genes Dev. 24:2169–2179 10.1101/gad.1945310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepperberg J.H., Moses M.J., Nath J. 1999. Colchicine effects on meiosis in the male mouse. II. Inhibition of synapsis and induction of nondisjunction. Mutat. Res. 429:93–105 10.1016/S0027-5107(99)00102-5 [DOI] [PubMed] [Google Scholar]
- Zhang X., Lei K., Yuan X., Wu X., Zhuang Y., Xu T., Xu R., Han M. 2009. SUN1/2 and Syne/Nesprin-1/2 complexes connect centrosome to the nucleus during neurogenesis and neuronal migration in mice. Neuron. 64:173–187 10.1016/j.neuron.2009.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.