Abstract
Two distinct preparations of amphiphilic diblock copolymer vesicles (i.e. polymersomes), composed of (poly(ethylene oxide)-poly(butadiene)) (PEO-PBD), with molecular weights of 1.8 kDa and 10.4 kDa, offering different hydrophobic membrane thicknesses, were used to encapsulate the oxygen (O2) storage and transport protein hemoglobin (Hb) for possible application as a red blood cell (RBC) substitute. Key biophysical properties as well as the kinetics of polymersome encapsulated Hb (PEH) interaction with physiologically important gaseous ligands (O2, carbon monoxide and nitric oxide) were measured as a function of the hydrophobic membrane thickness of the PEH particle. Taken together, the results of this work show that PEHs exhibit biophysical properties and retarded ligand binding/release kinetics (compared to cell-free Hb), which are similar to the behavior of RBCs. Therefore, PEHs have the potential to serve as safe and efficacious RBC substitutes for use in transfusion medicine.
INTRODUCTION
The results of previous work on acellular hemoglobin (Hb)-based oxygen (O2) carrier (HBOC) solutions, as red blood cell (RBC) substitutes for use in transfusion medicine, highlighted serious side-effects such as vasoconstriction (i.e. reduction in blood vessel diameter) and the development of systemic hypertension (i.e. high blood pressure).1–3 These side-effects are hypothesized to occur via scavenging of the gaseous signaling molecules nitric oxide (NO) and carbon monoxide (CO).1, 4, 5 In addition, the oversupply of O2 to surrounding tissues can trigger an autoregulatory induced vasoconstrictive response in order to regulate the tissue O2 supply.6 Therefore, it is apparent that the interaction of Hb with these gaseous ligands governs the overall toxicity of HBOCs in vivo.
Polymer vesicles (i.e. polymersomes) consisting of amphiphilic diblock copolymers containing poly(ethylene oxide) (PEO), the structural equivalent of poly(ethylene glycol) (PEG),7 as the hydrophilic block have been previously used to encapsulate Hb.8 The use of amphiphilic diblock copolymers is advantageous for vesicle formation, since these molecules can be synthesized with molecular weights (MWs) that are larger than natural lipids, which in turn facilitates control of the hydrophobic membrane thickness and size of the PEG corona.7, 9 These physical attributes in turn play a large role in regulating polymersome mechanical stability and circulatory lifetime in the blood. For example, the hydrophobic membrane thickness can be easily engineered from 3 to 40 nm through appropriate design of the diblock copolymer.10, 11 It is hypothesized that employing polymersomes to encapsulate Hb, i.e. polymersome encapsulated Hb (PEH), will enable regulation of the binding/release kinetics of the encapsulated Hb with O2, CO and NO by controlling gaseous ligand diffusion across the bilayer which will present a physical diffusion barrier, that can potentially negate or mitigate the overall toxicity of this type of HBOC in vivo.
In this work, two biocompatible and non-biodegradable PEO-poly(butadiene) (PEO-PBD) diblock copolymers were used to formulate PEHs for analysis. The two polymers varied greatly in their MWs, spanning 1.8 kDa to 10.4 kDa, hence yielding polymersomes with distinctly different hydrophobic membrane thicknesses. As a first step towards testing this hypothesis, key biophysical properties of the PEH dispersions, such as, particle size distribution, methemoglobin (metHb) level, O2-PEH equilibria, O2 affinity, cooperativity coefficient and gaseous ligand binding/release kinetics were evaluated as a function of the membrane thickness of the PEH particle. These experiments were geared towards exploring the ability of the PEH bilayer thickness to regulate gaseous ligand binding/release kinetics.
MATERIALS AND METHODS
Two biocompatible diblock copolymers composed of PBD-PEO were used in this study. Diblock copolymers were purchased from Polymer Source Inc. (Dorval QC, Canada). The physical properties of the two diblock copolymers are summarized in Table 1. For convenience, diblock copolymers will be referred to as PBD 1200 and PBD 6500, where the numeric coefficients denote the MW of the PBD block in daltons (Da).
Table 1.
Number-averaged MW (Mn), polydispersity index (Mw/Mn), PEG block MW, hydrophilic mass faction (fhydrophilic), and hydrophobic membrane thickness (d) of the biocompatible and non-biodegradable PBD-PEO copolymers used in this study. The magnitude of d was extrapolated from a mathematical correlation between d and the copolymer MW as reported in the literature.24
| Diblock copolymer | Mn (Da) | Mw/Mn | PEG Block MW (Da) | fhydrophilic | d (nm) |
|---|---|---|---|---|---|
| PBD(1200)-b-PEO(600) | 1800 | 1.17 | 600 | 0.33 | 4–5 |
| PBD(6500)-b-PEO(3900) | 10400 | 1.10 | 3900 | 0.38 | 12–13 |
The purification of human Hb from human RBCs, measurement of vesicle size distribution, O2-PEH equilibria, O2 affinity, cooperativity coefficient, Hb and metHb concentration in vesicle dispersions has been previously described in the literature.12
PEH preparation
1–2 grams of diblock polymer was completely dissolved in 30–40 mL of chloroform. The organic solvent was then removed to form a dry film using a rotary evaporator. The film was further dried under vacuum for 48 hrs and then hydrated with 20–40 mL of carbonyl Hb (HbCO) ([Hb] > 300 mg/mL) suspended in phosphate-buffered saline (PBS) (0.1 M, pH 7.4) inside a round bottom flask. The polymer/Hb solution was then incubated in a water bath sonicator for 30 mins followed by thoroughly mixing the contents of the flask at room temperature for 15 mins. This step was repeated 2–3 times.
Unencapsulated Hb was removed from the PEH solution at room temperature via diafiltration using a 500 kDa HF cartridge (Spectrum Laboratories, Rancho Dominguez, CA), using PBS as the diafiltration buffer. To ensure complete removal of free Hb from the PEH solution, the process was repeated 7–8 times using a 1:10 v/v (sonicated PEH: PBS) ratio until no free Hb was observed in the filtrate. The filtrate was assayed for the presence of Hb using UV-Vis spectroscopy and the Winterbourn equation.13 Finally, the PEH solution was concentrated using a 500 kDa HF cartridge. Strict aseptic conditions were maintained throughout the preparation of PEHs. Prior to all experiments, all tubing, filters and glassware used for the preparation of PEHs were immersed in 1 M NaOH solution overnight and rinsed with deionized water. The diafiltration and concentration of PEHs was performed inside a laminar flow hood.
Rapid kinetic measurements of PEH dispersions
All rapid kinetic measurements of gaseous ligand binding/release were performed using an Applied Photophysics SF-17 microvolume stopped-flow spectrophotometer (Applied Photophysics Ltd., Surrey, United Kingdom). The measurements were performed on PEH dispersions converted into the HbO2 form, prior to all experiments, using the protocols described by Rameez et al.12
RESULTS AND DISCUSSION
Biophysical characterization
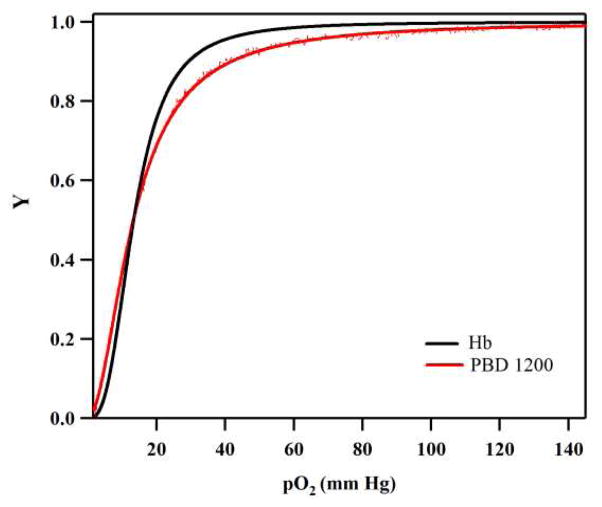
Figure 1 shows the O2-PEH equilibrium curve of a representative PEH dispersion (PBD 1200) prepared in this study compared to a control of human Hb. The O2 affinity (P50) and cooperativity coefficient (n) of both PEH dispersions was regressed from the O2-PEH equilibrium data fit to the Hill equation8 and found to be similar to that of unencapsulated human Hb (Table 2). The sigmoidal shape of the O2-PEH equilibrium curve qualitatively shows that the cooperative binding of O2 to Hb was not compromised by encapsulation inside the aqueous core of the vesicle. It was further observed that the P50 and n of the PEH dispersions did not vary much upon increasing the MW of the hydrophobic block of the diblock copolymer. Thus, it seems that increasing the MW of the hydrophobic block, which in turn increases the membrane thickness, does not have any effect on O2 binding equilibria. In addition, the metHb level (% of Hb in which the Fe atom in the heme ring is in the 3+ valence state instead of the 2+ valence state) of PEH dispersions prepared in this study was less than 1% compared to ~ 0.5% for purified human Hb. This shows that the metHb level did not increase significantly during the preparation of PEH dispersions.
Figure 1.
O2-PEH equilibrium curves of human Hb and polymersomes encapsulating human Hb (PBD 1200). Here, Y represents the fraction of Hb saturated with O2 and pO2 represents the partial pressure of O2.
Table 2.
Biophysical properties of various cellular HBOCs (RBCs, PEG-LEHs and PEHs).
| Type of Hb | Size (Diameter) | P50 (mm Hg) | n | koff, O2 (s −1) | kon, CO (106 M−1s−1) | kox, NO (106 M−1s−1) | References |
|---|---|---|---|---|---|---|---|
| Cell-free Hb | |||||||
| Human | 5.5 nm | 13.34 | 2.8 | 42.22 | 0.225 | 42 | * |
| RBCs | |||||||
| Human and Rat | 8 μm | 25.10 | 2.80 | 4.4 | 0.065 | 0.05 | 21, 25, 26 |
| Human | 8 μm | 20.00 | 2.18 | 16.67 | 0.130 | 0.03 | |
| PEG-LEHs | |||||||
| Human | 279 nm | 25–31 | 2.10 | 32.00 | 0.210 | 8.80 | 27, 28 |
| Human | 176 nm | 22.87 | 2.11 | 21.57 | 0.212 | 4.00 | 12 |
| PEHs | |||||||
| Human PBD 1200 | 380 nm | 13.20 | 1.91 | 14.24 | 0.211 | 2.87 | * |
| Human PBD 6500 | 417 nm | 12.13 | 1.95 | 4.16 | 0.206 | 2.20 | * |
represents results from this study.
The concentration of Hb in the PEH dispersions was ~1.0 g/dL. This forms the basis for one of the potential problems with PEH dispersions formulated in this study, the low Hb concentration in solution. This is mainly due to the fact that the PEO chains comprising the hydrophilic block of the diblock copolymer are also present in the inner leaflet of the PEH membrane. This reduces the aqueous core volume available for encapsulation of Hb, which results in the low Hb concentration in solution. To negate this potential problem, PEH dispersions with asymmetric membranes will be designed14 in the future. PEH particles composed of an asymmetric membrane will facilitate the self-assembly of an outer leaflet composed of an amphiphilic copolymer with long PEG chains, while the inner leaflet will be composed of an amphiphilic copolymer with short PEG chains. The asymmetric nature of the membrane should permit higher encapsulation of Hb within the aqueous core of the vesicle.
Rapid kinetic measurements of PEH dispersions
The ligand binding/release kinetics of Hb with various ligands, especially physiologically relevant ligands (O2, CO and NO) are just as important as equilibrium studies in providing information about the ability of these materials to store and transport gases in vivo.15
Reactions with O2
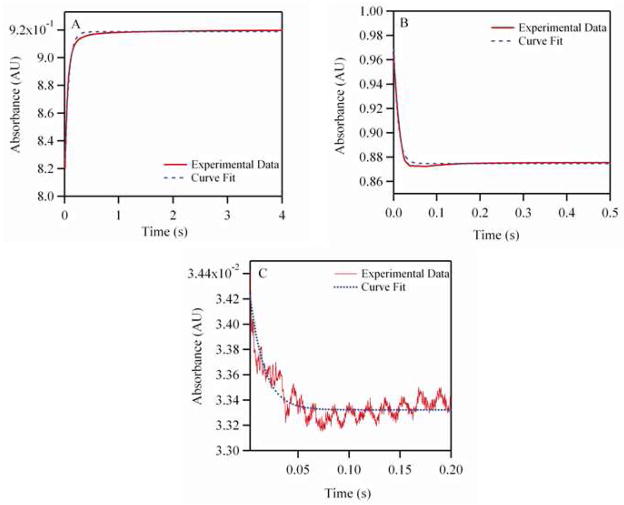
Figure 2A shows a representative time course for deoxygenation of oxygenated PBD 1200 encapsulating human Hb. The values for the O2 dissociation rate constant (koff, O2) are shown in Table 2. The values of koff, O2 for PEH dispersions was 3.5–10 fold lower than that of acellular Hb (Table 2). A similar level of retardation in O2 offloading has been observed in RBCs and PEG-conjugated liposome encapsulated Hbs (LEHs) as compared to acellular Hb (Table 2). This suggests that encapsulation of Hb inside the aqueous core of a polymersome can play a critical role in regulating the delivery of O2 as observed with RBCs. The values for O2 offloading were retarded to a greater extent when the membrane thickness of PEH particles was increased (PBD 1200 has a membrane thickness of 3–4 nm compared to PBD 6500 which has a membrane thickness of 12–13 nm). Hence, increasing the PEH membrane thickness increases the membrane resistance to O2 diffusion, thus retarding O2 offloading from the encapsulated Hb.
Figure 2.
Kinetic time courses for PEH (PBD 1200). (A) Represents deoxygenation of oxygenated PEH in the presence of 1.5 mg/mL of sodium dithionite. (B) Represents CO (464 μM) association with deoxygenated PEH. (C) Represents NO (25 μM) induced oxidation with oxygenated PEH. The experimental data shows an average of 7–10 kinetic traces. Deoxygenation and CO association reactions were monitored at 437.5 nm and 25°C, while NO induced oxidation reactions were monitored at 420 nm and 25°C. PEH solutions were made in oxygenated PBS (pH 7.4) for NO induced oxidation and deoxygenation experiments. In case of CO association experiments, PEH and CO solutions were made in PBS (pH 7.4) in the presence of 1.5 mg/mL of sodium dithionite.
Reactions with CO
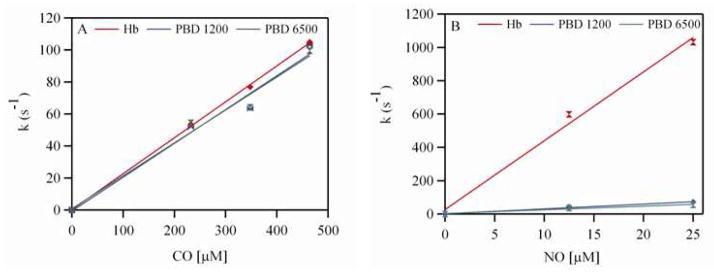
Figure 2B shows a representative time course for the association reaction between CO and deoxygenated PBD 1200 encapsulating human Hb. Figure 3A shows the dependence of the observed rates on CO concentration for both PBD 1200 and PBD 6500. The values of the CO association rate constants (kon, CO) are shown in Table 2. The kon, CO values for Hb and PEH dispersions were almost similar. Thus, encapsulation of Hb inside polymersomes did not affect the CO binding kinetics. A similar trend was observed for the encapsulation of Hb inside liposomes.12, 16 This can be attributed to the fact that the magnitude of the CO association rate constant is much smaller than those of O2 and NO.17
Figure 3.
Dependence of the observed rates on (A) CO concentration (0–464 μM) with 15 μM of human Hb and (B) NO concentrations (0–25 μM) with 0.5 μM of human Hb.
Reactions with NO
Figure 2C shows a representative time course for the NO dioxygenation reaction with oxygenated PBD 1200 encapsulating human Hb. Figure 3B shows the dependence of the observed rates of NO dioxygenation on NO concentration for both PEHs (PBD 1200 and PBD 6500). The values for the NO dioxygenation rate constants (kox, NO) are shown in Table 2.
The magnitude of kox, NO was significantly retarded for PEHs compared to cell-free Hb (Table 2). The kox, NO for PEHs was 6.5– 19 fold lower than that of cell-free human Hb. Moreover, the reduction in kox, NO was 1.5–4 fold less than PEG-conjugated LEHs reported in the literature (Table 2). However, kox, NO for PEHs is still higher than that of RBCs. This is expected given the smaller size of PEHs as compared to RBCs, which provides a smaller intracellular diffusion barrier to NO dioxygenation. Thus increasing the membrane thickness of the vesicle did not have as pronounced a retardation effect on the magnitude of the NO dioxygenation rate constant as was observed for the O2 offloading rate constant. Thus, the major source of retardation in the magnitude of the NO dioxygenation rate constant is due to the encapsulation of concentrated Hb inside the vesicle core, which provides for a high intracellular diffusion barrier for NO to bind Hb. This effect becomes more pronounced once the size of the particle is increased as observed in the case of RBCs (Table 2). However, the kox, NO values for PEHs in this study are comparable to a recombinant Hb with low NO dioxygenation activity (2.20–2.87 μM−1s−1 compared to 2 μM−1s−1) which elicited no vasoactivity when transfused in vivo.18 This in turn suggests the development of little to no vasoactivity of these PEHs if administered in vivo.
It has been shown that the intracellular diffusion barrier, which is due to the RBC size and high intracellular Hb concentration, facilitates a reduction in the magnitude of the NO binding rate constant.16 In addition, various other transport mechanisms have been proposed to explain the retardation of NO binding to RBCs. Some studies have demonstrated that the diffusion barrier against NO transport from the endothelium to the RBC column in the center of the blood vessel is due to the presence of an RBC-free plasma layer adjacent to the blood vessel wall.19,20 Other work has shown that the presence of an extracellular diffusion barrier due to the undisturbed layer around the RBC retards NO scavenging.21,22 Therefore regardless of the exact mechanism for retardation of NO binding, all these studies suggest that encapsulation of Hb in a membrane coated particle similar to that of a RBC plays a critical role in regulating NO transport to encapsulated Hb.
CONCLUSIONS
The results of this work show that PEHs yield low metHb levels, appropriate O2 binding equilibria and retarded ligand binding/release kinetics (compared to cell-free Hb), which is similar to the behavior of RBCs. In addition, engineering the hydrophobic membrane thickness of this class of particle regulates O2 offloading from the encapsulated Hb. The moderate O2 release rates exhibited by PEHs can be beneficial for their potential use as a HBOC in transfusion medicine, since the oversupply of O2 can trigger autoregulatory-induced vasoconstriction.23 Similarly, encapsulation of Hb inside polymer vesicles also retards NO binding to Hb, similar to encapsulation of Hb in other cellular HBOCs. Hence, through appropriate selection of amphiphilic diblock copolymers for subsequent formulation of PEHs, it is possible to regulate gaseous ligand binding/release kinetics which can potentially mitigate the side-effects observed with previous generations of acellular HBOCs.
Acknowledgments
This work was supported by National Institutes of Health grants R01HL078840 and R01DK070862 to A.F.P. We also acknowledge Dr. Christopher M. Hadad, Department of Chemistry, The Ohio State University, Columbus OH, for his willingness to let us use the UV-visible stopped-flow spectrophotometer in his laboratory.
References
- 1.Sakai H, Hara H, Yuasa M, Tsai AG, Takeoka S, Tsuchida E, Intaglietta M. Am J Physiol Heart Circ Physiol. 2000;279:H908–H915. doi: 10.1152/ajpheart.2000.279.3.H908. [DOI] [PubMed] [Google Scholar]
- 2.Tsai AG, Cabrales P, Manjula BN, Acharya SA, Winslow RM, Intaglietta M. Blood. 2006;108:3603–3610. doi: 10.1182/blood-2006-02-005272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alayash AI. Nat Rev Drug Discov. 2004;3:152–159. doi: 10.1038/nrd1307. [DOI] [PubMed] [Google Scholar]
- 4.Rohlfs RJ, Bruner E, Chiu A, Gonzales A, Gonzales ML, Magde D, Magde MD, Vandegriff KD, Winslow RM. J Biol Chem. 1998;273:12128–12134. doi: 10.1074/jbc.273.20.12128. [DOI] [PubMed] [Google Scholar]
- 5.Goda N, Suzuki K, Naito M, Takeoka S, Tsuchida E, Ishimura Y, Tamatani T, Suematsu M. J Clin Invest. 1998;101:604–612. doi: 10.1172/JCI1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harder DR, Narayanan J, Birks EK, Liard JF, Imig JD, Lombard JH, Lange AR, Roman R. J Circ Res. 1996;79:54–61. doi: 10.1161/01.res.79.1.54. [DOI] [PubMed] [Google Scholar]
- 7.Lee JC, Bermudez H, Discher BM, Sheehan MA, Won YY, Bates FS, Discher DE. Biotechnol Bioeng. 2001;73:135–145. doi: 10.1002/bit.1045. [DOI] [PubMed] [Google Scholar]
- 8.Rameez S, Alosta H, Palmer AF. Bioconjug Chem. 2008;19:1025–1032. doi: 10.1021/bc700465v. [DOI] [PubMed] [Google Scholar]
- 9.Ahmed F, Pakunlu RI, Srinivas G, Brannan A, Bates F, Klein ML, Minko T, Discher DE. Mol Pharm. 2006;3:340–350. doi: 10.1021/mp050103u. [DOI] [PubMed] [Google Scholar]
- 10.Photos PJ, Bacakova L, Discher B, Bates FS, Discher DE. J Control Release. 2003;90:323–334. doi: 10.1016/s0168-3659(03)00201-3. [DOI] [PubMed] [Google Scholar]
- 11.Bermudez H, Brannan AK, Hammer DA, Bates FS, Discher DE. Macromolecules. 2002;35:8203–8208. [Google Scholar]
- 12.Rameez S, Palmer AF. Langmuir. 2011;27:8829–8840. doi: 10.1021/la201246m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greenwald AR, editor. CRC Handbook of Methods for Oxygen Radical Research. CRC Press; Boca Raton, FL: 1985. pp. 137–141. [Google Scholar]
- 14.Stoenescu R, Meier W. Chem Commun. 2002;24:3016–3017. doi: 10.1039/b209352a. [DOI] [PubMed] [Google Scholar]
- 15.Antonini E, Brunori M. Hemoglobin and Myoglobin in their Reactions with Ligands. North-Holland; Amsterdam: 1971. [Google Scholar]
- 16.Sakai H, Sato A, Masuda K, Takeoka S, Tsuchida E. J Biochem. 2008;283:1508–1517. doi: 10.1074/jbc.M707660200. [DOI] [PubMed] [Google Scholar]
- 17.Olson JS, Foley EW, Maillett DH, Paster EV. Methods Mol Med. 2003:65–91. doi: 10.1385/1-59259-373-9:065. [DOI] [PubMed] [Google Scholar]
- 18.Doherty DH, Doyle MP, Curry SR, Vali RJ, Fattor TJ, Olson JS, Lemon DD. Nat Biotech. 1998;16:672–676. doi: 10.1038/nbt0798-672. [DOI] [PubMed] [Google Scholar]
- 19.Butler AR, Megson IL, Wright PG. Biochimica et Biophysica Acta - General Subjects. 1998;1425:168–176. doi: 10.1016/s0304-4165(98)00065-8. [DOI] [PubMed] [Google Scholar]
- 20.Liao JC, Hein WT, Vaughn MW, Huang KT, Kuo L. Proc Natl Acad Sci. 1999;96:8757–8761. doi: 10.1073/pnas.96.15.8757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu X, Miller MJS, Joshi MS, Sadowska-Krowicka H, Clark DA, Lancaster JR. J Biochem. 1998;273:18709–18713. doi: 10.1074/jbc.273.30.18709. [DOI] [PubMed] [Google Scholar]
- 22.Liu X, Samouilov A, Lancaster JR, Zweier JL. J Biochem. 2002;277:26194–26199. doi: 10.1074/jbc.M201939200. [DOI] [PubMed] [Google Scholar]
- 23.Harder DR, Narayanan J, Birks EK, Liard JF, Imig JD, Lombard JH, Lange AR, Roman RJ. Circ Res. 1996;79:54–61. doi: 10.1161/01.res.79.1.54. [DOI] [PubMed] [Google Scholar]
- 24.Srinivas G, Discher DE, Klein ML. Nat Mater. 2004;3:638–644. doi: 10.1038/nmat1185. [DOI] [PubMed] [Google Scholar]
- 25.Vandegriff KD, Olson JS. J Biochem. 1984;259:12609–12618. [PubMed] [Google Scholar]
- 26.Palmer AF, Sun G, Harris DR. Biotechnol Prog. 2009;25:189–199. doi: 10.1002/btpr.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sakai H, Okuda N, Sato A, Yamaue T, Takeoka S, Tsuchida E. Am J Physiol Heart Circ Physiol. 2010;298:H956–H965. doi: 10.1152/ajpheart.00741.2009. [DOI] [PubMed] [Google Scholar]
- 28.Sakai H, Sato A, Sobolewski P, Takeoka S, Frangos JA, Kobayashi K, Intaglietta M, Tsuchida E. Biochim Biophys Acta-Proteins & Proteomics. 2008;1784:1441–1447. doi: 10.1016/j.bbapap.2008.03.007. [DOI] [PubMed] [Google Scholar]