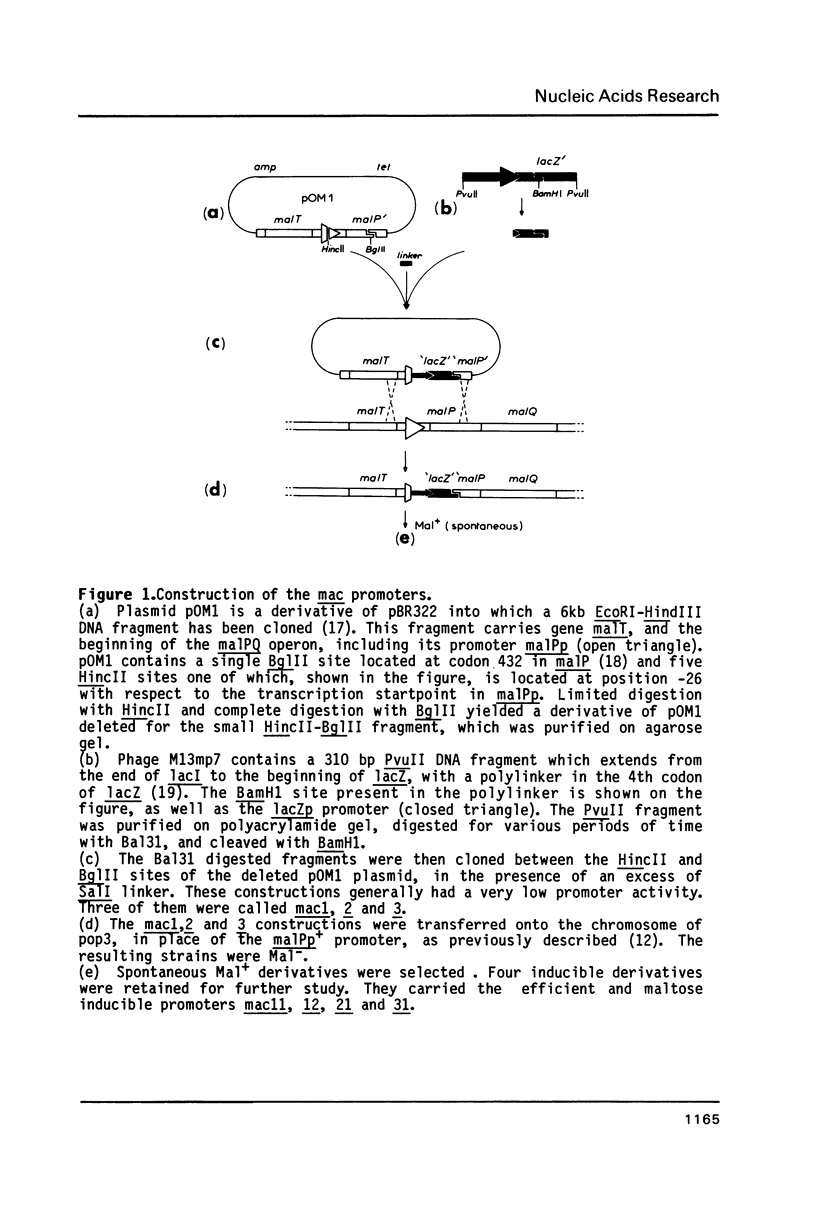
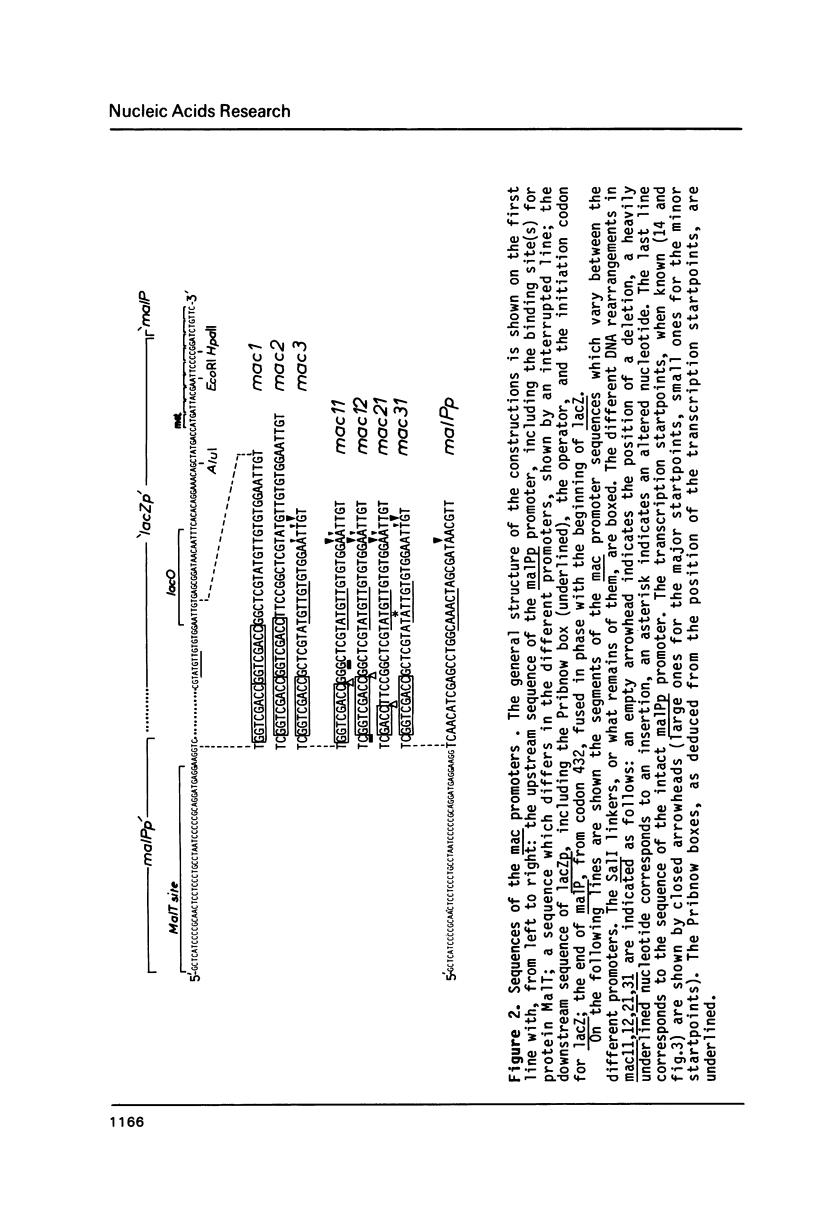
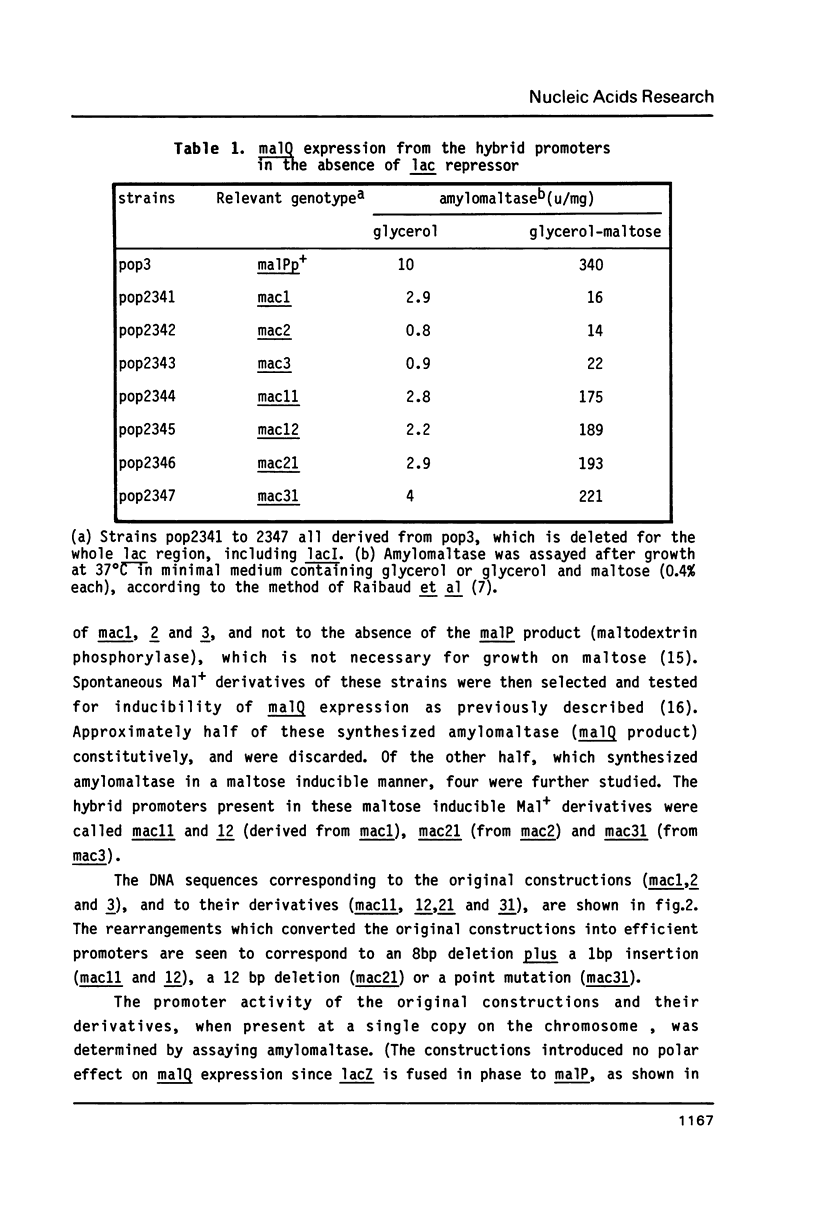
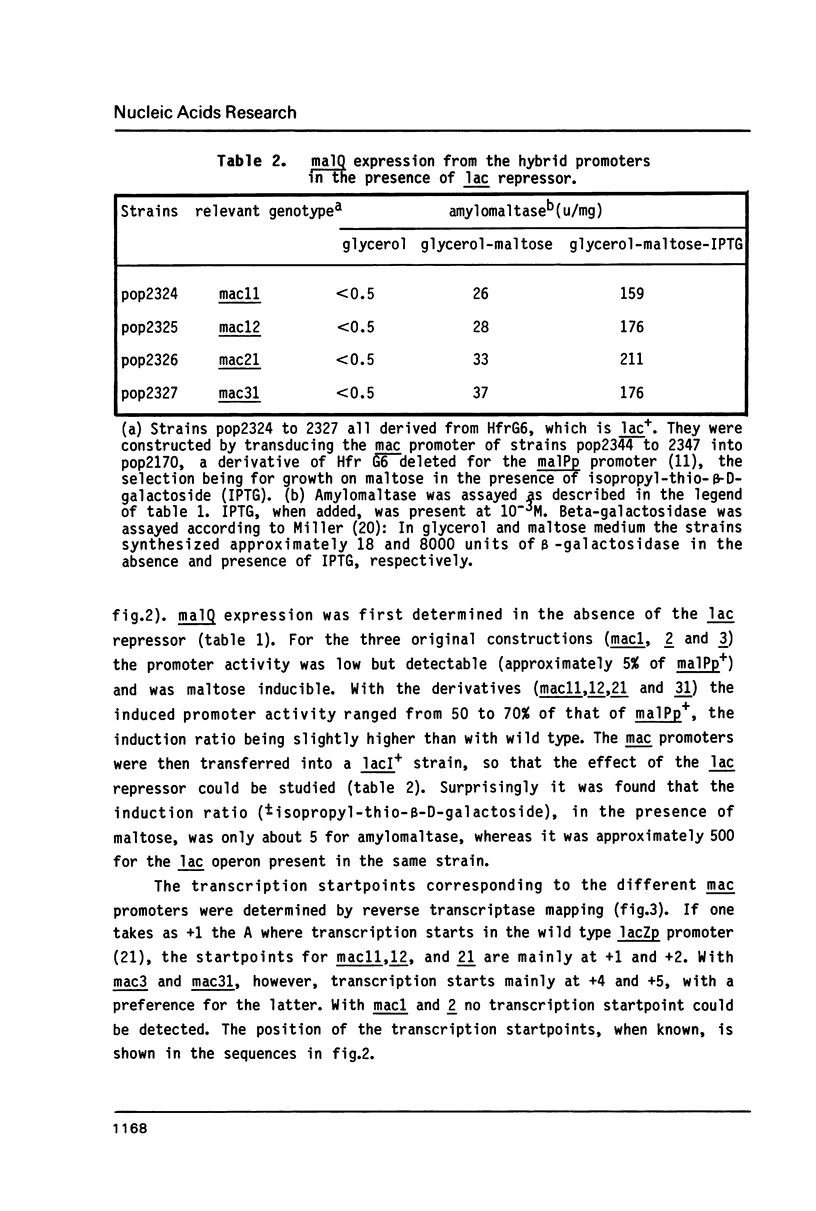
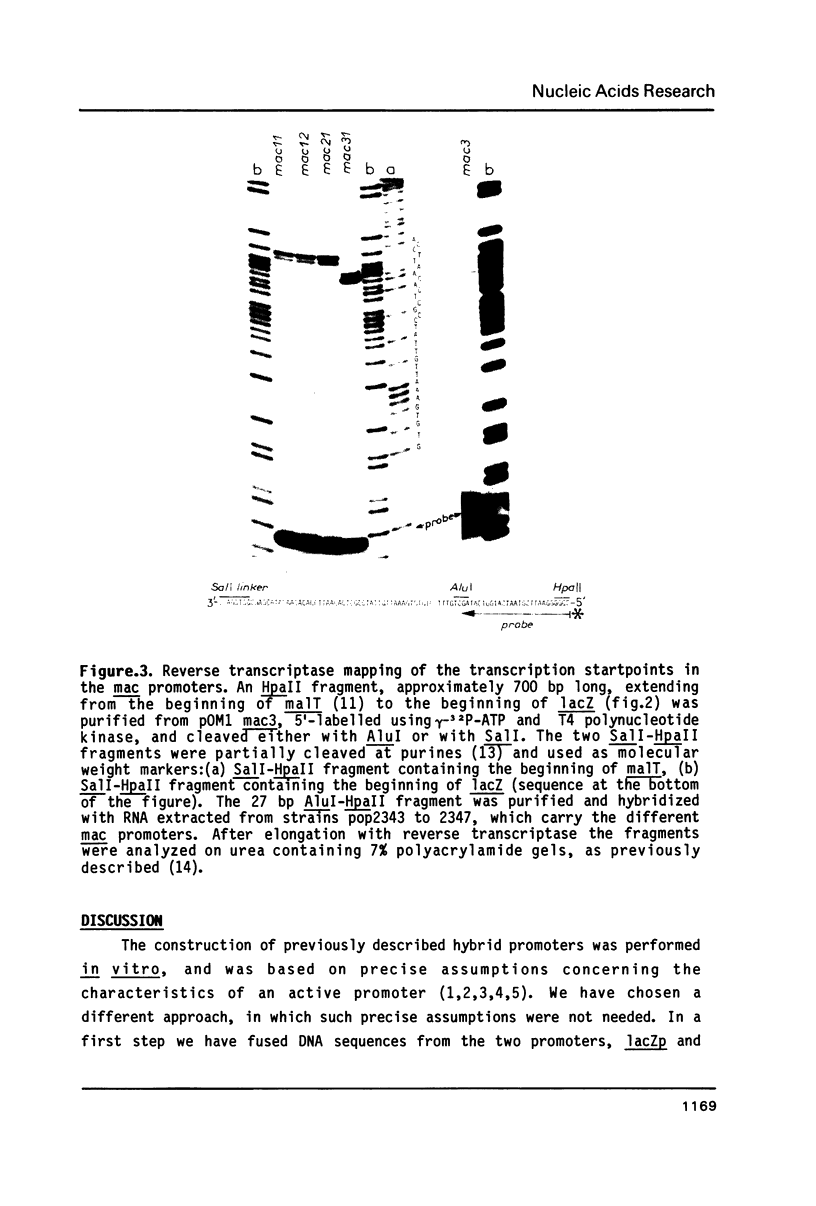
Abstract
Using in vitro techniques we have fused upstream sequences from the malPp promoter (normally activated by the MalT protein) to downstream sequences from the lacZp promoter (normally repressed by the LacI protein). Several hybrid promoters were thus obtained, which were controlled by the MalT protein, but were poorly active. More efficient promoters were then isolated using in vivo selection. Three main conclusions could be derived from the analysis of all of these hybrid promoters. Firstly, the MalT protein seems able to force RNA polymerase to start transcription at any DNA sequence, albeit with a low efficiency. Secondly, the strength of the hybrid promoters is considerably increased if a Pribnow Box is positioned at a precise location with respect to the MalT binding site. Thirdly, the presence of the lac operator, even when properly positioned with respect to the transcription startpoint, does not suffice to permit full repression by the lacI product.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amann E., Brosius J., Ptashne M. Vectors bearing a hybrid trp-lac promoter useful for regulated expression of cloned genes in Escherichia coli. Gene. 1983 Nov;25(2-3):167–178. doi: 10.1016/0378-1119(83)90222-6. [DOI] [PubMed] [Google Scholar]
- Bedouelle H., Schmeissner U., Hofnung M., Rosenberg M. Promoters of the malEFG and malK-lamB operons in Escherichia coli K12. J Mol Biol. 1982 Nov 15;161(4):519–531. doi: 10.1016/0022-2836(82)90405-3. [DOI] [PubMed] [Google Scholar]
- Casadaban M. J. Transposition and fusion of the lac genes to selected promoters in Escherichia coli using bacteriophage lambda and Mu. J Mol Biol. 1976 Jul 5;104(3):541–555. doi: 10.1016/0022-2836(76)90119-4. [DOI] [PubMed] [Google Scholar]
- Débarbouillé M., Raibaud O. Expression of the Escherichia coli malPQ operon remains unaffected after drastic alteration of its promoter. J Bacteriol. 1983 Mar;153(3):1221–1227. doi: 10.1128/jb.153.3.1221-1227.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Débarbouillé M., Shuman H. A., Silhavy T. J., Schwartz M. Dominant constitutive mutations in malT, the positive regulator gene of the maltose regulon in Escherichia coli. J Mol Biol. 1978 Sep 15;124(2):359–371. doi: 10.1016/0022-2836(78)90304-2. [DOI] [PubMed] [Google Scholar]
- Gutierrez C., Raibaud O. Point mutations that reduce the expression of malPQ, a positively controlled operon of Escherichia coli. J Mol Biol. 1984 Jul 25;177(1):69–86. doi: 10.1016/0022-2836(84)90058-5. [DOI] [PubMed] [Google Scholar]
- Hofnung M., Schwartz M. Mutations allowing growth on maltose of Escherichia coli K 12 strains with a deleted malT gene. Mol Gen Genet. 1971;112(2):117–132. doi: 10.1007/BF00267490. [DOI] [PubMed] [Google Scholar]
- Majors J. Initiation of in vitro mRNA synthesis from the wild-type lac promoter. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4394–4398. doi: 10.1073/pnas.72.11.4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matney T. S., Goldschmidt E. P., Erwin N. S., Scroggs R. A. A preliminary map of genomic sites for F-attachment in Escherichia coli K12. Biochem Biophys Res Commun. 1964 Oct 14;17(3):278–281. doi: 10.1016/0006-291x(64)90397-3. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Messing J., Crea R., Seeburg P. H. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981 Jan 24;9(2):309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raibaud O., Débarbouillé M., Schwartz M. Use of deletions created in vitro to map transcriptional regulatory signals in the malA region of Escherichia coli. J Mol Biol. 1983 Jan 25;163(3):395–408. doi: 10.1016/0022-2836(83)90065-7. [DOI] [PubMed] [Google Scholar]
- Raibaud O., Mock M., Schwartz M. A technique for integrating any DNA fragment into the chromosome of Escherichia coli. Gene. 1984 Jul-Aug;29(1-2):231–241. doi: 10.1016/0378-1119(84)90183-5. [DOI] [PubMed] [Google Scholar]
- Raibaud O., Schwartz M. Positive control of transcription initiation in bacteria. Annu Rev Genet. 1984;18:173–206. doi: 10.1146/annurev.ge.18.120184.001133. [DOI] [PubMed] [Google Scholar]
- Raibaud O., Schwartz M. Restriction map of the Escherichia coli malA region and identification of the malT product. J Bacteriol. 1980 Aug;143(2):761–771. doi: 10.1128/jb.143.2.761-771.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reznikoff W. S., Miller J. H., Scaife J. G., Beckwith J. R. A mechanism for repressor action. J Mol Biol. 1969 Jul 14;43(1):201–213. doi: 10.1016/0022-2836(69)90089-8. [DOI] [PubMed] [Google Scholar]
- Reznikoff W. S., Winter R. B., Hurley C. K. The location of the repressor binding sites in the lac operon. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2314–2318. doi: 10.1073/pnas.71.6.2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell D. R., Bennett G. N. Construction and analysis of in vivo activity of E. coli promoter hybrids and promoter mutants that alter the -35 to -10 spacing. Gene. 1982 Dec;20(2):231–243. doi: 10.1016/0378-1119(82)90042-7. [DOI] [PubMed] [Google Scholar]
- Schwartz M. Expression phénotypique et localisation génétique de mutations affectant le métabolisme du maltose chez Escherichia coli K 12. Ann Inst Pasteur (Paris) 1967 Jun;112(6):673–698. [PubMed] [Google Scholar]
- Stefano J. E., Ackerson J. W., Gralla J. D. Alterations in two conserved regions of promoter sequence lead to altered rates of polymerase binding and levels of gene expression. Nucleic Acids Res. 1980 Jun 25;8(12):2709–2723. doi: 10.1093/nar/8.12.2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yansura D. G., Henner D. J. Use of the Escherichia coli lac repressor and operator to control gene expression in Bacillus subtilis. Proc Natl Acad Sci U S A. 1984 Jan;81(2):439–443. doi: 10.1073/pnas.81.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer H. A., Comstock L. J., Vasser M. The tac promoter: a functional hybrid derived from the trp and lac promoters. Proc Natl Acad Sci U S A. 1983 Jan;80(1):21–25. doi: 10.1073/pnas.80.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]