Abstract
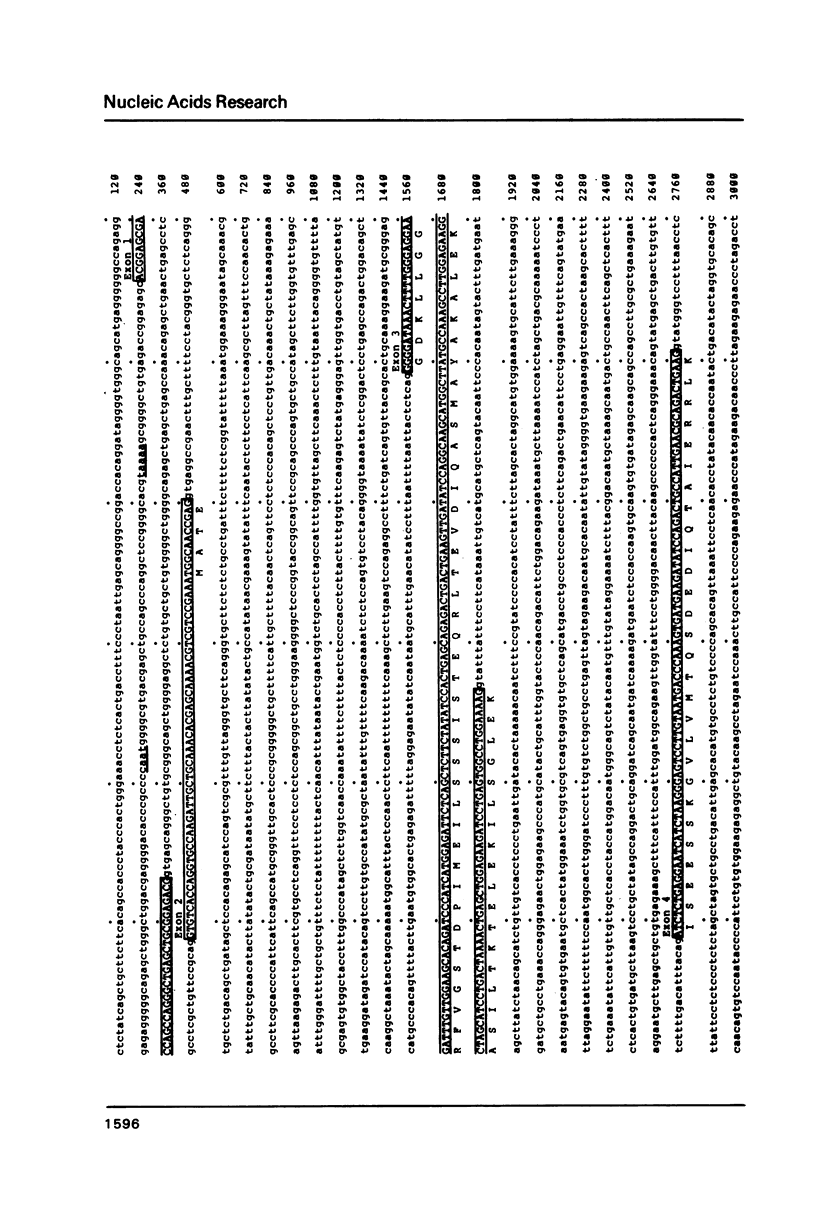
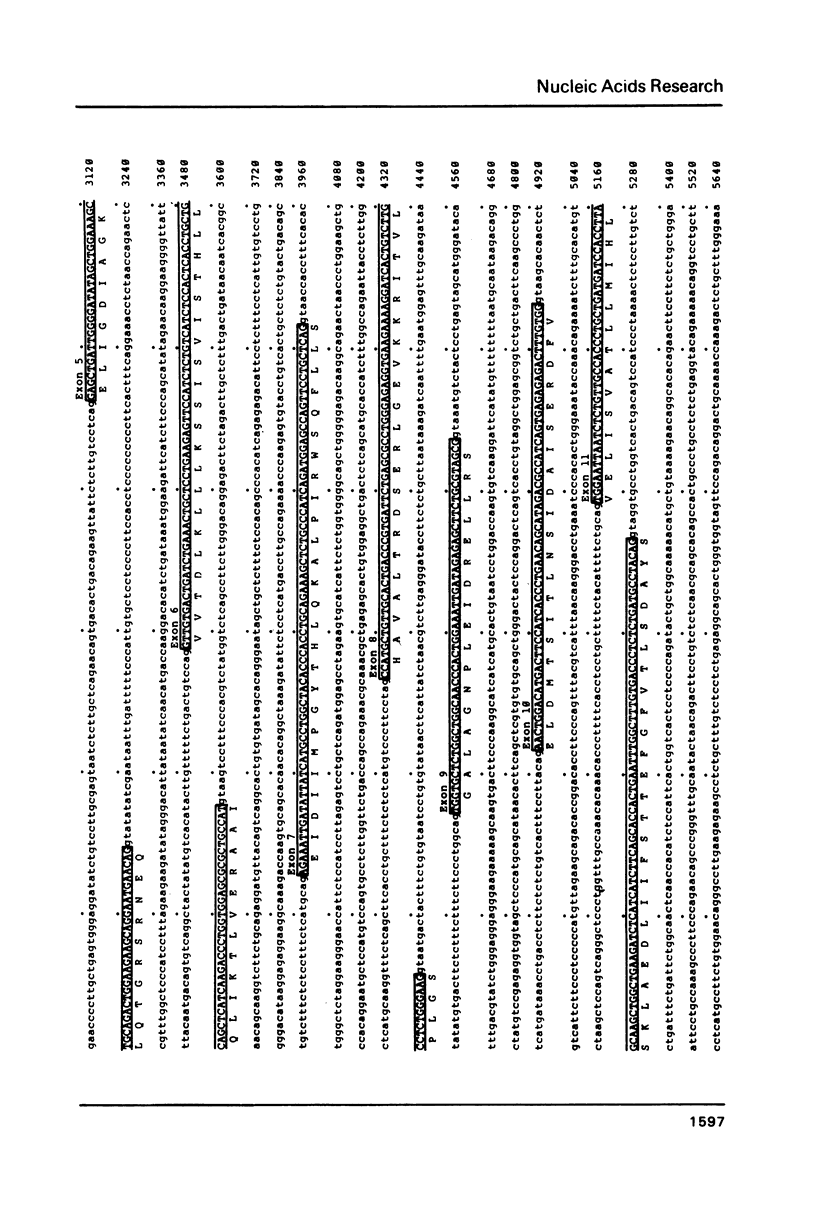
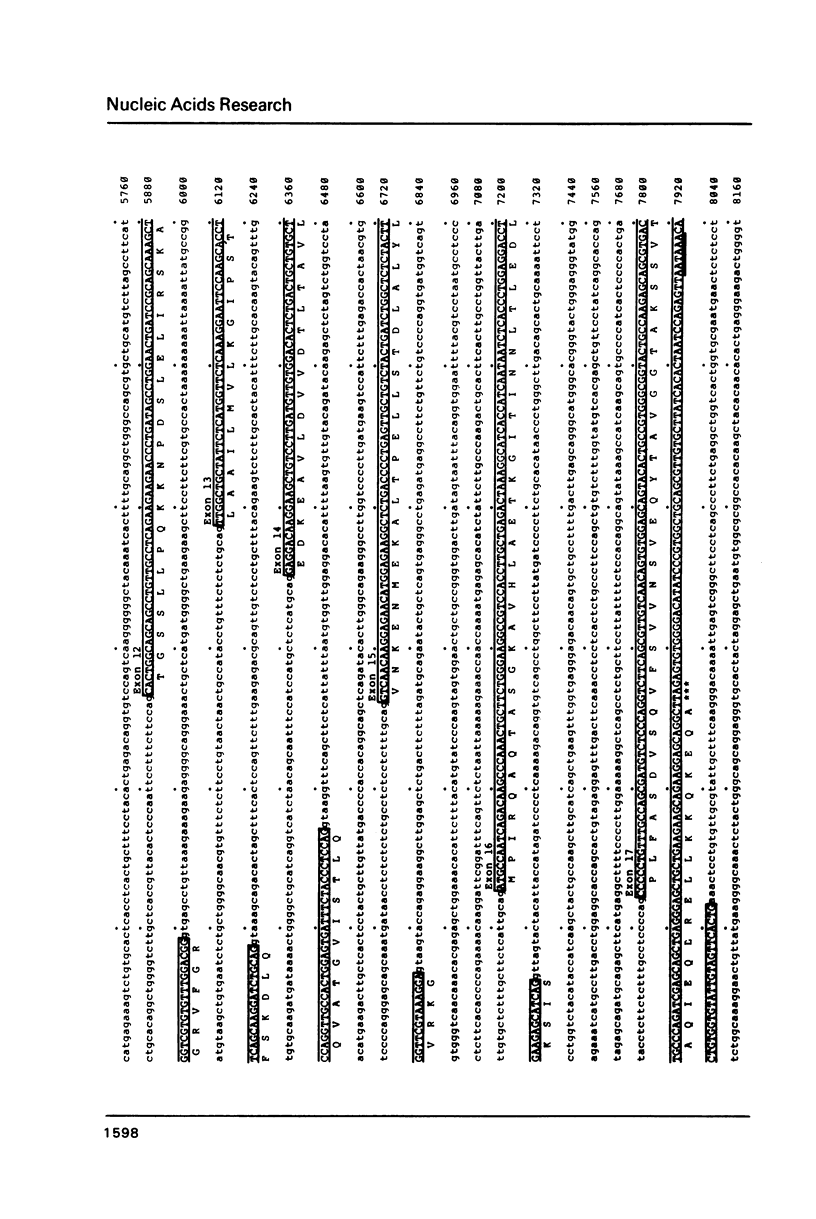
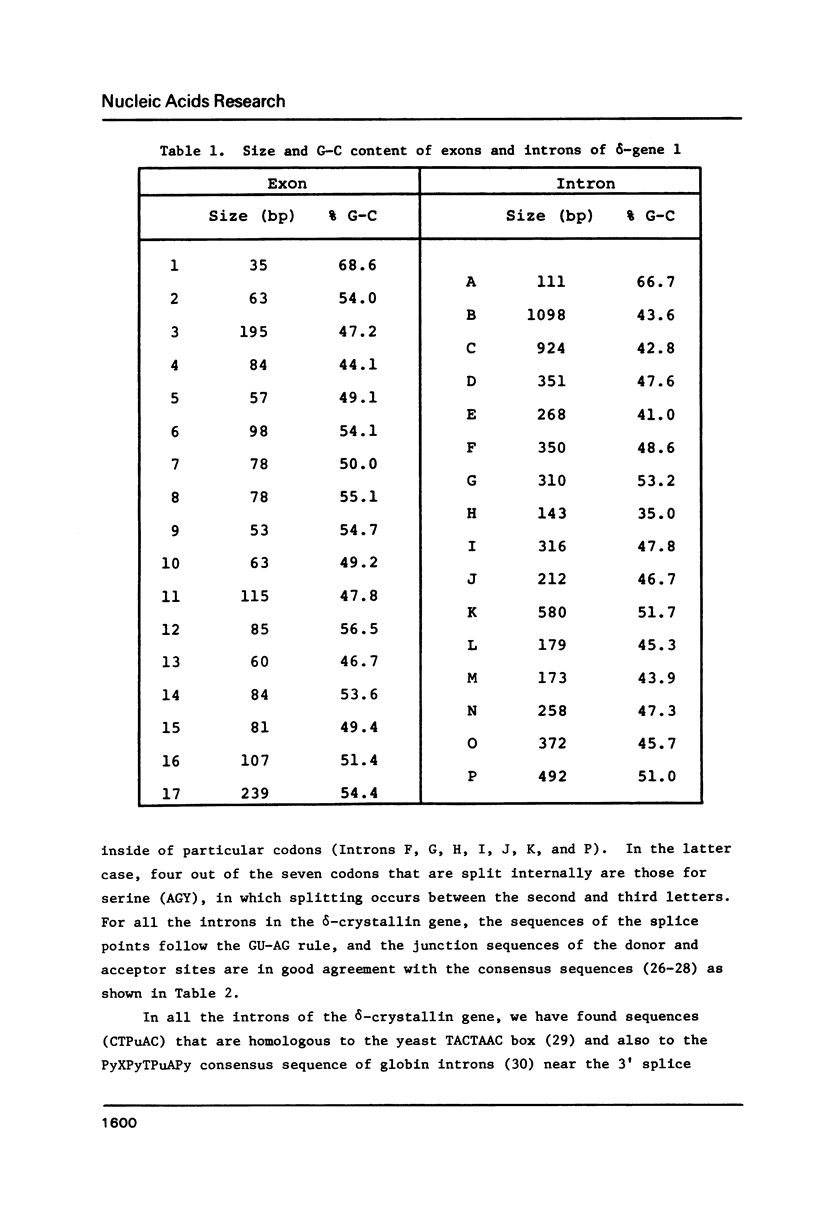
We have determined the complete nucleotide sequence of one of the two non-allelic delta-crystallin genes in the chicken, arbitrarily designated delta-gene 1, using a genomic clone (lambda g delta 106) containing the entire gene sequence. By comparison of the genomic sequence and the delta-crystallin cDNA sequence previously determined, we have identified exon sequences in the genomic sequence. Thus, the presence of 17 exons and 16 introns in the gene has been clarified. The delta-crystallin polypeptide deduced from the exon sequences consists of 465 amino acids which is larger, by 19 amino acid residues, than the polypeptide deduced from the cDNA sequence previously reported. Re-examination of the cDNA sequence using the same cDNA clone previously used shows that the present exon sequences are correct and the molecular weight of the deduced delta-crystallin polypeptide is 50,615 daltons instead of the previously reported value of 48,447 daltons. In addition, some structural features of the delta-crystallin gene including putative expression signals are discussed.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhat S. P., Jones R. E., Sullivan M. A., Piatigorsky J. Chicken lens crystallin DNA sequences show at least two delta-crystallin genes. Nature. 1980 Mar 20;284(5753):234–238. doi: 10.1038/284234a0. [DOI] [PubMed] [Google Scholar]
- Bhat S. P., Piatigorsky J. Molecular cloning and partial characterization of delta-crystallin cDNA sequences in a bacterial plasmid. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3299–3303. doi: 10.1073/pnas.76.7.3299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloemendal H. The vertebrate eye lens. Science. 1977 Jul 8;197(4299):127–138. doi: 10.1126/science.877544. [DOI] [PubMed] [Google Scholar]
- Bower D. J., Errington L. H., Wainwright N. R., Sime C., Morris S., Clayton R. M. Cytoplasmic RNA sequences complementary to cloned chick delta-crystallin cDNA show size heterogeneity. Biochem J. 1982 Feb 1;201(2):339–344. doi: 10.1042/bj2010339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breathnach R., Chambon P. Organization and expression of eucaryotic split genes coding for proteins. Annu Rev Biochem. 1981;50:349–383. doi: 10.1146/annurev.bi.50.070181.002025. [DOI] [PubMed] [Google Scholar]
- Harding J. J., Dilley K. J. Structural proteins of the mammalian lens: a review with emphasis on changes in development, aging and cataract. Exp Eye Res. 1976 Jan;22(1):1–73. doi: 10.1016/0014-4835(76)90033-6. [DOI] [PubMed] [Google Scholar]
- Hawkins J. W., Nickerson J. M., Sullivan M. A., Piatigorsky J. The chicken delta-crystallin gene family. Two genes of similar structure in close chromosomal approximation. J Biol Chem. 1984 Aug 10;259(15):9821–9825. [PubMed] [Google Scholar]
- Jones R. E., Bhat S. P., Sullivan M. A., Piatigorsky J. Comparison of two delta-crystallin genes in the chicken. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5879–5883. doi: 10.1073/pnas.77.10.5879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh A., Yashida K. Delta crystallin synthesis during chick lens differentiation. Exp Eye Res. 1973 Mar;15(3):353–360. doi: 10.1016/0014-4835(73)90150-4. [DOI] [PubMed] [Google Scholar]
- Langford C. J., Gallwitz D. Evidence for an intron-contained sequence required for the splicing of yeast RNA polymerase II transcripts. Cell. 1983 Jun;33(2):519–527. doi: 10.1016/0092-8674(83)90433-6. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Moormann R. J., den Dunnen J. T., Mulleners L., Andreoli P., Bloemendal H., Schoenmakers J. G. Strict co-linearity of genetic and protein folding domains in an intragenically duplicated rat lens gamma-crystallin gene. J Mol Biol. 1983 Dec 25;171(4):353–368. doi: 10.1016/0022-2836(83)90034-7. [DOI] [PubMed] [Google Scholar]
- Mount S. M. A catalogue of splice junction sequences. Nucleic Acids Res. 1982 Jan 22;10(2):459–472. doi: 10.1093/nar/10.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickerson J. M., Piatigorsky J. Sequence of a complete chicken delta-crystallin cDNA. Proc Natl Acad Sci U S A. 1984 May;81(9):2611–2615. doi: 10.1073/pnas.81.9.2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickerson J. M., Piatigorsky J. The nucleic acid and deduced protein sequence of cDNA clones for delta-crystallin of the chicken lens. FEBS Lett. 1982 Aug 2;144(2):289–292. doi: 10.1016/0014-5793(82)80656-x. [DOI] [PubMed] [Google Scholar]
- Norrander J., Kempe T., Messing J. Construction of improved M13 vectors using oligodeoxynucleotide-directed mutagenesis. Gene. 1983 Dec;26(1):101–106. doi: 10.1016/0378-1119(83)90040-9. [DOI] [PubMed] [Google Scholar]
- Papaconstantinou J. Molecular aspects of lens cell differentiation. Science. 1967 Apr 21;156(3773):338–346. doi: 10.1126/science.156.3773.338. [DOI] [PubMed] [Google Scholar]
- Piatigorsky J. Delta crystallins and their nucleic acids. Mol Cell Biochem. 1984;59(1-2):33–56. doi: 10.1007/BF00231304. [DOI] [PubMed] [Google Scholar]
- Piatigorsky J. Lens differentiation in vertebrates. A review of cellular and molecular features. Differentiation. 1981;19(3):134–153. doi: 10.1111/j.1432-0436.1981.tb01141.x. [DOI] [PubMed] [Google Scholar]
- Piatigorsky J., Zelenka P., Simpson R. T. Molecular weight and subunit structure of delta-crystallin from embryonic chick lens fibers. Exp Eye Res. 1974 May;18(5):435–446. doi: 10.1016/0014-4835(74)90080-3. [DOI] [PubMed] [Google Scholar]
- RABAEY M. Electrophoretic and immunoelectrophoretic studies on the soluble proteins in the developing lens of birds. Exp Eye Res. 1962 Jun;1:310–316. doi: 10.1016/s0014-4835(62)80017-7. [DOI] [PubMed] [Google Scholar]
- Reszelbach R., Shinohara T., Piatigorsky J. Resolution of two distinct embryonic chick delta-crystallin bands by polyacrylamide gel electrophoresis in the presence of sodium dodecyl sulfate and urea. Exp Eye Res. 1977 Dec;25(6):583–593. doi: 10.1016/0014-4835(77)90137-3. [DOI] [PubMed] [Google Scholar]
- Ruskin B., Krainer A. R., Maniatis T., Green M. R. Excision of an intact intron as a novel lariat structure during pre-mRNA splicing in vitro. Cell. 1984 Aug;38(1):317–331. doi: 10.1016/0092-8674(84)90553-1. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P. A. Speculations on RNA splicing. Cell. 1981 Mar;23(3):643–646. doi: 10.1016/0092-8674(81)90425-6. [DOI] [PubMed] [Google Scholar]
- Yasuda K., Kondoh H., Okada T. S., Nakajima N., Shimura Y. Organization of delta-crystallin genes in the chicken. Nucleic Acids Res. 1982 May 11;10(9):2879–2891. doi: 10.1093/nar/10.9.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda K., Nakajima N., Isobe T., Okada T. S., Shimura Y. The nucleotide sequence of a complete chicken delta-crystallin cDNA. EMBO J. 1984 Jun;3(6):1397–1402. doi: 10.1002/j.1460-2075.1984.tb01983.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwaan J., Ikeda A. Macromolecular events during differentiation of the chicken lens. Exp Eye Res. 1968 Apr;7(2):301–311. doi: 10.1016/s0014-4835(68)80081-8. [DOI] [PubMed] [Google Scholar]


