Abstract
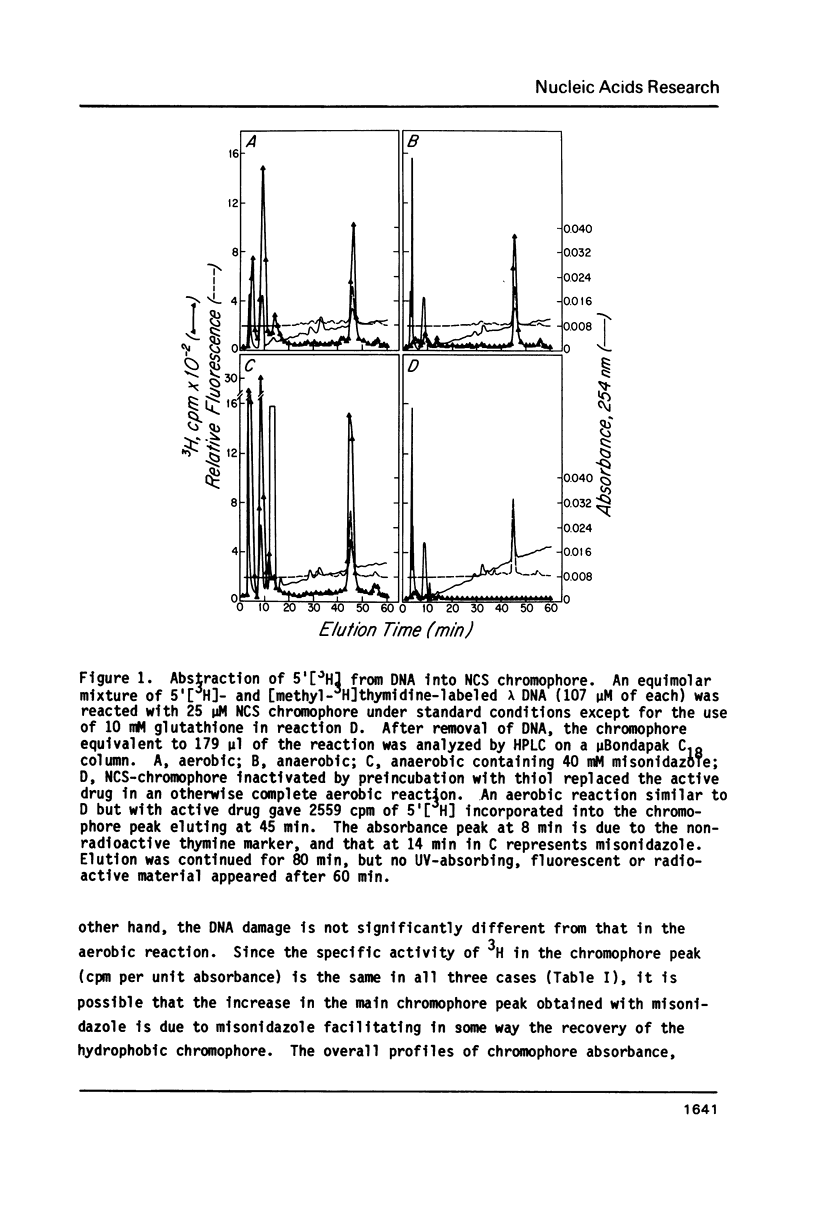
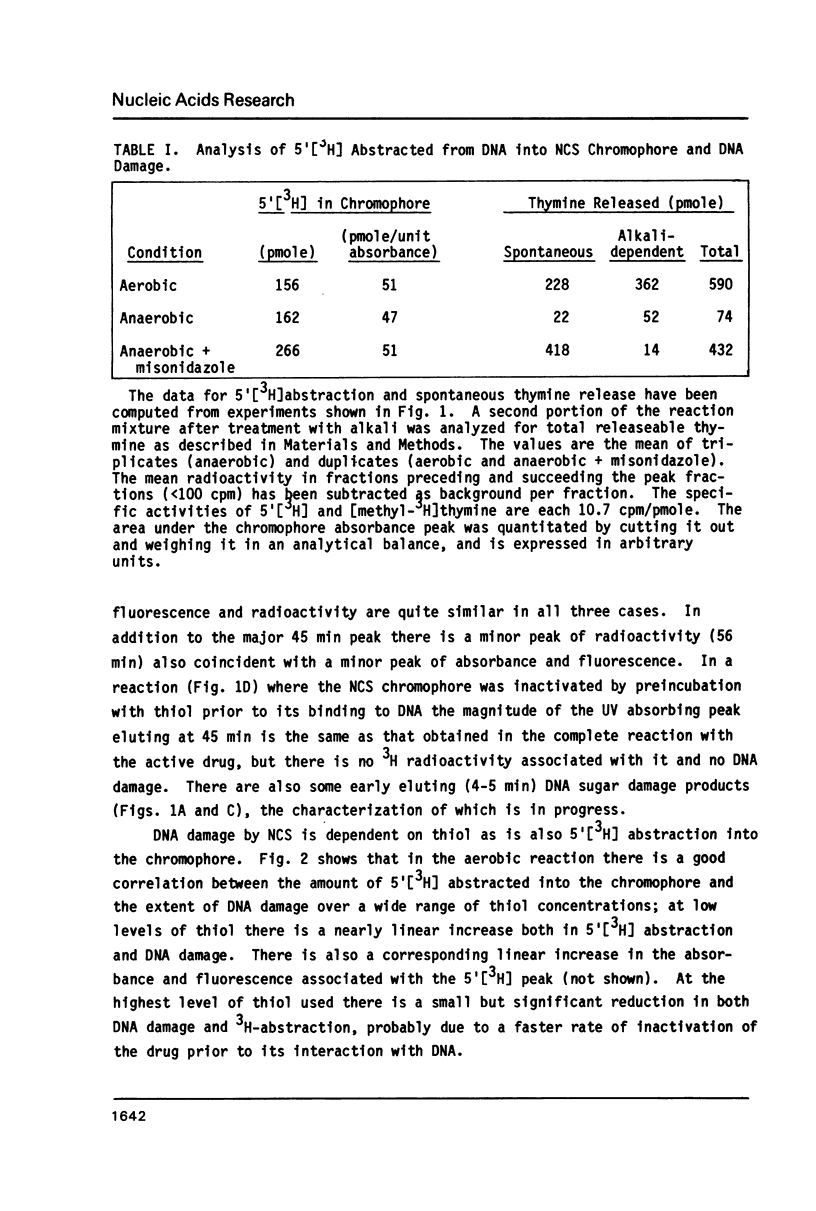
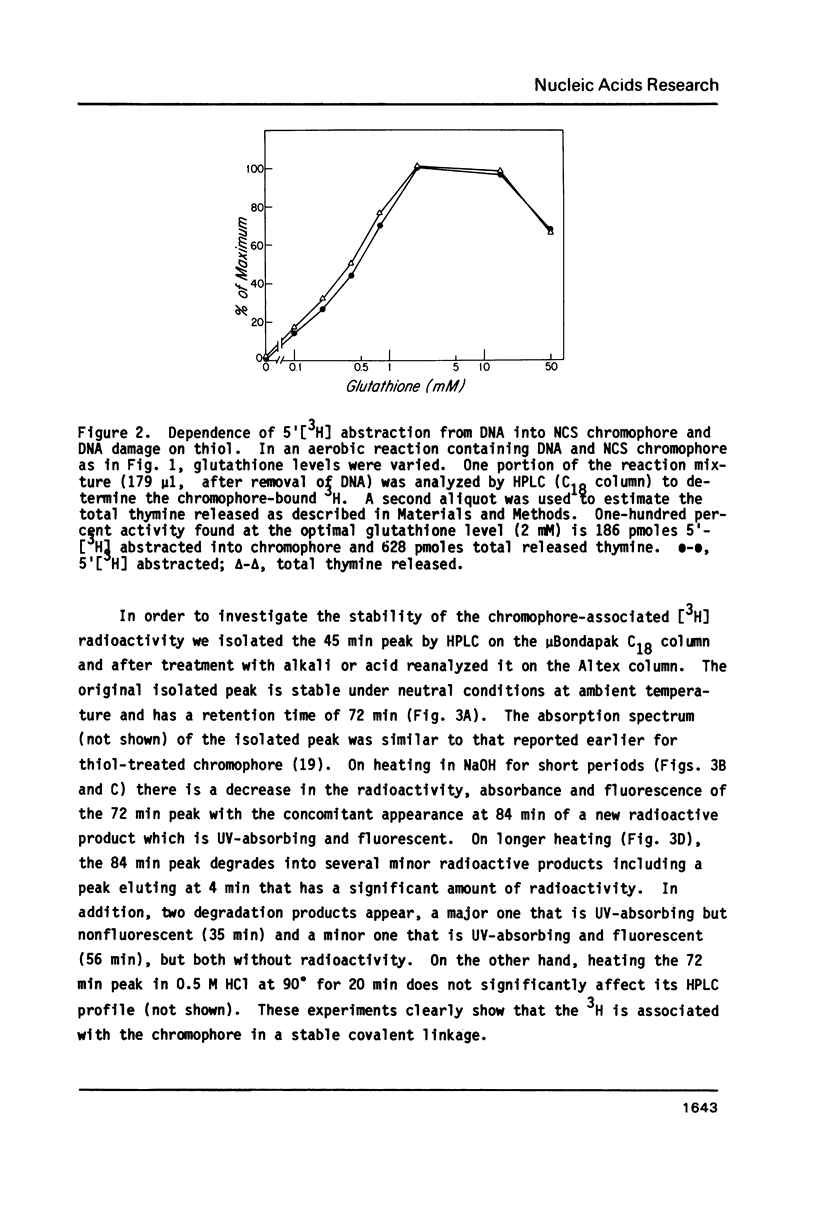
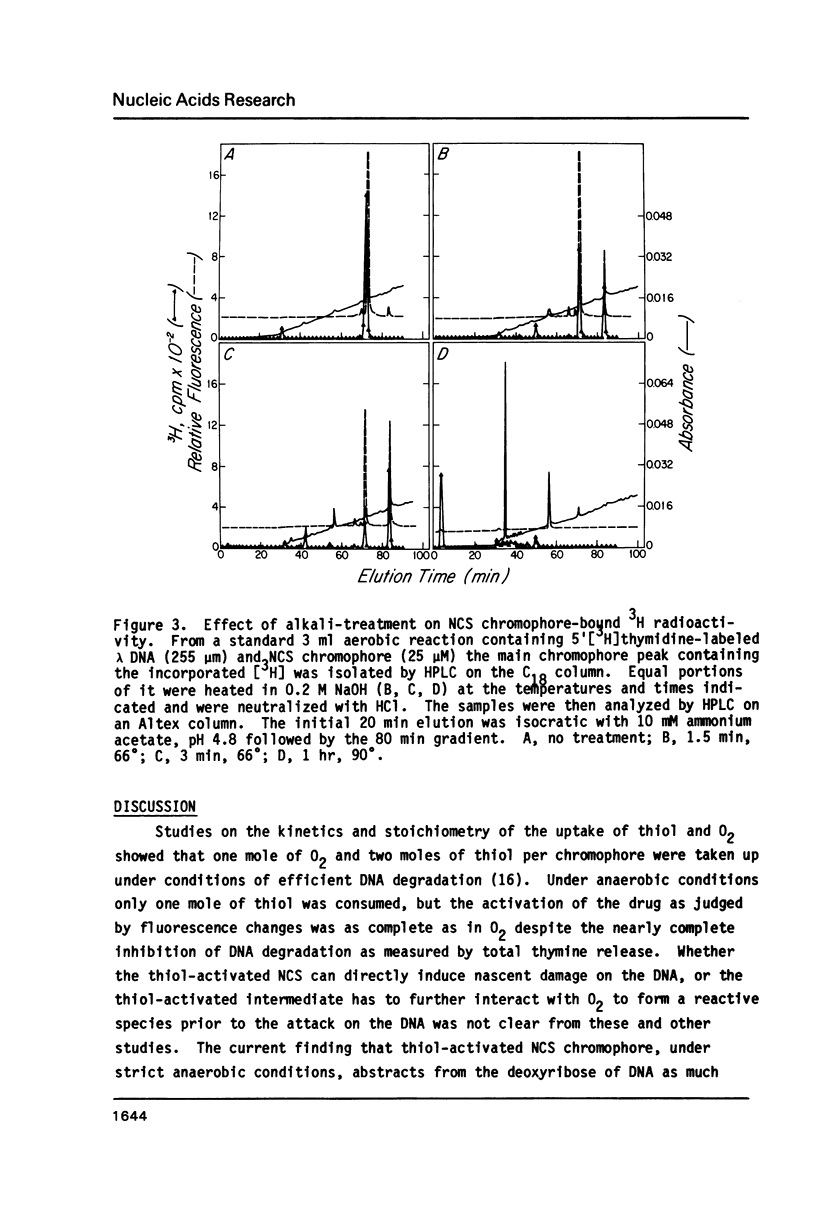
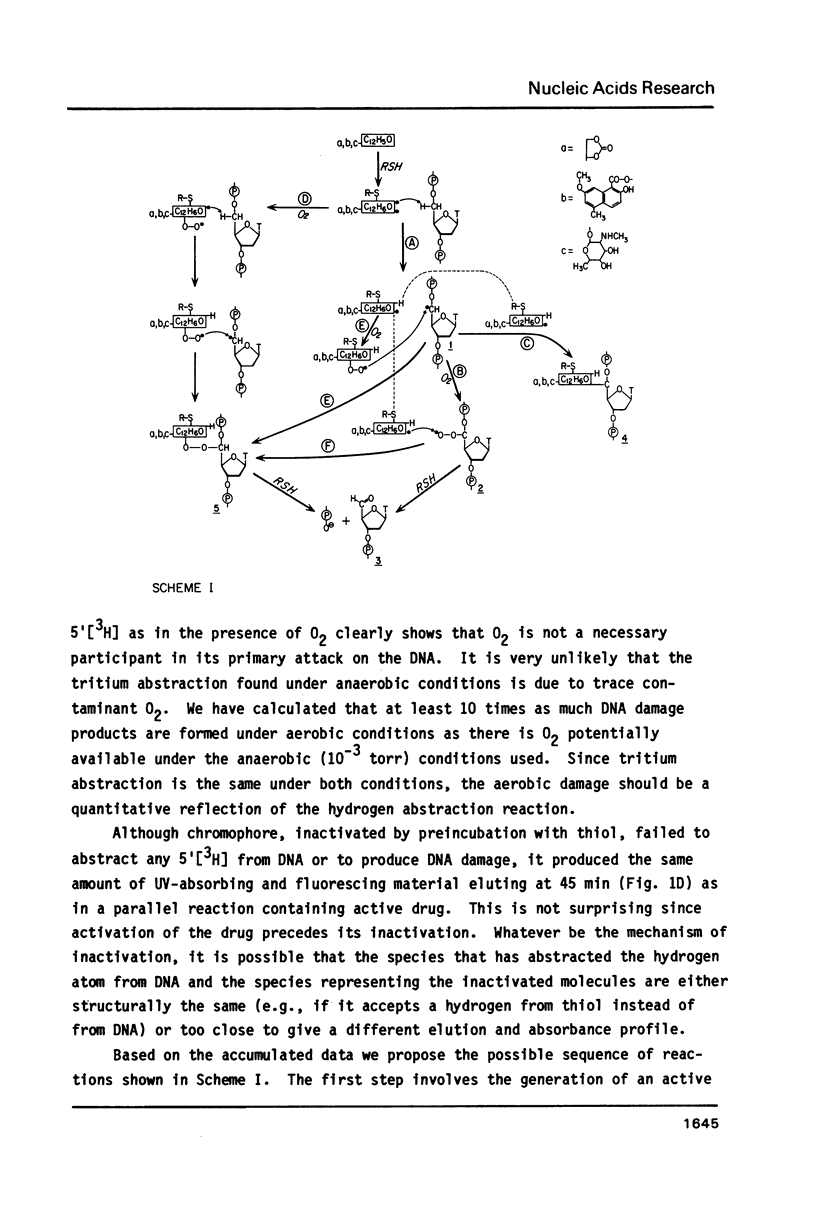
Thiol-activated neocarzinostatin chromophore abstracts tritium from the 5', but not from the 1' or 2' positions of deoxyribose in DNA and incorporates it into a stable, non-exchangeable form. The abstracted tritium remains covalently associated with the chromophore or its degradation product after treatment with acid or alkali, respectively. Drug activation and the consequent hydrogen abstraction reaction, presumably generating a carbon-centered radical at C-5', do not require molecular oxygen but have a dose-dependent relation with thiol. Under aerobic conditions, where base release and DNA strand breaks with nucleoside 5'-aldehyde at the 5'-ends are produced, hydrogen abstraction from C-5' parallels these parameters of DNA damage. It is possible to formulate a reaction scheme in which the carbon- centered radical at C-5' is an intermediate in the formation of the various DNA damage products found under both aerobic and anaerobic conditions.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bose K. K., Tatsumi K., Strauss B. S. Apurinic/apyrimidinic endonuclease sensitive sites as intermediates in the in vitro degradation of deoxyribonucleic acid by neocarzinostatin. Biochemistry. 1980 Oct 14;19(21):4761–4766. doi: 10.1021/bi00562a007. [DOI] [PubMed] [Google Scholar]
- Burger R. M., Peisach J., Horwitz S. B. Effects of O2 on the reactions of activated bleomycin. J Biol Chem. 1982 Apr 10;257(7):3372–3375. [PubMed] [Google Scholar]
- Charnas R. L., Goldberg I. H. Neocarzinostatin abstracts a hydrogen during formation of nucleotide 5'-aldehyde on DNA. Biochem Biophys Res Commun. 1984 Jul 31;122(2):642–648. doi: 10.1016/s0006-291x(84)80081-9. [DOI] [PubMed] [Google Scholar]
- Chin D. H., Carr S. A., Goldberg I. H. Incorporation of 18O2 into thymidine 5'-aldehyde in neocarzinostatin chromophore-damaged DNA. J Biol Chem. 1984 Aug 25;259(16):9975–9978. [PubMed] [Google Scholar]
- Favaudon V., Charnas R. L., Goldberg I. H. Poly(deoxyadenylic-deoxythymidylic acid) damage by radiolytically activated neocarzinostatin. Biochemistry. 1985 Jan 15;24(2):250–259. doi: 10.1021/bi00323a003. [DOI] [PubMed] [Google Scholar]
- Giloni L., Takeshita M., Johnson F., Iden C., Grollman A. P. Bleomycin-induced strand-scission of DNA. Mechanism of deoxyribose cleavage. J Biol Chem. 1981 Aug 25;256(16):8608–8615. [PubMed] [Google Scholar]
- Hensens O. D., Dewey R. S., Liesch J. M., Napier M. A., Reamer R. A., Smith J. L., Albers-Schönberg G., Goldberg I. H. Neocarzinostatin chromophore: presence of a highly strained ether ring and its reaction with mercaptan and sodium borohydride. Biochem Biophys Res Commun. 1983 Jun 15;113(2):538–547. doi: 10.1016/0006-291x(83)91759-x. [DOI] [PubMed] [Google Scholar]
- Ishida R., Takahashi T. In vitro release of thymine from DNA by neocarzinostatin. Biochem Biophys Res Commun. 1976 Jan 12;68(1):256–261. doi: 10.1016/0006-291x(76)90037-1. [DOI] [PubMed] [Google Scholar]
- Kappen L. S., Goldberg I. H. Deoxyribonucleic acid damage by neocarzinostatin chromophore: strand breaks generated by selective oxidation of C-5' of deoxyribose. Biochemistry. 1983 Oct 11;22(21):4872–4878. doi: 10.1021/bi00290a002. [DOI] [PubMed] [Google Scholar]
- Kappen L. S., Goldberg I. H., Liesch J. M. Identification of thymidine-5'-aldehyde at DNA strand breaks induced by neocarzinostatin chromophore. Proc Natl Acad Sci U S A. 1982 Feb;79(3):744–748. doi: 10.1073/pnas.79.3.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappen L. S., Goldberg I. H. Nitroaromatic radiation sensitizers substitute for oxygen in neocarzinostatin-induced DNA damage. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3312–3316. doi: 10.1073/pnas.81.11.3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappen L. S., Napier M. A., Goldberg I. H. Roles of chromophore and apo-protein in neocarzinostatin action. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1970–1974. doi: 10.1073/pnas.77.4.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobb R. R., Fett J. W. Purification of two distinct growth factors from bovine neural tissue by heparin affinity chromatography. Biochemistry. 1984 Dec 18;23(26):6295–6299. doi: 10.1021/bi00321a001. [DOI] [PubMed] [Google Scholar]
- Napier M. A., Goldberg I. H. Neocarzinostatin chromophore. Assignment of spectral properties and structural requirements for binding to DNA. Mol Pharmacol. 1983 Mar;23(2):500–510. [PubMed] [Google Scholar]
- Napier M. A., Holmquist B., Strydom D. J., Goldberg I. H. Neocarzinostatin: spectral characterization and separation of a non-protein chromophore. Biochem Biophys Res Commun. 1979 Jul 27;89(2):635–642. doi: 10.1016/0006-291x(79)90677-6. [DOI] [PubMed] [Google Scholar]
- Ohtsuki K., Ishida N. The biological effect of a nonprotein component removed from neocarzinostatin (NCS). J Antibiot (Tokyo) 1980 Jul;33(7):744–750. doi: 10.7164/antibiotics.33.744. [DOI] [PubMed] [Google Scholar]
- Poon R., Beerman T. A., Goldberg I. H. Characterization of DNA strand breakage in vitro by the antitumor protein neocarzinostatin. Biochemistry. 1977 Feb 8;16(3):486–493. doi: 10.1021/bi00622a023. [DOI] [PubMed] [Google Scholar]
- Povirk L. F., Dattagupta N., Warf B. C., Goldberg I. H. Neocarzinostatin chromophore binds to deoxyribonucleic acid by intercalation. Biochemistry. 1981 Jul 7;20(14):4007–4014. doi: 10.1021/bi00517a009. [DOI] [PubMed] [Google Scholar]
- Povirk L. F., Goldberg I. H. Covalent adducts of DNA and the nonprotein chromophore of neocarzinostatin contain a modified deoxyribose. Proc Natl Acad Sci U S A. 1982 Jan;79(2):369–373. doi: 10.1073/pnas.79.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Povirk L. F., Goldberg I. H. Neocarzinostatin chromophore-DNA adducts: evidence for a covalent linkage to the oxidized C-5' of deoxyribose. Nucleic Acids Res. 1982 Oct 25;10(20):6255–6264. doi: 10.1093/nar/10.20.6255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Povirk L. F., Goldberg I. H. Stoichiometric uptake of molecular oxygen and consumption of sulfhydryl groups by neocarzinostatin chromophore bound to DNA. J Biol Chem. 1983 Oct 10;258(19):11763–11767. [PubMed] [Google Scholar]
- Suzuki H., Miura K., Kumada Y., Takeuchi T., Tanaka N. Biological activities of non-protein chromophores of antitumor protein antibiotics: auromycin and neocarzinostatin. Biochem Biophys Res Commun. 1980 May 14;94(1):255–261. doi: 10.1016/s0006-291x(80)80214-2. [DOI] [PubMed] [Google Scholar]
- Von Sonntag C., Schulte-Frohlinde D. Radiation-induced degradation of the sugar in model compounds and in DNA. Mol Biol Biochem Biophys. 1978;27:204–226. [PubMed] [Google Scholar]
- Wu J. C., Kozarich J. W., Stubbe J. The mechanism of free base formation from DNA by bleomycin. A proposal based on site specific tritium release from Poly(dA.dU). J Biol Chem. 1983 Apr 25;258(8):4694–4697. [PubMed] [Google Scholar]


