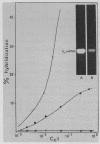
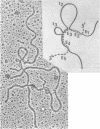
Abstract
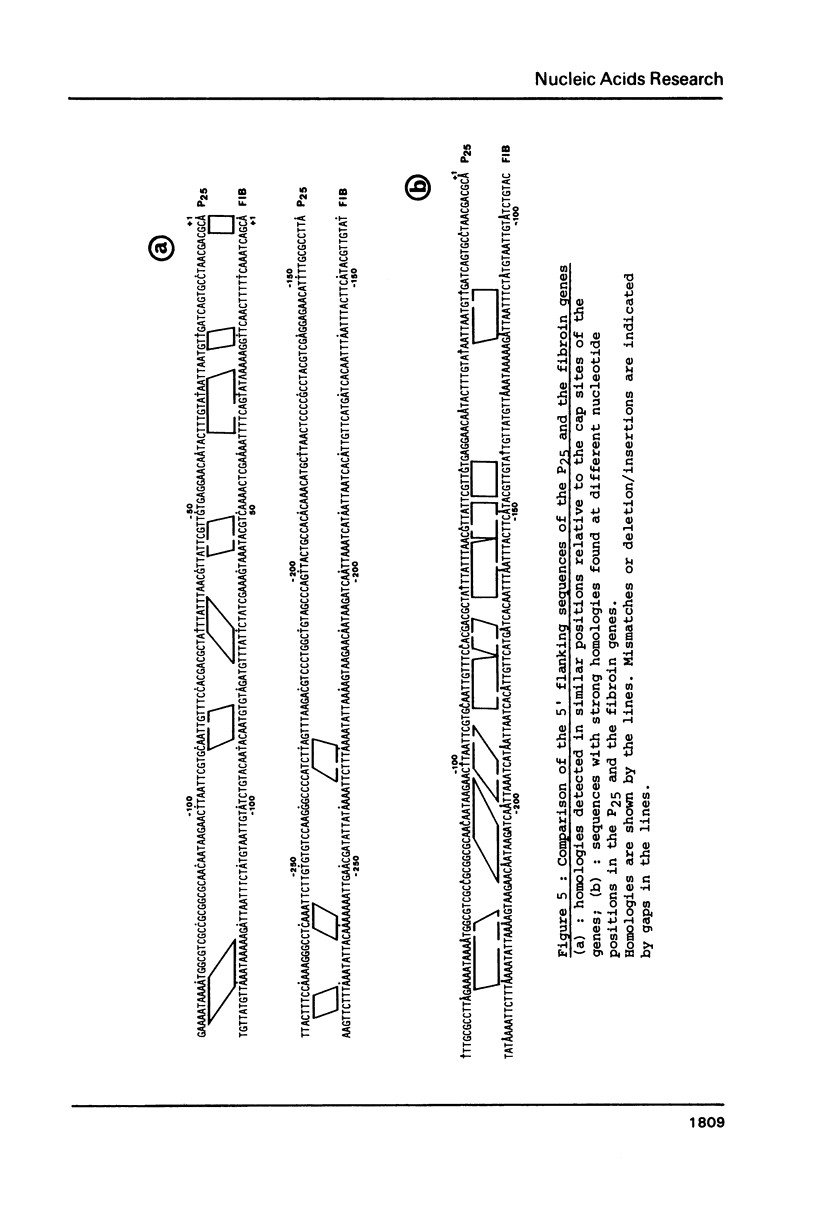
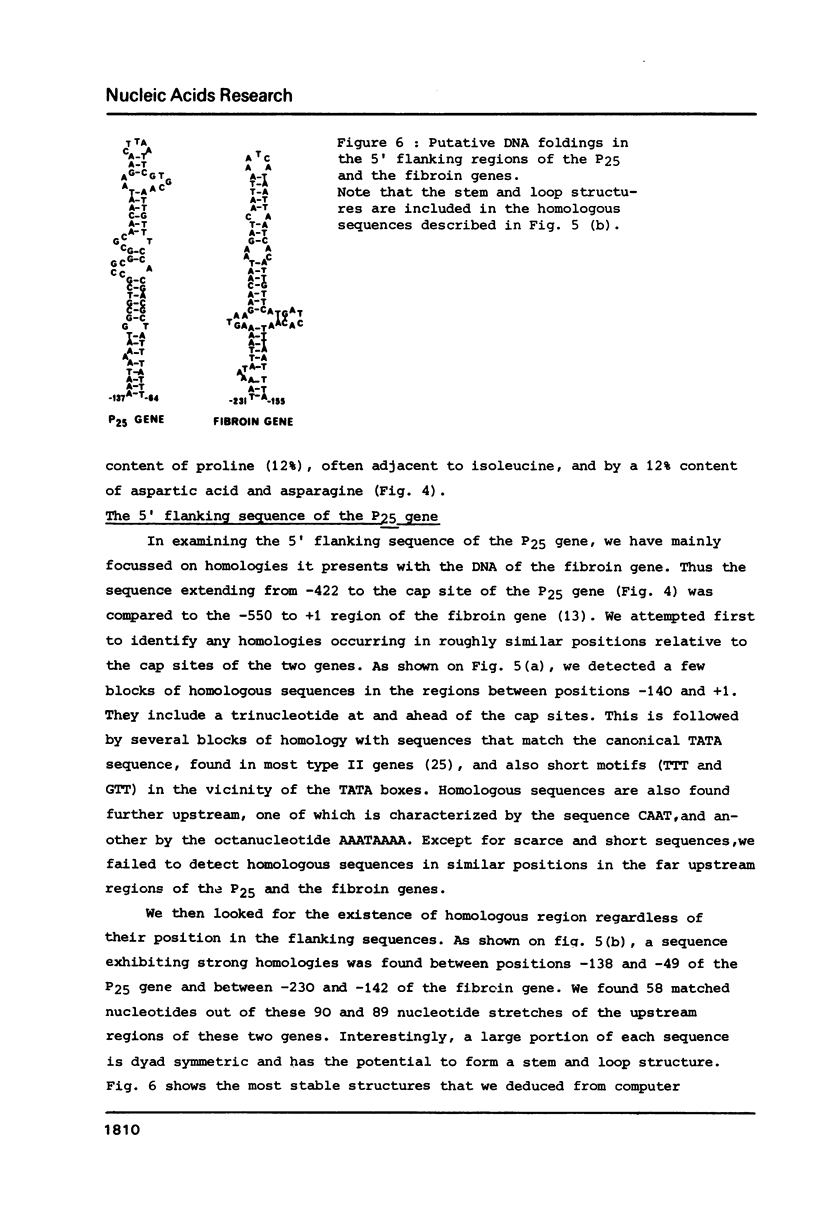
We have cloned a large portion of the P25 gene of Bombyx mori encoding the 25,000 dalton polypeptide which associates with fibroin to constitute the major silk protein. Its structure has been investigated by restriction mapping R-loop analysis, S1 nuclease protection experiments and nucleotide sequencing of the region spanning the 5' end of the gene and its flanking DNA. This has permitted a comparative sequence analysis of the DNA from the P25 and fibroin genes. The genes demonstrate no relatedness in their coding regions but they exhibit large blocks of sequence homology in their 5' flanking regions. In particular, the DNA upstream of the P25 gene possesses a sequence very similar to a region of fibroin 5' flanking DNA that is known to possess transcription modulation signals. The functional significance of these homologous regions is discussed with regard to the highly coordinated expression of these two genes.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benoist C., Chambon P. In vivo sequence requirements of the SV40 early promotor region. Nature. 1981 Mar 26;290(5804):304–310. doi: 10.1038/290304a0. [DOI] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Breathnach R., Chambon P. Organization and expression of eucaryotic split genes coding for proteins. Annu Rev Biochem. 1981;50:349–383. doi: 10.1146/annurev.bi.50.070181.002025. [DOI] [PubMed] [Google Scholar]
- Couble P., Garel A., Prudhomme J. C. Complexity and diversity of polyadenylated mRNA in the silk gland of Bombyx mori: changes related to fibroin production. Dev Biol. 1981 Feb;82(1):139–149. doi: 10.1016/0012-1606(81)90435-8. [DOI] [PubMed] [Google Scholar]
- Couble P., Moine A., Garel A., Prudhomme J. C. Developmental variations of a nonfibroin mRNA of Bombyx mori silkgland, encoding for a low-molecular-weight silk protein. Dev Biol. 1983 Jun;97(2):398–407. doi: 10.1016/0012-1606(83)90096-9. [DOI] [PubMed] [Google Scholar]
- Davidson E. H., Jacobs H. T., Britten R. J. Very short repeats and coordinate induction of genes. Nature. 1983 Feb 10;301(5900):468–470. doi: 10.1038/301468a0. [DOI] [PubMed] [Google Scholar]
- Dean D. C., Knoll B. J., Riser M. E., O'Malley B. W. A 5'-flanking sequence essential for progesterone regulation of an ovalbumin fusion gene. Nature. 1983 Oct 6;305(5934):551–554. doi: 10.1038/305551a0. [DOI] [PubMed] [Google Scholar]
- Donahue T. F., Daves R. S., Lucchini G., Fink G. R. A short nucleotide sequence required for regulation of HIS4 by the general control system of yeast. Cell. 1983 Jan;32(1):89–98. doi: 10.1016/0092-8674(83)90499-3. [DOI] [PubMed] [Google Scholar]
- Eickbush T. H., Kafatos F. C. A walk in the chorion locus of Bombyx mori. Cell. 1982 Jun;29(2):633–643. doi: 10.1016/0092-8674(82)90179-9. [DOI] [PubMed] [Google Scholar]
- Gage L. P., Manning R. F. Determination of the multiplicity of the silk fibroin gene and detection of fibroin gene-related DNA in the genome of Bombyx mori. J Mol Biol. 1976 Mar 5;101(3):327–348. doi: 10.1016/0022-2836(76)90151-0. [DOI] [PubMed] [Google Scholar]
- Gillies S. D., Morrison S. L., Oi V. T., Tonegawa S. A tissue-specific transcription enhancer element is located in the major intron of a rearranged immunoglobulin heavy chain gene. Cell. 1983 Jul;33(3):717–728. doi: 10.1016/0092-8674(83)90014-4. [DOI] [PubMed] [Google Scholar]
- Grez M., Land H., Giesecke K., Schütz G., Jung A., Sippel A. E. Multiple mRNAs are generated from the chicken lysozyme gene. Cell. 1981 Sep;25(3):743–752. doi: 10.1016/0092-8674(81)90182-3. [DOI] [PubMed] [Google Scholar]
- Grosschedl R., Birnstiel M. L. Spacer DNA sequences upstream of the T-A-T-A-A-A-T-A sequence are essential for promotion of H2A histone gene transcription in vivo. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7102–7106. doi: 10.1073/pnas.77.12.7102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosveld G. C., Rosenthal A., Flavell R. A. Sequence requirements for the transcription of the rabbit beta-globin gene in vivo: the -80 region. Nucleic Acids Res. 1982 Aug 25;10(16):4951–4971. doi: 10.1093/nar/10.16.4951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury G., Gruss P. Enhancer elements. Cell. 1983 Jun;33(2):313–314. doi: 10.1016/0092-8674(83)90410-5. [DOI] [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984 Jan 25;12(2):857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maekawa H., Suzuki Y. Repeated turn-off and turn-on of fibroin gene transcription during silk gland development of Bombyx mori. Dev Biol. 1980 Aug;78(2):394–406. doi: 10.1016/0012-1606(80)90343-7. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellon P., Parker V., Gluzman Y., Maniatis T. Identification of DNA sequences required for transcription of the human alpha 1-globin gene in a new SV40 host-vector system. Cell. 1981 Dec;27(2 Pt 1):279–288. doi: 10.1016/0092-8674(81)90411-6. [DOI] [PubMed] [Google Scholar]
- Muskavitch M. A., Hogness D. S. An expandable gene that encodes a Drosophila glue protein is not expressed in variants lacking remote upstream sequences. Cell. 1982 Jul;29(3):1041–1051. doi: 10.1016/0092-8674(82)90467-6. [DOI] [PubMed] [Google Scholar]
- Okamoto H., Ishikawa E., Suzuki Y. Structural analysis of sericin genes. Homologies with fibroin gene in the 5' flanking nucleotide sequences. J Biol Chem. 1982 Dec 25;257(24):15192–15199. [PubMed] [Google Scholar]
- Osley M. A., Hereford L. Identification of a sequence responsible for periodic synthesis of yeast histone 2A mRNA. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7689–7693. doi: 10.1073/pnas.79.24.7689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papanicolaou C., Gouy M., Ninio J. An energy model that predicts the correct folding of both the tRNA and the 5S RNA molecules. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):31–44. doi: 10.1093/nar/12.1part1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R. A regulatory upstream promoter element in the Drosophila hsp 70 heat-shock gene. Cell. 1982 Sep;30(2):517–528. doi: 10.1016/0092-8674(82)90249-5. [DOI] [PubMed] [Google Scholar]
- Sabatini D. D., Kreibich G., Morimoto T., Adesnik M. Mechanisms for the incorporation of proteins in membranes and organelles. J Cell Biol. 1982 Jan;92(1):1–22. doi: 10.1083/jcb.92.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimura K., Kikuchi A., Ohtomo K., Katagata Y., Hyodo A. Studies on silk fibroin of Bombyx mori. I. Fractionation of fibroin prepared from the posterior silk gland. J Biochem. 1976 Oct;80(4):693–702. doi: 10.1093/oxfordjournals.jbchem.a131328. [DOI] [PubMed] [Google Scholar]
- Tsuda M., Suzuki Y. Faithful transcription initiation of fibroin gene in a homologous cell-free system reveals an enhancing effect of 5' flanking sequence far upstream. Cell. 1981 Nov;27(1 Pt 2):175–182. doi: 10.1016/0092-8674(81)90371-8. [DOI] [PubMed] [Google Scholar]
- Tsuda M., Suzuki Y. Transcription modulation in vitro of the fibroin gene exerted by a 200-base-pair region upstream from the "TATA" box. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7442–7446. doi: 10.1073/pnas.80.24.7442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujimoto Y., Suzuki Y. Structural analysis of the fibroin gene at the 5' end and its surrounding regions. Cell. 1979 Feb;16(2):425–436. doi: 10.1016/0092-8674(79)90018-7. [DOI] [PubMed] [Google Scholar]
- Tsujimoto Y., Suzuki Y. The DNA sequence of Bombyx mori fibroin gene including the 5' flanking, mRNA coding, entire intervening and fibroin protein coding regions. Cell. 1979 Oct;18(2):591–600. doi: 10.1016/0092-8674(79)90075-8. [DOI] [PubMed] [Google Scholar]
- Wasylyk B., Wasylyk C., Augereau P., Chambon P. The SV40 72 bp repeat preferentially potentiates transcription starting from proximal natural or substitute promoter elements. Cell. 1983 Feb;32(2):503–514. doi: 10.1016/0092-8674(83)90470-1. [DOI] [PubMed] [Google Scholar]
- Wellauer P. K., Dawid I. B. The structural organization of ribosomal DNA in Drosophila melanogaster. Cell. 1977 Feb;10(2):193–212. doi: 10.1016/0092-8674(77)90214-8. [DOI] [PubMed] [Google Scholar]