Abstract
Tumor angiogenesis, the building of blood vessels in an expanding tumor mass, is an elegantly coordinated process that dictates tumor growth and progression. Stromal components of the tumor microenvironment, such as myofibroblasts and the extracellular matrix, collaborate with tumor cells in regulating development. Such myofibroblasts and the extracellular matrix have ever-expanding roles in the angiogenic process as well. This review summarizes how stromal myofibroblasts and the extracellular matrix can modulate tumor angiogenesis, highlighting recent findings.
Keywords: myofibroblasts, extracellular matrix, angiogenesis, stroma
Introduction
Angiogenesis, the process of building new blood vessels from existing ones, is a pivotal step in tumor development. The rate of tumor growth and progression relies on the tumor vasculature to provide a steady supply of nutrients and oxygen and to remove waste products from the growing tumor. Without a tumor blood supply, incipient neoplasms cannot grow beyond 2 mm3.1-3 Termed as the “angiogenic switch,” the change from avascular neoplastic growth to vascularized tumor growth is believed to be mediated by a net shift in favor of pro-angiogenic factors over anti-angiogenic factors in the microenvironment. In addition to being a nutrient and oxygen supply, the tumor vasculature is used by metastatic tumor cells as an entry point into systemic circulation. Finally, growth of micrometastases into to full metastatic disease is also dictated by angiogenesis.
A dynamic microcosm of cellular and non-cellular players exists within the growing tumor mass. Non-tumor stromal cells, such as stromal myofibroblasts, perivascular cells, and inflammatory cells, assist in the overall growth and progression of the tumor.4-6 Furthermore, the extracellular matrix (ECM) surrounding these cellular components provides contextual cues for tumor growth and progression. Although the vasculature is technically a stromal component of the tumor mass, it will be considered as a separate entity in the purposes of this review.
Stromal myofibroblasts and the ECM impart substantial, often pleiotropic, influences on tumor angiogenesis. As vessels innervate the tumor mass, a variety of myofibroblast- and ECM-derived signaling cues and proteolytic factors help recruit blood vessels, assist negotiations with the microenvironment, and stabilize the newly formed vessels. In this review, we focus our attention on complex interplay among myofibroblasts, ECM, and the vasculature within tumor microenvironment, highlighting recent discoveries in this area of active investigation.
The Extracellular Matrix Is a Rich Reservoir of Pro- and Anti-angiogenic Cues
The extracellular matrix is a proteinaceous network of macromolecules that provide structural support to its surrounding cells. The ECM can be broadly categorized into the basement membrane, a specialized 50-nm-thick sheet of ECM molecules on which endothelial cell or epithelial cells reside, and the interstitial matrix, a network of ECM molecules in which cells can be found embedded. Although collagen IV is the major component of basement membranes, fibrillar collagens, such as collage I, II, and III, can be mostly found in interstitial matrices.7 Along with collagen, other ECM molecules, including laminins, heparan sulfate proteoglycans (HSPGs), and fibronectins, come together to form a mesh-like network. Matricellular molecules, such as tenascins, entactin, thrombospondins, and SPARC (secreted protein, acidic and rich in cysteine), can be found embedded within the ECM.
During tumor development and angiogenesis, the ECM is far more than structural scaffolding. It provides contextual cues to endothelial cells through integrin signaling, affecting processes such as proliferation, differentiation, migration, and survival. Collectively, integrins recognize a variety of ECM and matricellular protein molecules, including collagens, laminins, fibronectins, thrombospondins, and tenascins. Although integrins themselves do not have enzymatic capabilities, integrin activation relays to downstream signaling pathways, such as focal adhesion kinase (FAK), the Src family of kinases, and integrin-linked kinase (ILK).8 With integrin binding sites removed or cryptic ones exposed by the action of matrix remodeling proteins, integrin signaling is a dynamic sensor to a continuously remodeling microenvironment.9
Endothelial cells express a limited number of integrins, including α1β1, α2β1 αvβ3, αvβ5, αvβ8, and α5β1. Genetic ablation of integrin subunits αv, β1, β3, β5, or β8 resulted in vascular defects with lethal consequences.10-14 Expression of select integrins coincides with various steps of angiogenesis, suggesting specific functions at these particular steps.15 Furthermore, although it is well known that integrin signaling controls various processes in endothelial cells, it can also affect tumor angiogenesis by acting on mural cells.16
The ECM also serves as a rich reservoir for pro-angiogenic and anti-angiogenic factors. Regulating the bioavailability of pro-angiogenic and anti- angiogenic factors is another way the ECM participates in tumor angiogenesis. Vascular endothelial growth factor (VEGF) and basic fibroblasts growth factor (bFGF) are often sequestered by HSPGs in the ECM, limiting their signaling capacity to the local vicinity of their source. Activity from transforming growth factor β (TGFβ) and insulin-like growth factors (IGFs) can be fine-tuned by latent TGFβ binding proteins (LTBP) and IGF binding proteins (IGFBPs), respectively. Both LTBPs and IGFBPs possess domains that bind ECM proteins. Proteolytic activity from matrix remodeling proteins can liberate these angiogenic growth factors.17
Many matricellular proteins in the ECM also exhibit pro-angiogenic or anti- angiogenic activity through a wide range of mechanisms. SPARC can inhibit tumor angiogenesis by directly binding to angiogenic factors or by altering ECM assembly.18 Although thrombospondins-1 and -2 can bind to VEGF as well, they can also act directly on endothelial cells to inhibit the angiogenic process via interactions with CD36, CD47, and integrins.19 In contrast, tenascin-C (tnC) promotes angiogenesis. TnC expression is associated with vascular sprouts in astrocytomas, and loss of TnC resulted in less angiogenic tumors.20,21 Osteopontin can promote an autocrine VEGF signaling loop in endothelial cells.22,23
ECM proteins themselves harbor cryptic anti-angiogenic domains. The non- collagenous (NC1) domains of collagen molecules have potent anti-angiogenic properties. Endostatin, derived from proteolytic cleavage of collagen XVIII, acts by inhibiting endothelial proliferation and migration.24 It may also act as a feedback inhibitor of certain MMPs.25 Arresten, canstatin, and tumstatin are derived from NC1 domains of collagen IV α1, α2, and α3 chains, respectively. Although the mode of angiogenic inhibition in arresten and tumstatin is mediated by integrin interactions and blocking VEGF or bFGF-stimulated ERK and Akt signaling, canstatin, through interactions with integrin αvβ3 and αvβ5 and the FasL receptor, potentiates endothelial apoptosis.26,27 Proteolysis of other ECM molecules such as perlecan, fibronectin, and fibulins can produce fragments with anti-angiogenic properties.28
Several classes of proteases participate in ECM remodeling. The most prominent class is matrix metalloproteinases (MMPs). The angiogenic contribution of MMPs is best illustrated in xenograft studies using MMP-deficient hosts. Here, tumors grew at a slower pace with impairments in angiogenesis.29-32 In particular, MMPs-2, -3, -7, -9, and -16 are believed to play key roles in regulating tumor angiogenesis.33 Regulation of VEGF bioavailability is mostly attributed to the action of MMP-9 and MMP-7.34,35
MMP activity can be fine-tuned in the tumor microenvironment by endogenously produced tissue inhibitors of metalloproteinases (TIMPs). In mammals, there are 4 TIMPs (TIMPs 1-4). Because MMPs are largely a pro-angiogenic force, TIMPs are, therefore, largely anti-angiogenic.36 Aside from directly inhibiting MMP activity, TIMPs can have non–MMP-dependent effects on angiogenesis. TIMP-2 can inhibit angiogenesis by inhibiting FGF2-dependent endothelial proliferation via interactions with a3b1 integrin.37,38 TIMP-3 has been found to disrupt the VEGF binding to VEGFR-2.39
ADAMs (a disintegrin and metalloproteinases) and ADAMTS (a disintegrin and metalloproteinase with thrombospondin motifs) are additional classes of metalloproteinases whose role in tumor angiogenesis is still emerging. In addition to having proteolytic activity for various ECM molecules, ADAMs and ADAMTSs possess a variety of signaling and binding domains, allowing for an expanded repertoire of functional capabilities. These include disintegrin domains, EGF-like domains, and, more specifically in ADAMTSs, thrombospondin type I sequence repeat (TSR) domains.40 In general, although ADAMs are membrane-bound, ADAMTS are associated with the ECM through its TSR domain. Some ADAM/ADAMTS members lack proteolytic activity.
A pro-angiogenic role of ADAMs has been implicated through the use of an ADAM-specific inhibitor.41 A splice isoform of ADAM-9, known as ADAM-9-S, can be secreted and can mediate stromal-tumor interactions and degrade laminins at the invasive front of the tumor.42 More recently, loss of ADAM-17 cells has been demonstrated to affect cell proliferation and vessel formation in vitro.43 Furthermore, its proteolytic activity is key to tumor necrosis factor α (TNFα) and TGFβ shedding, which may have secondary effects on angiogenesis.44 ADAM-10 acts as a sheddase for Delta, a key ligand in the Notch-Delta pathway, and may affect tumor angiogenesis in this manner.45
So far, only 6 ADAMTSs (ADAMTS-1, -2, -8, -9, -12, -15) are known to affect angiogenesis, and all of them appear to exert anti-angiogenic effects.46-49 This is attributed, in part, to their TSP domains, which may directly interact with endothelial cells or sequester the 165-kDa isoform of VEGF. Caution must be taken to not overgeneralize the role of ADAMs and ADAMTSs in tumor angiogenesis, as the potential role of many family members remains unknown.
Tumor angiogenesis is also regulated by the ECM architecture. ECM stiffness, density, and patterning have been implicated in modulating endothelial cell survival, sprouting, and migration.50-53 Although the pro-angiogenic properties of syndecan-1 has been attributed to integrin-based signaling to endothelial cells, it may also contribute in part by reorganizing the structure of fibrillar collagen to promote directional endothelial cell migration.54,55 Needless to say, ECM architecture is not a stagnant feature of the microenvironment, as active remodeling of such ECM parameters by endothelial cells has been captured via in vivo imaging methods.56
Stromal Myofibroblasts Control Tumor Angiogenesis through Direct and Indirect Interactions
As one of the first cell types recruited to an incipient tumor, stromal myofibroblasts produce a vast array of secreted factors that modulate both tumor and endothelial behavior. Characterized by their spindle-shaped morphological characteristics and expression of smooth muscle actin (αSMA), stromal myofibroblasts are a principal source for growth factors, chemokines, ECM molecules, and matrix-remodeling proteins within the tumor microenvironment.
Not surprisingly, stromal myofibroblasts are often found at the leading edge of the tumor, a place where tumor–host interactions, such as angiogenesis and local ECM remodeling, are most robust.57 Several studies show increased angiogenesis in xenograft models where tumor cells were co-inoculated with stromal myofibroblasts.58,59 The importance of stromal myofibroblast in tumor angiogenesis is further highlighted in a study demonstrating that recruited myofibroblasts act as a secondary source of VEGF and compensate for the loss of VEGF in tumor cells.60
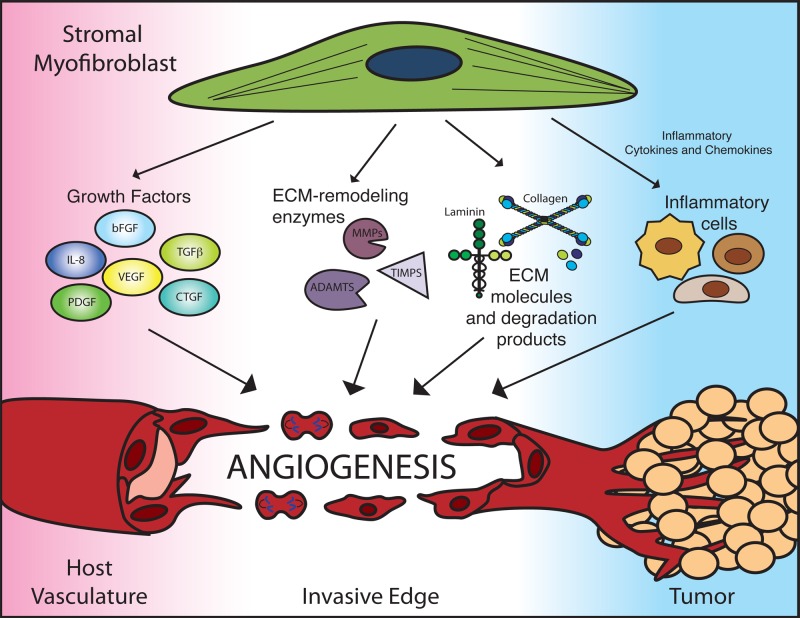
Stromal myofibroblasts participate in tumor angiogenesis through a multi-prong approach (Fig. 1). First, they provide a repertoire of secreted pro-angiogenic growth factors, including VEGF, bFGF, TGFβ, platelet-derived growth factors (PDGFs), hepatocyte growth factor (HGF), connective tissue growth factor (CTGF), and interleukin-8 (IL-8).5,60-63 Combined with other sources of pro-angiogenic growth factors in the tumor, myofibroblast-derived pro-angiogenic factors can tip the angiogenic balance in favor of tumor angiogenesis.
Figure 1.
Stromal myofibroblasts modulate angiogenesis with a multiprong approach. Stromal myofibroblasts are a prominent source of angiogenic growth factors, extracellular matrix (ECM) remodeling factors, and ECM components. Additionally, myofibroblast-derived inflammatory cytokines and chemokines recruit infiltrating immune cells, such as macrophages, neutrophils, and T-cells, which can have secondary effects on angiogenesis. ADAMTS, a disintegrin and metalloproteinases with thrombospondin motifs; bFGF, basic fibroblast growth factor; CTGF, connective tissue growth factor; IL-8, interleukin-8; MMPs, matrix metalloproteinases; PDGF, platelet-derived growth factor; TGFb, transforming growth factor b; TIMPs, tissue inhibitor of MMP; VEGF, vascular endothelial growth factor.
Stromal myofibroblasts are a key source of matrix remodeling proteins within the tumor microenvironment, including MMP-1, MMP-2, MMP-3, MMP-7, MMP-11, and MT-MMP1.64-67 Induction of MMPs in stromal myofibroblasts by tumor-derived factor EMMPRIN further stimulates tumor angiogenesis.68,69 In colorectal cancer, ADAMTS-12 expression is selectively robust in myofibroblasts.70 Furthermore, in the liver, ADAM-9, ADAM-12, ADAM-28, ADAMTS-1, and ADAMTS-2 are up-regulated following activation of hepatic stellate cells, which are stromal myofibroblasts of the liver, either in hepatocellular carcinoma or cirrhotic livers.30,71
In addition to recruiting existing endothelial cells to proliferate, migrate, and form blood vessels, stromal myofibroblasts may elicit endothelial progenitor cells to build blood vessels de novo, a process called vasculogenesis. Secretion of stromal-derived-factor 1 by stromal myofibroblasts (SDF1/CXCL12) stimulates recruitment of Sca1+CD31+ EPCs to the primary tumor.72
Modulation of the inflammatory response within the tumor microenvironment by stromal myofibroblasts is an additional mechanism to amplify its angiogenic agenda. Stromal myofibroblasts express an array of pro-inflammatory cytokines and chemokines, a process that recruits immune cells to the local microenvironment.72-76 Following recruitment, the secretory milieu of macrophages, neutrophils, and mast cells includes pro-angiogenic factors, such as VEGF, or ECM remodeling proteins, such as MMP-9 and MMP-13. A more detailed overview on the influence of inflammatory cells on tumor angiogenesis can be found in Noonan et al 77 and Zumsteg and Christofori.78
The genetic landscape of stromal myofibroblasts has gained considerable interest in recent years. Mutations in stromal cells have been implicated in tumor initiation.79,80 Stromal-specific mutations of key tumor-suppressive genes, such as TP53 and PTEN, have been identified in patient samples.81,82 Loss of stromal p53 has been identified critical step in tumor progression in a murine model of prostate cancer.83 In addition having a direct effect on tumor cells, p53 in stromal myofibroblasts can modulate tumor angiogenesis as well. p53 regulates the expression of TSP-1 and SDF-1, two potent regulators of angiogenesis, in stromal fibroblasts.84,85 As mentioned already, stromal myofibroblasts are a prominent source of VEGF and bFGF, two angiogenic factors regulated by p53.86,87 Dissecting the functional consequences of specific mutations in stromal myofibroblasts will be an exciting area to watch in cancer biology.
Fibrosis and the Desmoplastic Reaction in Tumor Angiogenesis
In many solid tumors, dense fibrotic tissue, rich in myofibroblasts and ECM molecules, often encapsulates proliferating tumor mass. Termed the stromal or desmoplastic response, it is a prominent feature in many cancers, including those of the breast and pancreas. Given their well-characterized role in fibrosis, stromal myofibroblasts are believed to be the predominant orchestrators of the desmoplastic response.88
The underlying cause of the desmoplastic response in cancer remains largely speculative. Some hypothesize that it is a host defense mechanism against incipient neoplasms, where the fibrotic tissue essentially isolates the neoplastic cells from the rest of the organ.87 In contrast, because of similarities between wound healing and the stromal response, others have suggested that the tumor cells hijack the wound-healing mechanism to support their own growth.89,90 In concordance with this notion, exposing fibroblasts to tumor cells induces changes in fibroblast gene expression that promote invasion and angiogenesis.91,92
Whether the desmoplastic response promotes or inhibits tumor angiogenesis is up for debate. On one hand, the desmoplastic response coincides with the invasive front of the tumor. An area of active angiogenesis, the invasive front is rich in angiogenic growth factors and ECM remodeling proteins. Furthermore, increased angiogenesis is a feature of fibrosis in certain organs.93,94 On the other hand, excessive deposition of ECM creates a physical barrier that impedes angiogenesis.95
Studies targeting the desmoplastic process have suggested that an overtly fibrotic microenvironment is counterproductive to angiogenesis. In pancreatic cancer, tumor cell–derived sonic hedgehog (Shh) was found to stimulate myofibroblast activation and desmoplasia formation in pancreatic cancer.96-98 Administration of an inhibitor of sonic hedgehog signaling led to reductions in myofibroblast proliferation, type I collagen, and desmoplastic formation. These changes were correlated with increased angiogenesis and enhanced perfusion throughout the tumor.99 In another murine model, pharmacological inhibition or genetic deletion of fibroblast activation protein (FAP), a serine protease expressed on fibroblasts and pericytes, has resulted in tumors with increased collagen deposition and decreased angiogenesis.100
Endothelial Cells in the Tumor Microenvironment Can Affect Neighboring Stromal Components
Stromal myofibroblasts and the ECM are important agents in modulating tumor angiogenesis. They participate in every step of angiogenesis and can be responsible for mediating resistance to chemotherapeutic, anti-angiogenic, and other targeted therapies. However, a clearer understanding of how tumor cells, endothelial cells, and stromal cells co-exist and collaborate is needed.
Although this review has focused on the unidirectional communication from stromal myofibroblast/ECM to endothelium in tumor angiogenesis, one must not forget that endothelial cells may reciprocally affect myofibroblasts and the ECM as well. The most parsimonious manner by which endothelial cells can interact with its stromal partners is through ECM remodeling. During tumor angiogenesis, endothelial cells also produce ECM remodeling proteins to assist in their own navigation through the ECM. Successful establishment of tumor vasculature will reoxygenate hypoxic areas, altering the expression profiles of cells within that area, including that of stromal myofibroblasts. In recent years, it has been demonstrated that endothelial cells can act as niche cells to brain tumor stem cells.101 Thus, it is conceivable that paracrine signals from vascular cells can alter other cells in the tumor stroma as well. Furthermore, recruitment of bone marrow–derived myofibroblast progenitors, termed fibrocytes, may rely on coordinated presentation of adhesion molecules on the endothelium, perhaps using mechanisms similar to that of leukocyte extravasation. Additionally, endothelial cells themselves can serve as a source of stromal myofibroblasts in the tumor microenvironment by undergoing endothelial-to-mesenchymal transition.102 Indeed, the influence of the endothelial cells on its surrounding stromal neighbors remains an underexplored area.
Acknowledgments
The authors thank Vesselina Cooke for her critical reading of this manuscript.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work has been supported by NIH grants CA 125550, CA-155370, CA151925, DK 055001, DK 55001 and the Champalimaud Metastasis Programme.
References
- 1. Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182-6 [DOI] [PubMed] [Google Scholar]
- 2. Gimbrone MAJ, Leapman SB, Cotran RS, Folkman J. Tumor dormancy in vivo by prevention of neovascularization. J Exp Med. 1972;136:261-76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Folkman J, Kalluri R. Cancer without disease. Nature. 2004;427:787. [DOI] [PubMed] [Google Scholar]
- 4. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646-74 [DOI] [PubMed] [Google Scholar]
- 5. Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6:392-401 [DOI] [PubMed] [Google Scholar]
- 6. Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. van der Rest M, Garrone R. Collagen family of proteins. FASEB J. 1991;5:2814-23 [PubMed] [Google Scholar]
- 8. Giancotti FG, Ruoslahti E. Integrin signaling. Science. 1999;285:1028-32 [DOI] [PubMed] [Google Scholar]
- 9. Xu J, Rodriguez D, Petitclerc E, et al. Proteolytic exposure of a cryptic site within collagen type IV is required for angiogenesis and tumor growth in vivo. J Cell Biol. 2001;154:1069-79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hodivala-Dilke KM, McHugh KP, Tsakiris DA, et al. β3-integrin-deficient mice are a model for Glanzmann thrombasthenia showing placental defects and reduced survival. J Clin Invest. 1999;103:229-38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bader BL, Rayburn H, Crowley D, Hynes RO. Extensive vasculogenesis, angiogenesis, and organogenesis precede lethality in mice lacking all αv integrins. Cell. 1998;95:507-19 [DOI] [PubMed] [Google Scholar]
- 12. Huang XZ, Wu JF, Ferrando R, et al. Fatal bilateral chylothorax in mice lacking the integrin α9β1. Mol Cell Biol. 2000;20:5208-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Reynolds LE, Wyder L, Lively JC, et al. Enhanced pathological angiogenesis in mice lacking β3 integrin or β3 and β5 integrins. Nat Med. 2002;8:27-34 [DOI] [PubMed] [Google Scholar]
- 14. Zhu J, Motejlek K, Wang D, et al. β8 integrins are required for vascular morphogenesis in mouse embryos. Development. 2002;129:2891-903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stupack DG, Cheresh DA. Integrins and angiogenesis. Curr Top Dev Biol. 2004;64:207-38 [DOI] [PubMed] [Google Scholar]
- 16. Abraham S, Kogata N, Fassler R, et al. Integrin β1 subunit controls mural cell adhesion, spreading, and blood vessel wall stability. Circ Res. 2008;102:562-70 [DOI] [PubMed] [Google Scholar]
- 17. Lee S, Jilani SM, Nikolova GV, et al. Processing of VEGF-A by matrix metalloproteinases regulates bioavailability and vascular patterning in tumors. J Cell Biol. 2005;169:681-91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Clark CJ, Sage EH. A prototypic matricellular protein in the tumor microenvironment —where there’s SPARC, there’s fire. J Cell Biochem. 2008;104:721-32 [DOI] [PubMed] [Google Scholar]
- 19. Bornstein P. Thrombospondins function as regulators of angiogenesis. J Cell Commun Sig. 2009;3:189-200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tanaka K, Hiraiwa N, Hashimoto H, et al. Tenascin-C regulates angiogenesis in tumor through the regulation of vascular endothelial growth factor expression. Int J Cancer. 2004;108:31-40 [DOI] [PubMed] [Google Scholar]
- 21. Zagzag D, Friedlander DR, Miller DC, et al. Tenascin expression in astrocytomas correlates with angiogenesis. Cancer Res. 1995;55:907-14 [PubMed] [Google Scholar]
- 22. Dai J, Peng L, Fan K, et al. Osteopontin induces angiogenesis through activation of PI3K/AKT and ERK1/2 in endothelial cells. Oncogene. 2009;28:3412-22 [DOI] [PubMed] [Google Scholar]
- 23. Chakraborty G, Jain S, Kundu GC. Osteopontin promotes vascular endothelial growth factor–dependent breast tumor growth and angiogenesis via autocrine and paracrine mechanisms. Cancer Res. 2008;68:152-61 [DOI] [PubMed] [Google Scholar]
- 24. O’Reilly MS, Boehm T, Shing Y, et al. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88:277-85 [DOI] [PubMed] [Google Scholar]
- 25. Kim YM, Jang JW, Lee OH, et al. Endostatin inhibits endothelial and tumor cellular invasion by blocking the activation and catalytic activity of matrix metalloproteinase 2. Cancer Res. 2000;60:5410-3 [PubMed] [Google Scholar]
- 26. Cooke VG, Kalluri R. Molecular mechanism of type IV collagen-derived endogenous inhibitors of angiogenesis. Methods Enzymol. 2008;444:1-19 [DOI] [PubMed] [Google Scholar]
- 27. Magnon C, Galaup A, Mullan B, et al. Canstatin acts on endothelial and tumor cells via mitochondrial damage initiated through interaction with αvβ3 and αvβ5 integrins. Cancer Res. 2005;65:4353-61 [DOI] [PubMed] [Google Scholar]
- 28. Nyberg P, Xie L, Kalluri R. Endogenous inhibitors of angiogenesis. Cancer Res. 2005;65:3967-79 [DOI] [PubMed] [Google Scholar]
- 29. Itoh T, Tanioka M, Yoshida H, et al. Reduced angiogenesis and tumor progression in gelatinase A-deficient mice. Cancer Res. 1998;58:1048-51 [PubMed] [Google Scholar]
- 30. Lederle W, Hartenstein B, Meides A, et al. MMP13 as a stromal mediator in controlling persistent angiogenesis in skin carcinoma. Carcinogenesis. 2010;31:1175-84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Huang S, Van Arsdall M, Tedjarati S, et al. Contributions of stromal metalloproteinase-9 to angiogenesis and growth of human ovarian carcinoma in mice. J Natl Cancer Inst. 2002;94:1134-42 [DOI] [PubMed] [Google Scholar]
- 32. Fang J, Shing Y, Wiederschain D, et al. Matrix metalloproteinase-2 is required for the switch to the angiogenic phenotype in a tumor model. Proc Nat Acad Sci U S A. 2000;97:3884-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jodele S, Blavier L, Yoon JM, DeClerck YA. Modifying the soil to affect the seed: role of stromal-derived matrix metalloproteinases in cancer progression. Cancer Metastasis Rev. 2006;25: 35-43 [DOI] [PubMed] [Google Scholar]
- 34. Hawinkels LJ, Zuidwijk K, Verspaget HW, et al. VEGF release by MMP-9 mediated heparan sulphate cleavage induces colorectal cancer angiogenesis. Eur J Cancer. 2008;44:1904-13 [DOI] [PubMed] [Google Scholar]
- 35. Ito TK, Ishii G, Chiba H, et al. The VEGF angiogenic switch of fibroblasts is regulated by MMP-7 from cancer cells. Oncogene. 2007;26:7194-203 [DOI] [PubMed] [Google Scholar]
- 36. Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141:52-67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Murphy AN, Unsworth EJ, Stetler-Stevenson WG. Tissue inhibitor of metalloproteinases-2 inhibits bFGF-induced human microvascular endothelial cell proliferation. J Cell Physiol. 1993;157:351-8 [DOI] [PubMed] [Google Scholar]
- 38. Seo DW, Li H, Guedez L, et al. TIMP-2 Mediated Inhibition of Angiogenesis: An MMP-Independent Mechanism. Cell. 2003;114:171-80 [DOI] [PubMed] [Google Scholar]
- 39. Qi JH, Ebrahem Q, Moore N, et al. A novel function for tissue inhibitor of metalloproteinases-3 (TIMP3): inhibition of angiogenesis by blockage of VEGF binding to VEGF receptor-2. Nat Med. 2003;9:407-15 [DOI] [PubMed] [Google Scholar]
- 40. Rocks N, Paulissen G, El Hour M, et al. Emerging roles of ADAM and ADAMTS metalloproteinases in cancer. Biochimie. 2008;90:369-79 [DOI] [PubMed] [Google Scholar]
- 41. Trochon V, Li H, Vasse M, et al. Endothelial metalloprotease-disintegrin protein (ADAM) is implicated in angiogenesis in vitro. Angiogenesis. 1998;2:277-85 [DOI] [PubMed] [Google Scholar]
- 42. Mazzocca A, Coppari R, De Franco R, et al. A secreted form of ADAM9 promotes carcinoma invasion through tumor-stromal interactions. Cancer Res. 2005;65:4728-38 [DOI] [PubMed] [Google Scholar]
- 43. Göoz P, Göoz M, Baldys A, Hoffman S. ADAM-17 regulates endothelial cell morphology, proliferation, and in vitro angiogenesis. Biochem Biophys Res Commun. 2009;380:33-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Black RA, White JM. ADAMs: focus on the protease domain. Curr Op Cell Biol. 1998;10:654-9 [DOI] [PubMed] [Google Scholar]
- 45. Qi H, D Rand M, Wu X, et al. Processing of the notch ligand delta by the metalloprotease Kuzbanian. Science. 1999;283:91-4 [DOI] [PubMed] [Google Scholar]
- 46. Vazquez F, Hastings G, Ortega MA, et al. METH-1, a human ortholog of ADAMTS-1, and METH-2 are members of a new family of proteins with angio-inhibitory activity. J Biol Chem. 1999;274:23349-57 [DOI] [PubMed] [Google Scholar]
- 47. Dubail J, Kesteloot F, Deroanne C, et al. ADAMTS-2 functions as anti-angiogenic and anti-tumoral molecule independently of its catalytic activity. Cell Mol Life Sci. 2010;67: 4213-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lo PH, Lung HL, Cheung AK, et al. Extracellular protease ADAMTS9 suppresses esophageal and nasopharyngeal carcinoma tumor formation by inhibiting angiogenesis. Cancer Res. 2010;70:5567-76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. El Hour M, Moncada-Pazos A, Blacher S, et al. Higher sensitivity of Adamts12-deficient mice to tumor growth and angiogenesis. Oncogene. 2010;29:3025-32 [DOI] [PubMed] [Google Scholar]
- 50. Korff T, Augustin HG. Tensional forces in fibrillar extracellular matrices control directional capillary sprouting. J Cell Sci. 1999;112: 3249-58 [DOI] [PubMed] [Google Scholar]
- 51. Krishnan L, Underwood CJ, Maas S, et al. Effect of mechanical boundary conditions on orientation of angiogenic microvessels. Cardiovasc Res. 2008;78:324-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bauer AL, Jackson TL, Jiang Y. Topography of extracellular matrix mediates vascular morphogenesis and migration speeds in angiogenesis. PLoS Comput Biol. 2009;5:e1000445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Myers KA, Applegate KT, Danuser G, et al. Distinct ECM mechanosensing pathways regulate microtubule dynamics to control endothelial cell branching morphogenesis. J Cell Biol. 2011;192:321-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Maeda T, Desouky J, Friedl A. Syndecan-1 expression by stromal fibroblasts promotes breast carcinoma growth in vivo and stimulates tumor angiogenesis. Oncogene. 2006;25:1408-12 [DOI] [PubMed] [Google Scholar]
- 55. Yang N, Mosher R, Seo S, Beebe D, Friedl A. Syndecan-1 in breast cancer stroma fibroblasts regulates extracellular matrix fiber organization and carcinoma cell motility. Am J Pathol. 2011;178:325-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kirkpatrick ND, Andreou S, Hoying JB, et al. Live imaging of collagen remodeling during angiogenesis. Am J Physiol Heart Circ Physiol. 2007;292:H3198-206 [DOI] [PubMed] [Google Scholar]
- 57. Granot D, Addadi Y, Kalchenko V, et al. In vivo imaging of the systemic recruitment of fibroblasts to the angiogenic rim of ovarian carcinoma tumors. Cancer Res. 2007;67:9180-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Orimo A, Gupta PB, Sgroi DC, et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335-48 [DOI] [PubMed] [Google Scholar]
- 59. Tuxhom JA, McAlhany SJ, Dang TD, et al. Stromal Cells promote angiogenesis and growth of human prostate tumors in a differential reactive stroma (DRS) xenograft model. Cancer Res. 2002;62:3298-307 [PubMed] [Google Scholar]
- 60. Dong J, Grunstein J, Tejada M, et al. VEGF-null cells require PDGFRα signaling-mediated stromal fibroblast recruitment for tumorigenesis. EMBO J. 2004;23:2800-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Crawford Y, Kasman I, Yu L, et al. PDGF-C mediates the angiogenic and tumorigenic properties of fibroblasts associated with tumors refractory to anti-VEGF treatment. Cancer Cell. 2009;15:21-34 [DOI] [PubMed] [Google Scholar]
- 62. Hlatky L, Tsionou C, Hahnfeldt P, et al. Mammary fibroblasts may influence breast tumor angiogenesis via hypoxia-induced vascular endothelial growth factor up-regulation and protein expression. Cancer Res 1994;54:6083-6 [PubMed] [Google Scholar]
- 63. Fang J, Yan L, Shing Y, Moses MA. HIF-1α-mediated up-regulation of vascular endothelial growth factor, independent of basic fibroblast growth factor, is important in the switch to the angiogenic phenotype during early tumorigenesis. Cancer Res. 2001;61:5731-5 [PubMed] [Google Scholar]
- 64. Bisson C, Blacher S, Polette M, et al. Restricted expression of membrane type 1-matrix metalloproteinase by myofibroblasts adjacent to human breast cancer cells. Int J Cancer. 2003;105:7-13 [DOI] [PubMed] [Google Scholar]
- 65. Basset P, Bellocq JP, Wolf C, et al. A novel metalloproteinase gene specifically expressed in stromal cells of breast carcinomas. Nature. 1990;348:699-704 [DOI] [PubMed] [Google Scholar]
- 66. Poulsom R, Pignatelli M, Stetler-Stevenson WG, et al. Stromal expression of 72 kDa type IV collagenase (MMP-2) and TIMP-2 mRNAs in colorectal neoplasia. Am J Pathol. 1992;141:389-96 [PMC free article] [PubMed] [Google Scholar]
- 67. Poulsom R, Hanby AM, Pignatelli M, et al. Expression of gelatinase-A and TIMP-2 mRNAs in desmoplastic fibroblasts in both mammary carcinomas and basal cell carcinomas of the skin. J Clin Pathol. 1993;46:429-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Tang Y, Nakada MT, Kesavan P, et al. Extracellular matrix metalloproteinase inducer stimulates tumor angiogenesis by elevating vascular endothelial cell growth factor and matrix metalloproteinases. Cancer Res. 2005;65:3193-9 [DOI] [PubMed] [Google Scholar]
- 69. Sameshima T, Nabeshima K, Toole BP, et al. Glioma cell extracellular matrix metalloproteinase inducer (EMMPRIN)(CD147) stimulates production of membrane-type matrix metalloproteinases and activated gelatinase A in co-cultures with brain-derived fibroblasts. Cancer Lett. 2000;157:177-84 [DOI] [PubMed] [Google Scholar]
- 70. Moncada-Pazos A, Obaya AJ, Fraga MF, et al. The ADAMTS12 metalloprotease gene is epigenetically silenced in tumor cells and transcriptionally activated in the stroma during progression of colon cancer. J Cell Sci. 2009;122:2906-13 [DOI] [PubMed] [Google Scholar]
- 71. Schwettmann L, Wehmeier M, Jokovic D, et al. Hepatic expression of A Disintegrin And Metalloproteinase (ADAM) and ADAMs with thrombospondin motives (ADAM-TS) enzymes in patients with chronic liver diseases. J Hepatol. 2008;49:243-50 [DOI] [PubMed] [Google Scholar]
- 72. Orimo A, Gupta PB, Sgroi DC, et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335-48 [DOI] [PubMed] [Google Scholar]
- 73. Allinen M, Beroukhim R, Cai L, et al. Molecular characterization of the tumor microenvironment in breast cancer. Cancer Cell. 2004;6:17-32 [DOI] [PubMed] [Google Scholar]
- 74. Orimo A, Tomioka Y, Shimizu Y, et al. Cancer-associated myofibroblasts possess various factors to promote endometrial tumor progression. Clin Cancer Res. 2001;7:3097. [PubMed] [Google Scholar]
- 75. Erez N, Truitt M, Olson P, Hanahan D. Cancer-Associated Fibroblasts Are Activated in Incipient Neoplasia to Orchestrate Tumor-Promoting Inflammation in an NF-[kappa] B-Dependent Manner. Cancer Cell. 2010;17:135-47 [DOI] [PubMed] [Google Scholar]
- 76. Silzle T, Kreutz M, Dobler MA, et al. Tumor-associated fibroblasts recruit blood monocytes into tumor tissue. Eur J Immunol. 2003;33:1311-20 [DOI] [PubMed] [Google Scholar]
- 77. Noonan DM, De Lerma Barbaro A, Vannini N, et al. Inflammation, inflammatory cells and angiogenesis: decisions and indecisions. Cancer Metastasis Rev. 2008;27:31-40 [DOI] [PubMed] [Google Scholar]
- 78. Zumsteg A, Christofori G. Corrupt policemen: inflammatory cells promote tumor angiogenesis. Curr Op Oncol. 2009;21:60-70 [DOI] [PubMed] [Google Scholar]
- 79. Bhowmick NA, Chytil A, Plieth D, et al. TGF-ß signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science. 2004;303:848-51 [DOI] [PubMed] [Google Scholar]
- 80. Katajisto P, Vaahtomeri K, Ekman N, et al. LKB1 signaling in mesenchymal cells required for suppression of gastrointestinal polyposis. Nat Genet. 2008;40:455-9 [DOI] [PubMed] [Google Scholar]
- 81. Kurose K, Gilley K, Matsumoto S, Watson PH, Zhou XP, Eng C. Frequent somatic mutations in PTEN and TP53 are mutually exclusive in the stroma of breast carcinomas. Nat Genet. 2002;32:355-7 [DOI] [PubMed] [Google Scholar]
- 82. Wernert N, Locherbach C, Wellmann A, et al. Presence of genetic alterations in microdissected stroma of human colon and breast cancers. Anticancer Res. 2001;21:2259-64 [PubMed] [Google Scholar]
- 83. Hill R, Song Y, Cardiff RD, et al. Selective evolution of stromal mesenchyme with p53 loss in response to epithelial tumorigenesis. Cell. 2005;123:1001-11 [DOI] [PubMed] [Google Scholar]
- 84. Dameron KM, Volpert OV, Tainsky MA, et al. Control of angiogenesis in fibroblasts by p53 regulation of thrombospondin-1. Science. 1994;265:1582-4 [DOI] [PubMed] [Google Scholar]
- 85. Addadi Y, Moskovits N, Granot D, et al. p53 status in stromal fibroblasts modulates tumor growth in an SDF1-dependent manner. Cancer Res. 2010;70:9650-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Pal S, Datta K, Mukhopadhyay D. Central role of p53 on regulation of vascular permeability factor/vascular endothelial growth factor (VPF/VEGF) expression in mammary carcinoma. Cancer Res. 2001;61:6952-7 [PubMed] [Google Scholar]
- 87. Ueba T, Nosaka T, Takahashi JA, et al. Transcriptional regulation of basic fibroblast growth factor gene by p53 in human glioblastoma and hepatocellular carcinoma cells. Proc Nat Acad Sci U S A. 1994;91:9009-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Desmoulière A, Guyot C, Gabbiani G. The stroma reaction myofibroblast: a key player in the control of tumor cell behavior. Int J Dev Biol. 2004;48:509-18 [DOI] [PubMed] [Google Scholar]
- 89. Dvorak HF. Tumors: wounds that do not heal: similarities between tumor stroma generation and wound healing. N Eng J Med. 1986;315:1650-9 [DOI] [PubMed] [Google Scholar]
- 90. Schäfer M, Werner S. Cancer as an overhealing wound: an old hypothesis revisited. Nat Rev Mol Cell Biol. 2008;9:628-38 [DOI] [PubMed] [Google Scholar]
- 91. Eck SM, Cote AL, Winkelman WD, et al. CXCR4 and MMP-1 are elevated in breast carcinoma-associated fibroblasts and in normal mammary fibroblasts exposed to factors secreted by breast cancer cells. Mol Cancer Res. 2009;7:1033-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Sato N, Maehara N, Goggins M. Gene expression profiling of tumor–stromal interactions between pancreatic cancer cells and stromal fibroblasts. Cancer Res. 2004;64:6950-9 [DOI] [PubMed] [Google Scholar]
- 93. Cosgrove GP, Brown KK, Schiemann WP, et al. Pigment epithelium-derived factor in idiopathic pulmonary fibrosis: a role in aberrant angiogenesis. Am J Respir Crit Care Med. 2004;170:242-51 [DOI] [PubMed] [Google Scholar]
- 94. Corpechot C, Barbu V, Wendum D, et al. Hypoxia-induced VEGF and collagen I expressions are associated with angiogenesis and fibrogenesis in experimental cirrhosis. Hepatology. 2002;35: 1010-21 [DOI] [PubMed] [Google Scholar]
- 95. Barsky SH, Nelson LL, Levy VA. Tumour desmoplasia inhibits angiogenesis. Lancet. 1987;2:1336-7 [DOI] [PubMed] [Google Scholar]
- 96. Yauch RL, Gould SE, Scales SJ, et al. A paracrine requirement for hedgehog signalling in cancer. Nature. 2008;455:406-10 [DOI] [PubMed] [Google Scholar]
- 97. Tian H, Callahan CA, DuPree KJ, et al. Hedgehog signaling is restricted to the stromal compartment during pancreatic carcinogenesis. Proc Nat Acad Sci U S A. 2009;106:4254-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Bailey JM, Swanson BJ, Hamada T, et al. Sonic hedgehog promotes desmoplasia in pancreatic cancer. Clin Cancer Res. 2008;14:5995-6004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Olive KP, Jacobetz MA, Davidson CJ, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Santos AM, Jung J, Aziz N, et al. Targeting fibroblast activation protein inhibits tumor stromagenesis and growth in mice. J Clin Invest. 2009;119:3613-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Gilbertson RJ, Rich JN. Making the tumour’s bed: glioblastoma stem cells and their vascular niche. Nat Rev Cancer. 2007;7:733-6 [DOI] [PubMed] [Google Scholar]
- 102. Zeisberg EM, Potenta S, Xie L, et al. Discovery of endothelial to mesenchymal transition as a source for carcinoma-associated fibroblasts. Cancer Res. 2007;67:10123-8 [DOI] [PubMed] [Google Scholar]