Abstract
Mutations of the CUL4B ubiquitin ligase gene are causally linked to syndromic X-linked mental retardation (XLMR). However, the pathogenic role of CUL4B mutations in neuronal and developmental defects is not understood. We have generated mice with targeted disruption of Cul4b, and observed embryonic lethality with pronounced growth inhibition and increased apoptosis in extra-embryonic tissues. Cul4b, but not its paralog Cul4a, is expressed at high levels in extra-embryonic tissues post implantation. Silencing of CUL4B expression in an extra-embryonic cell line resulted in the robust accumulation of the CUL4 substrate p21Cip1/WAF and G2/M cell cycle arrest, which could be partially rescued by silencing of p21Cip1/WAF. Epiblast-specific deletion of Cul4b prevented embryonic lethality and gave rise to viable Cul4b null mice. Therefore, while dispensable in the embryo proper, Cul4b performs an essential developmental role in the extra-embryonic tissues. Our study offers a strategy to generate viable Cul4b-deficient mice to model the potential neuronal and behavioral deficiencies of human CUL4B XLMR patients.
Keywords: cullin 4B, knockout, extra-embryonic tissue, ubiquitin, X-inactivation
Introduction
Mental retardation (MR) is a disability characterized by limitations of intellectual and adaptive skills. To date, over 900 genetic mutations are linked to MR, highlighting the enormous heterogeneity of this clinical condition 1. Of the known genetic lesions, the X-chromosome harbors over 100 MR genes, far more than those found on autosomes. Recently, multiple mutations of the human CUL4B ubiquitin ligase gene (Xq24) were identified as being causally associated with X-linked MR (XLMR) 2, 3, 4, 5. CUL4B-deficient patients display clinical symptoms that include growth retardation, macrocephaly, hypogonadism, obesity, and ataxia 2, 3, 4, 5.
CUL4B is a member of the cullin-RING ubiquitin ligase (CRL) family, which is the largest E3 ligase subtype in mammals that controls a wide spectrum of cellular processes including cell cycle, DNA replication and repair, signaling, transcription, and embryonic development (reviewed in 6, 7, 8). CRLs are multimeric E3 ligase complexes that assemble on the elongated cullin scaffold: the RING domain adaptor Rbx1/Roc1 recruits the E2 ubiquitin-conjugating enzyme and interacts with the C-terminal cullin homology domain, while the cullin N-terminus associates with a cullin-specific adaptor protein to recruit a large family of substrate receptors, each of which can bind multiple substrates. The CUL4 family is unique among the eight cullins as there are two CUL4 members, CUL4A and CUL4B, that share about 82% sequence identity. CUL4A and CUL4B assemble structurally similar E3 complexes using the same adaptors (Rbx1 and DDB1) and DCAF substrate receptors, and function redundantly to target critical cell cycle regulators, chromatin modifiers and transcription factors for ubiquitination and degradation, thereby supporting growth and survival (reviewed in 8, 9). Among the CUL4 substrates that control cell cycle progression, the cyclin-dependent kinase inhibitor p21 was recently shown to be targeted for ubiquitination and degradation by both CUL4A and CUL4B under normal conditions as well as post-UV irradiation in cell lines 10, 11. However, primary mouse embryonic fibroblasts preferentially utilize CUL4A to restrict p21 levels 12, suggesting context-dependent degradation of p21 by the two CUL4 ubiquitin ligases. Cul4a null mice are viable and display no gross phenotypic abnormalities throughout their lifespan except for meiotic defects in male mice 12, 13, 14. Despite the largely overlapping functions of CUL4A and CUL4B, recent studies also revealed distinct roles for the two CUL4 family members in DNA-damage response, male meiosis, and response to environmental toxins 12, 13, 14, 15. While CUL4B compensates for the loss of CUL4A 12, CUL4A expression, which is unaffected by CUL4B mutations 16, is apparently unable to rescue the neuronal and developmental deficiencies found in CUL4B patients with XLMR. Notably, the loss of CUL4B function is not lethal in humans 2, 3, 4, 5.
Like human CUL4B, the mouse Cul4b gene also resides on X-chromosome. Here, we generated the Cul4b knockout mice to determine the physiological functions of CUL4B and the pathological basis of CUL4B deficiency in XLMR and the associated developmental defects. While human CUL4B patients can survive to adulthood, Cul4b knockout mice die as early as E7.5, consistent with the observed embryonic lethality upon inactivation of Cul4b by gene trapping 17. The developmental defects of Cul4b null embryos were attributed exclusively to the degeneration of extra-embryonic tissues, while the epiblast remained largely unaffected by Cul4b deletion. Embryos with conditional deletion of Cul4b in the epiblast were viable and displayed no overt developmental defects compared to their wild-type littermates. Our results reveal an essential developmental role of CUL4B in the extra-embryonic tissues and highlight the differential requirement between mice and humans for CUL4B during embryogenesis.
Results
Generation of Cul4b null mice
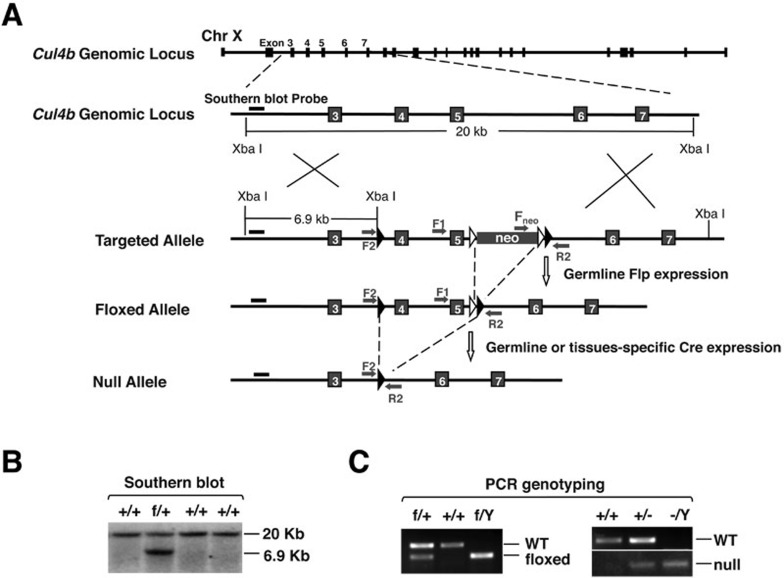
To investigate the physiological role of CUL4B, we generated conditional Cul4b knockout mice using the Cre/lox strategy. Exons 4 and 5, which span the DDB1-binding site 18, were floxed by loxP homologous recombination sites in embryonic stem (ES) cells (Figure 1A). Deletion of exons 4-5 by Cre recombinase removes the DDB1-binding site in the N terminus of CUL4B. Moreover, splicing of exon 3 to 6 in the recombined Cul4b transcript would result in a reading-frame shift and a premature stop codon, thus generating a putative 78-amino acid fragment of CUL4B lacking both the DDB1-binding domain and the cullin homology domain that is necessary for Rbx1 binding and subsequent recruitment of the E2 ubiquitin-conjugating enzyme. The targeting event was confirmed by Southern blotting using probes external to the floxed Cul4b sequences (Figure 1B). Mouse ES cells bearing floxed Cul4b were microinjected into blastocysts to derive chimeras from which germline transmission of the mutant allele was achieved. Hemizygous Cul4bf/Y and homozygous Cul4bf/f mice were healthy and phenotypically indistinguishable from their wild-type littermates. Cul4bf/Y and Cul4bf/f mice were maintained on a C57BL/6J-129 mixed background and their offspring were genotyped by PCR (Figure 1C, left panel). Cul4bf/+ mice subsequently interbred to Vav-iCre mice that express the Cre recombinase in hematopoietic stem cells 19. We validated the resulting hematopoietic-specific null allele of Cul4b by PCR-based genotyping of genomic DNA from peripheral blood cells from the offspring of the Cul4bf/+×Vav-iCre+/− mating (Figure 1C, right panel).
Figure 1.
Generation of floxed and knockout alleles of Cul4b in mice. (A) Genomic structure of the Cul4b gene on the X-chromosome and the targeted alleles. LoxP sites (filled arrowheads) were introduced into the intron regions flanking exons 4-5 of the targeting construct for homologous recombination in ESCs. Frt recombination sites (empty arrowheads) were engineered for removal of the neomycin selection marker by germline Flp expression following crossing to ACT-FLPe transgenic mice. Arrows, PCR primers for genotyping; Bars, probe for Southern blotting. (B) Generation of targeted ES cells. G418-resistant ES cell clones were screened by Southern blot analysis. DNA was digested with the XbaI restriction enzyme and hybridized to the probes as indicated in A. +/+, wild-type; f/+, floxed heterozygous allele. (C) PCR genotyping of the indicated alleles using tail DNA from wild-type to Cul4bf/+ breeding (left panel) or peripheral blood cell DNA from Vav-iCre to Cul4bf/+ breeding (right panel).
Embryonic lethality of Cul4b−/Y mice
To model XLMR patients with CUL4B deficiency in mice, we initially attempted to generate Cul4b null animals by crossing the floxed mice with EIIA-Cre transgenic mice 20. This approach was unsuccessful due to the mosaic pattern of expression from the EIIA promoter (see Supplementary information, Data S1 and Table S1). To bypass the mosaicism inherent to the EIIA-Cre mice, heterozygous Cul4bf/+ female mice were crossed to chicken-actin (CAG)-Cre mice that drive Cre-mediated recombination at the early zygote stage 21. As shown in Table 1, no viable hemizygous Cul4b−/Y pups were generated among a total of 77 offspring from the mating between male CAG-Cre+/− and female Cul4bf/+ mice. Hemizygous Cul4b−/Y embryos were still present at days E7.5 and E8.5 while no Cul4b−/Y embryos were identified beyond E9.5, suggesting that Cul4b null embryos lost viability prior to day E9.5 (Table 1).
Table 1. Genotype analysis of E7.5-E12.5 embryos and 2-week pups from matings between CAG-Cre(♂) and Cul4bf/+(♀).
| Age |
Genotype |
Total |
||
|---|---|---|---|---|
| Cul4bwt | Cul4b+/− | Cul4b−/Y | ||
| E7.5 | 25 | 6 | 4 | 35 |
| E8.5 | 50 | 12 | 5 | 67 |
| E9.5-12.5 | 34 | 6 | 0 | 40 |
| 2 weeks | 77 | 0 | 0 | 77 |
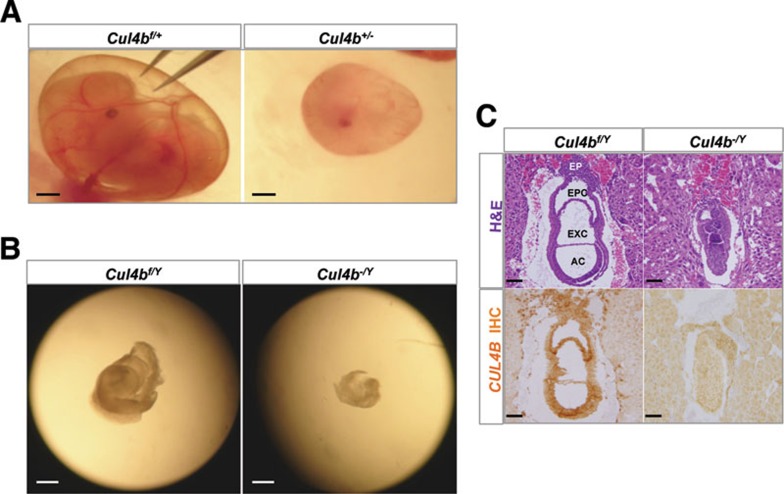
Notably, the heterozygous Cul4b+/− embryos were also not viable, but they exhibited a less severe phenotype than the Cul4b−/Y embryos: among a total of 40 embryos dissected at E9.5-E12.5, six were Cul4b+/− heterozygotes (Table 1). At day E12.5, the yolk sacs of Cul4b+/− embryos had underdeveloped branching vitelline vessels compared to controls (Figure 2A), suggesting an insufficient supply of nutrients to the developing embryo. No Cul4b+/− embryos were identified beyond E13.5. Since the paternal X chromosome undergoes imprinted inactivation in the extra-embryonic tissues in mice 22, the Cul4b+ allele in the Cul4b+/− heterozygous embryos is transcriptionally silenced, accounting for their lost viability (Supplementary information, Figure S1). It is noteworthy that Cul4b+/− embryos survived an additional 2-3 days compared to the Cul4b−/Y hemizygous embryos. Incomplete (or escape from) X-chromosome inactivation may have produced residual CUL4B protein, which may account for the limited proliferation and survival of Cul4b+/− embryos prior to E12.5 23, 24. Another possible explanation is that the genes on the paternal X chromosome are transcriptionally active at the two-cell stage and gradually silenced by imprinted X-inactivation as pre-implantation development continues 25. As such, Cul4b+/− embryos likely express a limited amount of CUL4B in the zygote whereas Cul4b−/Y embryos do not.
Figure 2.
Cul4b deletion resulted in embryonic lethality. (A) Angiogenic defects of the Cul4b−/Y yolk sac. Note the poor vasculature and lack of branching vitelline vessels in Cul4b−/Y yolk sac compared with those in the Cul4bf/Y embryos at day E12.5. (B) Morphology of the dissected E8.5 embryos from Cul4bf/Y and CAG-Cre mating. (C) Histological and immunohistochemical analysis of sagittal sections of E7.5 Cul4b−/Y and control Cul4bf/Y embryos. AC: amniotic cavity; EXC: exocoelom cavity; EPC: ectoplacental cavity. Bars: 1.0 mm, 0.2 mm and 50 μm in A, B, and C, respectively.
While we were unable to recover live heterozygous Cul4b+/− pups from the matings between male CAG-Cre and female Cul4bf/f mice, the reciprocal cross (male Cul4bf/Y X female CAG-Cre+/−) gave rise to 23 live-born Cul4b+/− mice in a total of 77 pups from 9 matings (Table 2). These results indicate that the Cul4b null allele cannot be transmitted from the mother to the next generation, in contrast to human CUL4B patients who inherit X-linked CUL4B mutations maternally. Given that the paternally-inherited X chromosome is inactivated in murine extra-embryonic tissues 22, Cul4b+/− embryos bearing the maternal mutant allele are deficient for CUL4B ubiquitin ligase activity in the extra-embryonic lineages (Supplementary information, Figure S1). The resulting extra-embryonic tissue defects likely account for the inability of heterozygous females to transmit the Cul4b null allele to their offspring.
Table 2. Crossing directions between CAG-Cre+/− and Cul4b mutant mice affect productivity of the females and genotype of the offspring.
| Male | Female | Cul4b+/− | Cul4b−/Y | No. of litters | No. of pups | Average litter size |
|---|---|---|---|---|---|---|
| CAG-Cre+/− | Cul4bf/+ | 0 | 0 | 19 | 77 | 4.1 |
| CAG-Cre+/− | Cul4bf/f | 0 | 0 | 6 | 17 | 2.8 |
| Cul4bf/Y | CAG-Cre+/− | 23 | NA | 9 | 77 | 8.6 |
| C57BL/6J | Cul4b+/− | 0 | 0 | 17 | 44 | 2.6 |
To evaluate the gross morphological changes in Cul4b−/Y embryos, a litter of E8.5 embryos were dissected from pregnant Cul4bf/f mice bred with CAG-Cre mice. Although all the deciduas were of normal appearance and size (Supplementary information, Figure S2A and 2B), 2 out of 11 embryos showed severe morphological abnormalities and were dramatically reduced in size (Figure 2B, Supplementary information, Figure S2C). PCR genotyping confirmed that these abnormal embryos were indeed Cul4b−/Y hemizygous knockouts (Supplementary information, Figure S2D). Notably, heterozygous Cul4b+/− embryos at this stage were normal-sized and morphologically indistinguishable from the wild-type embryos.
The E7.5 deciduas were dissected and embedded for histological analysis. H&E staining showed normal developmental appearance of wild-type embryos with the standard formation of the amniotic cavity, exocoelom, and ectoplacental cavities. Primitive streaks composed of different tissue layers were also readily recognizable in these embryos (Figure 2C). In contrast, Cul4b−/Y embryos displayed compact, indiscernible structures, underdeveloped cavities, and absent ectoplacental cones (EPCn) (Figure 2C). Primitive streak layers were absent in these embryos, and the embryonic ectoderm was disorganized and reduced in size. Collectively, these data indicate that Cul4b is essential for embryonic development.
Growth arrest and degeneration of Cul4b null embryos
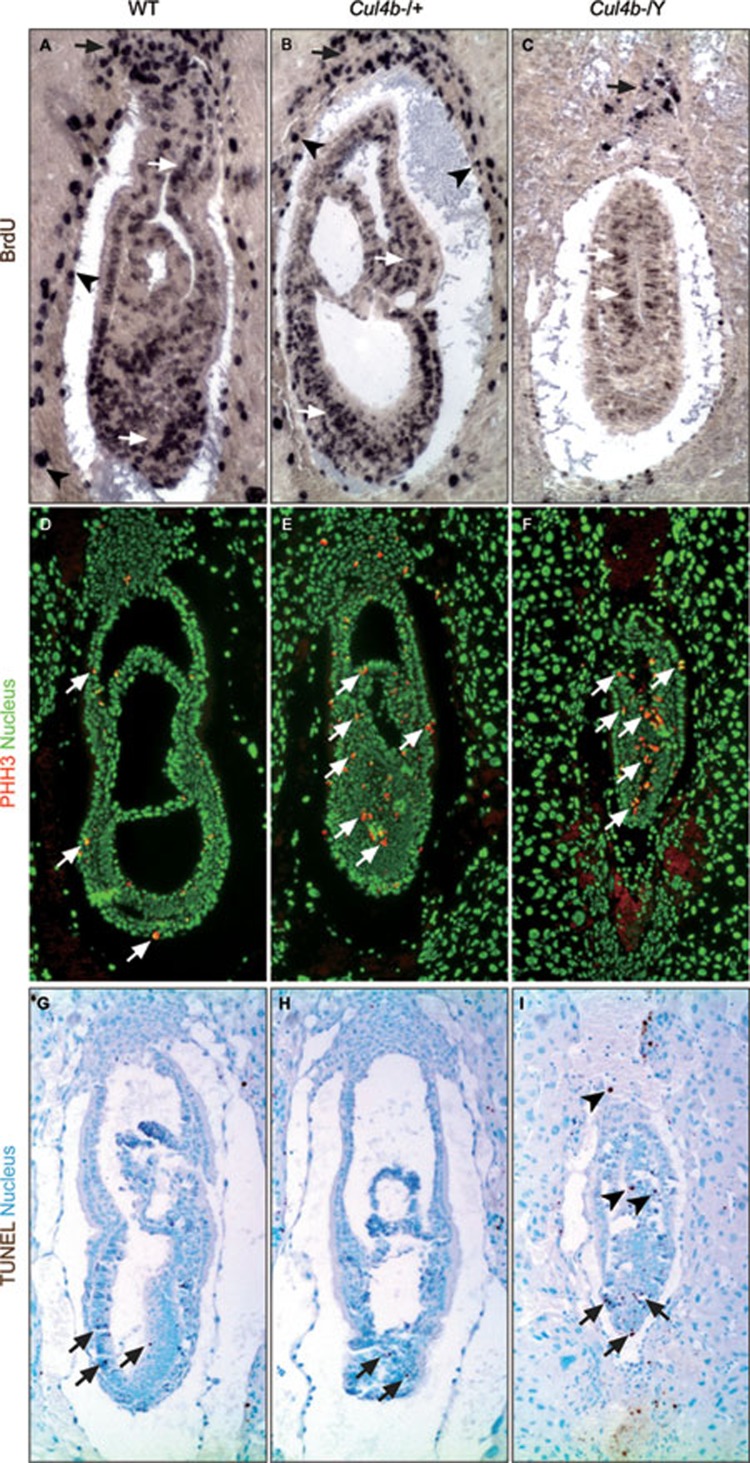
To explore the underlying cause of embryonic lethality of Cul4b−/Y mice, we evaluated cell proliferation and apoptosis in these mutants. 5-bromo-2′-deoxyuridine (BrdU) labeling revealed dramatically reduced numbers of proliferating cells throughout the E7.5 Cul4b−/Y conceptuses (Figure 3A, white arrows). Notably, far fewer proliferating cells were detected in the placental cone (black arrow) and trophectoderm-derived tissues (Figure 3C, compared to 3A and 3B). At this stage, no difference in BrdU labeling was detected between wild-type and heterozygous conceptuses (Figure 3A and 3B). On the other hand, immunofluorescent (IF) staining of phospho-Histone H3 (PHH3), a well-established mitotic marker, revealed only a few mitotic cells in control embryos, whereas a marked increase in this cell population was evident in Cul4b−/Y embryos (Figure 3D-3F). Considering the drastic decrease in the proliferation of Cul4b−/Y extra-embryonic cells (Figure 3A-3C), accumulation of PHH3-positive cells indicates that these mutant cells have arrested at G2/M. Furthermore, TUNEL assays revealed increased apoptosis in both the embryonic and extra-embryonic ectoderms of Cul4b−/Y embryos, while very few TUNEL-positive cells were visible in the Cul4b+/+ or Cul4b+/− embryos (Figure 3G-3I). Together, these data indicate that CUL4B is essential for maintaining growth and survival during embryogenesis.
Figure 3.
Proliferation and apoptosis status of Cul4b-null embryos. (A-C) BrdU assays revealed many S phase cells in the ectoplacental cone (black arrows), the parietral extra-embryonic endoderm and underlying endometrium (arrowheads), and in the embryo proper (white arrows) in both wild-type and heterozygous embryos (A, B). Reduction of proliferating cells was detected in those structures in Cul4b-null embryos (C). (D-F) PHH3 IF staining revealed an increase in PHH3-positive cells in the mutant embryos (compare C to A, arrows). (G-I) TUNEL assays revealed a limited number of apoptotic cells present in either wild-type or heterozygous embryos, primarily in the embryonic ectoderm (G, H, arrows). Dramatic increase in TUNEL-positive cells was evident throughout the Cul4b-null embryos (I, arrows). The genotype of individual embryos was determined by immunohistochemical staining with the anti-CUL4B antibody.
Dynamic expression of Cul4b and Cul4a during early embryonic development
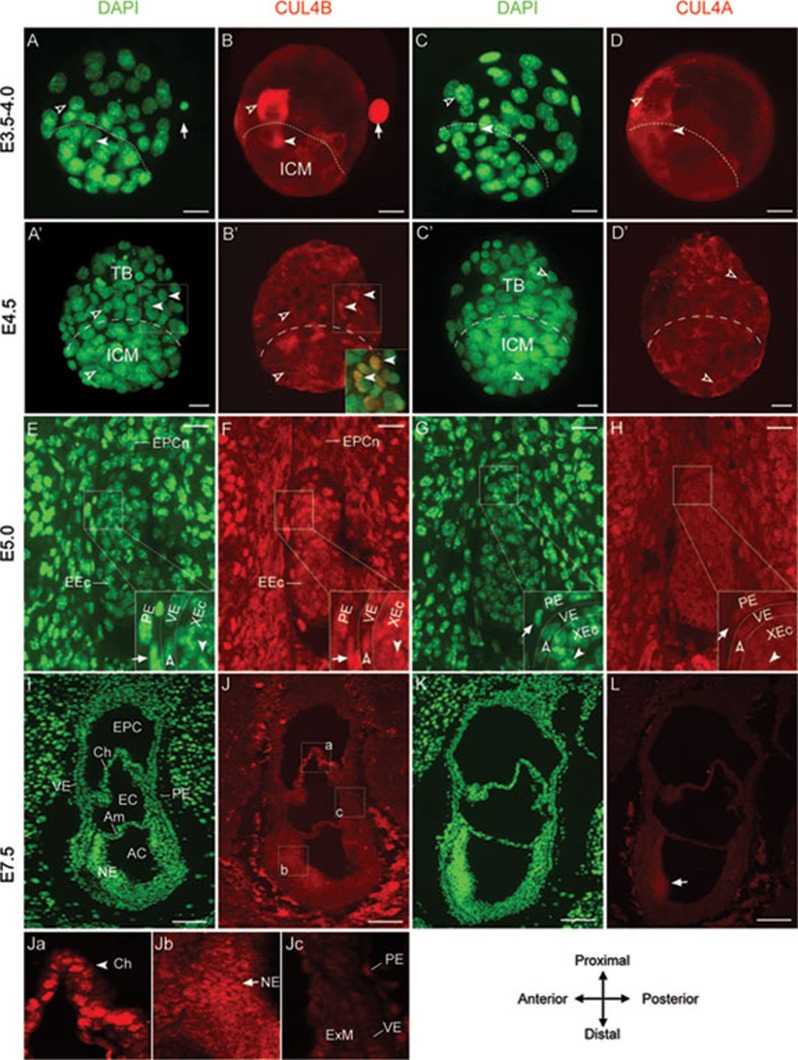
While we previously showed that Cul4b compensates for the loss of Cul4a in Cul4a−/− mice 12, the embryonic lethality of Cul4b−/Y mice indicates that Cul4a is unable to rescue Cul4b deficiency. We set out to investigate whether the lethality of Cul4b−/Y mice results from the differential expression patterns of the two CUL4 proteins, which were recently demonstrated as the cause of the meiotic defects in Cul4a−/− male mice 13, 14. In unhatched E3.5-4.0 blastocysts, both CUL4 proteins were detected in the inner cell mass (ICM) as well as in trophoblast (TB) cells (open arrowheads, Figure 4A-4D). However, CUL4B was highly expressed in the secondary polar body, where CUL4A was absent. In peri-implantation blastocysts (E4.5), both CUL4 proteins were present at comparable levels in the cytoplasm of most ICM and TB cells (Figure 4A′-4D′, open arrowheads). However, prominent nuclear CUL4B staining was evident in some TB cells (Figure 4B′, inset, arrowheads). Why these cells exhibit differential staining patterns and whether CUL4B exerts different functions in these cells remain unclear. Upon implantation, the two CUL4 proteins continue to exhibit differential expression patterns. At E5.0, CUL4B was predominantly expressed in the extra-embryonic ectoderm (Figure 4F, arrowhead) as well as the parietal extra-emrbyonic endoderm (PE) and visceral extra-embryonic endoderm (VE, Figure 4F, arrow and open arrowhead, respectively). In contrast, CUL4A levels were ubiquitously low throughout the embryo (Figure 4G and 4H). At E7.5, CUL4B was mainly detected in the chorionic membrane (Figure 4Ja, arrowhead), which originates partially from the extra-embryonic ectoderm 26. CUL4B was also detected at high levels in the neuroectoderm (Figure 4Jb, arrow), and at lower levels in other extra-embryonic tissues (Figure 4Jc). Conversely, CUL4A appeared to be present at low levels in all cell types, except for slightly higher expression in the newly formed neural plate (Figure 4K and 4L, arrow). Collectively, CUL4A is expressed at marginal levels post implantation in the extra-embryonic tissues, while CUL4B appears to be the predominant CUL4 form at these embryonic stages. These findings are consistent with the requirement of CUL4B for survival, while CUL4A likely does not accumulate above the threshold level required to sustain CUL4 ubiquitin ligase activity following CUL4B deletion.
Figure 4.
Expression of CUL4A and CUL4B during early embryonic development. (A, B) Immunofluorescent (IF) staining in unhatched (E3.5-4.0) blastocysts revealed expression of CUL4B in the inner cell mass (ICM, arrowheads), the trophoblast (TB, open arrowheads), and the secondary polar body (arrows). (C, D) Low-level expression of CUL4A in the ICM and TB cells. (A′-D′) In hatched blastocysts (E4.5), CUL4B was detected in the cytoplasm of most ICM and TB cells (B′, open arrowheads), but distinct nuclear staining was evident in some TB cells (B′, arrowheads). Inset in B′ shows the merged image of the boxed area indicated in A′ and B′ at higher magnification. Arrowheads indicate cells with strong nuclear staining (yellow). At this stage, TB cells are more readily distinguishable from ICM cells as they migrate further away, whereas the latter tend to cluster together. CUL4A, on the other hand, continued to show low cytoplasmic staining in all cells (C′, D′). (E, F) At E5.0, CUL4B IF showed prominent expression in the parietal extraembryonic endoderm (PE, arrows), visceral extraembryonic endoderm (VE, open arrowheads) and extra-embryonic ectoderm (XEc, arrowheads). CUL4B is also expressed in the ectoplacental cone (EPCn) and embryonic ectoderm (EEc) at lower levels. (G, H) Weak expression of CUL4A was detected in all tissue layers of E5.0 embryos. (I, J) At E7.5, prominent nuclear staining of CUL4B was evident in the chorionic membrane (Ch, arrowhead in Ja) and neuroectoderm (NE, arrow in Jb). CUL4B was also detected in other tissue layers including PE, VE, and extra-embryonic mesoderm (ExM) (Jc). Ja-Jc shows the boxed regions in J at a higher magnification. EPC, ectoplacental cavity; EC, exocoelomic cavity; AC, amniotic cavity; Am, amniotic membrane. (K, L) Expression of CUL4A was weak throughout the E7.5 embryo with slightly elevated levels in the neuroectoderm (L, arrow). Orientation of E7.5 embryo sections is indicated. Scale bars: 15 μm in A-D′, 25 μm in E-H, 50 μm in I, L.
Epiblast-specific deletion of floxed Cul4b prevents embryonic lethality and generates live Cul4b null mice
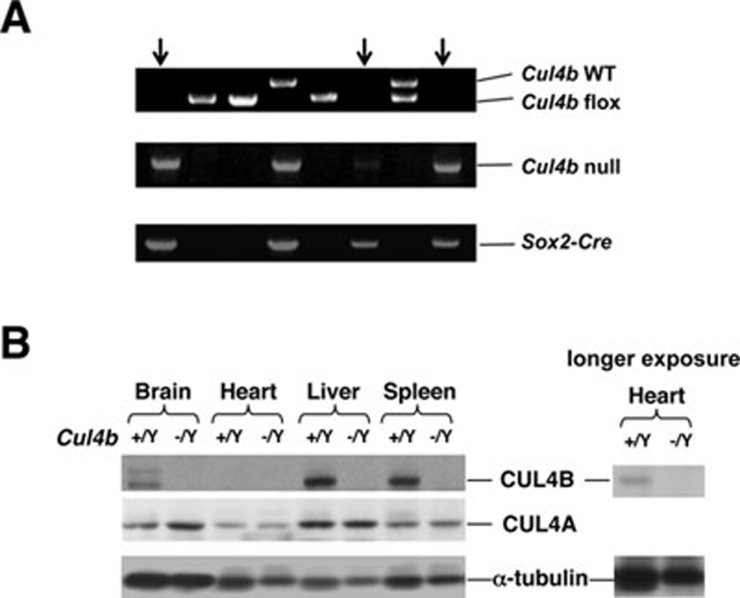
Given the essential role of extra-embryonic tissues in supporting embryonic development, we hypothesized that the degeneration of extra-embryonic tissues resulting from Cul4b deletion may be the cause of death in Cul4b null embryos. We employed a conditional knockout system in which floxed heterozygous Cul4bf/+ or homozygous Cul4bf/f females were crossed to Sox2-Cre males. Sox2-Cre mice express Cre exclusively in the epiblast 27, and have been utilized previously to circumvent placental defects caused by gene knockout and rescue early embryonic lethality. Indeed, a normal Mendelian ratio of live-born Cul4b−/Y null and Cul4b+/− mice was obtained (Table 3, Figure 5A). These Cul4b−/Y mice were healthy and showed no overt developmental abnormalities. Immunoblotting detected no CUL4B protein from several organs of the Cul4b-null animals, including the brain, heart, liver, and spleen, thus verifying the complete deletion of Cul4b in these mice (Figure 5B). CUL4A was detected at comparable or slightly increased levels in the Cul4b-null organs compared with those in their wild-type littermates. Our results confirm the essential role of Cul4b in the development of extra-embryonic tissues that lack compensatory Cul4a expression (Figure 4). In contrast, Cul4b is dispensable for the development of the embryo proper, likely due to the complementing role of CUL4A that is expressed in the epiblast.
Table 3. Genotyping analysis of offspring from breedings between Sox2-Cre+/− (♂) and Cul4b mutant (♀) mice.
| Breeding | Cul4b+/− | Cul4b−/Y | No. of litters | No. of pups | Average litter size |
|---|---|---|---|---|---|
| Sox2-Cre+/− (♂) X Cul4bf/+(♀) | 4 | 5 | 4 | 31 | 7.8 |
| Sox2-Cre+/− (♂) X Cul4bf/f(♀) | 22 | 18 | 10 | 82 | 8.2 |
Figure 5.
Epiblast-specific deletion of Cul4b gave rise to live Cul4b-null animals. (A) PCR genotyping indicated three Cul4bf/YSox2-Cre animals with Cul4b deletion (marked by arrows). (B) Western blotting to detect Cul4b deletion in brain, heart, liver, and spleen extracts from Cul4b−/Y null mice. Note the comparable expression of Cul4a in wild-type and Cul4b−/Y tissues.
Massive accumulation of the p21 cyclin-dependent kinase inhibitor and cell cycle arrest upon Cul4b inactivation
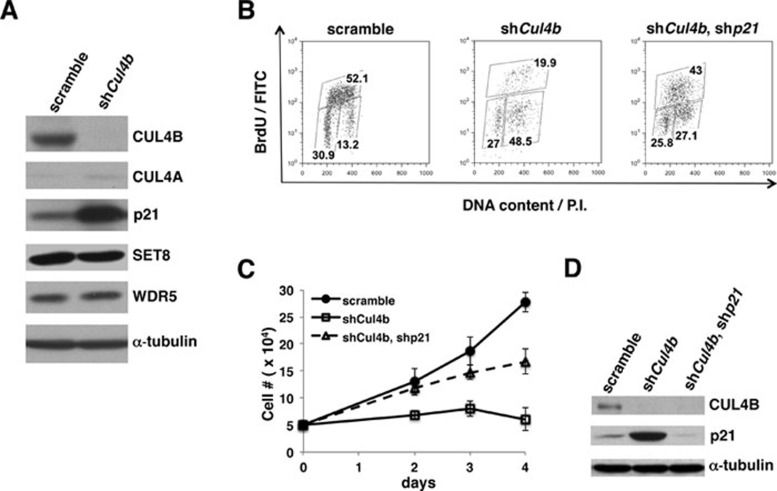
To gain insight into the molecular mechanisms underlying the developmental defects resulting from Cul4b deletion, we examined the protein levels of known CUL4 substrates in an extra-embryonic XEN cell line following Cul4b silencing by RNAi. Kunath et al. 28 showed that XEN cells could be reproducibly derived from mouse blastocysts and express lineage markers of the extra-embryonic endoderm. As shown in Figure 6A, p21 protein levels were robustly (16-fold) elevated upon lentiviral shRNA-mediated knockdown of endogenous Cul4b in XEN cells. Additional CUL4 substrates were also examined, including SET8 and WDR5, which all remained at similar levels compared to control (scrambled) shRNA-expressing cells. Notably, low levels of CUL4A were detected in Cul4b knockdown XEN cells (Figure 6A), consistent with the IF staining of CUL4A in wild-type extra-embryonic tissues (Figure 4). In agreement with the observed G2 growth arrest of Cul4b−/Y embryos (Figure 3C), BrdU staining and FACS analysis also indicated a profound G2/M arrest in Cul4b knockdown XEN cells compared with those infected with scrambled shRNA (Figure 6B, left and middle panels). Cul4b-knockdown XEN cells rapidly ceased to proliferate (Figure 6C). Annexin V staining and FACS analysis also revealed a 5-fold increase in apoptosis in Cul4b knockdown XEN cells (Supplementary information, Figure S3), recapitulating the increased apoptosis observed in Cul4b−/Y embryos (Figure 3I). To determine if p21 accumulation contributes to the growth arrest phenotype, we silenced p21 expression by lentiviral shp21 in Cul4bk/d XEN cells (Figure 6D). As seen in Figure 6B (right panel), p21 knockdown partially rescued G2 arrest following Cul4b silencing (27.1% vs 48.5% of G2 population), with resumption of DNA replication (43% vs 19.9% of S phase population). It is noteworthy that silencing of p21 did not achieve 100% recovery of G2-arrested Cul4bk/d XEN cells as compared to the scrambled shRNA-infected control cells. These results suggest that p21 is a major but not the sole effector responsible for the proliferation defect in Cul4bk/d XEN cells. Consistent with the cell cycle analysis, silencing of p21 in Cul4bk/d cells overcame the growth arrest and restored cellular proliferation, albeit at a slower rate than in control cells (Figure 6C). We also silenced CUL4A expression by lentiviral shRNA in XEN cells, but observed only a marginal effect on p21 upregulation and no growth inhibition (Supplementary information, Figure S4 and data not shown). Collectively, these results indicate a specific role for CUL4B in the targeted degradation of p21 in extra-embryonic tissue-derived cells. Further experiments should define the physiological function of CUL4B-mediated p21 destruction in the proliferation and survival of developing extra-embryonic tissues.
Figure 6.
p21 accumulation and G2/M cell cycle arrest upon silencing of Cul4b expression in extra-embryonic XEN cells. (A) XEN cells were infected with lentiviral shCul4b or scrambled shCul4b. CUL4A, CUL4B, and a panel of CUL4 substrates including p21, SET8, and WDR5 were detected by western blotting using the appropriate antibodies. (B-D) G2/M arrest of XEN cells following Cul4b knockdown by lentiviral shRNA and partial rescue by p21 silencing. XEN cells infected with lentiviral scramble shRNA, shCUL4B or both shCUL4B and shp21 were isolated by puromycin selection and FACS. Cells were fixed, stained with anti-BrdU antibody and propidium iodide, and analyzed by flow cytometry (B), counted to determine their growth rates (C), or subjected to western blotting with antibodies against CUL4B, p21, and actin (loading control). The percentages of G1, S, and G2/M cells in scramble- or shCul4b-expressing XEN cells are indicated.
Discussion
The two CUL4 family members have overlapping as well as divergent functions during embryonic development. We previously showed that Cul4a−/− mice (exons 17-19 deleted) were viable and displayed no developmental abnormalities 12. Kopanja et al. 13 also showed that another Cul4a−/− strain (exons 4-8 deleted) was viable and appeared phenotypically normal. Silencing of Cul4b expression in primary Cul4a−/− mouse embryonic fibroblasts led to the rapid loss of viability, indicating that CUL4B compensates for the essential functions of CUL4A to support growth and survival 12.
There is currently no evidence that suggests the selective usage of different DCAF substrate receptors by CUL4A or CUL4B, as they both employ the DDB1 adaptor to recruit DCAFs. However, an emerging mechanism that distinguishes the two CUL4 proteins is the non-overlapping expression pattern of CUL4A and CUL4B in specific tissues and organs during development. For example, Cul4b expression is silenced in the pachytene-diplotene stages of meiotic prophase, accounting for the reproductive defects and male infertility of the Cul4a−/− mice 14. Another difference is the predominant cytoplasmic vs nuclear distribution of CUL4A and CUL4B, respectively, suggesting another mechanism of substrate preference between the two CUL4 proteins 29, 30, although a subpopulation of CUL4A also resides in the nucleus to target transcription factors and nucleotide excision repair proteins (reviewed in 6, 7, 8). Moreover, in response to environmental toxins, the extreme N terminus of CUL4B binds to the aryl hydrocarbon receptor (AhR) to form a distinct E3 ligase that catalyzes the ubiquitination and degradation of the estrogen receptor, progesterone receptor, and AhR itself 15.
Here, we showed that the differential expression of CUL4A and CUL4B in extra-embryonic tissues underlies the functional divergence of the two CUL4 proteins. Cul4b−/Y embryos suffer lethality during early post-implantation development due to defects in extra-embryonic tissues, which express low levels of Cul4a (Figure 4). Deletion of Cul4b in the Cul4bf/Y CAG-Cre embryos resulted in overall inactivation of CUL4 ubiquitin ligase activity and induction of growth arrest in extra-embryonic tissues, which were subsequently unable to support the viability of the embryo. We further showed that inactivation of Cul4b in an extra-embryonic cell line led to the dramatic accumulation of the p21 cyclin-dependent kinase inhibitor and G2/M arrest (Figure 6). In contrast, the epiblast expresses both Cul4a and Cul4b (Figure 4), and, as a result, Cul4a compensates for the loss of Cul4b to maintain viability of the embryo proper. In support of the notion that the differential expression of Cul4a and Cul4b in the extra-embryonic tissues is the underlying cause of lethality in Cul4b−/Y embryos, Cul4bf/Y or Cul4bf/f mice crossed with Sox2-Cre are viable and develop to adulthood without overt phenotypic deficiencies. However, detailed neurological, behavioral and reproductive analyses have yet to be carried out. Previous studies demonstrated robust ubiquitin-dependent proteolysis in the trophoblast but not the inner cell mass of blastocysts, suggesting a critical role for proteolysis in the extra-embryonic tissue development in mice 31. Our results support this notion by further revealing the essential role of the CUL4B ubiquitin ligase in the extra-embryonic tissues, but not in the epiblast where there is a functional overlap of co-expressed CUL4A and CUL4B.
It is important to note that the Cul4b null allele cannot be passed from a heterozygous mother to its male offspring due to paternal X-chromosome inactivation (imprinting) in the extra-embryonic tissues, as evidenced by the absence of Cul4b+/− mice from the CAG-Cre (male) x Cul4bf/+ (female) mating (Table 1). Consistent with the defects found in the extra-embryonic tissues of the Cul4b+/− embryos, there was also a lack of vasculature in the mutant yolk sacs compared to the well-organized yolk sac vasculature in wild-type Cul4bf/+ embryos (Figure 2A). Since murine yolk sac hematopoiesis begins on E7.5 with the migration of hemangioblast precursors from the primitive streak into the mesodermal layer of the visceral yolk sac 32, defects in the mutant yolk sac vasculature are likely secondary, and result from the defective interaction between the mutant visceral endoderm and hemangioblasts. Heterozygous animals were derived by mating male Cul4bf/Y with female CAG-Cre (Table 2) to ensure that the wild-type Cul4b allele is maternally inherited. In contrast, X-inactivation in human extra-embryonic placental tissue occurs randomly and the paternal X-chromosome can remain active in a CUL4B heterozygous child 33. Thus, the mutated CUL4B allele can be maternally transmitted to the next generation in humans.
To understand the cellular pathways that are deregulated upon Cul4b ablation in extra-embryonic tissues, we silenced Cul4b expression with lentiviral shRNA in XEN cells and observed rapid growth arrest at G2/M phase. This cell cycle arrest corresponded with a 16-fold increase in p21 protein levels, while levels of several other CUL4 substrates were not affected (Figure 6A). Unfortunately, we were unable to detect p21 specifically by immunohistochemistry in Cul4b−/Y mouse extra-embryonic tissues using commercially available anti-p21 antibodies as cross-reacting signals were observed in the control p21−/− specimens (also personal communications with Andrew Koff, Memorial Sloan-Kettering Cancer Center). Previous studies on G2 arrest induced by DNA damage or mitogen starvation revealed that p21 directly interacts with cyclin A- and cyclin B-associated CDK1 kinase to block G2/M progression 34, 35, 36, 37. Consistent with these studies, accumulation of high levels of p21 following the depletion of the CUL4B ubiquitin ligase led to growth arrest at G2 phase in the extra-embryonic lineages. ShRNA-mediated silencing of p21 largely rescued the G2 arrest of Cul4b-knockdown XEN cells and resumed cell proliferation, indicating that p21 is indeed a major target of CUL4B. However, the inability to achieve full rescue by p21 silencing suggests that other yet-to-be identified CUL4B substrates likely also contribute to the cell cycle arrest that is triggered by CUL4B inactivation. Spruce et al. 38 recently demonstrated that inhibition of p21 expression by miRNA was required to maintain the trophoblast stem cell compartment. Our findings suggest that p21 accumulation is a significant factor that contributes to the developmental defects of the extra-embryonic tissues and embryonic lethality of Cul4b-null mice.
Human CUL4B patients survive to adulthood with multiple neuronal and developmental defects. Although Cul4b−/Y mice derived from the Cul4bf/Y × Sox2-Cre cross can survive with no overt developmental abnormalities, neurological and behavioral analyses have yet to be performed on these mice. We posit that germline Cul4b-deficient mice may model XLMR in patients, although the different epigenetic regulations between humans and mice may limit the recapitulation of developmental defects observed in CUL4B patients. Future studies should compare the temporal and spatial expression profiles of human and mouse CUL4A and CUL4B during development and organogenesis, especially in the affected tissues and organs of CUL4B XLMR patients. Moreover, the comprehensive proteomic profiling of accumulated substrates following Cul4b abrogation could potentially lead to the delineation of cellular pathways that contribute to the clinical symptoms of XLMR with CUL4B mutations, and offer insight into potential therapeutic strategies. Our study elucidates the roles of CUL4B during embryonic development and provides a strategy to generate viable Cul4b−/Y animals, thus setting the stage to probe the physiological and pathological functions of the CUL4B ubiquitin ligase.
Materials and Methods
Cells, plasmids, and antibodies
XEN cells were a kind gift from Dr Sundeep Kalantry (University of Michigan, USA). Lentiviruses encoding shCul4b, shCul4a, or scrambled shCul4b control were generated for infection of XEN cells and were described previously 12. Antibodies purchased from commercial sources include those against CUL4A (Bethyl Laboratories), CUL4B (Proteintech group), PHH3 (Millipore), p21 (Santa Cruz), WDR5 (R&D Systems), SET8 (Cell Signaling), and α-tubulin (Sigma Aldrich).
Generation of floxed Cul4b mice
To generate the floxed Cul4b allele, a murine Cul4b BAC clone was used to generate the final targeting construct. Targeted disruption of the Cul4b locus was accomplished by placing a LoxP site and an XbaI cutting site 135 bp upstream of exon 4 and another LoxP site and the neomycin resistance cassette 66 bp downstream of exon 5. The 5′ and 3′ homologous arms were 4.3 kb and 4 kb, respectively. Following electroporation and G418 selection, clones that underwent homologous recombination were identified by Southern blotting after XbaI digestion using a 5′-flanking probe. Cul4b-targeted mouse ES cells were injected into blastocysts to generate chimeras, from which germline transmission of the targeted Cul4b allele was accomplished and verified by PCR (Supplementary information, Data S1). The deleted Cul4b allele was created by germline-induced deletion of exons 4 and 5 by crossing floxed Cul4b mice with the CAG-Cre or SOX2-Cre transgenic lines.
Histology, immunofluorescence, and immunohistochemistry
Embryos were collected from timed pregnancy at various stages of gestation for further analysis. Fresh deciduas were fixed in 4% paraformaldehyde overnight at 4 °C, dehydrated and embedded in paraffin. Whole mount IF on blastocysts was performed as previously described 39. Standard IF was performed on PFA-fixed paraffin sections (6 μm) (E5.0) or frozen sections (E7.5) following procedures as previously described 40. Immunohistochemistry was performed on paraffin-embedded embryo sections as previously described in detail 12.
Proliferation and TUNEL assay
7.5 d.p.c. pregnant female mice were pulse-labeled via intraperitoneal injection of BrdU solution (100 μg BrdU per gram body weight) 1 h prior to embryo harvest. BrdU assays were performed on PFA-fixed, paraffin-embedded 10-mm sections using BrdU Labeling and Detection Kit (Roche Applied Science, Indianapolis, IN, USA) following the manufacturer's instructions. TUNEL assay were performed on paraffin sections using the TACS® 2 TdT-DAB In Situ Apoptosis Detection Kit (Trevigen, Gaithersburg, MD, USA).
Acknowledgments
We thank Sundeep Kalantry (University of Michigan, USA) for the XEN cells, Wei Gu (Columbia Universtity, USA) for help on generation of the germline-transmitted Cul4bf/+ mice and Willie Mark (Sloan-Kettering Cancer Center, USA) for the CAG-Cre transgenic mice, and Jennifer Lee and Brent Bany for critical reading of the manuscript. This work is supported by National Institutes of Health Grants CA098210 and CA118085 to PZ, ES016597 to LM, and GM58726 and CA08748 to EL. PZ is supported in part by the Irma T Hirschl Career Scientist Award and the Tri-Institutional Stem Cell Initiative. EL is also supported in part by NYSTEM (CO2433).
Footnotes
(Supplementary information is linked to the online version of the paper on the Cell Research website.)
Supplementary Material
Genotyping analysis of offspring from breeding between EIIA-Cre+/+ (♂) and Cul4bf/+ (♀) mice and mosaic mice
Extraembryonic tissues of heterozygous Cul4b+/− embryos exhibited imprinted paternal X chromosome inactivation.
Normal morphology and size of the conceptuses.
Evaluation of early stage apoptosis by annexin V-FITC/PI staining.
Silencing of Cul4a expression has in minimal or no effect on CUL4 substrates in the extraembryonic XEN cells.
References
- Rejeb I, Ben Jemaa L, Chaabouni H. X linked mental retardation. Tunis Med. 2009;87:311–318. [PubMed] [Google Scholar]
- Tarpey PS, Raymond FL, O'Meara S, et al. Mutations in CUL4B, which encodes a ubiquitin E3 ligase subunit, cause an X-linked mental retardation syndrome associated with aggressive outbursts, seizures, relative macrocephaly, central obesity, hypogonadism, pes cavus, and tremor. Am J Hum Genet. 2007;80:345–352. doi: 10.1086/511134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Y, Liu Q, Chen B, et al. Mutation in CUL4B, which encodes a member of cullin-RING ubiquitin ligase complex, causes X-linked mental retardation. A J Hum Genet. 2007;80:561–566. doi: 10.1086/512489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isidor B, Pichon O, Baron S, David A, Le Caignec C. Deletion of the CUL4B gene in a boy with mental retardation, minor facial anomalies, short stature, hypogonadism, and ataxia. Am J Med Genet A. 2010;152A:175–180. doi: 10.1002/ajmg.a.33152. [DOI] [PubMed] [Google Scholar]
- Badura-Stronka M, Jamsheer A, Materna-Kiryluk A, et al. A novel nonsense mutation in CUL4B gene in three brothers with X-linked mental retardation syndrome. Clin Genet. 2009;77:141–144. doi: 10.1111/j.1399-0004.2009.01331.x. [DOI] [PubMed] [Google Scholar]
- Lee J, Zhou P. Cullins and Cancer. Genes Cancer. 2010;1:690–699. doi: 10.1177/1947601910382899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarikas A, Hartmann T, Pan ZQ. The cullin protein family. Genome Biol. 2011;12:220. doi: 10.1186/gb-2011-12-4-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson S, Xiong Y. CRL4s: the CUL4-RING E3 ubiquitin ligases. Trends Biochem Sci. 2009;34:562–570. doi: 10.1016/j.tibs.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Zhou P. DCAFs, the missing link of the CUL4-DDB1 ubiquitin ligase. Mol Cell. 2007;26:775–780. doi: 10.1016/j.molcel.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Abbas T, Sivaprasad U, Terai K, et al. PCNA-dependent regulation of p21 ubiquitylation and degradation via the CRL4Cdt2 ubiquitin ligase complex. Genes Dev. 2008;22:2496–2506. doi: 10.1101/gad.1676108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Starostina NG, Kipreos ET. The CRL4Cdt2 ubiquitin ligase targets the degradation of p21Cip1 to control replication licensing. Genes Dev. 2008;22:2507–2519. doi: 10.1101/gad.1703708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Lee S, Zhang J, et al. CUL4A abrogation augments DNA damage response and protection against skin carcinogenesis. Mol Cell. 2009;34:451–460. doi: 10.1016/j.molcel.2009.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopanja D, Roy N, Stoyanova T, et al. Cul4A is essential for spermatogenesis and male fertility. Dev Biol. 2011;352:278–287. doi: 10.1016/j.ydbio.2011.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y, Lin C, Kim ST, et al. The E3 ubiquitin ligase Cullin 4A regulates meiotic progression in mouse spermatogenesis. Dev Biol. 2011;356:51–62. doi: 10.1016/j.ydbio.2011.05.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtake F, Baba A, Takada I, et al. Dioxin receptor is a ligand-dependent E3 ubiquitin ligase. Nature. 2007;446:562–566. doi: 10.1038/nature05683. [DOI] [PubMed] [Google Scholar]
- Kerzendorfer C, Whibley A, Carpenter G, et al. Mutations in Cullin 4B result in a human syndrome associated with increased camptothecin-induced topoisomerase I-dependent DNA breaks. Hum Mol Genet. 2010;19:1324–1334. doi: 10.1093/hmg/ddq008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox BJ, Vollmer M, Tamplin O, et al. Phenotypic annotation of the mouse X chromosome. Genome Res. 2010;20:1154–1164. doi: 10.1101/gr.105106.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angers S, Li T, Yi X, et al. Molecular architecture and assembly of the DDB1-CUL4A ubiquitin ligase machinery. Nature. 2006;443:590–593. doi: 10.1038/nature05175. [DOI] [PubMed] [Google Scholar]
- de Boer J, Williams A, Skavdis G, et al. Transgenic mice with hematopoietic and lymphoid specific expression of Cre. Eur J Immunol. 2003;33:314–325. doi: 10.1002/immu.200310005. [DOI] [PubMed] [Google Scholar]
- Lakso M, Pichel JG, Gorman JR, et al. Efficient in vivo manipulation of mouse genomic sequences at the zygote stage. Proc Natl Acad Sci USA. 1996;93:5860–5865. doi: 10.1073/pnas.93.12.5860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai K, Miyazaki J. A transgenic mouse line that retains Cre recombinase activity in mature oocytes irrespective of the cre transgene transmission. Biochem Biophys Res Commun. 1997;237:318–324. doi: 10.1006/bbrc.1997.7111. [DOI] [PubMed] [Google Scholar]
- Takagi N, Sasaki M. Preferential inactivation of the paternally derived X chromosome in the extraembryonic membranes of the mouse. Nature. 1975;256:640–642. doi: 10.1038/256640a0. [DOI] [PubMed] [Google Scholar]
- Lee JT. Disruption of imprinted X inactivation by parent-of-origin effects at Tsix. Cell. 2000;103:17–27. doi: 10.1016/s0092-8674(00)00101-x. [DOI] [PubMed] [Google Scholar]
- Sado T, Wang Z, Sasaki H, Li E. Regulation of imprinted X-chromosome inactivation in mice by Tsix. Development. 2001;128:1275–1286. doi: 10.1242/dev.128.8.1275. [DOI] [PubMed] [Google Scholar]
- Payer B, Lee JT, Namekawa SH. X-inactivation and X-reactivation: epigenetic hallmarks of mammalian reproduction and pluripotent stem cells. Hum Genet. 2011;130:265–280. doi: 10.1007/s00439-011-1024-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira PN, Dobreva MP, Graham L, et al. Amnion formation in the mouse embryo: the single amniochorionic fold model. BMC Dev Biol. 2011;11:48. doi: 10.1186/1471-213X-11-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi S, Lewis P, Pevny L, McMahon AP. Efficient gene modulation in mouse epiblast using a Sox2Cre transgenic mouse strain. Mech Dev. 2002;119 (Suppl 1):S97–S101. doi: 10.1016/s0925-4773(03)00099-6. [DOI] [PubMed] [Google Scholar]
- Kunath T, Arnaud D, Uy GD, et al. Imprinted X-inactivation in extra-embryonic endoderm cell lines from mouse blastocysts. Development. 2005;132:1649–1661. doi: 10.1242/dev.01715. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Xiong Y. X-Linked mental retardation gene CUL4B targets ubiquitylation of H3K4 methyltransferase component WDR5 and regulates neuronal gene expression. Mol Cell. 2011;43:381–391. doi: 10.1016/j.molcel.2011.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Y, Mi J, Cui J, et al. Characterization of nuclear localization signal in the N terminus of CUL4B and its essential role in cyclin E degradation and cell cycle progression. J Biol Chem. 2009;284:33320–33332. doi: 10.1074/jbc.M109.050427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutovsky P, Motlik J, Neuber E, et al. Accumulation of the proteolytic marker peptide ubiquitin in the trophoblast of mammalian blastocysts. Cloning Stem Cells. 2001;3:157–161. doi: 10.1089/153623001753205115. [DOI] [PubMed] [Google Scholar]
- Chung YS, Zhang WJ, Arentson E, et al. Lineage analysis of the hemangioblast as defined by FLK1 and SCL expression. Development. 2002;129:5511–5520. doi: 10.1242/dev.00149. [DOI] [PubMed] [Google Scholar]
- van den Berg IM, Galjaard RJ, Laven JS, van Doorninck JH. XCI in preimplantation mouse and human embryos: first there is remodelling. Hum Genet. 2011;130:203–215. doi: 10.1007/s00439-011-1014-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits VA, Klompmaker R, Vallenius T, et al. p21 inhibits Thr161 phosphorylation of Cdc2 to enforce the G2 DNA damage checkpoint. J Biol Chem. 2000;275:30638–30643. doi: 10.1074/jbc.M005437200. [DOI] [PubMed] [Google Scholar]
- Baus F, Gire V, Fisher D, Piette J, Dulic V. Permanent cell cycle exit in G2 phase after DNA damage in normal human fibroblasts. EMBO J. 2003;22:3992–4002. doi: 10.1093/emboj/cdg387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medema RH, Klompmaker R, Smits VA, Rijksen G. p21waf1 can block cells at two points in the cell cycle, but does not interfere with processive DNA-replication or stress-activated kinases. Oncogene. 1998;16:431–441. doi: 10.1038/sj.onc.1201558. [DOI] [PubMed] [Google Scholar]
- Niculescu AB., 3rd, , Chen X, Smeets M, et al. Effects of p21(Cip1/Waf1) at both the G1/S and the G2/M cell cycle transitions: pRb is a critical determinant in blocking DNA replication and in preventing endoreduplication. Mol Cell Biol. 1998;18:629–643. doi: 10.1128/mcb.18.1.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spruce T, Pernaute B, Di-Gregorio A, et al. An early developmental role for miRNAs in the maintenance of extraembryonic stem cells in the mouse embryo. Dev Cell. 2010;19:207–219. doi: 10.1016/j.devcel.2010.07.014. [DOI] [PubMed] [Google Scholar]
- Riley JK, Carayannopoulos MO, Wyman AH, Chi M, Moley KH. Phosphatidylinositol 3-kinase activity is critical for glucose metabolism and embryo survival in murine blastocysts. J Biol Chem. 2006;281:6010–6019. doi: 10.1074/jbc.M506982200. [DOI] [PubMed] [Google Scholar]
- Yin Y, Lin C, Ma L. MSX2 promotes vaginal epithelial differentiation and wolffian duct regression and dampens the vaginal response to diethylstilbestrol. Mol Endocrinol. 2006;20:1535–1546. doi: 10.1210/me.2005-0451. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Genotyping analysis of offspring from breeding between EIIA-Cre+/+ (♂) and Cul4bf/+ (♀) mice and mosaic mice
Extraembryonic tissues of heterozygous Cul4b+/− embryos exhibited imprinted paternal X chromosome inactivation.
Normal morphology and size of the conceptuses.
Evaluation of early stage apoptosis by annexin V-FITC/PI staining.
Silencing of Cul4a expression has in minimal or no effect on CUL4 substrates in the extraembryonic XEN cells.