Abstract
Arodyn (Ac[Phe1,2,3,Arg4,D-Ala8]Dyn A(1-11)-NH2) is an acetylated dynorphin A (Dyn A) analog that is a potent and selective κ opioid receptor antagonist (Bennett et al. J. Med. Chem. 2002, 45, 5617), and its analog [NMePhe1]arodyn shows even higher affinity and selectivity for κ opioid receptors (Bennett et al., J. Pep. Res. 2005, 65, 322). However, the latter compound is prone to deletion of the Ac-NMePhe moiety from the N-terminus of the peptide during acidic cleavage as described in the accompanying paper. Several stable analogs of [NMePhe1]arodyn and [NMePhe1,Trp3]arodyn where the acetyl group was substituted with a heteroatom-containing group were evaluated for their opioid receptor affinity, selectivity, and efficacy. Methoxycarbonyl derivatives exhibited the highest κ opioid receptor affinity among the analogs. Additional CH3OCO[NMePhe1]arodyn analogs where position 3 was substituted with other aromatic or non-aromatic residues were also evaluated for κ receptor affinity, selectivity, and efficacy. [CH3OCO-NMePhe1]arodyn has similar κ opioid receptor affinity as [NMePhe1]arodyn, retains high κ opioid receptor selectivity, and is a potent κ opioid receptor antagonist.
Keywords: N-methylamino acid, dynorphin analogs, arodyn, κ opioid receptor
Introduction
Clinically used drugs that interact with opioid receptors are primarily mu (μ) opioid receptor agonists (e.g. morphine) that are used as analgesics, but these agents are associated with serious side effects (respiratory depression, addiction liability and constipation).1 Therefore, there is considerable interest in developing agents for other opioid receptors, namely kappa (κ) and delta (δ) opioid receptors, as analgesics and for the treatment of other disorders. Peripherally selective κ opioid agonists have shown promise because of the analgesic activity of these compounds in visceral pain models, especially in conditions involving inflammation,2 without centrally mediated side effects (e.g. dysphoria3,4).
Kappa receptor antagonists were initially used only as pharmacological tools to study the pharmacology of κ opioid receptors, but recent reports sparked interest in their potential clinical applications. Kappa receptor antagonists have demonstrated antidepressant5 and antianxiety6,7 activity. They also have potential use in the treatment of cocaine8,9 and opioid dependence.10,11 Several selective nonpeptide κ opioid receptor antagonists have been discovered during the past two decades. The bivalent ligand nor-binaltorphimine (nor-BNI)12 is a potent and selective κ opioid receptor antagonist that has been used extensively in pharmacological studies.13 Extensive structure-activity relationship (SAR) studies of the δ opioid receptor antagonist naltrindole resulted in the identification of 5′-guanidinyl-naltrindole (5′-GNTI) as a κ opioid receptor selective antagonist.14 JDTic is a phenylpiperidine derivative that is also a potent antagonist for κ opioid receptors and shows very high κ opioid receptor affinity and selectivity.15 However, all three antagonists exhibit extremely long activity (ranging from several days to several weeks after a single dose),13,16-19 thus limiting their use as pharmacological tools and potentially as therapeutic agents.
The endogenous ligands for κ opioid receptors are peptides. We are exploring the structure-activity relationships (SAR) of the endogenous peptide Dyn A (Tyr-Gly-Gly-Phe-Leu-Arg-Arg-Ile-Arg-Pro-Lys-Leu-Lys-Trp-Asp-Asn-Gln), with an emphasis on analogs that are selective κ opioid receptor antagonists. It has been proposed that peptide and nonpeptide ligands bind to different domains of the κ opioid receptor,20-22 and therefore selective peptide antagonists are complementary to nonpeptide antagonists. Furthermore, peptide derivatives are expected to have shorter durations of action due to metabolism by proteases, and therefore peptide antagonists could overcome the problems associated with long-acting nonpeptide antagonists.
Several derivatives of Dyn A with antagonist activity have been reported.1 Early analogs generally exhibited weak antagonist activity and low κ opioid receptor selectivity, but recently more selective and potent analogs have been reported. [Pro3]Dyn A-(1-11)NH2 has high affinity and selectivity (Ki ratio (κ/μ/δ) = 1/2110/3260) for κ opioid receptors, but this ligand exhibits only weak antagonist potency in functional assays.23 Its Arg8 analog shows higher κ affinity without compromising the high κ selectivity.24 Dynantin ([(2S)-Mdp1]Dyn A-(1-11)NH2, Mdp = 2-methyl-3-(2′,6′-dimethyl-4-hydroxyphenyl)propionic acid), with modification of the first amino acid Tyr, was reported to be a potent κ receptor antagonist (Ke = 0.63 nM in the guinea pig ileum against Dyn A-(1-13)NH2) with high affinity (Ki (κ) = 0.82 nM) and selectivity (Ki ratio (κ/μ/δ) = 1/259/198) for κ opioid receptors.25
Our laboratory has identified several κ opioid receptor antagonists by modifying the N-terminal “message”26 sequence of Dyn A. These include three peptides lacking a basic N-terminal amine.27-29 One of these peptides is arodyn (1), which exhibits high κ opioid receptor affinity (Ki (κ) = 10 nM) and selectivity (Ki ratio (κ/μ/δ) = 1/174/583) and is a κ receptor antagonist.28 SAR studies of arodyn resulted in [NMePhe1]arodyn (2) which exhibits higher κ opioid receptor affinity (Ki (κ) = 4.6 nM) and selectivity (Ki ratio (κ/μ/δ) = 1/1100/>2170) than arodyn.30 This is one of the most selective peptide antagonists for κ opioid receptors.
[NMePhe1]arodyn and its analogs that are acetylated at the N-terminus, however, are all prone to loss of Ac-NMePhe under acidic cleavage conditions (see the accompanying paper). The yields of these peptides could be increased by modifying the cleavage cocktail and cleavage conditions, but even under optimized cleavage conditions, substantial degradation of these peptides still occurred. We showed that this degradation reaction could be suppressed by substituting the acetyl group with a heteroatom-containing group (e.g. methoxycarbonyl, phenyloxyacetyl, glycyl, and acetyl glycyl; see the accompanying article). Considering the remarkable pharmacological activity of [NMePhe1]arodyn, the pharmacological profiles of these stable analogs were examined. The SAR of arodyn indicated that Phe3 contributes to κ opioid receptor affinity, selectivity, and efficacy.30 Since the substitution of Phe1 with NMePhe can significantly increase κ opioid receptor affinity and selectivity, additional stable [NMePhe1]arodyn analogs in which position 3 was substituted with other aromatic or non-aromatic residues were also evaluated for κ opioid receptor affinity, selectivity, and opioid activity. Here we report the pharmacological results for these stable [NMePhe1]arodyn analogs.
Results and discussion
Analog design
We designed a number of analogs for evaluation of their pharmacological activity. First, the effects of substituting different heteroatom-containing groups at the N-terminus of the parent peptides [NMePhe1]arodyn (2) and [NMePhe1,Trp3]arodyn (3) were evaluated. [Trp3]arodyn (4), the Phe1 analog of [NMePhe1,Trp3]arodyn (3), was also prepared to evaluate the effects of N-methylation on the κ opioid receptor affinity and selectivity of [Trp3]arodyn. Thus, we synthesized [CH3OCO-NMePhe1,X3]arodyn (5 and 9), [PhOCH2CO-NMePhe1,X3]arodyn (6 and 10), [Gly-NMePhe1,X3]arodyn (7 and 11), and [Ac-Gly-NMePhe1,X3]arodyn (8 and 12) (where X = Phe or Trp, Table 1). Compounds 3, 4, and 9-12 were originally synthesized for the study of the side reaction (see the accompanying article). The methoxycarbonyl group was also incorporated in analogs where the third position was substituted with other aromatic residues (Tyr and 2-naphthylalanine (Nal(2′)) in compounds 13 and 14, respectively) or a non-aromatic residue (cyclohexylalanine (Cha) in compound 15). (The structures of Phe, NMePhe, Tyr, Trp, Nal(2′) and Cha are shown in Figure 1.) Their corresponding unstable acetyl analogs (compounds 16-18, Table 1) were synthesized for comparison of their κ opioid receptor affinities, selectivities, and efficacy.
Table 1.
Analytical data for the peptides
X-NMePhe-Phe-R-Arg-Leu-Arg-Arg-D-Ala-Arg-Pro-Lys-NH2
| Cmpd | X | R | HPLC (tR, (min))a | ESI-MS (m/z) | ||
|---|---|---|---|---|---|---|
| System 1b | System 2c | Calculated | Observed | |||
| 3 | Ac | Trp | 24.7 | 41.1 | [M+3H]3+=530.0 | [M+3H]3+=530.0 |
| 4 | Acd | Trp | 22.6 | 40.1 | [M+3H]3+=526.0 | [M+3H]3+=526.0 |
| 5 | CH3OCO | Phe | 26.3 | 44.2 | [M+3H]3+=522.6 | [M+3H]3+=522.5 |
| 6 | C6H5OCH2CO | Phe | 30.1 | 29.4e | [M+3H]3+=547.7 | [M+3H]3+=547.7 |
| 7 | Gly | Phe | 24.9 | 37.3 | [M+3H]3+=522.0 | [M+3H]3+=521.9 |
| 8 | Ac-Gly | Phe | 22.7 | 40.8 | [M+3H]3+=536.0 | [M+3H]3+=536.0 |
| 9 | CH3OCO | Trp | 26.3 | 43.6 | [M+3H]3+=535.3 | [M+3H]3+=535.3 |
| 10 | C6H5OCH2CO | Trp | 29.3 | 28.0e | [M+3H]3+=561.0 | [M+3H]3+=561.1 |
| 11 | Gly | Trp | 18.9 | 34.0 | [M+3H]3+=535.0 | [M+3H]3+=534.9 |
| 12 | Ac-Gly | Trp | 22.1 | 38.9 | [M+3H]3+=549.3 | [M+3H]3+=549.4 |
| 13 | CH3OCO | Tyr | 24.3 | 41.0 | [M+3H]3+=527.6 | [M+3H]3+=527.7 |
| 14 | CH3OCO | Nal(2′) | 29.3 | 29.1e | [M+3H]3+=539.3 | [M+3H]3+=539.4 |
| 15 | CH3OCO | Cha | 29.0 | 29.4e | [M+3H]3+=524.3 | [M+3H]3+=524.4 |
| 16 | Ac | Tyr | 22.8 | 38.4 | [M+3H]3+=523.0 | [M+3H]3+=522.9 |
| 17 | Ac | Nal(2′) | 28.1 | 28.4e | [M+3H]3+=534.0 | [M+3H]3+=533.9 |
| 18 | Ac | Cha | 27.3 | 27.8e | [M+3H]3+=519.3 | [M+3H]3+=519.3 |
The purity for all of the peptides by both methods was > 98%.
System 1: Solvent A = 0.1% aqueous TFA, solvent B = 0.1% TFA in acetonitrile.
System 2: Solvent A = 0.1% aqueous TFA, solvent B = 0.1% TFA in methanol. The gradient for both systems was 5-50% solvent B over 45 min at 1 mL/min.
Phe instead of NMePhe in position 1.
The gradient was 5-86% solvent B over 45 min at 1 mL/min.
Figure 1.
Structural comparison of Phe, NMePhe, Tyr, Trp, Nal(2′) and Cha
Chemistry
All peptides were synthesized on the Fmoc-PAL-PEG-PS (PAL-PEG-PS = Peptide Amide Linker-poly(ethylene glycol)-polystyrene) resin by solid-phase peptide synthesis (SPPS) using Fmoc-protected amino acids according to standard procedures.28,30 After completion of the peptide assembly and removal of the Fmoc group from the N-terminal residue, different acyl groups were coupled to the N-terminal free amine. After synthesizing the protected peptides on the resin, these peptides were cleaved with Reagent B (88% trifluoroacetic acid (TFA), 5% phenol, 5% water, and 2% triisopropylsilane (TIPS))31 or pure TFA (for compounds 2, 3, and 16-18; see the accompanying article) and purified by reversed-phase high-performance liquid chromatography (HPLC). The purity of the final purified peptides was >98% as verified by two different HPLC solvent systems (MeCN/water and MeOH/water containing 0.1% TFA). The molecular weights of these peptides were verified by electrospray ionization-mass spectrometry (ESI-MS). The analytical data for the peptides is presented in Table 1.
Pharmacology
The affinities of these peptides for rat κ and μ opioid receptors expressed on Chinese hamster ovary (CHO) cells were determined by equilibrium competitive inhibition of the radioligands [3H]diprenorphine and [3H]DAMGO ([D-Ala2,MePhe4,glyol]-enkephalin), respectively32 (Tables 2 and 3). Since all of the arodyn analogs reported to date show very low affinity for δ opioid receptors (Ki > 5 μM), only κ and μ opioid receptor affinities were evaluated.
Table 2.
Opioid receptor affinities and efficacies of arodyn (1) and its N-terminal substituted analogs
X-NMePhe-Phe-R-Arg-Leu-Arg-Arg-D-Ala-Arg-Pro-Lys-NH2a
| Peptide | X | R | Ki ± SEM (nM) | Ki ratio (μ/κ) |
AC % control @ 10 μMb |
|
|---|---|---|---|---|---|---|
| κ | μ | |||||
|
1,
arodyn c |
Acd | Phe | 10.0 ± 3.0 | 1750 ± 130 | 175 | 88 ± 8 |
| 2 e | Ac | Phe | 4.56 ± 0.45 | 5056 ± 790 | 1100 | 86 ± 1 |
| 5 | CH3OCO | Phe | 4.93 ± 0.15 | 1750 ± 100 | 355 | 74 ± 13 |
| 6 | C6H5OCH2CO | Phe | 7.34 ± 1.37 | 1450 ± 190 | 198 | 98 ± 21 |
| 7 | Gly | Phe | 28.9 ± 7.5 | 3070 ± 130 | 106 | 75 ± 8 |
| 8 | Ac-Gly | Phe | 8.04 ± 3.14 | >10,000 | >1240 | 82 ± 17 |
| 3 | Ac | Trp | 13.0 ± 2.6 | 2990 ± 700 | 230 | 105 ± 16 |
| 4 | Acd | Trp | 16.1 ± 2.1 | 1180 ± 290 | 73 | 108 ± 5 |
| 9 | CH3OCO | Trp | 9.52 ± 1.73 | 1280 ± 280 | 134 | 98 ± 13 |
| 10 | C6H5OCH2CO | Trp | 13.3 ± 3.6 | 1250 ± 140 | 94 | > 90 |
| 11 | Gly | Trp | 15.4 ± 4.2 | 502 ± 145 | 33 | 90 ± 8 |
| 12 | Ac-Gly | Trp | 21.9 ± 4.8 | 3760 ± 910 | 172 | 83 ± 7 |
Table 3.
Opioid receptor affinities and efficacy of [NMePhe1]arodyn analogs where position 3 was substituted with other aromatic and non-aromatic amino acids
X-NMePhe-Phe-R-Arg-Leu-Arg-Arg-D-Ala-Arg-Pro-Lys-NH2
| Peptide | X | R | Ki ± SEM (nM) | Ki ratio (μ/κ) |
AC % control @ 10 μMa |
|
|---|---|---|---|---|---|---|
| κ | μ | |||||
| 5 | CH3OCO | Phe | 4.93 ± 0.15 | 1750 ± 100 | 355 | 74 ± 13 |
| 9 | CH3OCO | Trp | 9.52 ± 1.73 | 1280 ± 280 | 134 | 98 ± 13 |
| 13 | CH3OCO | Tyr | 5.80 ± 1.27 | 1300 ± 340 | 224 | 58 ± 10 |
| 14 | CH3OCO | Nal(2′) | 24.4 ± 7.2 | 53.8 ± 13.1 | 2 | 109 ± 10 |
| 15 | CH3OCO | Cha | 3.60 ± 0.21 | 818 ± 253 | 227 | 55 ± 18 |
| 2 b | Ac | Phe | 4.56 ± 0.45 | 5056 ± 790 | 1100 | 86 ± 1 |
| 3 | Ac | Trp | 13.0 ± 2.6 | 2990 ± 700 | 230 | 105 ± 16 |
| 16 | Ac | Tyr | 37.3 ± 16.0 | 3370 ± 570 | 90 | 52 ± 11 |
| 17 | Ac | Nal(2′) | 27.0 ± 7.3 | 121 ± 35 | 4.5 | 101 ± 11 |
| 18 | Ac | Cha | 7.85 ± 2.74 | 5930 ±1920 | 755 | 52 ± 6 |
Relative to the full agonist Dyn A (1-13)NH2 (0% control, 100% inhibition).
From reference 30.
[NMePhe1]arodyn (2) was the most potent and selective arodyn analog in the initial SAR study.30 Substitution of the acetyl group in this compound with a methoxycarbonyl group resulted in compound 5 that has unchanged affinity for κ opioid receptors (Table 2). The μ opioid receptor affinity of this peptide increased by about 3-fold, however, and therefore the selectivity for κ over μ opioid receptors decreased by 3-fold. Compared with 2, κ opioid receptor affinity decreased somewhat when the N-terminal acetyl group of 2 was substituted with other acyl groups (compounds 6-8). Substitution of the N-terminal acetyl group in 2 with the bulky phenoxylacetyl group (compound 6) was well tolerated by κ opioid receptors while increasing μ opioid receptor affinity by 3.5-fold; therefore, the selectivity of 6 for κ opioid receptors is 6-fold less than 2. The largest decrease in κ opioid receptor affinity (6.3-fold) occurred when glycine was substituted for the acetyl group (compound 7), likely as a result of the introduction of the positively charged amine group at the N-terminus. This effect has also been observed for the des-acetyl derivative of arodyn, in which the κ opioid receptor affinity decreased 4-fold.30 The addition of this protonated amine also increases the affinity for μ opioid receptors slightly so that this compound shows only 106-fold selectivity for κ over μ opioid receptors. Acetylation of the N-terminal free amine of 7 to give 8 increased the affinity for κ opioid receptors 3.5-fold and decreased μ opioid receptor affinity (> 3-fold) compared to 7, so that the resulting compound exhibits very high κ opioid receptor selectivity (Ki ratio (μ/κ) > 1240) comparable to 2. This is one of the most selective peptide ligands for κ opioid receptors.
[NMePhe1,Trp3]arodyn (3) shows similar affinity and selectivity for κ opioid receptors as arodyn (1) (Table 2). However, this analog exhibits about 3-fold lower affinity than [NMePhe1]arodyn (2), suggesting that the larger aromatic group in position 3 in this peptide decreases κ opioid receptor affinity. Compared with 3, its Phe1 analog 4 shows similar affinity (Table 2) for κ opioid receptors; however, this compound exhibits about 3-fold higher affinity for μ opioid receptors, resulting in 3-fold lower selectivity for κ opioid receptors than compound 3. These results once again indicate that the N-methyl group on Phe1 increases κ opioid receptor selectivity, possibly due to the effect of methylation on orientation of the acetyl group. When the N-terminal acetyl group in 3 is replaced by a methoxycarbonyl group (compound 9), the affinity at κ opioid receptors increases slightly, but the affinity at μ opioid receptors increases 2-fold, resulting in somewhat lower κ opioid receptor selectivity compared to 3. Substitution of the N-terminal acetyl group with the larger phenoxylacetyl group (compound 10) did not affect κ opioid receptor affinity, but resulted in a 2-fold increase in affinity for μ opioid receptors; therefore, the selectivity of this peptide for κ opioid receptors is also lower than 3. The largest increase in μ opioid receptor affinity occurred when glycine was substituted for the acetyl group (compound 11) as a result of the introduction of the positively charged N-terminal amine group, resulting in a compound with limited selectivity (33-fold) for κ opioid receptors. Acetylation of the free amine in this peptide to give compound 12 decreased μ opioid receptor affinity so that 12 exhibits 5-fold higher κ opioid receptor selectivity than 11.
Thus the effects of the substitution in the two series of compounds (Phe3 and Trp3) are somewhat different. For example, compound 7 shows 6.3-fold lower affinity for κ opioid receptors than the methoxycarbonyl analog 5, while compound 11 has similar κ opioid receptor affinity as its corresponding methoxycarbonyl derivative 9. This substitution also has different effects on μ opioid receptor affinity. Compound 7 shows about 2-fold lower affinity for μ opioid receptors than its methoxycarbonyl analog 5, while compound 11 shows 2-fold higher affinity for μ opioid receptors than the corresponding methoxycarbonyl derivative 9. These results suggest that the residue in position 3 affects the orientation of the N-terminal substitutent and/or N-methyl group in the binding pocket, which in turn affects the pharmacological signature.
Based on these results, the acetyl group in the parent compounds can best be substituted with a methoxycarbonyl group to maintain affinity and selectivity for κ opioid receptors. The methoxycarbonyl group has similar size and other physicochemical properties as the acetyl group, and therefore the biological activity is preserved.
These arodyn analogs were evaluated for their efficacy at 10 μM to inhibit adenylyl cyclase (AC) using cloned rat κ opioid receptors stably expressed on CHO cells.33 The analogs exhibited negligible or low efficacy in this assay (≥74% of the control (≤26% inhibition) compared to Dyn A-(1-13)NH2, Table 2), similar to arodyn.
Stable N-terminal methyloxylcarbonyl analogs with various amino acids in position 3 were then prepared for comparison to the corresponding unstable acetylated NMePhe1 peptides (Table 3). The methoxycarbonyl analogs [CH3OCO-NMePhe1,Tyr3]arodyn (13) and [CH3OCO-NMePhe1,Cha3]arodyn (15) retain similar high affinity for κ opioid receptors as [CH3OCO-NMePhe1]arodyn (5), indicating that replacing Phe in position 3 with the phenolic amino acid Tyr or the saturated residue Cha (Figure 1) is tolerated by κ opioid receptors. [CH3OCO-NMePhe1,Nal(2′)3]arodyn (14), with Phe replaced by the bulky aromatic amino acid Nal(2′) (Figure 1), however, showed a 5-fold decrease in affinity for κ opioid receptors compared to 5. This is a larger decrease than observed for the Trp-containing analog [CH3OCO-NMePhe1,Trp3]arodyn (9). Interestingly, even though [CH3OCO-NMePhe1,Tyr3]arodyn (13) has similar affinity for κ opioid receptors as [CH3OCO-NMePhe1]arodyn (5), this is not the case for the corresponding acetylated analog [MePhe1,Tyr3]arodyn (16) which has 6-fold lower affinity for κ opioid receptors than the corresponding N-terminal methoxycarbonyl analog 15 (Table 3). In contrast, [MePhe1,Nal(2′)3]arodyn (17) has almost the same κ opioid receptor affinity as its methoxycarbonyl analog 14. These results once again suggest that the residue in 3 affects the orientation of the N-terminal substitutent in the binding pocket, which in turn affects receptor affinity.
The κ opioid receptor selectivity of these methoxycarbonyl analogs is not as high as [CH3OCO-MePhe1]arodyn (5, Table 3). [CH3OCO-MePhe1,Nal(2′)3]arodyn (14) unexpectedly shows relatively high μ opioid receptor affinity (Ki = 54 nM), and therefore this compound exhibits minimal selectivity (2-fold) for κ over μ opioid receptors. Similarly, its acetylated analog 17 also has relatively high affinity for μ opioid receptors (Table 3). Unlike the other arodyn analogs, these two peptides are the exceptions that exhibit significant affinity for μ opioid receptors. The bulky hydrophobic aromatic side chain in position 3 appears to be responsible for the increased affinity for μ opioid receptors, with the Nal(2′) peptides exhibiting >20-fold higher affinity for μ opioid receptors than the corresponding Trp-containing peptides. The detailed structural requirements for the μ opioid receptor affinity are currently under investigation in our laboratory.
These arodyn analogs were also evaluated for their efficacy to inhibit AC via κ opioid receptors. The four compounds with either Tyr or Cha in position 3 (13, 15, 16 and 18) exhibited significant efficacy in this assay (<60% control, >40% inhibition of AC relative to Dyn A-(1-13)NH2, Table 3) and are therefore partial agonists for κ opioid receptors.
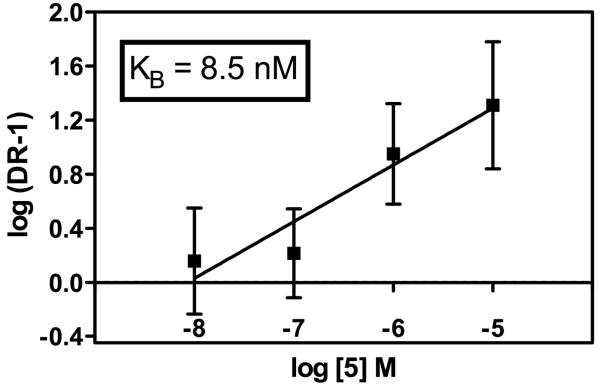
Because of its high affinity and selectivity for κ opioid receptors, [CH3OCO-NMePhe1]arodyn (5) was evaluated for its ability to antagonize Dyn A (1-13)NH2 in the AC assay. Schild analysis34 of peptide 5 vs Dyn A (1-13)NH2 (Figure 2) yielded a KB of 8.5 nM (pA2 = 8.07; 6.97-10.8 95% confidence interval), indicating that it is a potent κ opioid receptor antagonist.
Figure 2.
Schild analysis of peptide 5 as an antagonist of Dyn A-(1-13)NH2 at κ opioid receptors in the adenylyl cyclase assay. DR = dose ratio (EC50 of Dyn A-(1-13)NH2 in the presence of the indicated concentration of 5 divided by the EC50 of Dyn A-(1-13)NH2 in the absence of 5). Data shown are pooled data from three separate experiments.
Conclusions
[NMePhe1]arodyn (2) exhibits high affinity and selectivity for κ opioid receptors, but this peptide is unstable and prone to deletion of the Ac-NMePhe moiety under acidic conditions. By replacing the acetyl group with heteroatom-containing groups, stable analogs can be obtained (see accompanying article). Pharmacological evaluation showed that a methoxycarbonyl group could be substituted for the acetyl group in [NMePhe1]arodyn without loss of κ opioid receptor affinity. [CH3OCO-NMePhe1]arodyn (5) has similar κ opioid receptor affinity as [NMePhe1]arodyn (2) and retains high κ opioid receptor selectivity. For the [NMePhe1]arodyn analogs the N-terminal substituents C6H5OCH2CO and Ac-Gly (peptides 6 and 8, respectively) were well tolerated by κ opioid receptors, with only Gly causing a substantial (6.3-fold) decrease in κ opioid receptor affinity. [Ac-Gly-NMePhe1]arodyn (8) showed extremely high selectivity for κ over μ opioid receptors comparable to that of 2 due to its very low affinity for μ opioid receptors. In the [NMePhe1,Trp3]arodyn series of analogs, substitutents other than CH3OCO resulted in similar κ opioid receptor affinity (within 2-fold) compared to 9. As in the Phe3 series, the selectivity for κ over μ opioid receptors in this series of compounds was generally not as high as when CH3OCO was the N-terminal substitutent, except for [AcGly-NMePhe1,Trp3]arodyn (8), which retained very high κ opioid receptor selectivity comparable to [NMePhe1]arodyn (2).
The N-terminal methoxycarbonyl analogs with other aromatic or non-aromatic amino acids in position 3 generally retained similar affinity for κ opioid receptors as [CH3OCO-NMePhe1]arodyn (5). In contrast, [NMePhe1]arodyn (2) has higher κ opioid receptor affinity than the acetylated analogs where Phe3 was replaced with other aromatic or non-aromatic amino acids (Table 3). The selectivity of the methoxycarbonyl analogs for κ over μ opioid receptors was somewhat lower than 5. [CH3OCO-MePhe1,Nal(2′)3]arodyn (14) and its acetylated analog 17 unexpectedly showed relatively high μ opioid receptor affinity, and therefore they exhibit minimal selectivity for κ over μ opioid receptors (Table 3).
Most of the analogs described here, similar to arodyn, exhibited negligible or low efficacy in the AC assay. The exceptions were the Tyr3 and Cha3 analogs 13, 15, 16, and 18. [CH3OCO-NMePhe1]arodyn (5) antagonized Dyn A-(1-13)NH2 at κ opioid receptors in the AC assay with high potency (KB = 8.5 nM). Thus we have successfully identified stable [NMePhe1]arodyn analogs that are selective for κ opioid receptors and in the case of [CH3OCO-NMePhe1]arodyn (5) acts as a potent κ opioid receptor antagonist. [CH3OCO-NMePhe1]arodyn and selected analogs could be very useful pharmacological tools complementary to the non-peptide κ receptor selective antagonists to study the physiological processes mediated by κ opioid receptors and also as lead peptides for further modification.
Experimental section
Materials
Fmoc-Nal(2′)-OH was purchased from Peptides International (Louisville, KY). Fmoc-Cha-OH was purchased from Calbiochem-Novabiochem (San Diego, CA). The sources of other materials are the same as used in the accompanying manuscript.
Peptide synthesis and cleavage
Peptide Synthesis: All peptides were synthesized on the Fmoc-PAL-PEG-PS resin (0.16-0.24 mmol/g) by standard SPPS methods (see the accompanying article for details). For the the stable analogs, the peptides were cleaved from the resin and deprotected using Reagent B (85% TFA, 5% phenol, 5% water, and 2% triisopropylsilane)31 at room temperature for 2 h. For the unstable analogs (i.e. 2, 3, and 16-18), the peptides were cleaved from the resin using pure TFA without any scavenger at 4 °C for 3 h (see the accompanying article).
Purification and analysis of peptides
The crude peptides were purified by preparative reversed-phase HPLC (Shimadzu HPLC system equipped with a Shimadzu SPD-10A detector) on a Vydac C18 column (10 μm, 300 Å, 21 × 250 mm) equipped with a Vydac guard cartridge. For purification, a linear gradient of 15-50% MeCN containing 0.1% TFA over 35 min, at a flow rate of 20 mL/min, was used. The purification was monitored by UV absorbance at 214 nm.
The purity of the final peptides was verified by analytical HPLC in two different solvent systems using a Shimadzu HPLC system equipped with a Shimadzu SPD-10A detector and a Vydac C18 column (5 μm, 300 Å, 4.6 × 50 mm) equipped with a Vydac guard cartridge. In system 1, a linear gradient of 5-50% solvent B (solvent A, aqueous 0.1% TFA, and solvent B, MeCN containing 0.1% TFA) over 45 min, at a flow rate of 1 mL/min, was used with monitoring at 214 nm. In system 2, a linear gradient of 5-50% solvent B (solvent A aqueous 0.1% TFA, and solvent B MeOH containing 0.1% TFA) over 45 min, at a flow rate of 1 mL/min, was used with monitoring at 230 nm. However, for the more hydrophobic peptides (i.e. 6, 10, 14, 15, 17, and 18), a gradient of 5-86% solvent B (methanol containing 0.1% TFA) over 45 min was used as the second system for analysis. The final purity of all peptides in both analytical systems was >98% (Table 1). Interestingly, the retention times for the methoxycarbonyl analogs were longer than for the corresponding acetyl analogs. The molecular weights of the compounds were determined by ESI-MS (Waters, Q-TOF).
Radioligand binding assays
Radioligand binding assays were performed as previously described using cloned rat κ and μ opioid receptors stably expressed on CHO cells.32 [3H]Diprenorphine and [3H]DAMGO ([D-Ala2,MePhe4,glyol]enkephalin) were used as radioligands in the assays for κ and μ receptors, respectively. Nonspecific binding was determined in the presence of 10 μM unlabeled Dyn A-(1-13)NH2 or DAMGO for κ and μ receptors, respectively. IC50 values were determined by nonlinear regression analysis to fit a logistic equation to the competition data using GraphPad Prism software (GraphPad Software Co., San Diego, CA). Ki values were calculated from the IC50 values by the Cheng and Prusoff equation,35 using KD values of 0.45 and 0.49 nM for [3H]diprenorphine and [3H]DAMGO, respectively. The results presented (Tables 2 and 3) are the mean ± SEM from at least three separate assays.
Adenylyl cyclase assay
The AC assay was performed using cloned rat κ opioid receptors stably expressed on CHO cells as previously described.33 The inhibition of cAMP production was determined for each peptide at 10 μM compared untreated controls and the reference agonist Dyn A-(1-13)NH2; the results presented (Tables 2 and 3) are the mean ± SEM from at least three separate assays.
The antagonist activity of 5 was determined by measuring the EC50 of Dyn A-(13)NH2 in the AC assay33 in the absence and presence of 5 at different concentrations (10 nM-10 μM). The pA2 was determined by Schild analysis.34
Acknowledgements
The authors thank Miranda Thomas and Yanjun Cui in Dr. Thomas Murray’s laboratory for performing the pharmacological assays and Sandra Bartlett at the University of Kansas for helpful discussion and suggestions. The research was supported by grant R01DA018832 from the National Institute on Drug Abuse.
References
- 1.Aldrich JV, Vigil-Cruz SC. In: Burger’s Medicinal Chemistry & Drug Discovery. Abraham DJ, editor. Vol. 6. John Wiley & Sons, Inc.; New York: 2003. pp. 329–481. [Google Scholar]
- 2.Riviere PJM, Junien JL. In: Drug Development, Molecular Targets for GI Diseases. Gaginella TS, Guglietta A, editors. Humana Press; Totowa, NJ: 2000. pp. 203–238. [Google Scholar]
- 3.Pfeiffer A, Brantl V, Herz A, Emrich HM. Science. 1986;233:774–776. doi: 10.1126/science.3016896. [DOI] [PubMed] [Google Scholar]
- 4.Rimoy GH, Wright DM, Bhaskar NK, Rubin PC. Eur J Clin Pharmacol. 1994;46:203–207. doi: 10.1007/BF00192549. [DOI] [PubMed] [Google Scholar]
- 5.Mague SD, Pliakas AM, Todtenkopf MS, Tomasiewicz HC, Zhang Y, Stevens WC, Jr., Jones RM, Portoghese PS, Carlezon WA., Jr. J Pharmacol Exp Ther. 2003;305:323–330. doi: 10.1124/jpet.102.046433. [DOI] [PubMed] [Google Scholar]
- 6.Tsuda M, Suzuki T, Misawa M, Nagase H. Eur J Pharmacol. 1996;307:7–14. doi: 10.1016/0014-2999(96)00219-1. [DOI] [PubMed] [Google Scholar]
- 7.Agmo A, Belzung C. Neuropharmacology. 1998;37:223–232. doi: 10.1016/s0028-3908(98)00003-3. [DOI] [PubMed] [Google Scholar]
- 8.Beardsley PM, Howard JL, Shelton KL, Carroll FI. Psychopharmacology. 2005;183:118–126. doi: 10.1007/s00213-005-0167-4. [DOI] [PubMed] [Google Scholar]
- 9.Carey AN, Borozny K, Aldrich JV, McLaughlin JP. Eur J Pharmacol. 2007;569:84–89. doi: 10.1016/j.ejphar.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rothman RB, Gorelick DA, Heishman SJ, Eichmiller PR, Hill BH, Norbeck J, Liberto JG. J Subst Abuse Treat. 2000;18:277–281. doi: 10.1016/s0740-5472(99)00074-4. [DOI] [PubMed] [Google Scholar]
- 11.Carroll FI, Harris LS, Aceto MD. Eur J Pharmacol. 2005;524:89–94. doi: 10.1016/j.ejphar.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 12.Portoghese PS, Lipkowski AW, Takemori AE. J Med Chem. 1987;30:238–239. doi: 10.1021/jm00385a002. [DOI] [PubMed] [Google Scholar]
- 13.Metcalf MD, Coop A. Drug Addiction. Springer; New York: 2008. pp. 395–431. [Google Scholar]
- 14.Stevens WC, Jones RM, Subramanian G, Metzger TG, Ferguson DM, Portoghese PS. J Med Chem. 2000;43:2759–2769. doi: 10.1021/jm0000665. [DOI] [PubMed] [Google Scholar]
- 15.Thomas JB, Atkinson RN, Rothman RB, Fix SE, Mascarella SW, Vinson NA, Xu H, Dersch CM, Lu YF, Cantrell BE, Zimmerman DM, Carroll FI. J Med Chem. 2001;44:2687–2690. doi: 10.1021/jm015521r. [DOI] [PubMed] [Google Scholar]
- 16.Endoh T, Matsuura H, Tanaka C, Nagase H. Arch Int Pharmacodyn Ther. 1992;316:30–42. [PubMed] [Google Scholar]
- 17.Horan P, Taylor J, Yamamura HI, Porreca F. J Pharmacol Exp Ther. 1992;260:1237–1243. [PubMed] [Google Scholar]
- 18.Negus SS, Mello NK, Linsenmayer DC, Jones R, Portoghese PS. Psychopharmacology. 2002;163:412–419. doi: 10.1007/s00213-002-1038-x. [DOI] [PubMed] [Google Scholar]
- 19.Carroll I, Thomas JB, Dykstra LA, Granger AL, Allen RM, Howard JL, Pollard GT, Aceto MD, Harris LS. Eur J Pharmacol. 2004;501:111–119. doi: 10.1016/j.ejphar.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 20.Wang JB, Johnson PS, Wu JM, Wang WF, Uhl GR. J Biol Chem. 1994;269:25966–25969. [PubMed] [Google Scholar]
- 21.Xue JC, Chen C, Zhu J, Kunapuli S, DeRiel JK, Yu L, Liu-Chen LY. J Biol Chem. 1994;269:30195–30199. [PubMed] [Google Scholar]
- 22.Meng F, Hoversten MT, Thompson RC, Taylor L, Watson SJ, Akil H. J Biol Chem. 1995;270:12730–12736. doi: 10.1074/jbc.270.21.12730. [DOI] [PubMed] [Google Scholar]
- 23.Schlechtingen G, Zhang L, Maycock A, DeHaven RN, Daubert JD, Cassel J, Chung NN, Schiller PW, Goodman M. J Med Chem. 2000;43:2698–2702. doi: 10.1021/jm990442p. [DOI] [PubMed] [Google Scholar]
- 24.Schlechtingen G, DeHaven RN, Daubert JD, Cassel JA, Chung NN, Schiller PW, Taulane JP, Goodman M. J Med Chem. 2003;46:2104–2109. doi: 10.1021/jm020125+. [DOI] [PubMed] [Google Scholar]
- 25.Lu Y, Nguyen TMD, Weltrowska G, Berezowska I, Lemieux C, Chung NN, Schiller PW. J Med Chem. 2001;44:3048–3053. doi: 10.1021/jm0101186. [DOI] [PubMed] [Google Scholar]
- 26.Chavkin C, Goldstein A. Proc Natl Acad Sci USA. 1981;78:6543–6547. doi: 10.1073/pnas.78.10.6543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wan Q, Murray TF, Aldrich JV. J Med Chem. 1999;42:3011–3013. doi: 10.1021/jm9901071. [DOI] [PubMed] [Google Scholar]
- 28.Bennett MA, Murray TF, Aldrich JV. J Med Chem. 2002;45:5617–5619. doi: 10.1021/jm025575g. [DOI] [PubMed] [Google Scholar]
- 29.Vig BS, Murray TF, Aldrich JV. J Med Chem. 2003;46:1279–1282. doi: 10.1021/jm0256023. [DOI] [PubMed] [Google Scholar]
- 30.Bennett MA, Murray TF, Aldrich JV. J Pept Res. 2005;65:322–332. doi: 10.1111/j.1399-3011.2005.00216.x. [DOI] [PubMed] [Google Scholar]
- 31.Sole NA, Barany G. J Org Chem. 1992;57:5399–5403. [Google Scholar]
- 32.Arttamangkul S, Ishmael JE, Murray TF, Grandy DK, DeLander GE, Kieffer BL, Aldrich JV. J Med Chem. 1997;40:1211–1218. doi: 10.1021/jm960753p. [DOI] [PubMed] [Google Scholar]
- 33.Soderstrom K, Choi H, Berman FW, Aldrich JV, Murray TF. Eur J Pharmacol. 1997;338:191–197. doi: 10.1016/s0014-2999(97)81948-6. [DOI] [PubMed] [Google Scholar]
- 34.Schild HO. Br J Pharmacol. 1947;2:189–206. doi: 10.1111/j.1476-5381.1947.tb00336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheng Y, Prusoff WH. Biochem Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]