Abstract
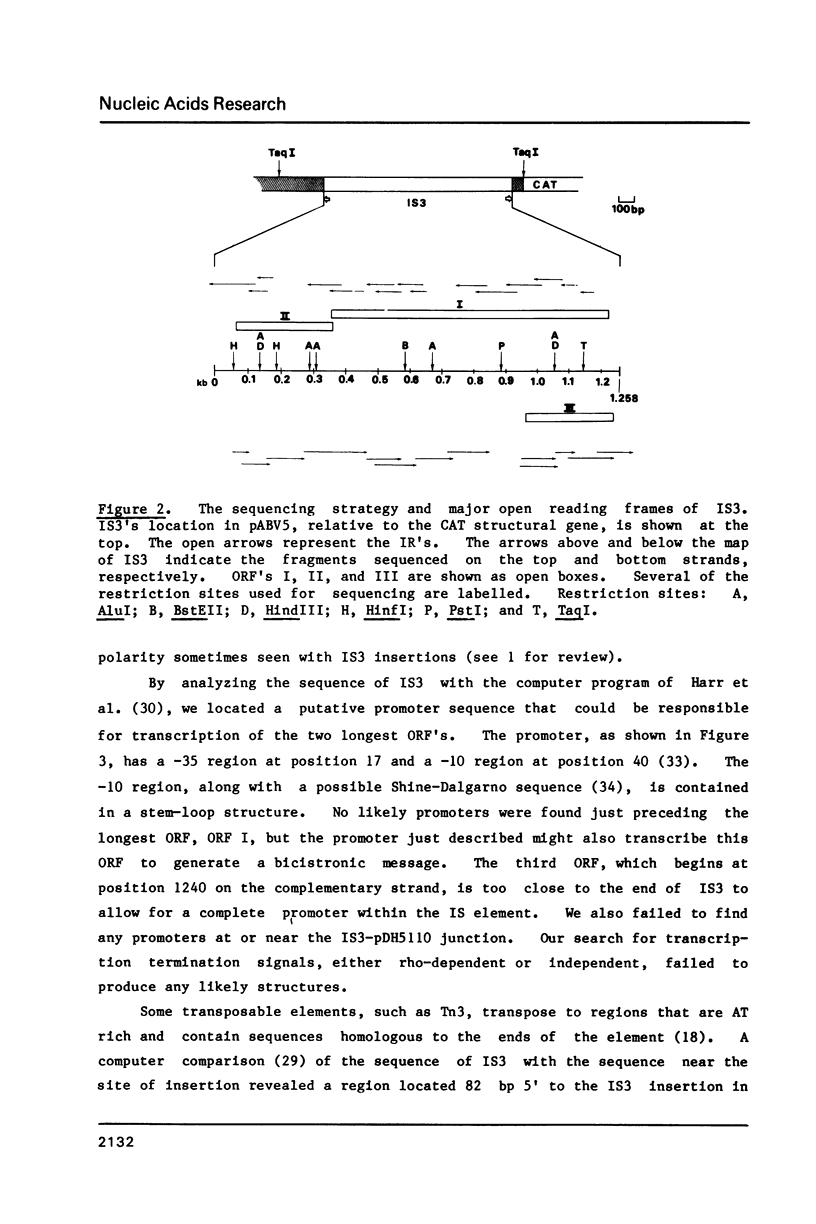
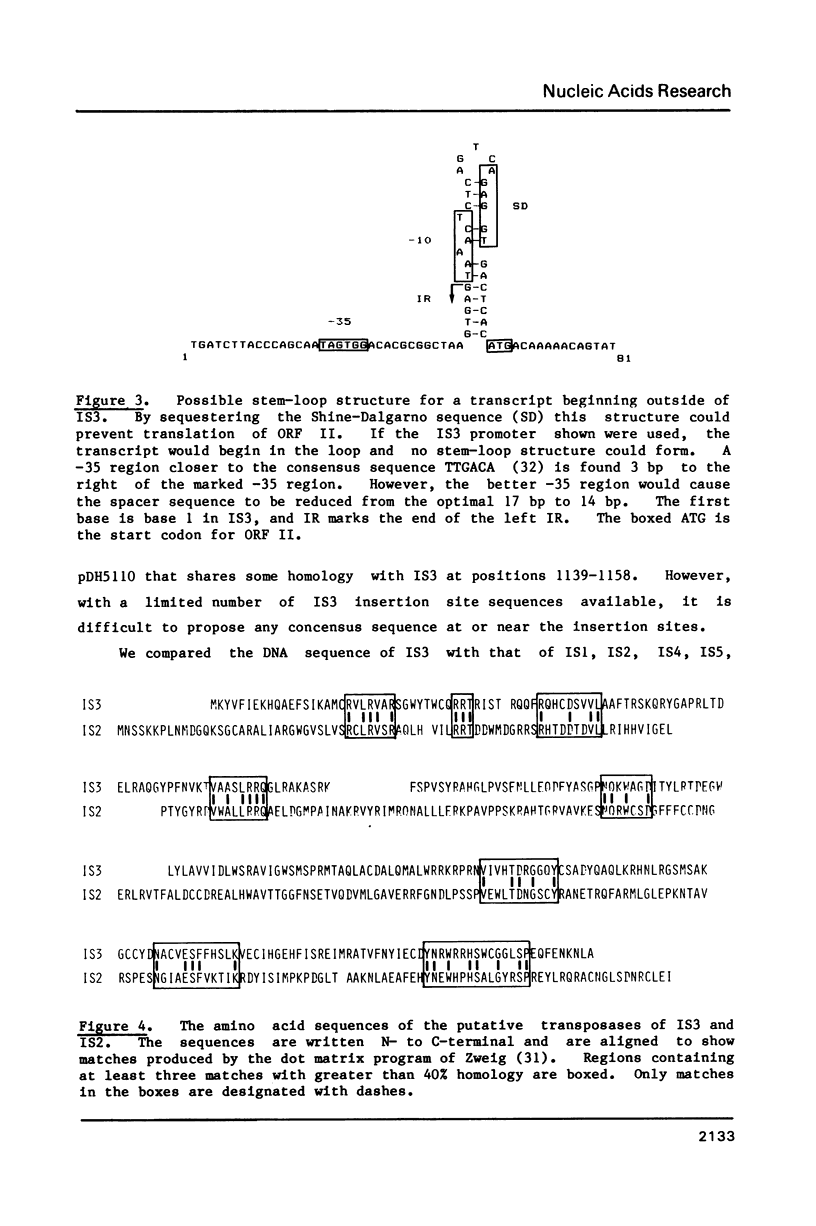
We have determined the nucleotide sequence of IS3. Our IS3 isolate has 39 bp inverted repeats (IR's) with 6 mismatches, and is 1258 bp long. IS3 contains a large open reading frame (ORF) of 288 codons with a smaller, partially overlapping ORF of 91 codons on the opposite strand in codon-codon register. The large ORF is preceded by and has a 4 bp overlap with a 99 codon ORF that has potential transcriptional and translational start signals. Thus, IS3 could encode a bicistronic mRNA. The Shine-Dalgarno sequence for this 99 codon ORF could be sequestered in a stem-loop structure, but only if the transcript began outside IS3, as was first seen with IS10. This could be a means for preventing fortuitous activation of IS3 by outside promoters. No DNA sequence homology was found between IS3 and other prokaryotic IS elements, but there is slight amino acid sequence homology and significant conservation of hydropathicity patterns between the putative transposases of IS3 and IS2.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appleyard R K. Segregation of New Lysogenic Types during Growth of a Doubly Lysogenic Strain Derived from Escherichia Coli K12. Genetics. 1954 Jul;39(4):440–452. doi: 10.1093/genetics/39.4.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerswald E. A., Ludwig G., Schaller H. Structural analysis of Tn5. Cold Spring Harb Symp Quant Biol. 1981;45(Pt 1):107–113. doi: 10.1101/sqb.1981.045.01.019. [DOI] [PubMed] [Google Scholar]
- Baughman G., Nomura M. Localization of the target site for translational regulation of the L11 operon and direct evidence for translational coupling in Escherichia coli. Cell. 1983 Oct;34(3):979–988. doi: 10.1016/0092-8674(83)90555-x. [DOI] [PubMed] [Google Scholar]
- Bernardi A., Bernardi F. Complete sequence of an IS element present in pSC101. Nucleic Acids Res. 1981 Jun 25;9(12):2905–2911. doi: 10.1093/nar/9.12.2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardi F., Bernardi A. Atypical deletions generated by mutated IS102 elements. Mol Gen Genet. 1984;195(3):452–458. doi: 10.1007/BF00341446. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunel F., Heusterspreute M., Merchez M., Ha Thi V., Pilaete M. F., Davison J. Gene rearrangements leading to the expression of an insertion-inactivated tetracycline resistance gene in pBR322. Plasmid. 1983 Mar;9(2):201–214. doi: 10.1016/0147-619x(83)90021-5. [DOI] [PubMed] [Google Scholar]
- Charlier D., Piette J., Glansdorff N. IS3 can function as a mobile promoter in E. coli. Nucleic Acids Res. 1982 Oct 11;10(19):5935–5948. doi: 10.1093/nar/10.19.5935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad B., Mount D. W. Microcomputer programs for DNA sequence analysis. Nucleic Acids Res. 1982 Jan 11;10(1):31–38. doi: 10.1093/nar/10.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalrymple B., Caspers P., Arber W. Nucleotide sequence of the prokaryotic mobile genetic element IS30. EMBO J. 1984 Sep;3(9):2145–2149. doi: 10.1002/j.1460-2075.1984.tb02104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DasSarma S., RajBhandary U. L., Khorana H. G. High-frequency spontaneous mutation in the bacterio-opsin gene in Halobacterium halobium is mediated by transposable elements. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2201–2205. doi: 10.1073/pnas.80.8.2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deonier R. C., Hadley R. G., Hu M. Enumeration and identification of IS3 elements in Escherichia coli strains. J Bacteriol. 1979 Mar;137(3):1421–1424. doi: 10.1128/jb.137.3.1421-1424.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler J. A., van Bree M. P. The nucleotide sequence and protein-coding capability of the transposable element IS5. Gene. 1981 Aug;14(3):155–163. doi: 10.1016/0378-1119(81)90111-6. [DOI] [PubMed] [Google Scholar]
- Fischhoff D. A., Vovis G. F., Zinder N. D. Organization of chimeras between filamentous bacteriophage f1 and plasmid pSC101. J Mol Biol. 1980 Dec 15;144(3):247–265. doi: 10.1016/0022-2836(80)90089-3. [DOI] [PubMed] [Google Scholar]
- Ghosal D., Sommer H., Saedler H. Nucleotide sequence of the transposable DNA-element IS2. Nucleic Acids Res. 1979 Mar;6(3):1111–1122. doi: 10.1093/nar/6.3.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glansdorff N., Charlier D., Zafarullah M. Activation of gene expression by IS2 and IS3. Cold Spring Harb Symp Quant Biol. 1981;45(Pt 1):153–156. doi: 10.1101/sqb.1981.045.01.024. [DOI] [PubMed] [Google Scholar]
- Halling S. M., Simons R. W., Way J. C., Walsh R. B., Kleckner N. DNA sequence organization of IS10-right of Tn10 and comparison with IS10-left. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2608–2612. doi: 10.1073/pnas.79.8.2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harr R., Häggström M., Gustafsson P. Search algorithm for pattern match analysis of nucleic acid sequences. Nucleic Acids Res. 1983 May 11;11(9):2943–2957. doi: 10.1093/nar/11.9.2943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S., Ptashne K., Cohen S. N., Davidson N. alphabeta sequence of F is IS31. J Bacteriol. 1975 Aug;123(2):687–692. doi: 10.1128/jb.123.2.687-692.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaurin B., Normark S. Insertion of IS2 creates a novel ampC promoter in Escherichia coli. Cell. 1983 Mar;32(3):809–816. doi: 10.1016/0092-8674(83)90067-3. [DOI] [PubMed] [Google Scholar]
- Joos H., Timmerman B., Montagu M. V., Schell J. Genetic analysis of transfer and stabilization of Agrobacterium DNA in plant cells. EMBO J. 1983;2(12):2151–2160. doi: 10.1002/j.1460-2075.1983.tb01716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jund R., Loison G. Activation of transcription of a yeast gene in E. coli by an IS5 element. Nature. 1982 Apr 15;296(5858):680–681. doi: 10.1038/296680a0. [DOI] [PubMed] [Google Scholar]
- Klaer R., Kühn S., Tillmann E., Fritz H. J., Starlinger P. The sequence of IS4. Mol Gen Genet. 1981;181(2):169–175. doi: 10.1007/BF00268423. [DOI] [PubMed] [Google Scholar]
- Kleckner N. Transposable elements in prokaryotes. Annu Rev Genet. 1981;15:341–404. doi: 10.1146/annurev.ge.15.120181.002013. [DOI] [PubMed] [Google Scholar]
- Kröger M., Hobom G. Structural analysis of insertion sequence IS5. Nature. 1982 May 13;297(5862):159–162. doi: 10.1038/297159a0. [DOI] [PubMed] [Google Scholar]
- Kupersztoch Y. M., Helinski D. R. A catenated DNA molecule as an intermediate in the replication of the resistance transfer factor R6K in Escherichia coli. Biochem Biophys Res Commun. 1973 Oct 15;54(4):1451–1459. doi: 10.1016/0006-291x(73)91149-2. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Malamy M. H., Fiandt M., Szybalski W. Electron microscopy of polar insertions in the lac operon of Escherichia coli. Mol Gen Genet. 1972;119(3):207–222. doi: 10.1007/BF00333859. [DOI] [PubMed] [Google Scholar]
- Malamy M. H. Frameshift mutations in the lactose operon of E. coli. Cold Spring Harb Symp Quant Biol. 1966;31:189–201. doi: 10.1101/sqb.1966.031.01.027. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McDonell M. W., Simon M. N., Studier F. W. Analysis of restriction fragments of T7 DNA and determination of molecular weights by electrophoresis in neutral and alkaline gels. J Mol Biol. 1977 Feb 15;110(1):119–146. doi: 10.1016/s0022-2836(77)80102-2. [DOI] [PubMed] [Google Scholar]
- Mollet B., Iida S., Shepherd J., Arber W. Nucleotide sequence of IS26, a new prokaryotic mobile genetic element. Nucleic Acids Res. 1983 Sep 24;11(18):6319–6330. doi: 10.1093/nar/11.18.6319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsubo H., Ohtsubo E. Nucleotide sequence of an insertion element, IS1. Proc Natl Acad Sci U S A. 1978 Feb;75(2):615–619. doi: 10.1073/pnas.75.2.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheim D. S., Yanofsky C. Translational coupling during expression of the tryptophan operon of Escherichia coli. Genetics. 1980 Aug;95(4):785–795. doi: 10.1093/genetics/95.4.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rak B., Lusky M., Hable M. Expression of two proteins from overlapping and oppositely oriented genes on transposable DNA insertion element IS5. Nature. 1982 May 13;297(5862):124–128. doi: 10.1038/297124a0. [DOI] [PubMed] [Google Scholar]
- Riggs D., Artz S. The hisD-hisC gene border of the Salmonella typhimurium histidine operon. Mol Gen Genet. 1984;196(3):526–529. doi: 10.1007/BF00436203. [DOI] [PubMed] [Google Scholar]
- Saedler H., Reif H. J., Hu S., Davidson N. IS2, a genetic element for turn-off and turn-on of gene activity in E. coli. Mol Gen Genet. 1974;132(4):265–289. doi: 10.1007/BF00268569. [DOI] [PubMed] [Google Scholar]
- Sanger F., Air G. M., Barrell B. G., Brown N. L., Coulson A. R., Fiddes C. A., Hutchison C. A., Slocombe P. M., Smith M. Nucleotide sequence of bacteriophage phi X174 DNA. Nature. 1977 Feb 24;265(5596):687–695. doi: 10.1038/265687a0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R. The use of thin acrylamide gels for DNA sequencing. FEBS Lett. 1978 Mar 1;87(1):107–110. doi: 10.1016/0014-5793(78)80145-8. [DOI] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. The 3'-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebenlist U., Simpson R. B., Gilbert W. E. coli RNA polymerase interacts homologously with two different promoters. Cell. 1980 Jun;20(2):269–281. doi: 10.1016/0092-8674(80)90613-3. [DOI] [PubMed] [Google Scholar]
- Simsek M., DasSarma S., RajBhandary U. L., Khorana H. G. A transposable element from Halobacterium halobium which inactivates the bacteriorhodopsin gene. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7268–7272. doi: 10.1073/pnas.79.23.7268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer H., Cullum J., Saedler H. Integration of IS3 into IS2 generates a short sequence duplication. Mol Gen Genet. 1979;177(1):85–89. doi: 10.1007/BF00267256. [DOI] [PubMed] [Google Scholar]
- Trinks K., Habermann P., Beyreuther K., Starlinger P., Ehring R. An IS4-encoded protein is synthesized in minicells. Mol Gen Genet. 1981;182(2):183–188. doi: 10.1007/BF00269656. [DOI] [PubMed] [Google Scholar]
- Tu C. P., Cohen S. N. 3'-end labeling of DNA with [alpha-32P]cordycepin-5'-triphosphate. Gene. 1980 Jul;10(2):177–183. doi: 10.1016/0378-1119(80)90135-3. [DOI] [PubMed] [Google Scholar]
- Walz A., Ratzkin B., Carbon J. Control of expression of a cloned yeast (Saccharomyces cerevisiae) gene (trp5) by a bacterial insertion element (IS2). Proc Natl Acad Sci U S A. 1978 Dec;75(12):6172–6176. doi: 10.1073/pnas.75.12.6172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W. L., Doolittle W. F. Structure of the archaebacterial transposable element ISH50. Nucleic Acids Res. 1983 Jun 25;11(12):4195–4199. doi: 10.1093/nar/11.12.4195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T., Tamura T., Yokota T., Takano T. Overlapping genes in the heat-labile enterotoxin operon originating from Escherichia coli human strain. Mol Gen Genet. 1982;188(2):356–359. doi: 10.1007/BF00332701. [DOI] [PubMed] [Google Scholar]
- Yousaf S. I., Carroll A. R., Clarke B. E. A new and improved method for 3'-end labelling DNA using [alpha-32P]ddATP. Gene. 1984 Mar;27(3):309–313. doi: 10.1016/0378-1119(84)90075-1. [DOI] [PubMed] [Google Scholar]
- Zweig S. E. Analysis of large nucleic acid dot matrices on small computers. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 2):767–776. doi: 10.1093/nar/12.1part2.767. [DOI] [PMC free article] [PubMed] [Google Scholar]


