Abstract
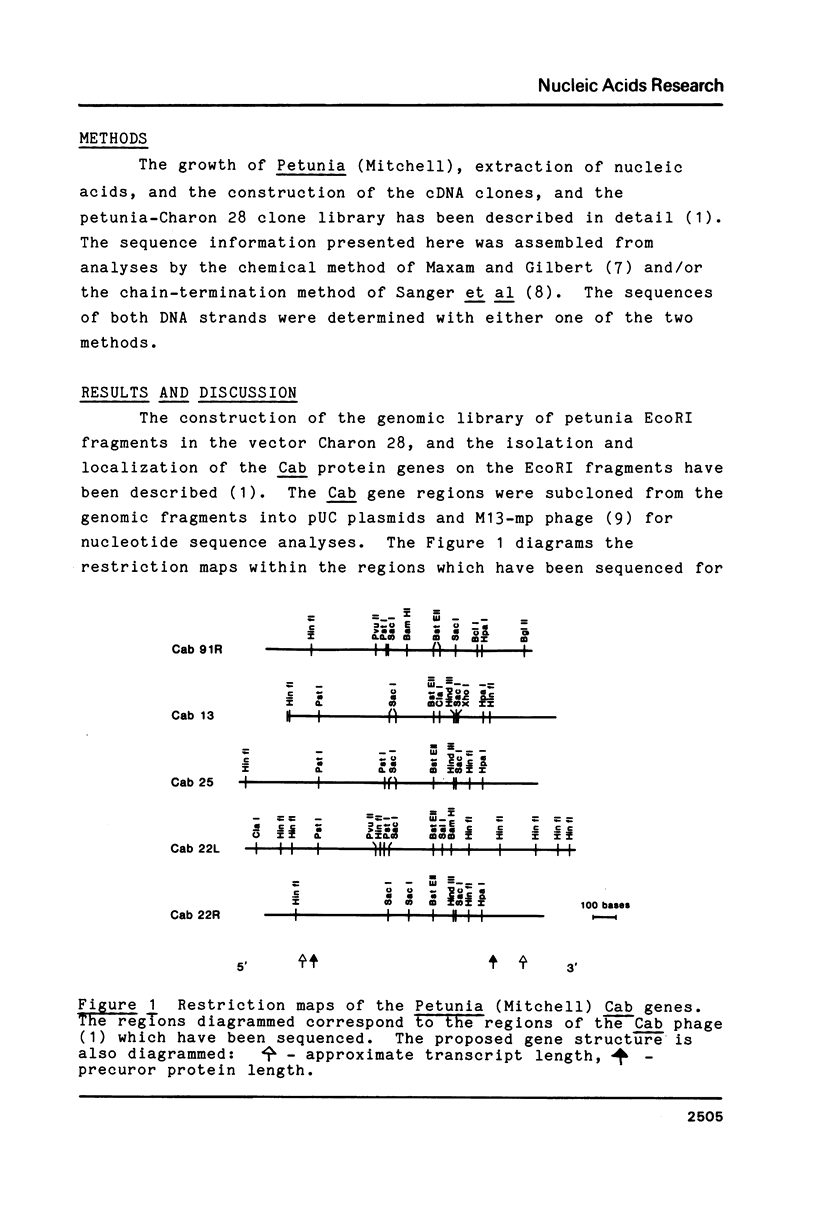
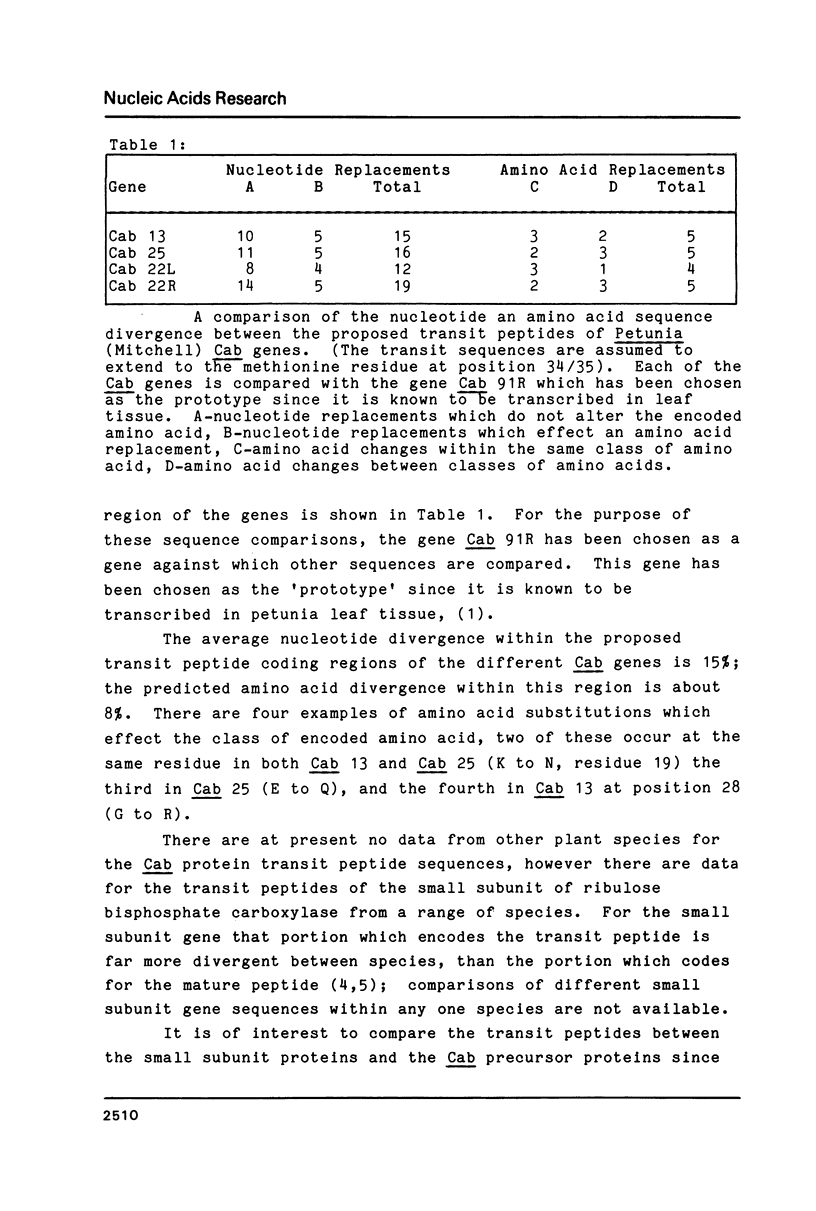
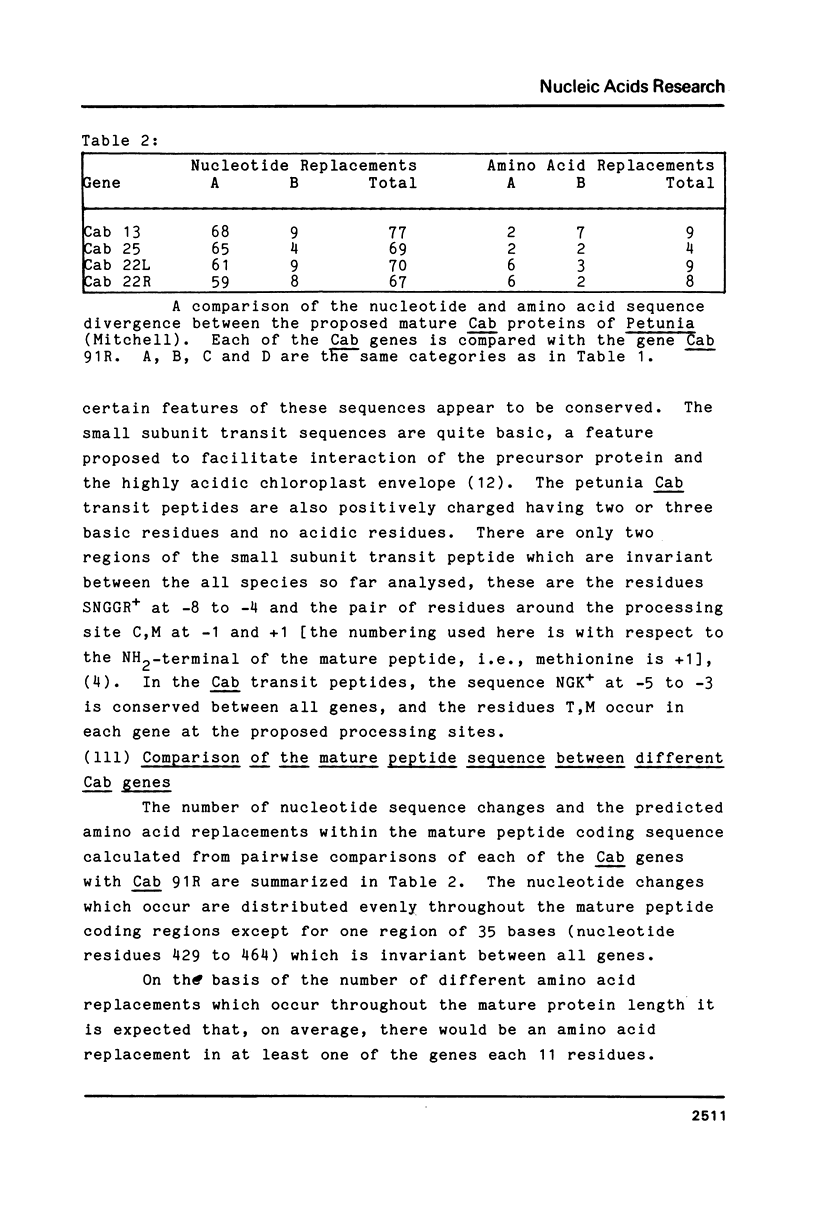
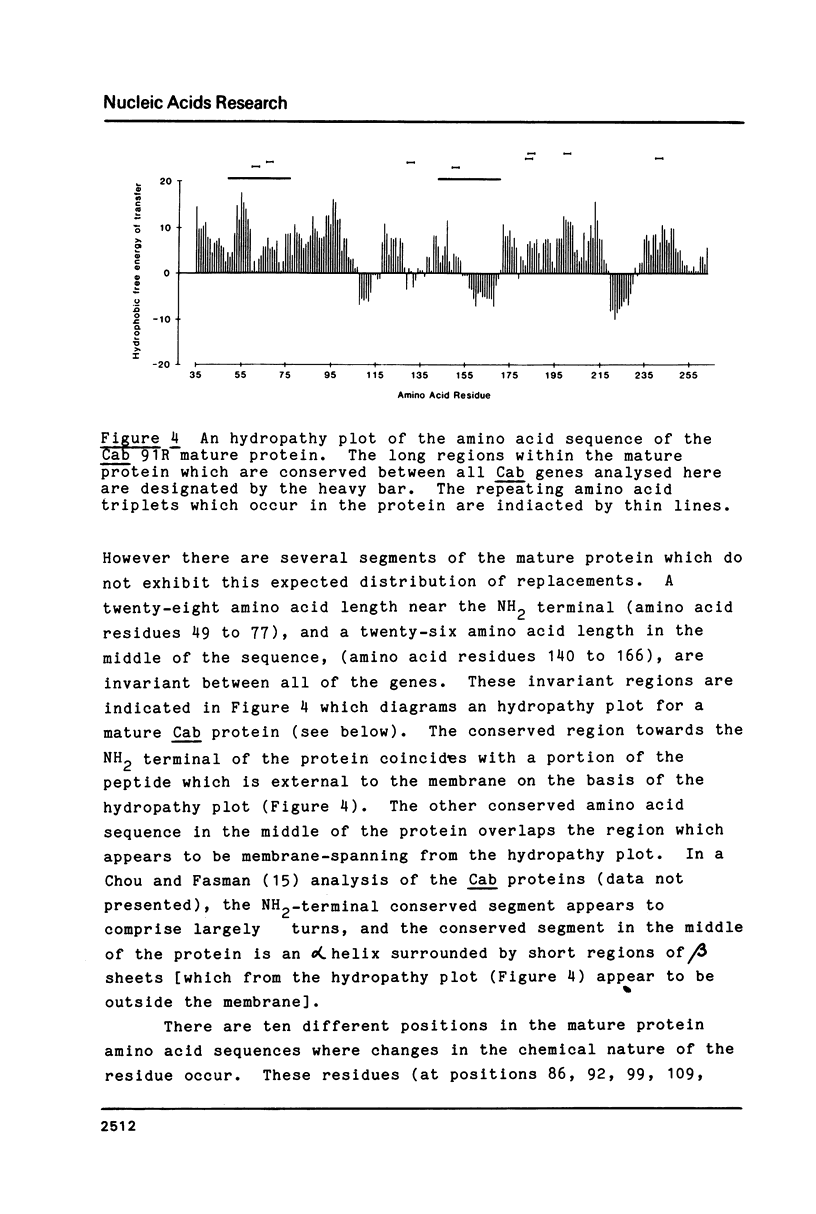
In Petunia (Mitchell) there are at least 16 genes which encode the chlorophyll a/b binding proteins; these genes have been classified into small multigene families based upon nucleotide sequence homology (1). A gene from each of five distinct Cab gene families is compared here. These genes have uninterrupted open reading frames of 266 or 267 amino acids corresponding to the Cab precursor proteins of sizes around 32000 daltons. A comparison of the amino acid sequences deduced here with published information from direct NH2-terminal analysis of a mature Cab protein in pea (10) suggests that a 34-36 amino acid transit peptide is cleaved from the NH2-terminal of the petunia precursor proteins. The proposed transit peptide sequences are more divergent than the mature peptide sequences between the Cab genes from different gene families. There are two regions within the mature Cab proteins which are conserved between all genes--a sequence of 28 amino acids near the NH2 terminal, and another sequence of 26 amino acids in the middle of the protein. The DNA sequences proximal to the Cab coding regions contain typical eukaryote promoter elements--TATA and CCAAT boxes, and in addition those genes which are known to be expressed in petunia leaf tissue also have an extensive region of homology (48 nucleotides) centered at approximately 130 nucleotides from the proposed transcription start sites.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berry-Lowe S. L., Mc Knight T. D., Shah D. M., Meagher R. B. The nucleotide sequence, expression, and evolution of one member of a multigene family encoding the small subunit of ribulose-1,5-bisphosphate carboxylase in soybean. J Mol Appl Genet. 1982;1(6):483–498. [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Empirical predictions of protein conformation. Annu Rev Biochem. 1978;47:251–276. doi: 10.1146/annurev.bi.47.070178.001343. [DOI] [PubMed] [Google Scholar]
- Chua N. H., Schmidt G. W. Post-translational transport into intact chloroplasts of a precursor to the small subunit of ribulose-1,5-bisphosphate carboxylase. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6110–6114. doi: 10.1073/pnas.75.12.6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coruzzi G., Broglie R., Cashmore A., Chua N. H. Nucleotide sequences of two pea cDNA clones encoding the small subunit of ribulose 1,5-bisphosphate carboxylase and the major chlorophyll a/b-binding thylakoid polypeptide. J Biol Chem. 1983 Feb 10;258(3):1399–1402. [PubMed] [Google Scholar]
- Dunsmuir P., Smith S. M., Bedbrook J. The major chlorophyll a/b binding protein of petunia is composed of several polypeptides encoded by a number of distinct nuclear genes. J Mol Appl Genet. 1983;2(3):285–300. [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Nevins J. R. The pathway of eukaryotic mRNA formation. Annu Rev Biochem. 1983;52:441–466. doi: 10.1146/annurev.bi.52.070183.002301. [DOI] [PubMed] [Google Scholar]
- Ryrie I. J., Anderson J. M., Goodchild D. J. The role of the light-harvesting chlorophyll a/b protein complex in chloroplast membrane stacking. Cation-induced aggregation of reconstituted proteoliposomes. Eur J Biochem. 1980 Jun;107(2):345–354. doi: 10.1111/j.1432-1033.1980.tb06035.x. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt G. W., Bartlett S. G., Grossman A. R., Cashmore A. R., Chua N. H. Biosynthetic pathways of two polypeptide subunits of the light-harvesting chlorophyll a/b protein complex. J Cell Biol. 1981 Nov;91(2 Pt 1):468–478. doi: 10.1083/jcb.91.2.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiekema W. J., Wimpee C. F., Tobin E. M. Nucleotide sequence encoding the precursor of the small subunit of ribulose 1,5-bisphosphate carboxylase from Lemna gibba L.G-3. Nucleic Acids Res. 1983 Nov 25;11(22):8051–8061. doi: 10.1093/nar/11.22.8051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. On the hydrophobic nature of signal sequences. Eur J Biochem. 1981 May 15;116(2):419–422. doi: 10.1111/j.1432-1033.1981.tb05351.x. [DOI] [PubMed] [Google Scholar]


