Abstract
Primary ovarian insufficiency (POI) is a critical fertility defect characterized by an anticipated and silent impairment of the follicular reserve, but its pathogenesis is largely unexplained. The frequent maternal inheritance of POI together with a remarkable dependence of ovarian folliculogenesis upon mitochondrial biogenesis and bioenergetics suggested the possible involvement of a generalized mitochondrial defect. Here, we verified the existence of a significant correlation between blood and ovarian mitochondrial DNA (mtDNA) content in a group of women undergoing ovarian hyperstimulation (OH), and then aimed to verify whether mtDNA content was significantly altered in the blood cells of POI women. We recruited 101 women with an impaired ovarian reserve: 59 women with premature ovarian failure (POF) and 42 poor responders (PR) to OH. A Taqman copy number assay revealed a significant mtDNA depletion (P<0.001) in both POF and PR women in comparison with 43 women of similar age and intact ovarian reserve, or 53 very old women with a previous physiological menopause. No pathogenic variations in the mitochondrial DNA polymerase γ (POLG) gene were detected in 57 POF or PR women with low blood mtDNA content. In conclusion, blood cell mtDNA depletion is a frequent finding among women with premature ovarian aging, suggesting that a still undetermined but generalized mitochondrial defect may frequently predispose to POI which could then be considered a form of anticipated aging in which the ovarian defect may represent the first manifestation. The determination of mtDNA content in blood may become an useful tool for the POI risk prediction.
Introduction
Primary ovarian insufficiency (POI) is a critical fertility defect characterized by an anticipated impairment of the follicular reserve [1], [2]. This process is generally silent without evident menstrual irregularity so that women are diagnosed with POI due to the premature cessation of menses (secondary amenorrhea, SA) before 40 years of age in the stage of complete follicular depletion (premature ovarian failure, POF or overt POI).
The cause of POI remains obscure in most women [3], [4]. A strong genetic component in idiopathic POI pathogenesis is revealed by the frequent familiarity (30–40% of total cases) for this disease [5], with mothers and daughters experiencing a premature ovarian aging and an anticipation of menopause. Among the familial cases, the maternal inheritance of the ovarian defect is prevalent (about 60%) [5], [6]. This observation together with the known association of POF with X-chromosomal abnormalities have prompted studies on the possible involvement of X-linked genes in POI pathogenesis [4], [7], [8]. However, the mitochondrial material of the zygote has a maternal origin and also mitochondrial diseases may have a maternal transmission. Noteworthy, mitochondrial defects are strongly associated with physiological or pathological tissue aging, and can lead to altered metabolism, impaired activity and/or accelerated degeneration [9], [10].
Mitochondrial biogenesis and bioenergetics play an essential role in oocyte maturation and embryo development [11], [12]. Mature oocytes require one of the highest number of mitochondria among all tissues in the body, and mitochondrial DNA copy number per oocyte has been shown to be strictly associated to the probability to develop a zygote [13], [14]. In line with these findings, intracytoplasmatic transfer of mitochondria markedly improves the chances of pregnancy [12], [13]. In addition, a marked mitochondrial depletion in the oocyte has been observed in women with poor recoveries of fertilizable oocytes after ovarian hyperstimulation [15] and, importantly, also in cases of ovarian insufficiency [16]. In addition, it has been reported that granulosa cells from women above 38 years of age have a diminished number of mitochondria when compared to those from younger fertile women [17]. The whole of these data indicate that a reduction in the number of intact mitochondria correlates with ovarian aging thus suggesting that a weakened respiratory chain activity may be involved in the anticipated impairment of ovarian reserve.
A correlation between mitochondrial diseases and POI has been observed both in animals and in humans [18]–[21]. In particular, germline mutations in the mitochondrial DNA (mtDNA) polymerase-γ (POLG; MIM 174763) gene cause a generalized mtDNA replication impairment and mitochondrial depletion and are associated with phenotypes variably including neurological and muscular defects, diabetes and POI.
The aim of the present study was to verify whether the content of mtDNA is significantly reduced in the blood cells of women with a prematurely impaired ovarian reserve. Therefore, we verified the possible existence of a correlation between blood and ovarian mtDNA content and then evaluated mtDNA content in peripheral blood cells of women with POF or an anticipated impairment of their ovarian reserve and in two control groups: one constituted by women with intact ovarian reserve and the second by very old women reporting a physiological menopause. Since responsiveness to ovarian hyper-stimulation is currently believed to be the most appropriate surrogate way to assess ovarian reserve [22], women belonging to the impaired and intact ovarian reserve groups were recruited among patients undergoing in vitro fertilization (IVF) cycles.
Materials and Methods
Approval for the study was obtained by the local Institution review board and all subjects gave their informed consent for granulosa cells (GCs) and/or blood sampling and genetic analysis.
Subjects
In a subgroup of 11 women undergoing in vitro fertilization (IVF) protocols we obtained both GCs and blood cells in order to determine the existence of a possible correlation between ovarian and blood mtDNA content. Then, blood samples were obtained in three groups of women of comparable young age and one group of old women with physiological menopause (Table 1). The first group was constituted by 59 women with idiopathic POF,17 with primary and 42 with secondary amenorrhea and FSH serum levels exceeding 40 IU/L on at least two determinations [23]. The other 2 groups were selected among women undergoing ovarian hyperstimulation for IVF cycles. One group was constituted by poor responder women developing few co-dominant follicles and retrieving few (<5) oocytes despite elevated gonadotropin dosages (>300 IU per day) (PR; n = 42). The other was the group of control women, selected among those undergoing ovarian hyperstimulation using gonadotropin dosages ≤250 IU and retrieving ≥5 oocytes (Normal Responders, NR, n = 43). In both of these groups the main indication for IVF were male and tubal factors (88% in NR and 74% in PR). Finally another group is represented by very old control women with physiological menopause beyond 48 years of age (CPM, n = 53). Patients with ovarian cysts and/or those who were operated for ovarian cysts were excluded from all groups. All patients were of Caucasian origin.
Table 1. Anagraphical, clinical and biochemical parameters in the four groups of subjects.
| Parameters | Group 1: POF (n = 59) | Group 2: PR (n = 42)(<5 eggs retrieved) | Group 3: NR (n = 43)(≥5 eggs retrieved) | Group 4: CPM (n = 53) |
| Age in years at blood samplingmean ±SE (range) | 29.2±1.8* (15–41) | 34.4±2.6 (27–39) | 33±2.9 (27–38) | 90±1.3 (82–105) |
| Age in years at menopausemean ±SE (range) | 28.6±1.7 (14–39) | – | – | 50.9±0.3 (48–58) |
| FSH (U/L) mean ±SE (range) | 94.8±6.2* (30.2–160.0) | 16.2±9.8 (5.1–53.0) | 6.7±2.0 (1.9–12.7) | 88.3±6.4 (69.0–105.2) |
| Ovarian volume (ml) | 4.2±2.9* | 5.4±2.8** | 8.0±3.7 | – |
| AFCa | 0–1* | 2.7±1.3** | 7.3±2.7 | – |
Antral Follicle Count per ovary.
p<0.03 vs PR and NR.
p<0.05 vs NR.
A blood sample was obtained from selected patients prior to the initiation of medical treatments (ovarian hyper-stimulation or hormone replacement therapy). Selection of patients with compromised ovarian reserve and controls was initially based on the outcome of previous ovarian hyperstimulation cycles, the antral follicle count (AFC on both ovaries <8) and the hormonal tests (day 3 serum FSH >12 U/L). The appropriateness of their inclusion was confirmed after the treatment cycle.
MtDNA Determination in Circulating Blood Cells
Whole blood samples for the determination of mtDNA content were collected in EDTA-containing tubes. In these samples, the hemogram parameters including white blood cell (NR:6,074±1,352; PR:6,116±1,320; POF: 7,433±1,783; CPM: 5,917±1,441) and platelet (NR:212,040±56,350; PR:219,036±52,389; POF:211,571±41,279; CPM: 213,844±61,953) counts were similar in the different groups, a finding against the potential interference by significant variations in platelet count. The blood samples were immediately stored at −20°C and thawed simultaneously for mtDNA content determination. Total genomic DNA was isolated from whole-blood specimens by the Wizard Genomic DNA Purification Kit (Promega). MtDNA content was determined utilizing a quantitative real-time PCR (QPCR) by the Taqman method (Applied Biosystem 9700 HT Sequention Detection System) similarly to previously described protocols [24]. Specific probes were used to amplify a fragment of the mitochondrial D-loop region, Hs02596861_s1 MT-7S (Applied Biosystems), or a fragment of the nuclear genomic RNAse P, 4316844 RNAse P VIC (Applied Biosystems). PCR reactions were performed according to standard conditions for Taqman (Applied Biosystems): 50°C for 2′; 95°C for 10′; 40 cycles at 95°C 15′′, 60°C 1′. PCR assays were performed in triplicate for each DNA sample. Real-time PCR efficiencies were calculated from the given slopes in LightCycler software. The corresponding real-time PCR efficiencies (E) of one cycle in the exponential phase was calculated according to the equation: E = 10[−1/slope]. Investigated genes showed high real-time PCR efficiency rates; for D-Loop, 1.90 and RNAse P, 1.89 in the investigated range from 0.40 to 100 ng DNA input (n = 5) with high linearity (Pearson correlation coefficient r>0.98). We then determined the expression of mtDNA copy number relative to nuclear DNA using the efficiency correction method as described by Pfaffl et co [25]:
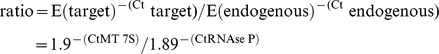 |
The primary endpoint of the study was the mtDNA content evaluation in women with intact and compromised ovarian reserve. Assumptions to determine the sample size included a type I and II error of 0.05 and 0.20, respectively.
Analysis of Mitochondrial DNA Polymerase γ (POLG) Gene
POLG gene was analyzed by PCR amplification using intronic primers as previously described [26], and subsequently sequenced by automated nucleotide sequencing with the Big Dye terminator Ready Reaction Kit version 3.1 on a 3100 Genetic analyzer automatic sequencer (Applied Biosystem, CA, USA). The analysis was limited to the exons 7,8,17,18,21 on the basis of the reported mutations in the POLG gene in association with POI [19], [21], [27], [28].
Results
In 11 women undergoing IVF protocols, the blood cell mtDNA/nDNA copy number correlated significantly (p = 0.008) with that of their GCs (Figure 1).
Figure 1. Correlation between peripheral blood and ovarian granulosa cell (GC) mitochondrial DNA content.
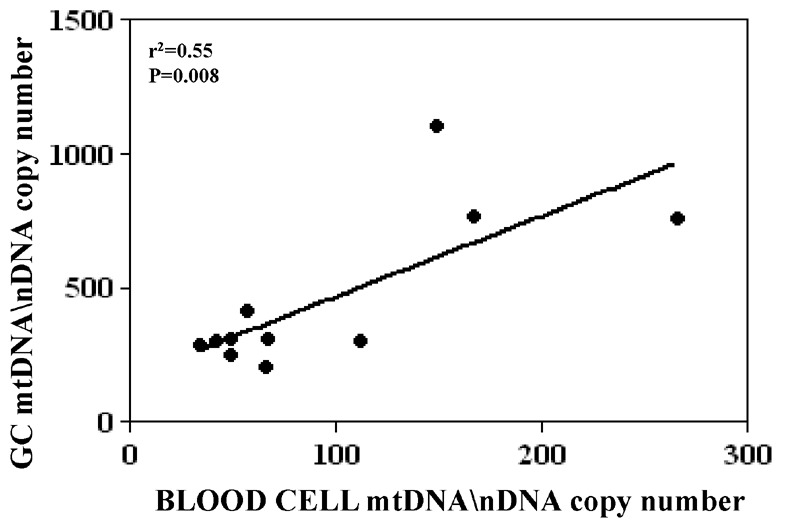
Total DNA isolated from whole blood and GCs of a total of 11 women has been quantified by real-time quantitative PCR analysis using the RNAse P as an endogenous control. Data were analyzed by using comparative Ct method [25] and are expressed as relative quantification of mitochondrial on nuclear DNA copy numbers (mtDNA/nDNA). Regression analysis was obtained by GraphPad Prism 5.0.
Age and BMI of NR, PR and POF women were comparable, though POF women tended to be younger (Table 1). CPM women were instead very old, 90±1.3 years, but had a BMI, 25±4 kg/ m2, similar to that of the other groups. Consistently with diagnosis, POF women had smaller ovaries without antral follicles in most cases. Antral follicle counts (AFC) and ovary volumes at ultrasound were lower in PR women, as compared to NR women.
Each DNA sample was run in at least three distinct Taqman assays in triplicate. Intra- and inter-assay coefficients of variability were <9.6% and <15.2%, respectively. Kurtosis and skewness indexes of the content in mtDNA were consistent with a normal distribution within the groups but variances differed. Data were thus compared using ANOVA test and Dunnett (T3) post-hoc test. The mean ±SE of the mtDNA/nDNA copy number was significantly lower in women with an impaired ovarian reserve (POF: 28.65±1.8; PR: 52.4±6.6; p<0.0001), in comparison to control women with an intact ovarian reserve (NR: 176.0±18.4) (Figure 2). In addition, the mtDNA/nDNA copy number was significantly higher in NR versus CPM (CPM: 69.6±3.1) (p<0.0001) and in CPM versus POF women (p<0.01). Instead, the Dunnett (T3) post-hoc test showed statistically significant differences for all comparisons, including PR versus POF (p = 0.005) or CPM versus PR (p = 0.014).
Figure 2. Relative quantification of mitochondrial on nuclear DNA copy number (mtDNA/nDNA) in peripheral blood cells.
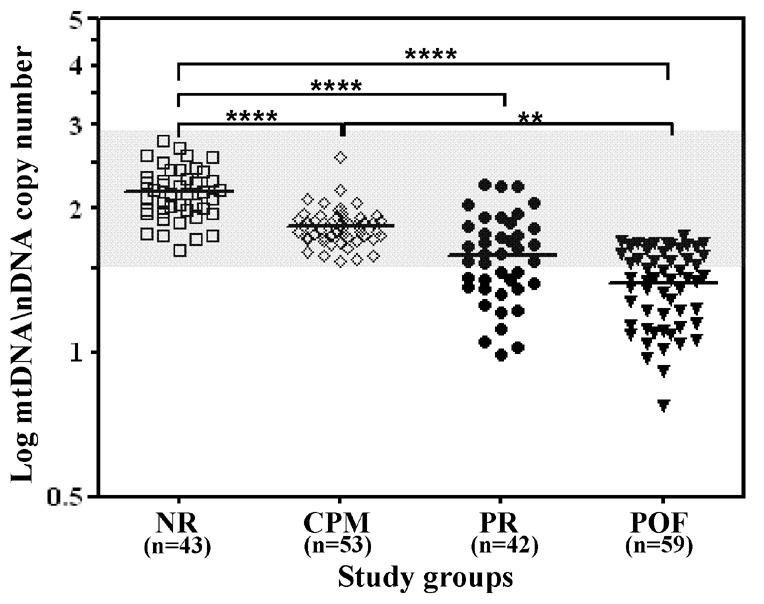
Total DNA isolated from whole blood has been quantified by real-time quantitative PCR analysis using the RNAse P as an endogenous control. The PCR data were analyzed by using a comparative Ct method [25] and values are here expressed as the Logarithm of mtDNA/nDNA copy number. Mean value for each group is graphically indicated by a line. The grey area is indicating the range observed in the NR group. The significant differences detected by one-way ANOVA test are indicated in the figure (****p<0.0001; **p<0.01).
Considering the range of mtDNA/nDNA copy number observed in CPM women, lower values were found in 0/43 (0%) NR women, 19/42 (45%) PR and 38/59 (64%) POF. The analysis of POLG gene did not reveal any pathogenic variation in the 57 (38 POF and 19 PR) women with a mtDNA/nDNA copy number below the lower limit observed in the control groups.
Discussion
In this study, we observed a significant correlation between ovarian and blood mtDNA content and then found a diminished mtDNA copy number in peripheral blood cells of women with an impaired ovarian reserve compared to controls. A biological grading clearly emerged showing the lowest levels in women with POF and the highest in those with conserved ovarian function. Interestingly, an intermediate level of mtDNA/nDNA copy number was found in women with a poor response to ovarian hyperstimulation (PR). The control groups of the present study consisted of women with a normal response to ovarian hyperstimulation (NR) and very old women with physiological menopause (CPM). Since aging is a condition associated with a progressive and generalized mitochondrial depletion [29]–[31], it is reasonable that mean mtDNA/nDNA copy number in old CPM women was found significantly lower than in young NR ones. However, the mtDNA amount of CPM women was always included within the range observed in NRs and significantly higher than that of young women with a premature impairment of their ovarian reserve. Therefore, a still undetermined mitochondrial DNA damage might represent a defect predisposing to premature ovarian insufficiency.
Since the reduction of the mtDNA/nDNA copy number was seen in the blood cells of subjects with compromised ovarian reserve and POF this defect appears as an intrinsic feature of the women. Though the defects of mitochondrial metabolism cause a wide range of human diseases affecting several tissues [32], the affected women in this study did not report any systemic manifestation at enrolment. One possible explanation for this incongruity is that the entity of the defect detected in this study is detrimental in the ovary while being tolerable for the other tissues. There is indeed evidence suggesting that biochemical dysfunction and consequent systemic manifestations occur only below a threshold level [32], [33] and ovarian tissue may be particularly susceptible to the risks associated with a relative mitochondrial DNA depletion. Consistently, folliculogenesis would require the bioenergetic support of a large number of mitochondria in both germinal and granulosa cells [16], [17]. Ultrastructural studies in human germinal cells showed that the number of mitochondria dramatically increases from about 10 in primordial germ cells to more than 100,000 in mature oocytes [34], [35]. This phenomenon correlating with a progressive increment of respiratory chain activity and oxygen consumption during follicle maturation [36]–[38]. In addition, Luoma et al. reported a relevant incidence of POF in women with POLG mutations, a protein essential for mtDNA replication [19]. Pagnamenta et al. recently confirmed this finding [21] and suggested to screen POF women for possible genetic mechanisms of mtDNA depletion even in the absence of evident manifestations such as the progressive external ophtalmoplegia (PEO). However, we could not find any pathogenic variations of POLG gene in POF or PR women with impaired mtDNA/nDNA copy number, thus suggesting that other mechanisms cause the mitochondrial DNA depletion in these women.
Based on these findings, POI might be interpreted as a condition of anticipated aging in which the ovarian defect may represent the first manifestation. In contrast, fertility beyond the age of 40 might be associated with longevity. Accordingly, centenarian women have been described to be four times more likely to have had children while in their forties than 73 year-old women [39]. This finding was recently confirmed by a comparative study of women from three large historical databases [40]. Interestingly, also the relatives of old mothers were shown to survive longer indicating the involvement of inheritable traits [41]. Our findings may then represent a suitable explanation for the correlation between late female fertility and longevity, and may also have implications regarding the theoretical basis of menopause and human lifespan. In line with this view stands the fact that a decreased fertility generally occurs before death in all female animals, and only human females go into menopause nowadays 30–50 years before death thanks to the recent progress of preventive medicine and therapeutics. These observations in humans are consistent with selection experiments in Drosophila in which the ability to produce eggs later in life is correlated with greater life expectancy [42].
Here, the difference in mtDNA content between POF/PR women and NR control women of similar age was highly significant with overlaps that were in several assays limited to about half of the cohorts. Since menopause has been repeatedly shown to arise earlier in women with a reduced responsiveness to ovarian hyperstimulation [43]–[45], blood cell mtDNA/nDNA copy number determination might represent a valuable test predicting the premature cessation of menses and ovarian failure in the occult phase of the disease, when the currently available tests are still not sufficiently efficient [1], [2], [4], [22].
In conclusion, the blood and ovarian mtDNA/nDNA copy numbers appear to be correlated and a diminished mtDNA content was found in peripheral blood cells of women with an impaired ovarian reserve. Therefore, POI may frequently occur as a consequence of a generalized and still undetermined mitochondrial defect in which the ovary appears as the first affected tissue. Finally, we propose the blood mtDNA/nDNA copy number determination as a promising tool for the prediction either of the POI risk or the poor response to ovarian hyperstimulation.
Acknowledgments
The Authors acknowledge the contribution of all participants to the Italian Network for the study of Ovarian Dysfunctions (NIDO) (listed by alphabetical order): Associazione Menopausa Precoce (http://www.menopausaprecoce.splinder.com/), Associazione Italiana Sindrome “X-Fragile” (http://www.xfragile.net/), ONDA (http://www.ondaosservatorio.it), M. Arosio (Milan), P. Beck-Peccoz (Milan), M. Biondi (Avellino), S. Bione (Pavia) V. Bruni (Florence), C. Brigante (Milan), S. Cannavò (Messina), L. Cavallo (Bari), M. Cisternino (Pavia), I. Colombo (Milan), S. Corbetta (Milan), P.G. Crosignani (Milan), M.G. D’Avanzo (Avellino), L. Dalprà (Monza), C. Danesino (Pavia), E. Di Battista (Genova), F. Di Prospero (Civitanova Marche-AN), E. Donti (Perugia), S. Einaudi (Torino), A. Falorni (Perugia), C. Foresta (Padova), F. Fusi (Milan), N. Garofalo (Palermo), I. Giotti (Florence), R. Lanzi (Milan), D. Larizza (Pavia), N. Locatelli (Milan), P. Loli (Milano), S. Madaschi (Milan), M. Maghnie (Genova), S. Maiore (Roma), F. Mantero (Padova), A. Marozzi (Milan), S. Marzotti (Perugia), N. Migone (Turin), R. Nappi (Pavia), D. Palli (Florence), M.G. Patricelli (Milan), C. Pisani (Pavia), P. Prontera (Perugia), F. Petraglia (Siena), G. Radetti (Bolzano), A. Renieri (Siena), I. Ricca (Pavia), A. Ripamonti (Milan), R. Rossetti (Milan), G. Russo (Milan), S. Russo (Varese), M. Tonacchera (Pisa), D. Toniolo (Milano), F. Torricelli (Florence), W. Vegetti (Milan), N. Villa (Monza), P. Vineis (London), M. Wasniewska (Messina), O. Zuffardi (Pavia).
Contributions
Conceived and designed the experiments: ES GR LP. Performed the experiments: ES CC M. Busnelli. Analyzed the data: M. Bonomi CC LP. Contributed reagents/materials/analysis tools: DM AP SB RR. Wrote the paper: M. Bonomi ES LP.
Funding Statement
The financial support of Telethon Foundation - Italy (Grant no. GGP09126A) and Istituto Auxologico Italiano, Italy (Ricerca Corrente Funds: 05C501) are gratefully acknowledged. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Contributor Information
the Italian Network for the study of Ovarian Dysfunctions:
M. Arosio, P. Beck-Peccoz, M. Biondi, S. Bione, V. Bruni, C. Brigante, S. Cannavo`, L. Cavallo, M. Cisternino, I. Colombo, S. Corbetta, P.G. Crosignani, M.G. D'Avanzo, L. Dalpra, C. Danesino, E. Di Battista, F. Di Prospero, E. Donti, S. Einaudi, A. Falorni, C. Foresta, F. Fusi, N. Garofalo, I. Giotti, R. Lanzi, D. Larizza, N. Locatelli, P. Loli, S. Madaschi, M. Maghnie, S. Maiore, F. Mantero, A. Marozzi, S. Marzotti, N. Migone, R. Nappi, D. Palli, M.G. Patricelli, C. Pisani, P. Prontera, F. Petraglia, G. Radetti, A. Renieri, I. Ricca, A. Ripamonti, R. Rossetti, G. Russo, S. Russo, M. Tonacchera, D. Toniolo, F. Torricelli, W. Vegetti, N. Villa, P. Vineis, M. Wasniewsk, and O. Zuffardi
References
- 1. Welt CK (2008) Primary ovarian insufficiency: a more accurate term for premature ovarian failure. Clin Endocrinol (Oxf) 68: 499–509. [DOI] [PubMed] [Google Scholar]
- 2. Nelson LM (2009) Clinical practice. Primary ovarian insufficiency. N Engl J Med 360: 606–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Simpson JL (2008) Genetic and phenotypic heterogeneity in ovarian failure: overview of selected candidate genes. Ann N Y Acad Sci 1135: 146–154. [DOI] [PubMed] [Google Scholar]
- 4. Persani L, Rossetti R, Cacciatore C (2010) The genes involved in human premature ovarian failure. J Mol Endocrinol 45: 257–279. [DOI] [PubMed] [Google Scholar]
- 5. Vegetti W, Marozzi A, Manfredini E, Testa G, Alagna F, et al. (2000) Premature ovarian failure. Mol Cell Endocrinol 161: 53–57. [DOI] [PubMed] [Google Scholar]
- 6. Davies CJ, Davison RM, Payne NN, Rodeck CH, Conway GS (2000) Female sex preponderance for idiopathic familial premature ovarian failure suggests an X chromosome defect. Hum Reprod 15: 2418–2422. [DOI] [PubMed] [Google Scholar]
- 7. Rizzolio F, Pramparo T, Sala C, Zuffardi O, Desantis L, et al. (2009) Epigenetic analysis of the Critical Region I for Premature Ovarian Failure (POF): demonstration of a highly heterochromatic domain on the long arm of the mammalian X chromosome. J Med Genet 46: 585–592. [DOI] [PubMed] [Google Scholar]
- 8. Persani L, Rossetti R, Cacciatore C, Bonomi M (2009) Primary ovarian insufficiency: X chromosome defects and autoimmunity. J Autoimmun 33: 35–41. [DOI] [PubMed] [Google Scholar]
- 9. Pang CY, Ma YS, Wei YU (2008) MtDNA mutations, functional decline and turnover of mitochondria in aging. Front Biosci 13: 3661–3675. [DOI] [PubMed] [Google Scholar]
- 10. Johannsen DL, Ravussin E (2009) The role of mitochondria in health and disease. Curr Opinion Pharmacol 9: 780–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. van Blerkom J, Davis PW, Lee J (1995) ATP content of human oocytes and developmental potential and outcome after in-vitro fertilization and embryo transfer. Hum Reprod 10: 415–424. [DOI] [PubMed] [Google Scholar]
- 12. Dumollard R, Duchen M, Carroll J (2007) The role of mitochondrial function in the oocyte and embryo. Curr Top Dev Biol 77: 21–49. [DOI] [PubMed] [Google Scholar]
- 13. May-Panloup P, Chretien MF, Malthiery Y, Reynier P (2007) Mitochondrial DNA in the oocyte and the developing embryo. Curr Top Dev Biol 77: 51–83. [DOI] [PubMed] [Google Scholar]
- 14. Wai T, Ao A, Zhang X, Cyr D, Dufort D, et al. (2010) The role of mitochondrial DNA copy number in mammalian fertility. Biol Reprod 83: 52–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Santos TA, El Shourbagy S, St John JC (2006) Mitochondrial content reflects oocyte variability and fertilization outcome. Fertil Steril 85: 584–591. [DOI] [PubMed] [Google Scholar]
- 16. May-Panloup P, Chrétien MF, Jacques C, Vasseur C, Malthièry Y, et al. (2005) Low oocyte mitochondrial DNA content in ovarian insufficiency. Hum Reprod 20: 593–597. [DOI] [PubMed] [Google Scholar]
- 17. Tatone C, Carbone MC, Falone S, Aimola P, Giardinelli A, et al. (2006) Age-dependent changes in the expression of superoxide dismutases and catalase are associated with ultrastructural modifications in human granulosa cells. Mol Hum Reprod 12: 655–660. [DOI] [PubMed] [Google Scholar]
- 18. Trifunovic A, Wredenberg A, Falkenberg M, Spelbrink JN, Rovio AT, et al. (2004) Premature aging in mice expressing defective mitochondrial DNA polymerase. Nature 429: 417–423. [DOI] [PubMed] [Google Scholar]
- 19. Luoma P, Melberg A, Rinne JO, Kaukonen JA, Nupponen NN, et al. (2004) Parkinsonism, premature menopause, and mitochondrial DNA polymerase gamma mutations: clinical and molecular genetic study. Lancet 364: 875–882. [DOI] [PubMed] [Google Scholar]
- 20. Kujoth GC, Hiona A, Pugh TD, Someya S, Panzer K, et al. (2005) Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science 309: 481–484. [DOI] [PubMed] [Google Scholar]
- 21. Pagnamenta AT, Taanman JW, Wilson CJ. Anderson NE, Marotta R, et al. (2006) Dominant inheritance of premature ovarian failure associated with mutant mitochondrial DNA polymerase gamma. Hum Reprod 21: 2467–2473. [DOI] [PubMed] [Google Scholar]
- 22. Broekmans FJ, Kwee J, Hendriks DJ, Mol BW, Lambalk CB (2006) A systematic review of tests predicting ovarian reserve and IVF outcome. Hum Reprod Update 12: 685–718. [DOI] [PubMed] [Google Scholar]
- 23. Rossetti R, Di Pasquale E, Marozzi A, Bione S, Toniolo D, et al. (2009) BMP15 mutations associated with primary ovarian insufficiency cause a defective production of bioactive protein. Hum Mutat 30: 804–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Trinei M, Berniakovich I, Pelicci PG, Giorgio M (2006) Mitochondrial DNA copy number is regulated by cellular proliferation: a role for Ras and p66(Shc). Biochim Biophys Acta 1757: 624–630. [DOI] [PubMed] [Google Scholar]
- 25. Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29: e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Van Goethem G, Dermaut B, Löfgren A, Martin JJ, Van Broeckhoven C (2001) Mutation of POLG is associated with progressive external ophthalmoplegia characterized by mtDNA deletions. Nat Genet 28: 211–2. [DOI] [PubMed] [Google Scholar]
- 27. Hudson G, Schaefer AM, Taylor RW, Tiangyou W, Gibson A, et al. (2007) Mutation of the linker region of the polymerase gamma-1 (POLG1) gene associated with progressive external ophthalmoplegia and Parkinsonism. Arch Neurol. 64: 553–7. [DOI] [PubMed] [Google Scholar]
- 28. Tong ZB, Sullivan SD, Lawless LM, Vanderhoof V, Zachman K, et al. (2010) Five mutations of mitochondrial DNA polymerase-gamma (POLG) are not a prevalent etiology for spontaneous 46, XX primary ovarian insufficiency. Fertil Steril. 94: 2932–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cree LM, Patel SK, Pyle A, Lynn S, Turnbull DM, et al. (2008) Age-related decline in mitochondrial DNA copy number in isolated human pancreatic islets. Diabetologia 51: 1440–1443. [DOI] [PubMed] [Google Scholar]
- 30. Short KR, Bigelow ML, Kahl J, Singh R, Coenen-Schimke J, et al. (2005) Decline in skeletal muscle mitochondrial function with aging in humans. Proc Natl Acad Sci 102: 5618–5623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Menshikova EV, Ritov VB, Fairfull L, Ferrell RE, Kelley DE, et al. (2006) Effects of exercise on mitochondrial content and function in aging human skeletal muscle. J Gerontol A Biol Sci Med Sci 61: 534–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schapira AH (2006) Mitochondrial disease. Lancet 368: 70–82. [DOI] [PubMed] [Google Scholar]
- 33. Spikings EC, Alderson J, John JC (2007) Regulated mitochondrial DNA replication during oocyte maturation is essential for successful porcine embryonic development. Biol Reprod 76: 327–735. [DOI] [PubMed] [Google Scholar]
- 34. Jansen RP, de Boer K (1998) The bottleneck: mitochondrial imperatives in oogenesis and ovarian follicular fate. Mol Cell Endocrinol 145: 81–88. [DOI] [PubMed] [Google Scholar]
- 35. Shoubridge EA, Wai T (2007) Mitochondrial DNA and the mammalian oocyte. Curr Top Dev Biol 77: 87–111. [DOI] [PubMed] [Google Scholar]
- 36. Boland NI, Humpherson PG, Leese HJ, Gosden RG (1993) Pattern of lactate production and steroidogenesis during growth and maturation of mouse ovarian follicles in vitro. Biol Reprod 48: 798–806. [DOI] [PubMed] [Google Scholar]
- 37. Wycherley G, Kane MT, Hynes AC (2005) Oxidative phosphorylation and the tricarboxylic acid cycle are essential for normal development of mouse ovarian follicles. Hum Reprod 20: 2757–2763. [DOI] [PubMed] [Google Scholar]
- 38. Harris SE, Leese HJ, Gosden RG, Picton HM (2009) Pyruvate and oxygen consumption throughout the growth and development of murine oocytes. Mol Reprod Dev 76: 231–238. [DOI] [PubMed] [Google Scholar]
- 39. Perls TT, Alpert L, Fretts RC (1997) Middle-aged mothers live longer. Nature 389: 133. [DOI] [PubMed] [Google Scholar]
- 40. Gagnon A, Smith KR, Tremblay M, Vézina H, Paré PP, et al. (2009) Is there a trade-off between fertility and longevity? A comparative study of women from three large historical databases accounting for mortality selection. Am J Hum Biol. 21: 533–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Smith KR, Gagnon A, Cawthon RM, Mineau GP, Mazan R, et al. (2009) Familial aggregation of survival and late female reproduction. J Gerontol A Biol Sci Med Sci 64: 740–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hutchinson EW, Shaw AJ, Rose MR (1991) Quantitative genetics of postponed aging in Drosophila melanogaster. II. Analysis of selected lines. Genetics 127: 729–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Farhi J, Homburg R, Ferber A, Orvieto R, Ben Rafael Z (1997) Non-response to ovarian stimulation in normogonadotrophic, normogonadal women: a clinical sign of impending onset of ovarian failure pre-empting the rise in basal follicle stimulating hormone levels. Hum Reprod 12: 241–243. [DOI] [PubMed] [Google Scholar]
- 44. De Boer EJ, den Tonkelaar I, te Velde ER, Burger CW, van Leeuwen FE, et al. (2003) Increased risk of early menopausal transition and natural menopause after poor response at first IVF treatment. Hum Reprod 18: 1544–1552. [DOI] [PubMed] [Google Scholar]
- 45. Lawson R, El-Toukhy T, Kassab A, Taylor A, Braude P, et al. (2003) Poor response to ovulation induction is a stronger predictor of early menopause than elevated basal FSH: a life table analysis. Hum Reprod 18: 527–533. [DOI] [PubMed] [Google Scholar]


