Abstract
Context
Schizophrenia is a devastating illness with an indeterminate pathophysiology. Several lines of evidence implicate dysfunction in the thalamus, a key node in the distributed neural networks underlying perception, emotion, and cognition. Existing evidence of aberrant thalamic function is based on indirect measures of thalamic activity, but dysfunction has not yet been demonstrated with a causal method.
Objective
Test the hypothesis that direct physiological stimulation of cortex will produce an abnormal thalamic response in individuals with schizophrenia.
Design
We stimulated the precentral gyrus with single-pulse transcranial magnetic stimulation (spTMS) and measured the response to this pulse in synaptically-connected regions (thalamus, medial superior frontal cortex [mSFG], insula) using concurrent functional magnetic resonance imaging (fMRI). The mean hemodynamic response from these regions was fit with the sum of two gamma functions and response parameters were compared across groups.
Setting
Academic research laboratory.
Participants
Patients with schizophrenia and sex- and age- matched psychiatrically healthy subjects were recruited from the community.
Main Outcome Measures
Peak amplitude of the thalamic hemodynamic response to spTMS of precentral gyrus.
Results
spTMS-evoked responses did not differ between groups at the cortical stimulation site. Compared to healthy subjects, schizophrenia patients showed a reduced response to spTMS in the thalamus (P=1.86 × 10−9) and mSFG (P=.02). Similar results were observed in the insula. Sham TMS indicated that these results could not be attributed to indirect effects of TMS coil discharge. Functional connectivity analyses revealed weaker thalamus-mSFG and thalamus-insula connectivity in schizophrenia patients compared to control subjects.
Conclusions
Individuals with schizophrenia showed reduced thalamic activation in response to direct perturbation delivered to the cortex. These results extend prior work implicating the thalamus in the pathophysiology of schizophrenia and suggest that the thalamus contributes to the patterns of aberrant connectivity characteristic of this disease.
Introduction
Schizophrenia is a devastating mental illness that has a significant impact on family, caregivers, society, and patients1–3. More than 2.4 million Americans suffer from schizophrenia4 and mortality rates are 2–3 times greater for patients compared to the population as a whole5,6. Although research has focused heavily on identifying diagnostic tools7 and treatments8 for the illness, schizophrenia is currently diagnosed based on clinical criteria9, treatments are often ineffective10, and the pathophysiology of the disease remains elusive. The work presented here builds on numerous prior studies that have implicated dysfunction of the thalamus in schizophrenia. In the section that follows we review the theoretical and empirical basis for this hypothesis of thalamic dysfunction in schizophrenia and conclude that much of the extant evidence is either indirect or subject to significant inferential limitations. For instance, some studies rely on cortical differences or effects measured at the scalp to draw inferences about thalamic dysfunction. Others infer functional differences on the basis of structural measures or rely on the assumption that patient and control groups perform behavioral tasks in a comparable manner. We designed the present experiment to circumvent these limitations, using single-pulse transcranial magnetic stimulation (spTMS) to directly stimulate cortex and concurrent functional magnetic resonance imaging (fMRI) to measure the resulting thalamic response.
Three lines of evidence suggest that schizophrenia is associated with thalamic dysfunction. First, aberrant scalp-recorded electrophysiological indices of sensory gating in schizophrenia have been interpreted as evidence for thalamic dysfunction, given nonhuman research confirming the critical role of the thalamus in conceptually similar processes11–15. Sensory gating deficits in schizophrenia have been demonstrated using P50 prepulse inhibition16,17, P300 auditory oddball18 and mismatch negativity tasks19,20,21. All of these paradigms have been interpreted as requiring thalamically mediated filtering of novel or salient stimuli. On this basis, some have suggested that hallucinations are a result of impaired thalamic filtering of salient and external from non-salient and internal speech22,23.
A second line of evidence comes from overnight electroencephalography (EEG) studies demonstrating sleep spindle deficits in individuals with schizophrenia. Sleep spindles are waxing and waning 12 to 16 Hz oscillations that are initiated by the thalamic reticular nucleus (TRN) and regulated by thalamo-reticular and thalamo-cortical circuits24,25. Individuals with schizophrenia display fewer and smaller sleep spindles. These metrics distinguish patients from healthy control subjects, medicated control subjects, and individuals with depression with high sensitivity and specificity26–28.
A third line of evidence implicating the thalamus in schizophrenia comes from studies that directly measured the thalamus using structural and functional neuroimaging techniques. Structural imaging studies have consistently identified decreases in thalamic grey matter29–31 and aberrant thalamic morphology32,33 in individuals with schizophrenia. In parallel, functional imaging studies have consistently found abnormal thalamic activation during sensory gating32,33, working memory34, and other executive function35–40 tasks.
Although the studies reviewed above have contributed to an important model of the pathophysiology of schizophrenia, most are subject to one of three key inferential limitations. One limitation is that studies employing scalp-recorded electrophysiology, as in studies of sensory gating and sleep, do not measure thalamic activity directly. A second is that structural imaging studies cannot address thalamic physiology, and are therefore unable to directly test hypotheses of thalamic dysfunction. A third is that most studies employing fMRI measure thalamus activity in the context of task performance and are therefore susceptible to detecting group differences in physiology that are mediated by performance differences (e.g., attention, compliance, comprehension, motivation, strategy) rather than differences in underlying disease-related neurobiology36–38.
The aim of the present study was to circumvent these limitations and to more directly test the hypothesis that the thalamus functions abnormally in schizophrenia. We used spTMS to present a direct physiological challenge to the cortex while we simultaneously measured the transynaptic response to this challenge in the thalamus with fMRI. TMS uses electromagnetic induction to non-invasively produce weak currents in the tissue underlying the TMS coil39. In addition to affecting the tissue that experiences the magnetic flux directly, depolarization at the stimulation site propagates to distal regions via synaptic transmission or spread of neural impulses40,41. Whereas repetitive TMS (rTMS) is thought to create a transient “virtual lesion” by overwhelming a brain region with noise42, or otherwise altering ongoing neural functioning43,44, spTMS transiently excites discrete cortical patches without producing prolonged changes in cortical excitability or function. spTMS permits concurrent measurement of both the local response and the response in distal regions that are functionally connected to the stimulation site41,45,46. In the present study, the cortical and thalamic responses to spTMS were measured using blood oxygen level dependent (BOLD) fMRI47. Although concurrent TMS-fMRI has been used to evaluate brain function in healthy individuals48,49 and a number of commentators have highlighted the potential benefits of using this technique to probe the neurobiology of schizophrenia50,51, to our knowledge, it has never before been applied to the study of any psychiatric illness.
Here, spTMS was delivered to the precentral gyrus, and the resulting hemodynamic response was parameterized (amplitude, peak latency, and width) in four regions-of-interest (ROIs). Hypothesis testing focused on group differences in peak amplitude in the thalamus. Differences in the hemodynamic response were also assessed in the cortex beneath the TMS coil (precentral gyrus), the medial superior frontal gyrus (mSFG), and the insula. Exploratory analyses were used to characterize group differences in the latter two cortical ROIs, and measures of functional connectivity were computed. Results were compared with sham TMS. A button-pressing (BP) task was also assessed to confirm that spTMS-evoked responses were qualitatively similar to those obtained with a standard motor task. We hypothesized and demonstrated that patients with schizophrenia show a reduced thalamic response to spTMS.
Methods
Procedures were approved by the University of Wisconsin-Madison Health Sciences Institutional Review Board. Written informed consent was obtained from all subjects.
Subjects
Fourteen healthy subjects and 14 subjects with schizophrenia, recruited from local mental health providers, through newspaper and Internet advertisements, and by word-of-mouth, participated in the study (Table 1). A psychiatrist interviewed all subjects to obtain psychiatric and medical histories and to exclude (healthy controls) or confirm (schizophrenia patients) diagnoses using DSMV-IV-TR criteria9 (see Author Appendix). The Structured Clinical Interview for DSM-IV Disorders52 was also administered. Symptom severity was evaluated using the Positive and Negative Syndrome Scale (PANSS)53.
Table 1.
Demographics and Clinical Characteristics of Healthy Control Subjects and Schizophrenia Patients
| Control Group N=14 |
Schizophrenia Group N=14 |
Analysisa | |
|---|---|---|---|
| Age (years)-Range | 20–45 | 25–48 | |
| Age (years)-Mean ± SD | 34 ± 8.04 | 32.93 ± 7.53 | n.s. |
| Male/Female | 10/4 | 10/4 | |
| Education (years, starting with high school ± SD) | 6.0 ± 2.51 | 5.21 ± 2.12 | n.s. |
| Positive and Negative | 70.1 ± 17.7 | ||
| Syndrome Scale (PANSS) score Mean ± SD | |||
| Positive Mean ± SD | 15.6 ± 6.0 | ||
| Negative Mean ± SD | 20.7 ± 6.0 | ||
| General Mean ± SD | 33.8 ± 10.5 |
Student’s t-test
Patients were diagnosed as paranoid (N=11), residual (N=1), catatonic (N=1), or undifferentiated (N=1) subtype. They were receiving second-generation (N=10), first- and second- generation (N=2), or first-generation (N=2) antipsychotic medications. All were outpatients with a stable chronic illness (M=11 years, SD=7).
Design Overview
The study consisted of two sessions occurring on separate days. During the first session, structural MRI images required for the subsequent spTMS/fMRI session were collected; first session data for some subjects was obtained from a prior study. The second session featured two challenges. The first was spTMS to the precentral gyrus of the left hemisphere. The second was a button-pressing (BP) task known to produce a well-characterized hemodynamic response54 (11 subjects performed this task during the first session).
Four criteria led us to select the precentral gyrus as the spTMS target. First, to ensure that spTMS-induced input to thalamus would be comparable across groups, we required a target that is not dysfunctional in schizophrenia. Thus we ruled out, for example, prefrontal cortex55,56. Second, we required a target easily accessible in the scanner and not covered in musculature57, ruling out the occipital and temporal lobes. Third, we preferred a target with a well-characterized hemodynamic response, one that has been studied in prior TMS-fMRI research, and whose activity is associated with robust thalamic activity46,58. Fourth, given the sleep spindle abnormality described above26,59 we preferred a target with robust projections to TRN60. The precentral gyrus satisfied all of these criteria.
Session 1: Structural MRI Data Acquisition
During the first session, T1-weighted high-resolution structural images (TE=3.2ms; TR=8.2ms; FOV=25.6cm; matrix=256×256; 156×1.0mm slices; no inversion recovery) were collected using a 3T General Electric Discovery 750 MR scanner (Waukesha, WI). Single-subject data were transferred to a Navigated Brain Stimulation (NBS i.e., “frameless stereotaxy”) system (Nexstim, Helsinki, Finland), and the TMS target (left precentral gyrus in the vicinity of the primary hand representation [“knob”]61) was identified.
Session 2: spTMS Targeting and fMRI Acquisition
Session 2 included 1) coregistering the subject’s head with the high-resolution T1 image to determine TMS positioning and 2) fMRI scanning. The order of functional scanning was: spTMS to precentral gyrus, BP task (if not obtained during the first session), spTMS to another TMS target (data not presented here), and sham TMS (see Author Appendix). Each time the TMS coil was relocated the subject was repositioned in the scanner. Following each scan with spTMS, a medium-resolution, structural scan was obtained. All MRI sessions occurred at the same time of day (early afternoon).
spTMS Targeting
NBS was used to coregister each subject to his/her own T1 (see Supplemental Figure 1 and Author Appendix). Stimulation intensity was determined by delivering spTMS at varying intensities (using a stair-casing procedure62) to the hand area of the precentral gyrus until an intensity was reached that evoked a contralateral motor response to 5 of 10 pulses. spTMS was delivered using a 70mm figure-eight coil and biphasic stimulator (Magstim Rapid 2, Wales, U.K). To avoid evoking motor responses in the scanner we used NBS to move the coil along the precentral gyrus until a location was identified that did not evoke a motor response. Because the NBS system is not MRI-compatible, the exact position of the TMS coil was traced onto a cap worn by the subject, allowing stimulation to be delivered to the same location using an MRI-compatible TMS coil in the scanner. The subject was then escorted to the scanner.
fMRI Acquisition
To minimize startle from the “click” associated with the TMS coil, subjects were fitted with pneumatic headphones (Avotec, Stuart, FL) through which white noise was played during the session. Volume was titrated to the maximum level that the subject could comfortably tolerate. Foam padding was used to minimize movement. An MRI-compatible TMS coil (Magstim, Wales, U.K. and Jali Medical, Woburn, MA) was attached to a custom, multi-jointed mount (see Supplemental Figure 1 and Author Appendix). An 8-foot radio frequency (RF) shielded cable, passed through a waveguide in the penetration panel, connected the TMS coil in the scanner to the stimulator in the control room.
The TMS coil was aligned to the coil tracing on the subject’s cap and single pulses were delivered to confirm that movements were not evoked. Subjects were instructed to remain calm, still, and awake with open eyes.
The first scan was a localizer image, followed by a higher order shim (TE=7.0ms; TR=1558ms; FOV=24cm; slice thickness=5.8mm) and field map (TE=7/10ms; TR=710ms; FOV=20cm; matrix=256×256; 25×1.0mm slices). This was followed by two 20-pulse runs of spTMS to precentral gyrus, (110% motor threshold; intertrial interval=16–24 seconds) and one 20-pulse run of the BP task. To minimize TMS artifacts, the pulse sequence for these echo-planar images (EPIs, TR=2000ms; TE=25ms; FOV=22.4cm; matrix=64×64; 35×3.0mm slices (0.6mm gap); flip=60°) was modified such that image acquisition occurred during the first 1770ms of the TR, thereby leaving a 230ms “silent” gap during which spTMS could be delivered (see Supplemental Figure 2). The same pulse sequence was used for the BP task, during which subjects were instructed to press a button with their right thumb as firmly and as quickly as possible following a 500ms tone (Current Designs, Philadelphia, PA). Stimuli were controlled by E-Prime (Psychology Software Tools, Sharpsburg, PA) and TTL pulses generated by the scanner. Finally, a medium-resolution structural scan (TR=4.3ms; TE=1.22; FOV=28cm; matrix=256×256; 60×2.6mm slices) was collected.
The subject was then slid out of the scanner and the TMS coil configured for sham stimulation. The procedure was repeated with two runs of sham TMS replacing spTMS (for details of sham TMS procedure, see Author Appendix). One subject in each group discontinued participation in the experiment prior to collection of sham TMS data.
Data Preprocessing
Except where otherwise noted, processing employed AFNI (http://afni.nimh.nih.gov)63 and in-house software. Functional images were first reconstructed on the scanner. Images were slice-time, motion and field map corrected using FSL (http://www.fmrib.ox.ac.uk/fsl)64. Data were masked to exclude extracerebral voxels and converted to percent signal change (PSC). Functional images were aligned to the high-resolution T1 using the transformation matrix generated by aligning the medium- to the high-resolution T1 and then applying the transformation matrix to the functional data (6 df, least-squares, sinc interpolation; resampledto 3×3×3.6mm).
For each subject, the hemodynamic response was modeled using a generalized least-squares fit with restricted maximum likelihood estimation of temporal autocorrelation. Hemodynamic responses were modeled using a set of triangular (“tent”) functions (9 tents; 0–16 s). Similar to other basis functions (e.g., finite impulse responses) this allowed the amplitude, peak latency and width of the hemodynamic response to vary.
Analysis
The aim of the present investigation was to test whether individuals with schizophrenia show an attenuated thalamic response to cortical spTMS. To accomplish this, we employed an a priori ROI-based strategy. This approach has two advantages. First, statistical power is maximized by eliminating the need to correct for thousands of voxelwise comparisons. Second, this strategy circumvents the assumption that the thalamus is anatomically similar in patients and controls. ROIs were prescribed in the axial plane on the high-resolution T1, referring to a human brain atlas65 as needed (see Supplemental Table 1 and Author Appendix).
In addition to interrogating the stimulation site and the thalamus, we analyzed two other regions. mSFG was selected for exploratory analyses after visual inspection of activation maps from the first eight subjects enrolled in the study revealed that it was consistently and robustly activated by spTMS. When these analyses revealed abnormal thalamo-cortical coupling in schizophrenia, we assessed the generality of this finding by interrogating the insula, for which thalamic dysconnectivity in schizophrenia has recently been reported66. Importantly, for each subject, the nearest border of the mSFG and insula ROIs was always located several centimeters from the site targeted for spTMS. Because direct electromagnetic induction is limited to an area of approximately 2cm2 and depth of 2cm67,68, the responses observed in these regions (and the thalamus) were necessarily due to synaptic transmission.
For each ROI, a mask was created containing the upper 5th percentile of voxels responsive to spTMS (determined using the t-statistic at the peak of each individual’s hemodynamic response (4–6 s)). The hemodynamic response from these voxels was parameterized using the sum of two gamma functions (in units of PSC). The gamma functions were up-sampled from 2000-millisecond (i.e., the original sampling rate) to 10-millisecond resolution69. This fit enabled us to extract model parameters corresponding to the amplitude, peak latency, and overall width (at approximately the full width at half maximum) of the hemodynamic response, and to compare them across groups.
For spTMS and sham TMS conditions, 1 of 2 runs was analyzed. Although a single 20-trial run is sufficient to produce a BP-evoked hemodynamic response54, the technical challenges inherent in measuring the spTMS-evoked response with fMRI led us to collect a second spTMS run as a back-up. For the analyses reported here, we selected the run in which the t-statistic (corresponding to the greatest difference from baseline) was most similar to that of the BP run (see Author Appendix for details about run selection, and results from analyses of “unselected” runs). To control for nonspecific TMS effects, thalamic voxels responsive to spTMS were also compared to sham TMS.
When analyses of the spTMS-evoked responses in mSFG and insula revealed group differences in amplitude in these regions, we assessed group differences in functional connectivity among ROIs. Specifically, time-series were averaged across the upper-5th-percentile of voxels within each ROI for each subject, demeaned, correlated, and then Z-transformed using Fisher’s technique.
Hypothesis Testing
Between-groups ANOVAs, Student’s t-tests, Pearson’s correlations, and discriminant analyses were performed with SPSS (SPSS, Inc, Chicago, IL). Gamma fit parameters were also assessed with non-parametric statistics, which yielded similar conclusions (not reported). Owing to non-normality of the connectivity metric, coefficients were rank-transformed prior to testing70,71. Hemodynamic response curves and their associated 95% confidence intervals (computed using group means and standard errors at each time-point) were plotted using in-house code written for R (http://r-project.org). Effect size is reported as partial eta squared (η2). The spTMS and BP task were not formally compared because we did not have theoretically motivated hypotheses involving this comparison.
Results
Cortical response to spTMS, but not the BP task, is similar across groups
The spTMS-evoked response did not differ between groups in the precentral gyrus (F(1,26)=.52, n.s., η2=.02). However, during the BP task, schizophrenia patients showed a wider hemodynamic response (F(1,26)=5.86, P=.02, η2=.18, Fig. 1). There were no group differences in BP reaction time (F(1,26)=2.05, n.s., η2=.07).
Figure 1. Cortical response to spTMS and button press task.
(A) Group averaged spTMS-evoked response in the cortical region underlying the TMS coil (precentral gyrus).
(B) Group averaged BP-evoked response in precentral gyrus.
* P<.05, shaded areas: 95% Confidence Intervals (CI). Inset: single-subject representation of ROI (yellow); voxels most responsive to condition (red). N=14 in each group.
Thalamic responses to spTMS and the BP task are abnormal in schizophrenia
In the spTMS condition, individuals with schizophrenia showed a smaller (F(1,26) =80.79, P=1.89×10−9, η2=.76) and earlier peaking (F(1,26)=4.39, P=.05, η2=.14) thalamic response. Indeed, every patient showed a peak that was numerically smaller than the least responsive member of the control group (Fig 2C, patients PSC range: 0.26%–0.80%, healthy subjects PSC range: 0.88%–1.67%). A formal discriminant analysis indicated that this measure did an excellent job classifying members of the two groups (χ2=36.0, P=1.95×10−9; leave-one-out cross-validation: sensitivity=85.7%; specificity=100.0%; overall classification accuracy=92.9%). This effect could not be accounted for by the peripheral consequences of spTMS stimulation because, compared to spTMS, sham TMS produced a nonexistent response (patients: F(1,12)=16.33, P=.002, η2=.58; healthy subjects: F(1,12)=135.50, P=6.8×10−8, η2=.92) that did not differ between groups (F(1,24)=3.0×l0−5, n.s., η2=1.0×10−6, Fig. 2). The BP task showed a similar, albeit weaker, pattern (F(1,26)=10.69, P=.003, η2=.29,Fig 2).
Figure 2. Thalamic response to spTMS and BP task.
(A) Group averaged spTMS-evoked response in thalamus
(B) Group averaged BP-evoked response in thalamus
Shaded areas: 95% CI; Red/blue dashed lines: sham TMS response in the same voxels.
(C,D) Dot plots illustrating single-subject peak percent signal change (extracted 3–6.5 seconds following spTMS delivery). The black line indicates the group mean, and the grey box bounding this line indicates SEM. * P<.05, ** P<1.9×10−9; (Note: Because peak latency varied across subjects, the group means shown in C,D necessarily differ from the maxima of the average HRF waveforms depicted in A,B. C,D represent data used for hypothesis testing.)
Inset: single-subject representation of ROI (yellow); voxels most responsive to condition (red). N=14 in each group, N=13 in each group for sham analysis.
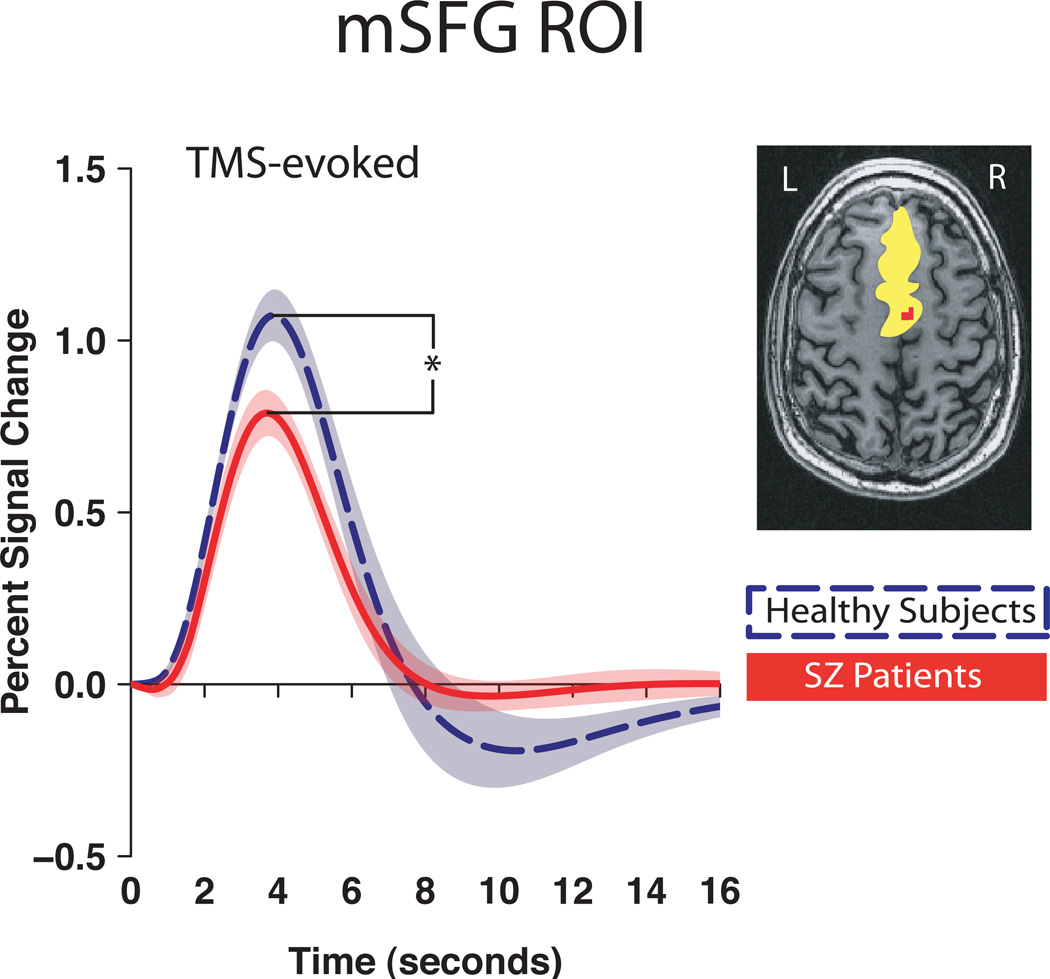
mSFG and insula responses to precentral gyrus spTMS are decreased in schizophrenia
The spTMS-evoked response was smaller in magnitude in the mSFG in patients with schizophrenia compared to healthy control subjects (F(1,26)=6.56, P=.02, η=.20, Fig. 3). To explore possible factors underlying this difference, we assessed functional connectivity between precentral gyrus and mSFG, between mSFG and thalamus, and between precentral gyrus and thalamus (using time series correlations). These analyses found no group differences in precentral gyrus-mSFG connectivity (F(1,26)=0.47, n.s., η2=.02) or in precentral gyrus-thalamus connectivity (F(1,26) 0.02, n.s, η2=.001), but did reveal that patients with schizophrenia had reduced coupling between thalamus and mSFG relative to healthy control subjects (F(1,26)=32.00, P=6.0×10−5, η2=.55, Table 2). Importantly, the lack of a group difference in coupling between precentral gyrus and mSFG was not simply a function of low overall connectivity between these regions; actual magnitudes of the correlations reflected a relatively high level of functional connectivity in both groups (Table 2).
Figure 3. mSFG response to spTMS of the precentral gyrus.
Group averaged spTMS-evoked response in mSFG * P<.05, shaded areas: 95% CI.
Inset: single-subject representation of ROI (yellow); voxels most responsive to condition (red). N=14 in each group.
Table 2.
Group Mean Correlation Coefficient (r) Between ROI Time Series
| Precentral Gyrus |
Thalamus | |
|---|---|---|
| Precentral | .35 n.s. | |
| Gyrus | .38 | |
| mSFG | .49 n.s. | .58 * |
| .43 | .35 |
Blue: healthy control subjects
Red: schizophrenia subjects
P < 6.0×10−6
Variation in thalamo-cortical coupling also predicted the magnitude of the spTMS-evoked mSFG response. Across groups, subjects with lower thalamus-mSFG coupling showed a smaller evoked response in mSFG (ρ(26)=.37, P=.05). Results for the insula were complementary to those for mSFG (see Supplemental Figure 3, Supplemental Table 2, and Author Appendix).
Control analyses
Disease chronicity and medication dosage, assessed using Chlorpromazine (CPZ) equivalencies72, did not predict any of the brain measure, Ps > .13. Across groups, variation in years of formal education did not predict any brain measure (Ps > .15). Likewise, accounting for variation in education did not substantively alter the significance of any group difference. Because the majority of the subjects (N=11) were diagnosed with paranoid schizophrenia and the remaining three subjects were diagnosed with residual, undifferentiated, and catatonic schizophrenia, it was not possible to meaningfully assess the effect of subtype. Nevertheless, analyses performed with these three individuals omitted did not alter any of our conclusions.
Thalamic deficits and symptom severity
There was a trend for patients with smaller thalamic responses to spTMS to show more severe positive symptoms on the PANSS (r(12)=−.49, P = .07). Relations with negative symptoms were not significant (r(12)=−.17, P = .57).
Comment
Schizophrenia is a severe mental illness whose neurobiology remains unclear1–3. There is considerable circumstantial evidence of a thalamic abnormality in schizophrenia, as assessed structurally29,30 and functionally32,33. The results presented here strengthen this hypothesis of thalamic dysfunction in schizophrenia with a procedure that supports causal inference: Subjects with schizophrenia evinced a smaller spTMS-evoked response in the thalamus compared to healthy control subjects. Analysis of a sham stimulation condition indicated that these effects could not be attributed to secondary consequences of spTMS. Additionally, because no group differences were found in response to spTMS in precentral gyrus, the results likely reflect local deficits in thalamic physiology, not downstream consequences of deficits in cortical function.
Abnormal thalamic functioning in schizophrenia is confirmed with spTMS-fMRI
The average thalamic spTMS-evoked response in patients with schizophrenia was less than half the magnitude of the average response in healthy subjects. Although this measure identified individuals from the two groups with 100% specificity, additional research is needed to determine whether the groups are better characterized as falling into one of two clusters, or as falling along a continuum on which patients with schizophrenia tend to have lower values. In terms of pathophysiology, the difference might be due to one of three factors: 1) a physiological abnormality in the stimulated cortical tissue, 2) deficient cortico-thalamic signal propagation, or 3) a physiological abnormality in the thalamus. The first possibility is ruled out by the fact that the response in cortical tissue underlying spTMS did not differ across groups. The second seems unlikely because the connectivity between the thalamus and precentral gyrus, that is, the degree of cortico-thalamic coupling, did not differ across groups. The most likely interpretation, therefore, is that our results reflect aberrant functioning of the thalamus itself, a claim consistent with evidence of structural abnormalities29–31,73,74 in the thalamus in subjects with schizophrenia.
mSFG and insula show decreased spTMS-evoked response in schizophrenia
Group differences in spTMS-evoked responses were also observed in mSFG and insula, cortical regions distal to the site of spTMS delivery. Such findings could be attributed to 1) a functional deficit in mSFG/insula, 2) a deficit in the region of stimulation (precentral gyrus) or its coupling with mSFG/insula or 3) an abnormality in a third area that is connected to both the precentral gyrus and the mSFG/insula (e.g., the thalamus) or the coupling with this third area. Again, the results of the functional connectivity analysis support the third possibility, revealing reduced coupling between thalamus and mSFG and thalamus and insula in patients with schizophrenia, but no difference in the degree of coupling between precentral gyrus and these regions. (Note that, in healthy control subjects, coupling between mSFG and thalamus was significantly stronger than coupling between precentral gyrus and thalamus (see Author Appendix). Although this specific pattern does not alter the reasoning behind our interpretation of the group difference in overall patterns of functional connectivity, it is an intriguing observation that may merit future investigation.)
Further consistent with the third scenario, when data from the groups were combined, the strength of the thalamus-mSFG coupling predicted the magnitude of the TMS-evoked responses in mSFG, and, likewise, the strength of thalamus-insula coupling predicted the magnitude of the TMS-evoked responses in insula. Taken together, these observations strongly suggest that the group difference in the magnitude of the mSFG and insula responses to spTMS reflect deficits centered on the thalamus or thalamo-cortical circuitry, rather than local cortical deficits. Thus, although numerous studies have shown aberrant activation in cortical areas in response to various tasks in schizophrenia37,75,76, our results suggest that such deficits could reflect underlying deficits in structures that are connected with the cortex, such as the thalamus77,78. Requiring further investigation is the extent to which the abnormal coupling between thalamus and mSFG and thalamus and insula can be attributed to dysfunction in thalamic activity per se, versus the integrity of structural connections between these regions79.
Clinical Significance
Numerically, there was no overlap in the amplitude of TMS-evoked thalamic response between schizophrenia and healthy subjects. Additionally, thalamic response amplitude in patients showed a trend toward predicting the severity of positive symptoms, a result that is in accord with similar relations observed with sleep spindle data26. Consequently, the results presented here not only confirm the thalamic abnormality in schizophrenia, but also show that it may be related to clinical symptoms.
Future Challenges
Several limitations of this investigation represent challenges for future research. First, although sleep spindle data suggest that thalamic deficits do not reflect group difference in medication26, it will be necessary to replicate our results while controlling for effects of medication. Additionally, further investigation with first-degree relatives will be necessary for understanding the potential genetic components of the abnormality in thalamus. Investigating first-episode schizophrenia patients will help discern whether the thalamic abnormality is present in early as well as in later stages of the illness. Future studies will be required to assess the influence of potentially important demographic and diagnostic variables (e.g., socioeconomic status, subtype).
A Deficit in Thalamic Reticular Nucleus?
The sleep spindle deficit in schizophrenia implicates a thalamic, and more specifically a thalamic reticular nucleus (TRN), abnormality in schizophrenia26. Because the TRN, a structure that surrounds the dorsal and lateral portions of the thalamus, is very thin (~1mm in cross-section80), it is not possible to resolve it with conventional fMRI techniques. There are two reasons, however, to believe that the activity we measured in the thalamus is heavily weighted by contributions of the TRN. First, the sole efferents of the TRN are inhibitory projections to the underlying thalamus. (Thus, the totality of the synaptic activity attributable to TRN output will be reflected in the BOLD signal from the principal thalamic nuclei that receive these outputs.) Second, synaptic activity in TRN is likely to be larger in magnitude than synaptic activity in principal thalamic nuclei. This is because there are 3.7 times more excitatory glutamatergic cortico-thalamic synapses onto TRN than onto principal thalamic nuclei, and because excitatory postsynaptic currents (EPSC’s) are 2.5 times larger in TRN than in thalamo-cortical neurons81. Consequently, it is plausible that the BOLD signal that we measured in the thalamus was heavily weighted by cortico-TRN-thalamic propagation of the spTMS-evoked response, and that the decreased spTMS-evoked thalamic response in subjects with schizophrenia may reflect a more specific abnormality in the TRN.
Animal studies support the idea that the TRN is necessary for sensory gating and attention modulation82,83, brain functions aberrant in schizophrenia19,84,85. More evidence gleaned from animal models and higher resolution imaging or postmortem studies in humans will be necessary to more fully test this hypothesis.
In summary, the present study implicates an abnormality in thalamus in the neurobiology of schizophrenia. This physiological abnormality cannot be attributed to differences in attention, compliance, or task performance that may exist between groups. Future studies will need to determine whether this deficit stems specifically from dysfunction of the TRN. More generally, this study underscores the value of concurrent spTMS-fMRI for probing the integrity of distributed neural circuits in psychiatric populations.
Supplementary Material
Acknowledgments
This work was funded by NIH grants R01-MH064498 (BRP), 20MH-077967-01A (GT), RC1MH090912-12 (MEM), and T31-GM007507 (Neuroscience Training Program). We thank Daniel Acheson, Tom Johnstone, John Ollinger, and Adam Riggall for programming; Eva Feredoes and Andrew Fox for advice; Michael Anderle, Rasmus Birn, Kristina Bolduc, Jenelle Fuller, Marti Garcia, Andy Mulder, and DJ Nephew for technical assistance. Contributions: B.R.P., F.F., and G.T. designed research; Y.G., F.F., F.J.L, M.J.P., S.S., and performed the experiments; Y.G., A.J.S., B.R.P., and M.E.M. analyzed the data; Y.G., A.J.S., and B.P.R. wrote the manuscript; all authors commented on the manuscript.
Footnotes
Portions of this research were presented at the annual meeting of the Society for Biological Psychiatry (San Francisco, CA), May 14, 2011.
Authors report no conflicts of interest or financial disclosures.
References
- 1.Nicholl D, Akhras KS, Diels J, Schadrack J. Burden of schizophrenia in recently diagnosed patients: healthcare utilisation and cost perspective. Curr Med Res Opin. 2010;26:943–955. doi: 10.1185/03007991003658956. [DOI] [PubMed] [Google Scholar]
- 2.Caqueo-Urizar A, Gutierrez-Maldonado J, Miranda-Castillo C. Quality of life in caregivers of patients with schizophrenia: a literature review. Health Qual Life Outcomes. 2009;7:84. doi: 10.1186/1477-7525-7-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Corring DJ. Quality of life: perspectives of people with mental illnesses and family members. Psychiatr Rehabil J. 2002;25:350–358. doi: 10.1037/h0095002. [DOI] [PubMed] [Google Scholar]
- 4.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 5.Osby U, Correia N, Brandt L, Ekbom A, Sparen P. Mortality and causes of death in schizophrenia in Stockholm county, Sweden. Schizophr Res. 2000;45:21–28. doi: 10.1016/s0920-9964(99)00191-7. [DOI] [PubMed] [Google Scholar]
- 6.Meltzer HY, Baldessarini RJ. Reducing the risk for suicide in schizophrenia and affective disorders. J Clin Psychiatry. 2003;64:1122–1129. doi: 10.4088/jcp.v64n0920. [DOI] [PubMed] [Google Scholar]
- 7.Schwarz E, Izmailov R, Spain M, Barnes A, Mapes JP, Guest PC, Rahmoune H, Pietsch S, Leweke FM, Rothermundt M, Steiner J, Koethe D, Kranaster L, Ohrmann P, Suslow T, Levin Y, Bogerts B, van Beveren NJ, McAllister G, Weber N, Niebuhr D, Cowan D, Yolken RH, Bahn S. Validation of a blood-based laboratory test to aid in the confirmation of a diagnosis of schizophrenia. Biomark Insights. 2010;5:39–47. doi: 10.4137/bmi.s4877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thaker GK. Schizophrenia endophenotypes as treatment targets. Expert Opin Ther Targets. 2007;11:1189–1206. doi: 10.1517/14728222.11.9.1189. [DOI] [PubMed] [Google Scholar]
- 9.Association AP. Diagnostic and Statistical Manual of Mental Disorders. 4 ed. Washington, DC: 2000. [Google Scholar]
- 10.Abbott A. Schizophrenia: The drug deadlock. Nature. 2010;468:158–159. doi: 10.1038/468158a. [DOI] [PubMed] [Google Scholar]
- 11.McCormick DA, Bal T. Sensory gating mechanisms of the thalamus. Curr Opin Neurobiol. 1994;4:550–556. doi: 10.1016/0959-4388(94)90056-6. [DOI] [PubMed] [Google Scholar]
- 12.Jones EG. The thalamus. 2nd ed. Cambridge; New York: Cambridge University Press; 2007. [Google Scholar]
- 13.Sherman SM, Guillery RW. Exploring the thalamus and its role in cortical function. 2nd ed. Cambridge, Mass: MIT Press; 2006. [Google Scholar]
- 14.Wolf R, Matzke K, Paelchen K, Dobrowolny H, Bogerts B, Schwegler H. Reduction of Prepulse Inhibition (PPI) after neonatal excitotoxic lesion of the ventral thalamus in pubertal and adult rats. Pharmacopsychiatry. 2010;43:99–109. doi: 10.1055/s-0029-1242823. [DOI] [PubMed] [Google Scholar]
- 15.Krause M, Hoffmann WE, Hajos M. Auditory sensory gating in hippocampus and reticular thalamic neurons in anesthetized rats. Biol Psychiatry. 2003;53:244–253. doi: 10.1016/s0006-3223(02)01463-4. [DOI] [PubMed] [Google Scholar]
- 16.Potter D, Summerfelt A, Gold J, Buchanan RW. Review of clinical correlates of P50 sensory gating abnormalities in patients with schizophrenia. Schizophr Bull. 2006;32:692–700. doi: 10.1093/schbul/sbj050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brockhaus-Dumke A, Schultze-Lutter F, Mueller R, Tendolkar I, Bechdolf A, Pukrop R, Klosterkoetter J, Ruhrmann S. Sensory gating in schizophrenia: P50 and N100 gating in antipsychotic-free subjects at risk, first-episode, and chronic patients. Biol Psychiatry. 2008;64:376–384. doi: 10.1016/j.biopsych.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 18.Kim DI, Mathalon DH, Ford JM, Mannell M, Turner JA, Brown GG, Belger A, Gollub R, Lauriello J, Wible C, O'Leary D, Lim K, Toga A, Potkin SG, Birn F, Calhoun VD. Auditory oddball deficits in schizophrenia: an independent component analysis of the fMRI multisite function BIRN study. Schizophr Bull. 2009;35:67–81. doi: 10.1093/schbul/sbn133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salisbury DF, Shenton ME, Griggs CB, Bonner-Jackson A, McCarley RW. Mismatch negativity in chronic schizophrenia and first-episode schizophrenia. Arch Gen Psychiatry. 2002;59:686–694. doi: 10.1001/archpsyc.59.8.686. [DOI] [PubMed] [Google Scholar]
- 20.Javitt DC. Intracortical mechanisms of mismatch negativity dysfunction in schizophrenia. Audiol Neurootol. 2000;5:207–215. doi: 10.1159/000013882. [DOI] [PubMed] [Google Scholar]
- 21.Javitt DC, Doneshka P, Grochowski S, Ritter W. Impaired mismatch negativity generation reflects widespread dysfunction of working memory in schizophrenia. Arch Gen Psychiatry. 1995;52:550–558. doi: 10.1001/archpsyc.1995.03950190032005. [DOI] [PubMed] [Google Scholar]
- 22.Behrendt RP, Young C. Hallucinations in schizophrenia, sensory impairment, and brain disease: a unifying model. Behav Brain Sci. 2004;27:771–787. doi: 10.1017/s0140525x04000184. discussion 787–830. [DOI] [PubMed] [Google Scholar]
- 23.Behrendt RP. Dysregulation of thalamic sensory "transmission" in schizophrenia: neurochemical vulnerability to hallucinations. J Psychopharmacol. 2006;20:356–372. doi: 10.1177/0269881105057696. [DOI] [PubMed] [Google Scholar]
- 24.Fuentealba P, Steriade M. The reticular nucleus revisited: intrinsic and network properties of a thalamic pacemaker. Prog Neurobiol. 2005;75:125–141. doi: 10.1016/j.pneurobio.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 25.Steriade M, Deschenes M, Domich L, Mulle C. Abolition of spindle oscillations in thalamic neurons disconnected from nucleus reticularis thalami. J Neurophysiol. 1985;54:1473–1497. doi: 10.1152/jn.1985.54.6.1473. [DOI] [PubMed] [Google Scholar]
- 26.Ferrarelli F, Peterson MJ, Sarasso S, Riedner BA, Murphy MJ, Benca RM, Bria P, Kalin NH, Tononi G. Thalamic dysfunction in schizophrenia suggested by whole-night deficits in slow and fast spindles. Am J Psychiatry. 2010;167:1339–1348. doi: 10.1176/appi.ajp.2010.09121731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferrarelli F, Huber R, Peterson MJ, Massimini M, Murphy M, Riedner BA, Watson A, Bria P, Tononi G. Reduced sleep spindle activity in schizophrenia patients. Am J Psychiatry. 2007;164:483–492. doi: 10.1176/ajp.2007.164.3.483. [DOI] [PubMed] [Google Scholar]
- 28.Vukadinovic Z. Sleep abnormalities in schizophrenia may suggest impaired tran-sthalamic cortico-cortical communication: towards a dynamic model of the illness. Eur J Neurosci. 2011;34:1031–1039. doi: 10.1111/j.1460-9568.2011.07822.x. [DOI] [PubMed] [Google Scholar]
- 29.Ellison-Wright I, Bullmore E. Anatomy of bipolar disorder and schizophrenia: a meta-analysis. Schizophr Res. 2010;117:1–12. doi: 10.1016/j.schres.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 30.Glahn DC, Laird AR, Ellison-Wright I, Thelen SM, Robinson JL, Lancaster JL, Bullmore E, Fox PT. Meta-analysis of gray matter anomalies in schizophrenia: application of anatomic likelihood estimation and network analysis. Biol Psychiatry. 2008;64:774–781. doi: 10.1016/j.biopsych.2008.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adriano F, Spoletini I, Caltagirone C, Spalletta G. Updated meta-analyses reveal thalamus volume reduction in patients with first-episode and chronic schizophrenia. Schizophr Res. 2010;123:1–14. doi: 10.1016/j.schres.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 32.Tregellas JR, Davalos DB, Rojas DC, Waldo MC, Gibson L, Wylie K, Du YP, Freedman R. Increased hemodynamic response in the hippocampus, thalamus and prefrontal cortex during abnormal sensory gating in schizophrenia. Schizophr Res. 2007;92:262–272. doi: 10.1016/j.schres.2006.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tregellas JR, Ellis J, Shatti S, Du YP, Rojas DC. Increased hippocampal, thalamic, and prefrontal hemodynamic response to an urban noise stimulus in schizophrenia. Am J Psychiatry. 2009;166:354–360. doi: 10.1176/appi.ajp.2008.08030411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bor J, Brunelin J, Sappey-Marinier D, Ibarrola D, d' Amato T, Suaud-Chagny MF, Saoud M. Thalamus abnormalities during working memory in schizophrenia. An fMRI study. Schizophr Res. 2011;125:49–53. doi: 10.1016/j.schres.2010.10.018. [DOI] [PubMed] [Google Scholar]
- 35.Andrews J, Wang L, Csernansky JG, Gado MH, Barch DM. Abnormalities of thalamic activation and cognition in schizophrenia. Am J Psychiatry. 2006;163:463–469. doi: 10.1176/appi.ajp.163.3.463. [DOI] [PubMed] [Google Scholar]
- 36.Walter H, Vasic N, Hose A, Spitzer M, Wolf RC. Working memory dysfunction in schizophrenia compared to healthy controls and patients with depression: evidence from event-related fMRI. Neuroimage. 2007;35:1551–1561. doi: 10.1016/j.neuroimage.2007.01.041. [DOI] [PubMed] [Google Scholar]
- 37.Minzenberg MJ, Laird AR, Thelen S, Carter CS, Glahn DC. Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Arch Gen Psychiatry. 2009;66:811–822. doi: 10.1001/archgenpsychiatry.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barch DM. The relationships among cognition, motivation, and emotion in schizophrenia: how much and how little we know. Schizophr Bull. 2005;31:875–881. doi: 10.1093/schbul/sbi040. [DOI] [PubMed] [Google Scholar]
- 39.Barker AT, Jalinous R, Freeston IL. Non-invasive magnetic stimulation of human motor cortex. Lancet. 1985;1:1106–1107. doi: 10.1016/s0140-6736(85)92413-4. [DOI] [PubMed] [Google Scholar]
- 40.Strafella AP, Paus T. Cerebral blood-flow changes induced by paired-pulse transcranial magnetic stimulation of the primary motor cortex. J Neurophysiol. 2001;85:2624–2629. doi: 10.1152/jn.2001.85.6.2624. [DOI] [PubMed] [Google Scholar]
- 41.Ferrarelli F, Haraldsson HM, Barnhart TE, Roberts AD, Oakes TR, Massimini M, Stone CK, Kalin NH, Tononi G. A [17F]-fluoromethane PET/TMS study of effective connectivity. Brain Res Bull. 2004;64:103–113. doi: 10.1016/j.brainresbull.2004.04.020. [DOI] [PubMed] [Google Scholar]
- 42.Pascual-Leone A, Bartres-Faz D, Keenan JP. Transcranial magnetic stimulation: studying the brain-behaviour relationship by induction of 'virtual lesions'. Philos Trans R Soc Lond B Biol Sci. 1999;354:1229–1238. doi: 10.1098/rstb.1999.0476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Roth well JC. Theta burst stimulation of the human motor cortex. Neuron. 2005;45:201–206. doi: 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- 44.Walsh V, Rushworth M. A primer of magnetic stimulation as a tool for neuropsychology. Neuropsychologia. 1999;37:125–135. [PubMed] [Google Scholar]
- 45.Bohning DE, Shastri A, Wassermann EM, Ziemann U, Lorberbaum JP, Nahas Z, Lomarev MP, George MS. BOLD-f MRI response to single-pulse transcranial magnetic stimulation (TMS) J Magn Reson Imaging. 2000;11:569–574. doi: 10.1002/1522-2586(200006)11:6<569::aid-jmri1>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 46.Hanakawa T, Mima T, Matsumoto R, Abe M, Inouchi M, Urayama S, Anami K, Honda M, Fukuyama H. Stimulus-response profile during single-pulse transcranial magnetic stimulation to the primary motor cortex. Cereb Cortex. 2009;19:2605–2615. doi: 10.1093/cercor/bhp013. [DOI] [PubMed] [Google Scholar]
- 47.Ogawa S, Lee TM, Nayak AS, Glynn P. Oxygenation-sensitive contrast in magnetic resonance image of rodent brain at high magnetic fields. Magn Reson Med. 1990;14:68–78. doi: 10.1002/mrm.1910140108. [DOI] [PubMed] [Google Scholar]
- 48.Heinen K, Ruff CC, Bjoertomt O, Schenkluhn B, Bestmann S, Blankenburg F, Driver J, Chambers CD. Concurrent TMS-fMRI reveals dynamic interhemispheric influences of the right parietal cortex during exogenously cued visuospatial attention. Eur J Neurosci. 2011;33:991–1000. doi: 10.1111/j.1460-9568.2010.07580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ruff CC, Blankenburg F, Bjoertomt O, Bestmann S, Freeman E, Haynes JD, Rees G, Josephs O, Deichmann R, Driver J. Concurrent TMS-fMRI and psychophysics reveal frontal influences on human retinotopic visual cortex. Curr Biol. 2006;16:1479–1488. doi: 10.1016/j.cub.2006.06.057. [DOI] [PubMed] [Google Scholar]
- 50.McClintock SM, Freitas C, Oberman L, Lisanby SH, Pascual-Leone A. Transcranial magnetic stimulation: a neuroscientific probe of cortical function in schizophrenia. Biol Psychiatry. 2011;70:19–27. doi: 10.1016/j.biopsych.2011.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Carter CS, Barch DM, Bullmore E, Breiling J, Buchanan RW, Butler P, Cohen JD, Geyer M, Gollub R, Green MF, Jaeger J, Krystal JH, Moore H, Nuechterlein K, Robbins T, Silverstein S, Smith EE, Strauss M, Wykes T. Cognitive Neuroscience Treatment Research to Improve Cognition in Schizophrenia II: Developing Imaging Biomarkers to Enhance Treatment Development for Schizophrenia and Related Disorders. Biol Psychiatry. 2011;70:7–12. doi: 10.1016/j.biopsych.2011.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.First MBSR, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Patient Edition. New York: New York State Psychiatric Institute; 2002. [Google Scholar]
- 53.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 54.Aguirre GK, Zarahn E, D'Esposito M. The variability of human, BOLD hemodynamic responses. Neuroimage. 1998;8:360–369. doi: 10.1006/nimg.1998.0369. [DOI] [PubMed] [Google Scholar]
- 55.Ferrarelli F, Massimini M, Peterson MJ, Riedner BA, Lazar M, Murphy MJ, Huber R, Rosanova M, Alexander AL, Kalin N, Tononi G. Reduced evoked gamma oscillations in the frontal cortex in schizophrenia patients: a TMS/EEG study. Am J Psychiatry. 2008;165:996–1005. doi: 10.1176/appi.ajp.2008.07111733. [DOI] [PubMed] [Google Scholar]
- 56.Pomarol-Clotet E, Canales-Rodriguez EJ, Salvador R, Sarro S, Gomar JJ, Vila F, Ortiz-Gil J, Iturria-Medina Y, Capdevila A, McKenna PJ. Medial prefrontal cortex pathology in schizophrenia as revealed by convergent findings from multimodal imaging. Mol Psychiatry. 2010;15:823–830. doi: 10.1038/mp.2009.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shackman AJ, McMenamin BW, Slagter HA, Maxwell JS, Greischar LL, Davidson RJ. Electromyogenic artifacts and electroencephalographic inferences. Brain Topogr. 2009;22:7–12. doi: 10.1007/s10548-009-0079-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Blankenburg F, Ruff CC, Bestmann S, Bjoertomt O, Eshel N, Josephs O, Weiskopf N, Driver J. Interhemispheric effect of parietal TMS on somatosensory response confirmed directly with concurrent TMS-fMRI. J Neurosci. 2008;28:13202–13208. doi: 10.1523/JNEUROSCI.3043-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ferrarelli F, Tononi G. The thalamic reticular nucleus and schizophrenia. Schizophr Bull. 2011;37:306–315. doi: 10.1093/schbul/sbq142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Leichnetz GR. Afferent and efferent connections of the dorsolateral precentral gyrus (area 4, hand/arm region) in the macaque monkey, with comparisons to area 8. J Comp Neurol. 1986;254:460–492. doi: 10.1002/cne.902540403. [DOI] [PubMed] [Google Scholar]
- 61.Yousry TA, Schmid UD, Alkadhi H, Schmidt D, Peraud A, Buettner A, Winkler P. Localization of the motor hand area to a knob on the precentral gyrus. A new landmark. Brain. 1997;120(Pt 1):141–157. doi: 10.1093/brain/120.1.141. [DOI] [PubMed] [Google Scholar]
- 62.Roth well JC, Hallett M, Berardelli A, Eisen A, Rossini P, Paulus W. Magnetic stimulation: motor evoked potentials. The International Federation of Clinical Neurophysiology. Electroencephalogr Clin Neurophysiol Suppl. 1999;52:97–103. [PubMed] [Google Scholar]
- 63.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 64.Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 65.Duvernoy HM, Bourgouin P. The human brain : surface, three-dimensional sectional anatomy with MRI, and blood supply. Wien; New York: Springer; 1999. 2nd, completely rev., and enl. ed. [Google Scholar]
- 66.Corradi-Dell'acqua C, Tomelleri L, Bellani M, Rambaldelli G, Cerini R, Pozzi-Mucelli R, Balestrieri M, Tansella M, Brambilla P. Thalamic-insular dysconnectivity in schizophrenia: Evidence from structural equation modeling. Hum Brain Mapp. 2011 doi: 10.1002/hbm.21246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Roth Y, Amir A, Levkovitz Y, Zangen A. Three-dimensional distribution of the electric field induced in the brain by transcranial magnetic stimulation using figure-8 and deep H-coils. J Clin Neurophysiol. 2007;24:31–38. doi: 10.1097/WNP.0b013e31802fa393. [DOI] [PubMed] [Google Scholar]
- 68.Wagner T, Rushmore J, Eden U, Valero-Cabre A. Biophysical foundations underlying TMS: setting the stage for an effective use of neurostimulation in the cognitive neurosciences. Cortex. 2009;45:1025–1034. doi: 10.1016/j.cortex.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Handwerker DA, Ollinger JM, D'Esposito M. Variation of BOLD hemodynamic responses across subjects and brain regions and their effects on statistical analyses. Neuroimage. 2004;21:1639–1651. doi: 10.1016/j.neuroimage.2003.11.029. [DOI] [PubMed] [Google Scholar]
- 70.Wager TD, Atlas LY, Leotti LA, Rilling JK. Predicting individual differences in placebo analgesia: contributions of brain activity during anticipation and pain experience. J Neurosci. 2011;31:439–452. doi: 10.1523/JNEUROSCI.3420-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Conover WJIR. Rank transformations as a bridge between parametric and nonparametric statistics. The American Statistician. 1981;35:124–129. [Google Scholar]
- 72.Andreasen NC, Pressler M, Nopoulos P, Miller D, Ho BC. Antipsychotic dose equivalents and dose-years: a standardized method for comparing exposure to different drugs. Biol Psychiatry. 2010;67:255–262. doi: 10.1016/j.biopsych.2009.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rose SE, Chalk JB, Janke AL, Strudwick MW, Windus LC, Hannah DE, McGrath JJ, Pantelis C, Wood SJ, Mowry BJ. Evidence of altered prefrontal-thalamic circuitry in schizophrenia: an optimized diffusion MRI study. Neuroimage. 2006;32:16–22. doi: 10.1016/j.neuroimage.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 74.Agarwal N, Rambaldelli G, Perlini C, Dusi N, Kitis O, Bellani M, Cerini R, Isola M, Versace A, Balestrieri M, Gasparini A, Mucelli RP, Tansella M, Brambilla P. Microstructural thalamic changes in schizophrenia: a combined anatomic and diffusion weighted magnetic resonance imaging study. J Psychiatry Neurosci. 2008;33:440–448. [PMC free article] [PubMed] [Google Scholar]
- 75.Glahn DC, Ragland JD, Abramoff A, Barrett J, Laird AR, Bearden CE, Velligan DI. Beyond hypofrontality: a quantitative meta-analysis of functional neuroimaging studies of working memory in schizophrenia. Hum Brain Mapp. 2005;25:60–69. doi: 10.1002/hbm.20138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Meyer-Lindenberg A, Miletich RS, Kohn PD, Esposito G, Carson RE, Quarantelli M, Weinberger DR, Berman KF. Reduced prefrontal activity predicts exaggerated striatal dopaminergic function in schizophrenia. Nat Neurosci. 2002;5:267–271. doi: 10.1038/nn804. [DOI] [PubMed] [Google Scholar]
- 77.Barth DS, MacDonald KD. Thalamic modulation of high-frequency oscillating potentials in auditory cortex. Nature. 1996;383:78–81. doi: 10.1038/383078a0. [DOI] [PubMed] [Google Scholar]
- 78.Rafal RD, Posner MI. Deficits in human visual spatial attention following thalamic lesions. Proc Natl Acad Sci USA. 1987;84:7349–7353. doi: 10.1073/pnas.84.20.7349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Oh JS, Kubicki M, Rosenberger G, Bouix S, Levitt JJ, McCarley RW, Westin CF, Shenton ME. Thalamo-frontal white matter alterations in chronic schizophrenia: a quantitative diffusion tractography study. Hum Brain Mapp. 2009;30:3812–3825. doi: 10.1002/hbm.20809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Morel A, Magnin M, Jeanmonod D. Multiarchitectonic and stereotactic atlas of the human thalamus. J Comp Neurol. 1997;387:588–630. doi: 10.1002/(sici)1096-9861(19971103)387:4<588::aid-cne8>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 81.Golshani P, Liu XB, Jones EG. Differences in quantal amplitude reflect GluR4-subunit number at corticothalamic synapses on two populations of thalamic neurons. Proc Natl Acad Sci USA. 2001;98:4172–4177. doi: 10.1073/pnas.061013698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.McAlonan K, Cavanaugh J, Wurtz RH. Attentional modulation of thalamic reticular neurons. J Neurosci. 2006;26:4444–4450. doi: 10.1523/JNEUROSCI.5602-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zikopoulos B, Barbas H. Circuits formultisensory integration and attentional modulation through the prefrontal cortex and the thalamic reticular nucleus in primates. Rev Neurosci. 2007;18:417–438. doi: 10.1515/revneuro.2007.18.6.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Freedman R, Olincy A, Ross RG, Waldo MC, Stevens KE, Adler LE, Leonard S. The genetics of sensory gating deficits in schizophrenia. Curr Psychiatry Rep. 2003;5:155–161. doi: 10.1007/s11920-003-0032-2. [DOI] [PubMed] [Google Scholar]
- 85.Luck SJ, Gold JM. The construct of attention in schizophrenia. Biol Psychiatry. 2008;64:34–39. doi: 10.1016/j.biopsych.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.