Abstract
The adult central nervous system (CNS) has a remarkable ability to repair itself. However, severe brain and spinal cord injuries cause lasting disability and there are only a few therapies that can prevent or restore function in such cases. In this review, we provide an overview of traumatic CNS injuries and discuss several emerging pharmacological options that have shown promise in preclinical and early clinical studies. We highlight therapies that modulate mammalian target of rapamycin (mTOR) signaling, a pathway that is well known for its roles in cell growth, metabolism and cancer. Interestingly, this pathway is also gaining newfound attention for its role in CNS repair and regeneration.
Keywords: Central nervous system, CNS, brain injury, TBI, spinal cord injury, SCI, mTOR pathway, JAK–STAT pathway, neuroprotection, axon regeneration
Damage to the CNS can be caused by traumatic injuries to the brain or the spinal cord. In most cases, spontaneous recovery occurs. For example, most individuals recover substantially after a first stroke and those with ‘incomplete’ spinal cord injuries (SCI) recover the ability to walk. However, severe and repeated injuries can cause lasting impairments and disabilities that threaten to surpass many diseases as the major cause of death and disability. In the USA, traumatic brain injury (TBI) is the leading cause of death for those under the age of 45, and an estimated 1.4 million are living with devastating consequences of TBI [1,2]. Common causes of traumatic CNS injury include motor vehicle accidents, falls, assaults, self-inflicted injuries and sports-related concussions. Moreover, mild brain injuries from improvised explosive devices are common during military conflicts. In fact, recent reports indicate that a large number of soldiers fighting in Iraq and Afghanistan have sustained mild TBIs [3]. In addition to brain injuries, approximately 2.5 million people worldwide suffer from injuries to the spinal cord and, in the USA, as many as 12 000–15 000 people suffer traumatic SCI each year [4]. Clearly, TBI and SCI are a pressing public health concern and medical issue. Despite considerable efforts to develop treatments for severe traumatic CNS injuries, therapies that prevent or restore functional loss after severe traumatic injuries are still lacking. Here, we review promising pharmacological agents and highlight emerging strategies for the treatment of such injuries.
CNS injuries
TBI, also known as intracranial injury, is generally classified as severe, moderate or mild, based on the severity and disruption of normal brain function. Given that TBI can cause a wide range of short- and long-term changes to behavioral and cognitive functions, diagnoses of moderate to severe TBI are currently done by traditional behavioral measures and functional MRI [5]. By contrast, mild TBI is difficult to diagnose because neurological imaging techniques are unable to detect minor changes in brain function. Additionally, repetitive mild TBI can have serious consequences, including epilepsy and increased incidence of age-related brain diseases, such as Alzheimer’s disease [6]. Thus, it is imperative that diagnoses of brain injuries are done as soon as warning signs or symptoms appear. Clinical symptoms of brain injuries can include cognitive impairments, such as loss of memory, attenuation of speech and deficits in learning ability. These clinical symptoms are often accompanied by behavioral and psychiatric symptoms, such as depression, anxiety, post-traumatic epilepsy (seizure), sleep disorders, irritability, aggression and motor deficits. Mild TBI (e.g. concussions) is defined by symptoms of headaches, dizziness, sleep disturbances and a transient loss of consciousness. Moderate to severe TBIs can display more dramatic impairments, such as slurred speech, repeated vomiting, loss of bladder control and seizures. Patients with TBI can also experience internal hemorrhaging [7]. To minimize the complications and maximize the quality of life, severe brain injuries require early treatment. Even after a long recovery period, stress conditions can reinvoke TBI-related symptoms [8]. Additionally, many of these symptoms have a delayed onset, which makes accurate diagnosis challenging. The US Food and Drug Administration (FDA) has not yet approved any drug or therapies that improve functional outcomes, such as amelioration of memory loss, cognitive deficits, paralysis, sensory loss and other sequelae, of TBI.
Whereas TBI injuries impair brain function, SCI also represent a significant portion of injuries to the CNS [4]. The spinal cord provides a route for communication between the brain and the body, extending down from the base of the brain along the back. Trauma to the spinal cord through injuries from motor vehicle accidents or falls can fracture or dislocate the vertebrate column. This can lead to compression, stretching or severing of the spinal cord and spinal roots situated in the spinal canal, as well as damage to neurons and glia in the spinal cord. The spinal cord is remarkably resistant to injury. For example, in the USA alone, over a million whiplash injuries occur every year, but fewer than 10 000 people suffer severe SCI sufficient to require hospitalization. If a person has residual motor or sensory function below the injury site, they are said to have a so-called ‘incomplete’ spinal cord injury and are likely to recover substantial function, including walking. Approximately half of SCI involve cervical (neck) spinal segments and the other half involve the thoracic and lumbar segments. SCI cause not only paralysis and sensory loss below the injury site, but also spasticity and neuropathic pain, as well as autonomic dysreflexia. Common clinical symptoms of SCI include neuropathic pain, loss of sensation, muscle spasticity, weakness and atrophy. Bladder and bowel dysfunction is common in patients with SCI. In addition to these symptoms, SCI can also affect a patient’s emotional status and many have depression and loss of self-esteem.
Cellular and molecular responses to CNS injury
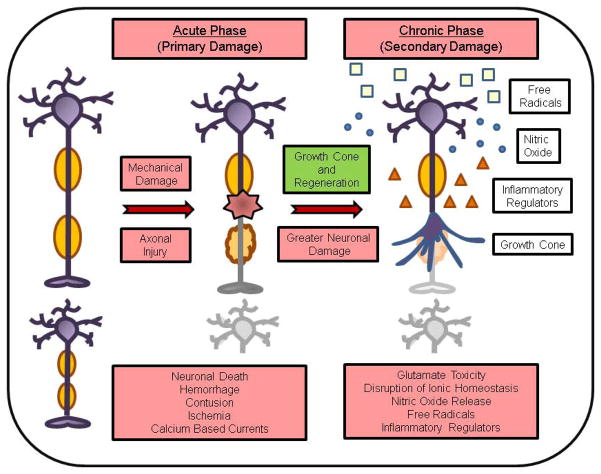
Functional recovery from a traumatic CNS injury is dependent on repair mechanisms that promote axonal regeneration, remyelination, and reconnection of the neuronal circuitry for restoration of neurotransmission (Figure 1). At the cellular level, a traumatic CNS injury causes primary and secondary damage [9,10]. The primary insult induces significant mechanical damage to neurons at the site of injury and to surrounding tissues, resulting in massive neuronal cell death. In this acute phase, the mechanical damage involves the immediate insult, diffuse axonal injury, hemorrhage, contusion and ischemia. At the molecular level, calcium has an important role in detection of, and response to, acute axonal injury. For instance, in cultured mammalian neurons subjected to axotomy, rapid calcium-based currents propagate retrograde signals from the axonal injury site to the cell body [11]. Studies in Caenorhabditis elegans, Drosophila, and mice suggest that calcium signals activate dual leucine kinase-1 (DLK-1), a member of the mitogen-activated protein kinase (MAPK) pathway, to initiate regeneration in response to the CNS injury [12]. In addition, retrograde transport of several other intracellular proteins functions as a signal for the neuronal cell body to generate a response to the injury. The primary damage is typically followed by a secondary phase, where greater neuronal damage occurs hours and days following the initial insult. The secondary injury develops over the post-traumatic period and is the result of a combination of vasogenic and cytotoxic edema, including glutamate excitotoxicity, disturbance of ionic homeostasis, lipid peroxidation, generation of nitric oxide and free radicals, and release of inflammatory regulators. Given that functional recovery is modest in severely injured patients because of the limited capacity of injured CNS neurons to regenerate, and that neurogenesis (birth of new neurons) is restricted to certain areas of the adult CNS [13], prevention of secondary injury is crucially important. For the much larger population of chronically impaired patients with SCI, regenerative and neural replacement therapies are needed.
Figure 1.
[LM2][LM3]Mechanisms involved in functional recovery from a traumatic central nervous system (CNS) injury.
Current and experimental CNS therapies
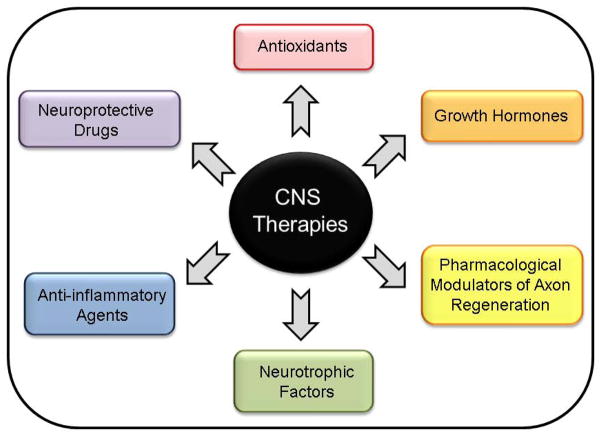
Pharmacological therapies for TBI are still at the preclinical stage or under clinical investigation, and include neuroprotective drugs, antioxidants, neurotrophic factors, growth hormones and anti-inflammatory agents (Figure 2). Although these therapies have shown significant promise in preclinical studies, only a few agents have demonstrated efficacy in clinical trials [14–17]. During the 1990s, the dominant theory of secondary injury after trauma to the brain was glutamate excitotoxicity, which can damage cells, disrupt metabolism and contribute to further ionic disequilibrium [18]. However, all trials of glutamate receptor blockers have so far failed to show any benefit in patients with TBI even though these drugs continue to show neuroprotection in preclinical studies [14,17]. The reasons attributed to the failure of these clinical trials include insufficient mechanistic understanding of secondary injuries, lack of adequate animal models and experimental design in preclinical studies. These disappointing results have led to a reappraisal of clinical trial study design, as well as recommendations for improving future data analysis and standardization of data collection processes. Other neuroprotective compounds, including erythropoietin (EPO) and tetracycline derivatives, have proved efficacious in early stages of clinical investigation and several phase II and III trials are ongoing [14–16]. Numerous trials are also underway to evaluate the efficacy of neurotrophic factors [e.g. nerve growth factor (NGF)], hormone therapies (e.g. progesterone) and anti-inflammatory agents (e.g. apolipoprotein E-mimetics) [16]. Although a selected few agents appear promising, none of the pharmacotherapies tested so far have successfully reached phase III randomized controlled clinical trials [16,19]. The FDA has yet to approve drugs or therapies that improve the functional outcome of patients with TBI.
Figure 2.
[LM4]Current pharmacological therapies under investigation for the treatment of traumatic brain injury (TBI). Abbreviation: CNS, central nervous system.
The ultimate challenge for scientists and clinicians investigating SCI is to discover and develop effective therapies to prevent secondary injury, and to restore motor, sensory and autonomic functions. At present, patients undergo rehabilitation after SCI, aimed at teaching them how to manage their impairments and reduce disability. Although many solutions have been developed to prevent SCI-related consequences and improve quality of life and independence, no therapies are currently available that restore function [4,20–22]. Methylprednisolone sodium succinate (MPSS), a synthetic glucocorticoid, is widely used to treat acute SCI world-wide and is FDA approved for the treatment of various inflammatory conditions. In addition to its well-known anti-inflammatory actions, MPSS also has neuroprotective and antioxidant properties. The second National Acute Spinal Cord Injury Study (NASCIS) found that, when administered intravenously (i.v.) within 8 h of the injury for a period of 24 h, MPSS improved neurological recovery by 20% in patients with SCI [23]. MPSS is the only treatment shown to be beneficial for treatment of acute SCI in clinical trials [23,24]. Other promising pharmacological therapies for acute SCI include EPO and minocycline, both of which have reported benefits in animal studies of SCI [21,25]. These agents are currently being investigated in clinical trials for the treatment of SCI. Several other drugs that are FDA approved for other indications are also being considered for treatment of acute SCI [25]. Riluzole is an anticonvulsant agent used for treatment of amyotrophic lateral sclerosis (ALS), a neurodegenerative motor neuron disease [26]. In animal studies, a combination of riluzole plus MPSS promotes functional recovery compared with either drug alone [27]. A phase I clinical trial is being planned to assess the safety and efficacy of riluzole for acute SCI (NCT00876889). Lithium, an FDA-approved drug for the treatment of bipolar disorders, can increase the levels of neurotrophic factors, stimulate neurogenesis, promote axon growth and provide neuroprotection [28]. A phase II trial is currently comparing lithium to MPSS using umbilical cord blood cell transplants (NCT01046786). It remains to be seen whether these newer pharmacological agents will effectively prevent the secondary injury process and restore function in patients with SCI.
Targeting mTOR as an emerging pharmacological strategy
Original studies on axonal regeneration revealed that, unlike the peripheral nervous system (PNS), the adult human CNS environment contains extracellular inhibitory factors that limit the ability of injured CNS neurons to repair themselves after a traumatic injury [29–31]. Owing to the modest effect of current pharmacological therapies that target extracellular inhibitory factors [e.g. anti-NOGO-A monoclonal antibody (ATI355), MAG antagonist (GSK249320) and Rho GTPase antagonist (cethrin)], more recent research has focused on the role of intrinsic signaling mechanisms in promoting functional recovery after a traumatic CNS injury. These studies have led to the current view that stimulation of intrinsic signaling pathways might overcome the inhibitory extracellular CNS environment to promote functional recovery following injury [32].
The mTOR pathway
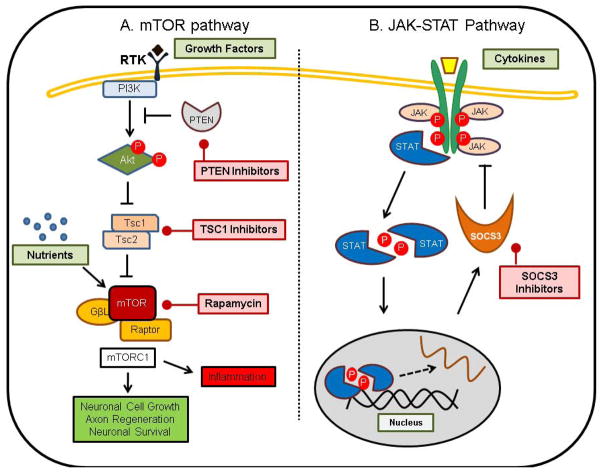
The phosphatidylinositol 3′-kinase (PI3K)–Akt–mTOR pathway responds to growth factors and mitogenic signals (Figure 3) [33,34], and emerging evidence suggests that modulation of this pathway promotes axonal regeneration and neuronal survival [32,35]. Briefly, growth factors and mitogen signals activate receptor tyrosine kinases present on the cell membrane that, in turn, activate PI3K, catalyzing the conversion of phosphatidylinositol 4,5-bisphosphate (PIP2) to phosphatidylinositol 3,4,5-triphosphate (PIP3). Phosphatase and tensin homolog deleted on chromosome ten (PTEN) is responsible for negative regulation of PIP2 to PIP3 conversion. PIP3 recruits Akt, protein kinase B (PKB) and 3-phosphoinositide-dependent protein kinase 1 (PDK1) to the cell membrane. As a result, Akt is phosphorylated and activated by PDK1. Once activated, Akt inactivates tuberous sclerosis protein complex (TSC), a heterodimer comprising tuberous sclerosis protein 1 (TSC1) and TSC2, by directly phosphorylating TSC2. TSC2 acts as a GTPase-activating protein (GAP) for Ras homolog enriched in brain protein (Rheb), which is immediately upstream of the mTOR and is responsible for stimulation of its activity. mTOR forms two functionally distinct complexes, referred to as mTORC1 and mTORC2. These complexes phosphorylate downstream substrates to regulate various growth-related cell processes. Known substrates of these two complexes include ribosomal protein S6 kinase 1 (p70S6K), inhibitor of eukaryotic translation initiation factor 4E (4E-BP1) and CLIP-170 for mTORC1 and Akt and protein kinase C (PKC)α for mTORC2. mTORC1 controls transcription, translation, ribosome biogenesis and autophagy, whereas mTORC2 regulates cell survival and actin dynamics.
Figure 3.
[LM5]Strategies for treatment of traumatic central nervous system (CNS) injuries. (a) The phosphatidylinositol 3-kinase (PI3K)–Akt–mammalian target of rapamycin (mTOR) pathway. (b) The Janus kinase/signal transducer and activator of transcription (JAK---STAT) pathway. Abbreviations: PTEN, phosphatase and tensin homolog deleted on chromosome ten; RTK, XXX[LM6]; SOCS3, suppressor of cytokine signaling 3; TSC1/2, tuberous sclerosis protein 1/2.
mTOR has an important role in several physiological functions of the nervous system, including regulation of neuronal cell growth, survival, axonal and dendritic development during differentiation, and synaptic plasticity [36,37]. Neuronal mTOR regulates protein synthesis in cell bodies and axons, which is important for cellular growth. It also controls the size of cell soma. The formation of a new growth cone requires mTOR activity, which is also responsible for axonal protein synthesis. Generation of a new growth cone is dependent on this axonal protein synthesis. Moreover, mTOR function has been implicated in dendrite development and morphogenesis of dendritic spines, which also requires protein synthesis and actin organization [38]. mTOR is required for neurotrophin-induced dendrite and spine development through activation of the PI3K–Akt pathway and Ras [39,40]. In addition, other factors, such as the extracellular protein Reelin, induce mTOR activity via the PI3K–Akt pathway, thereby promoting dendritic outgrowth in dissociated neurons [41,42]. Finally, mTOR function is also linked to various forms of synaptic plasticity. These mTOR-dependent physiological functions are also important during CNS repair and regeneration; therefore, mTOR is likely to have an instrumental role in the functional recovery process following a traumatic CNS injury.
Stimulation of the mTOR pathway
Several recent studies have revealed that activation of the intrinsic growth signal in adult CNS neurons can significantly reduce neuronal death and promote repair and regeneration. In a rat model of TBI, phosphorylation of mTOR and its downstream targets (p70S6K, S6 and 4E-BP1) increased within 30 min of a moderate injury to the parietal cortex and lasted up to 24 h [43]. This increase in phosphorylation and activation of mTOR pathway could provide a mechanism to respond to, and recover from, the TBI. In fact, Park et al. demonstrated that activation of mTOR signaling pathway in adult retinal ganglion cells (RGCs) induced robust axon regeneration after optical nerve injury [35]. Moreover, a follow-up study from the same research group using corticospinal tract (CST) axonal injury models demonstrated that mTOR activity was also a crucial regulator of the regenerative capacity in corticospinal neurons [44]. The CST is essential for voluntary movements and CST axons are often injured during SCI. These investigators showed that conditional deletion of PTEN enhances axon regeneration from both spared axons (compensatory sprouting) and injured axons (regenerative growth). The regenerating injured axons were able to pass through the lesion site and form synapses past these sites. Therefore, upregulation of mTOR pathway might be sufficient to promote axon regeneration in the adult CNS after brain injuries and SCI [35,44].
ATP is an important regulator of signaling pathways and is thought to have a central role in recovery after CNS injuries; therefore, Hu et al. investigated the role of ATP-induced alterations in mTOR signaling pathway for repair of SCI using a rat compression model [45]. Exogenous administration of ATP significantly increased protein and mRNA levels as well as the phosphorylation and activation of mTOR pathway components in the spinal cord. Activation of mTOR pathway was associated with improvements in locomotor function recovery after the injury and increases in expression of neuronal genes, nestin, neuronal nuclei (NeuN) and neurofilament 200 (NF200). These findings suggest that stimulation of mTOR pathway provides beneficial effects for locomotor functional defects associated with SCI. In another study, Ning et al. depleted PTEN in SMN7-deficient mice (an animal model of spinal muscular atrophy) using a adeno-associated virus serotype 6 (AAV6)-mediated knockdown approach. Depletion of PTEN in spinal motor neurons rescued axonal growth defect and prevented motor neuron cell death [46]. Park et al. further demonstrated that TSC1 knockout does not mimic the effects of PTEN knockout, suggesting that other mTOR-independent pathways are involved in stimulating axon regeneration [35]. Nevertheless, mTOR activity is crucial for axonal regenerative response in adult CNS injuries.
In addition to neuroprotection and axon regeneration, stimulation of mTOR activity might be beneficial in other aspects associated with TBI. For instance, mTOR activity reduces the cytotoxic effect of TBI-induced glutamate excitotoxicity. mTOR activity is required for glutamate transporter expression in cultured astrocytes [47]. Given that the amount of glutamate transporter is associated with glutamate clearance, it is possible that pharmacological activation of mTOR reduces glutamate-induced excitotoxicity induced by TBI. Moreover, mTOR activity is required for insulin-induced neuronal differentiation of neural progenitors in primary culture [48]. These data suggest that modulation of mTOR promotes neurogenesis. Therefore, pharmacological activation of mTOR pathway might significantly improve the condition of patients with traumatic CNS injuries.
Inhibition of the mTOR pathway
Interestingly, inhibition of mTOR with rapamycin in animal models of TBI is beneficial for ameliorating TBI-associated symptoms, including epilepsy and adverse inflammatory responses [49–53]. TBI-induced epilepsy is characterized by numerous abnormalities, including molecular, cellular and synaptic activities in the brain [53]. mTOR might also be involved in these cellular processes, which cause epileptogenesis following brain injuries. First, mTOR regulates various cellular functions, such as protein synthesis and synaptic plasticity, that might cause abnormally excited electrical signals, thereby contributing to epileptogenesis in the injured brain. Second, TSC is one of the most common genetic causes of epilepsy [54]. It is caused by mutation of the genes TSC1/2, which encode the upstream negative regulators of mTOR [33]. The role of mTOR in epilepsy pathogenesis has been demonstrated using the mTOR inhibitor rapamycin in a variety of mouse models of epilepsy [53,55,56]. Therefore, pharmacological inhibition of mTOR to alleviate TBI-induced epilepsy could represent a novel anti-epileptogenic therapy for patients with TBI.
In addition to epilepsy, the secondary brain injury is frequently associated with neuro-inflammatory responses owing to activation of immune cells, such as microglia and astrocytes. These cause secondary neuronal damage by releasing cytotoxic molecules, including reactive oxygen species (ROS) and cytokines [57]. In addition, early inflammatory response (within hours) contributes to the later stages of brain injuries [58]. As a potent immunosuppressant, rapamycin has been investigated for its neuroprotective effects in closed head injury TBI models [50]. Rapamycin injection within 4 h following brain injury reduced microglia and/or macrophage activation, increased survival of neurons and significantly improved brain functional recovery [50]. Taken together, these encouraging preclinical studies suggest that pharmacological modulation of mTOR pathway is an attractive therapeutic strategy for treatment of traumatic CNS injuries.
mTOR-independent signaling pathways
Although the mTOR pathway is important for axonal regeneration, other mTOR-independent pathways are also essential for this process. Given that TSC1 knockout did not mimic the effects of PTEN knockout, Park and colleagues suggested that other mTOR-independent downstream targets of PTEN, such as 3′PIs, glycogen synthase kinase 3 (GSK-3), collapsin response modifier protein 2 (CRMP2), adenomatous polyposis coli (APC) and MAP1b are involved in axon regeneration [59]. Moreover, deletion of suppressor of cytokine signaling 3 (SOCS3), a negative regulator of the Janus kinase/signal transducer and activator of transcription (JAK–STAT) pathway, also promotes axon regeneration of injured optic nerves [60]. The JAK–STAT pathway is activated by cytokines, including ciliary neurotrophic factor (CNTF) and interleukin 6 (IL-6) (Figure 3) [61]. These cytokines are upregulated in response to CNS injury and can have a role in transmitting the injury signal and promoting axon regeneration [62]. Exogenous CNTF treatment dramatically increased the axon regeneration of injured optic nerves in SOCS3-deleted RGCs [60]. The effect on axon regeneration was comparable to that observed in RGCs of PTEN-knockout mice. CNTF has been investigated for use as an anti-obesity and type 2 diabetes mellitus drug [63]; it is possible that exogenous CNTF treatment in combination with SOCS3 inhibitors could be used for treatment of CNS injuries.
Microtubule dynamics are integral for axon growth and regeneration. In the case of SCI, the two main processes that impede SCI repair are hypertrophic scar formation and modest ability to regrow injured axons, both of which require correct microtubule dynamics. Notably, two recent reports demonstrate that paclitaxel at low concentrations might be beneficial for CNS injuries [64–66]. Paclitaxel is a microtubule-stabilizing drug approved by the FDA for treatment of cancer. At high concentrations, it stabilizes microtubules, which prevents the dynamics necessary for cell division; thus, it is very effective as an anticancer drug. However, at low concentrations, paclitaxel promotes polymerization at microtubule plus-ends within the growth cone and provides a means for axon regeneration after injury. Hellal and colleagues demonstrated that low-dose paclitaxel stimulated intrinsic axon growth and decreased scar formation to facilitate axon regeneration in SCI [64]. Paclitaxel also provided neuroprotection against ischemia and/or reperfusion-induced cell death in brain injury [67]. These studies have made a strong case for the use of microtubule stabilizers in the management of traumatic CNS injuries. The challenge with paclitaxel is that it does not cross the blood–brain barrier (BBB); however, chemically modified derivatives of this drug or chemically modified nanoparticle carriers could be used to optimize delivery across the BBB [68]. Overall, it is evident that both mTOR-dependent and -independent pathways have essential roles in repair and regeneration following CNS injury. Therefore, pharmacological modulation of these pathways is emerging as a new strategy for treatment of traumatic CNS injuries (Figure 3).
Perspectives and concluding remarks
Modulation of intrinsic signaling pathways, in particular, the mTOR signaling and the JAK–STAT pathways, is central for promoting CNS repair and regeneration. Stimulation of the mTOR signaling pathway provides neuroprotection, promotes neurogenesis and axon regeneration while reducing the cytotoxic effects of TBI-induced glutamate release. However, inhibition of mTOR activity with rapamycin also provides neuroprotection. It might be that a delicate balance between inhibition and activation of mTOR pathway exists to maintain neuronal survival while promoting axonal regeneration. Undoubtedly, this paradoxical effect needs further investigation and clarification in preclinical and clinical studies. SF1670 is a small molecule inhibitor of PTEN originally developed for treatment of type 2 diabetes mellitus [69], and this, or other novel PTEN inhibitors, could be used to investigate this further. Unlike PTEN inhibitors, TSC1 inhibitors might not be as beneficial because knockout of TSC1 did not have the same dramatic effect on axon regeneration as in PTEN-knockout mice [35]. Finally, given that hyperactivation of the PI3K–Akt–mTOR pathway is found in many different cancers [34], risks associated with the use of PTEN or TSC1 inhibitors for TBI or SCI treatment should be evaluated further. Pharmacological activation of the JAK–STAT pathway can be accomplished with cytokines such as CNTF or SOCS3 inhibitors. Because CNTF treatment dramatically increased axon regeneration of injured optic nerves in SOCS3-deleted RGCs, CNTF alone and in combination with SOCS3 inhibitors should be evaluated further in animal models and clinical trials. However, activation of the JAK–STAT pathway can also cause cancer (importantly, a recent study demonstrated that deletion of PTEN and SOCS3 together is able to sustain axon regeneration [70]). Therefore, combination therapies of both the mTOR pathway and JAK–STAT pathway could enhance functional recovery in injured patients.
Gene therapy has also gained considerable attention in recent years and is being increasingly explored to promote neuronal growth and/or minimize neuronal death post-injury [71]. Viral vector-mediated delivery of growth factors or cytokines that stimulate the mTOR and JAK–STAT pathways directly into the injury site could be used to stimulate the intrinsic regenerative capacity of injured neurons and promote axon regeneration. These approaches have been studied in animal models [71]. Although gene therapy studies remain in the preclinical stage of research, as more is understood about the signaling pathways that are affected during brain injuries and SCIs, this approach could become a viable option for treatment. However, gene therapy is still controversial because the benefits versus risks are not yet known.
Despite the limitations and controversies, the number of therapeutic strategies to promote CNS repair and regeneration is increasing, and the emerging pharmacological approaches appear promising. Repair and regeneration of the injured CNS will be one of the most challenging tasks to face the medical community over the next decade or so. Understanding the physiology of the CNS as well as the pathophysiology of TBI and SCI will be crucial for the development of effective CNS therapies.
Highlights.
We provide a brief overview of CNS injuries and current therapies
Currently few therapies are available to treat severe CNS injuries
Emerging evidence suggests intrinsic signaling pathways are central for CNS repair and regeneration
mTOR pathway plays an important role in several physiological functions of the CNS
Modulation of mTOR pathway could be an effective novel approach for treatment of CNS injuries
Acknowledgments
AAD is supported by a Postdoctoral Fellowship from the New Jersey Commission on Spinal Cord Injury. CKT is supported by a grant from the New Jersey Commission on Brain Injury. GD and TMK are supported in part by a research grant from the New Jersey Governor’s Council for Medical Research and Treatment of Autism.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Thurman DJ, et al. Traumatic brain injury in the United States: a public health perspective. J Head Trauma Rehabil. 1999;14:602–615. doi: 10.1097/00001199-199912000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Bruns J, Jr, Hauser WA. The epidemiology of traumatic brain injury: a review. Epilepsia. 2003;44 (Suppl 10):2–10. doi: 10.1046/j.1528-1157.44.s10.3.x. [DOI] [PubMed] [Google Scholar]
- 3.Okie S. Traumatic brain injury in the war zone. N Engl J Med. 2005;352:2043–2047. doi: 10.1056/NEJMp058102. [DOI] [PubMed] [Google Scholar]
- 4.Thuret S, et al. Therapeutic interventions after spinal cord injury. Nat Rev Neurosci. 2006;7:628–643. doi: 10.1038/nrn1955. [DOI] [PubMed] [Google Scholar]
- 5.Provenzale JM. Imaging of traumatic brain injury: a review of the recent medical literature. AJR Am J Roentgenol. 2010;194:16–19. doi: 10.2214/AJR.09.3687. [DOI] [PubMed] [Google Scholar]
- 6.Fandino-Rivera J. Traumatic brain injury and ischemic stroke: a delayed sequela? Rev Neurol. 2004;38:912–915. [PubMed] [Google Scholar]
- 7.Simpson JR. Mild traumatic brain injury and postconcussion syndrome: the new evidence base for diagnosis and treatment. J Am Acad Psychiatry Law. 2011;39:133–134. [Google Scholar]
- 8.Risdall JE, Menon DK. Traumatic brain injury. Philos Trans R Soc B. 2011;366:241–250. doi: 10.1098/rstb.2010.0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McDonald JW, Sadowsky C. Spinal-cord injury. Lancet. 2002;359:417–425. doi: 10.1016/S0140-6736(02)07603-1. [DOI] [PubMed] [Google Scholar]
- 10.Chua KS, et al. A brief review of traumatic brain injury rehabilitation. Ann Acad Med Singapore. 2007;36:31–42. [PubMed] [Google Scholar]
- 11.Mandolesi G, et al. Acute physiological response of mammalian central neurons to axotomy: ionic regulation and electrical activity. FASEB J. 2004;18:1934–1936. doi: 10.1096/fj.04-1805fje. [DOI] [PubMed] [Google Scholar]
- 12.Ferguson TA, et al. Armed response: how dying cells influence T-cell functions. Immunol Rev. 2011;241:77–88. doi: 10.1111/j.1600-065X.2011.01006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lie DC, et al. Neurogenesis in the adult brain: new strategies for central nervous system diseases. Annu Rev Pharmacol Toxicol. 2004;44:399–421. doi: 10.1146/annurev.pharmtox.44.101802.121631. [DOI] [PubMed] [Google Scholar]
- 14.Narayan RK, et al. Clinical trials in head injury. J Neurotrauma. 2002;19:503–557. doi: 10.1089/089771502753754037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schouten JW. Neuroprotection in traumatic brain injury: a complex struggle against the biology of nature. Curr Opin Crit Care. 2007;13:134–142. doi: 10.1097/MCC.0b013e3280895d5c. [DOI] [PubMed] [Google Scholar]
- 16.Maas AI, et al. Clinical trials in traumatic brain injury: past experience and current developments. Neurotherapeutics. 2010;7:115–126. doi: 10.1016/j.nurt.2009.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Royo NC, et al. Pharmacology of traumatic brain injury. Curr Opin Pharmacol. 2003;3:27–32. doi: 10.1016/s1471-4892(02)00006-1. [DOI] [PubMed] [Google Scholar]
- 18.Park E, PhD, et al. Traumatic brain injury: can the consequences be stopped? Can Med Assoc J. 2008;178:1163–1170. doi: 10.1503/cmaj.080282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stein DG, Wright DW. Progesterone in the clinical treatment of acute traumatic brain injury. Expert Opin Invest Drugs. 2010;19:847–857. doi: 10.1517/13543784.2010.489549. [DOI] [PubMed] [Google Scholar]
- 20.Rossignol S, et al. Spinal cord injury: time to move? J Neurosci. 2007;27:11782–11792. doi: 10.1523/JNEUROSCI.3444-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baptiste DC, Fehlings MG. Emerging drugs for spinal cord injury. Expert Opin Emerg Drugs. 2008;13:63–80. doi: 10.1517/14728214.13.1.63. [DOI] [PubMed] [Google Scholar]
- 22.Rabchevsky AG, Kitzman PH. Latest approaches for the treatment of spasticity and autonomic dysreflexia in chronic spinal cord injury. Neurotherapeutics. 2011;8:274–282. doi: 10.1007/s13311-011-0025-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bracken MB, et al. A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal-cord injury. Results of the Second National Acute Spinal Cord Injury Study. N Engl J Med. 1990;322:1405–1411. doi: 10.1056/NEJM199005173222001. [DOI] [PubMed] [Google Scholar]
- 24.Bracken MB, et al. Administration of methylprednisolone for 24 or 48 hours or tirilazad mesylate for 48 hours in the treatment of acute spinal cord injury. Results of the Third National Acute Spinal Cord Injury Randomized Controlled Trial National Acute Spinal Cord Injury Study. JAMA. 1997;277:1597–1604. [PubMed] [Google Scholar]
- 25.Rabchevsky AG, et al. Pharmacological interventions for spinal cord injury: where do we stand? How might we step forward? Pharmacol Ther. 2011;132:15–29. doi: 10.1016/j.pharmthera.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 26.Wagner ML, Landis BE. Riluzole: a new agent for amyotrophic lateral sclerosis. Ann Pharmacother. 1997;31:738–744. doi: 10.1177/106002809703100614. [DOI] [PubMed] [Google Scholar]
- 27.Mu X, et al. Riluzole and methylprednisolone combined treatment improves functional recovery in traumatic spinal cord injury. J Neurotrauma. 2000;17:773–780. doi: 10.1089/neu.2000.17.773. [DOI] [PubMed] [Google Scholar]
- 28.Young W. Review of lithium effects on brain and blood. Cell Transplant. 2009;18:951–975. doi: 10.3727/096368909X471251. [DOI] [PubMed] [Google Scholar]
- 29.David S, Aguayo AJ. Axonal elongation into peripheral nervous system ‘bridges’ after central nervous system injury in adult rats. Science. 1981;214:931–933. doi: 10.1126/science.6171034. [DOI] [PubMed] [Google Scholar]
- 30.Yiu G, He Z. Glial inhibition of CNS axon regeneration. Nat Rev Neurosci. 2006;7:617–627. doi: 10.1038/nrn1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McKerracher L, Winton MJ. Nogo on the go. Neuron. 2002;36:345–348. doi: 10.1016/s0896-6273(02)01018-8. [DOI] [PubMed] [Google Scholar]
- 32.Liu K, et al. Neuronal intrinsic mechanisms of axon regeneration. Annu Rev Neurosci. 2011;34:131–152. doi: 10.1146/annurev-neuro-061010-113723. [DOI] [PubMed] [Google Scholar]
- 33.Tsang C, et al. Targeting mammalian target of rapamycin (mTOR) for health and diseases. Drug Discov Today. 2007;12:112–124. doi: 10.1016/j.drudis.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 34.Don AS, Zheng XF. Recent clinical trials of mTOR-targeted cancer therapies. Rev Recent Clin Trials. 2011;6:24–35. doi: 10.2174/157488711793980147. [DOI] [PubMed] [Google Scholar]
- 35.Park KK, et al. Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science. 2008;322:963–966. doi: 10.1126/science.1161566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jaworski J, Sheng M. The growing role of mTOR in neuronal development and plasticity. Mol Neurobiol. 2006;34:205–219. doi: 10.1385/MN:34:3:205. [DOI] [PubMed] [Google Scholar]
- 37.Swiech L, et al. Role of mTOR in physiology and pathology of the nervous system. Biochim Biophys Acta. 2008;1784:116–132. doi: 10.1016/j.bbapap.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 38.Swiech L, et al. CLIP-170 and IQGAP1 cooperatively regulate dendrite morphology. J Neurosci. 2011;31:4555–4568. doi: 10.1523/JNEUROSCI.6582-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jaworski J, et al. Control of dendritic arborization by the phosphoinositide-3′-kinase-Akt-mammalian target of rapamycin pathway. J Neurosci. 2005;25:11300–11312. doi: 10.1523/JNEUROSCI.2270-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kumar V, et al. Regulation of dendritic morphogenesis by Ras-PI3K-Akt-mTOR and Ras-MAPK signaling pathways. J Neurosci. 2005;25:11288–11299. doi: 10.1523/JNEUROSCI.2284-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jossin Y, et al. Processing of Reelin by embryonic neurons is important for function in tissue but not in dissociated cultured neurons. J Neurosci. 2007;27:4243–4252. doi: 10.1523/JNEUROSCI.0023-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ventruti A, et al. Reelin deficiency causes specific defects in the molecular composition of the synapses in the adult brain. Neuroscience. 2011;189:32–42. doi: 10.1016/j.neuroscience.2011.05.050. [DOI] [PubMed] [Google Scholar]
- 43.Chen S, et al. Alterations in mammalian target of rapamycin signaling pathways after traumatic brain injury. J Cereb Blood Flow Metab. 2007;27:939–949. doi: 10.1038/sj.jcbfm.9600393. [DOI] [PubMed] [Google Scholar]
- 44.Liu K, et al. PTEN deletion enhances the regenerative ability of adult corticospinal neurons. Nat Neurosci. 2010;13:1075–1081. doi: 10.1038/nn.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hu LY, et al. ATP-mediated protein kinase B Akt/mammalian target of rapamycin mTOR/p70 ribosomal S6 protein p70S6 kinase signaling pathway activation promotes improvement of locomotor function after spinal cord injury in rats. Neuroscience. 2010;169:1046–1062. doi: 10.1016/j.neuroscience.2010.05.046. [DOI] [PubMed] [Google Scholar]
- 46.Ning K, et al. PTEN depletion rescues axonal growth defect and improves survival in SMN-deficient motor neurons. Hum Mol Genet. 2010;19:3159–3168. doi: 10.1093/hmg/ddq226. [DOI] [PubMed] [Google Scholar]
- 47.Wu X, et al. PI3K/Akt/mTOR signaling regulates glutamate transporter 1 in astrocytes. Biochem Biophys Res Commun. 2010;393:514–518. doi: 10.1016/j.bbrc.2010.02.038. [DOI] [PubMed] [Google Scholar]
- 48.Han J, et al. Mammalian target of rapamycin (mTOR) is involved in the neuronal differentiation of neural progenitors induced by insulin. Mol Cell Neurosci. 2008;39:118–124. doi: 10.1016/j.mcn.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 49.Pignataro G, et al. Neuroprotective, immunosuppressant and antineoplastic properties of mTOR inhibitors: current and emerging therapeutic options. Curr Opin Pharmacol. 2011;11:378–394. doi: 10.1016/j.coph.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 50.Erlich S, et al. Rapamycin is a neuroprotective treatment for traumatic brain injury. Neurobiol Dis. 2007;26:86–93. doi: 10.1016/j.nbd.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 51.Martel RR, et al. Inhibition of the immune response by rapamycin, a new antifungal antibiotic. Can J Physiol Pharmacol. 1977;55:48–51. doi: 10.1139/y77-007. [DOI] [PubMed] [Google Scholar]
- 52.Sunnen CN, et al. Inhibition of the mammalian target of rapamycin blocks epilepsy progression in NS-Pten conditional knockout mice. Epilepsia. 2011;52:2065–2075. doi: 10.1111/j.1528-1167.2011.03280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wong M. Mammalian target of rapamycin (mTOR) inhibition as a potential antiepileptogenic therapy: from tuberous sclerosis to common acquired epilepsies. Epilepsia. 2010;51:27–36. doi: 10.1111/j.1528-1167.2009.02341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sparagana SP. Tuberous sclerosis complex: from basic science to clinical phenotypes: International Review of Child Neurology Series. Neurology. 2006;67:546-a. [Google Scholar]
- 55.D’Arcangelo G. Rapamycin treatment suppresses epileptogenic activity in conditional Pten knockout mice. Cell Cycle. 2010;9:2487–2488. doi: 10.4161/cc.9.13.12272. [DOI] [PubMed] [Google Scholar]
- 56.Ljungberg MC, et al. Rapamycin suppresses seizures and neuronal hypertrophy in a mouse model of cortical dysplasia. Dis Model Mech. 2009;2:389–398. doi: 10.1242/dmm.002386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shohami E, et al. Cytokine production in the brain following closed head injury: dexanabinol (HU-211) is a novel TNF-α inhibitor and an effective neuroprotectant. J Neuroimmunol. 1997;72:169–177. doi: 10.1016/s0165-5728(96)00181-6. [DOI] [PubMed] [Google Scholar]
- 58.DeLegge MH, Smoke A. Neurodegeneration and Inflammation. Nutr Clin Pract. 2008;23:35–41. doi: 10.1177/011542650802300135. [DOI] [PubMed] [Google Scholar]
- 59.Park KK, et al. PTEN/mTOR and axon regeneration. Exp Neurol. 2010;223:45–50. doi: 10.1016/j.expneurol.2009.12.032. [DOI] [PubMed] [Google Scholar]
- 60.Smith PD, et al. SOCS3 deletion promotes optic nerve regeneration in vivo. Neuron. 2009;64:617–623. doi: 10.1016/j.neuron.2009.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cattaneo E, et al. Signalling through the JAK-STAT pathway in the developing brain. Trends Neurosci. 1999;22:365–369. doi: 10.1016/s0166-2236(98)01378-2. [DOI] [PubMed] [Google Scholar]
- 62.Zigmond RE, et al. Changes in neuropeptide phenotype after axotomy of adult peripheral neurons and the role of leukemia inhibitory factor. Perspect Dev Neurobiol. 1996;4:75–90. [PubMed] [Google Scholar]
- 63.Matthews VB, Febbraio MA. CNTF: a target therapeutic for obesity-related metabolic disease? J Mol Med. 2008;86:353–361. doi: 10.1007/s00109-007-0286-y. [DOI] [PubMed] [Google Scholar]
- 64.Hellal F, et al. Microtubule stabilization reduces scarring and causes axon regeneration after spinal cord injury. Science. 2011;331:928–931. doi: 10.1126/science.1201148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sengottuvel V, Fischer D. Facilitating axon regeneration in the injured CNS by microtubules stabilization. Commun Integr Biol. 2011;4:391–393. doi: 10.4161/cib.4.4.15552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sengottuvel V, et al. Taxol facilitates axon regeneration in the mature CNS. J Neurosci. 2011;31:2688–2699. doi: 10.1523/JNEUROSCI.4885-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Qi SH, et al. Neuroprotection of paclitaxel against cerebral ischemia/reperfusion-induced brain injury through JNK3 signaling pathway. J Recept Signal Transduct Res. 2011;31:402–407. doi: 10.3109/10799893.2011.621070. [DOI] [PubMed] [Google Scholar]
- 68.Juillerat-Jeanneret L. The targeted delivery of cancer drugs across the blood-brain barrier: chemical modifications of drugs or drug-nanoparticles? Drug Discov Today. 2008;13:1099–1106. doi: 10.1016/j.drudis.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 69.Rosivatz E, et al. A small molecule inhibitor for phosphatase and tensin homologue deleted on chromosome 10 (PTEN) ACS Chem Biol. 2006;1:780–790. doi: 10.1021/cb600352f. [DOI] [PubMed] [Google Scholar]
- 70.Sun F, et al. Sustained axon regeneration induced by co–deletion of PTEN and SOCS3. Nature. 2011:LM1. doi: 10.1038/nature10594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bo X, et al. Gene therapy approaches for neuroprotection and axonal regeneration after spinal cord and spinal root injury. Curr Gene Ther. 2011;11:1a01–115. doi: 10.2174/156652311794940773. [DOI] [PubMed] [Google Scholar]