Abstract
Here we report a unique role for MHC II–peptide complexes in controlling immune responses of naïve CD8 T cells. Compared with CD8 T cells from WT mice, CD8 T cells isolated from MHC II−/− mice hyperproliferated under lymphopenic conditions, differentiated into effector cells producing proinflammatory cytokines, and mediated more severe tissue inflammation. The elevated responses of MHC II−/− CD8 T cells were due to the absence of MHC II, but not CD4, T cells. The hyperreactivity appeared to be a feature of mature T cells, given its absence in CD8 single positive thymocytes derived from MHC II−/− mice. Expression of the MHC II ligand LAG3 was markedly enhanced during in vivo activation of MHC II−/− CD8 T cells, and blockade of MHC II–LAG3 interactions further enhanced T-cell expansion. Importantly, CD8 T cells isolated from H-2M−/− mice expressing WT levels of MHC II also displayed hyperresponsiveness similar to that of MHC II−/− CD8 T cells, suggesting that peptides presented on MHC II are involved in the control of CD8 T-cell responses. Our results uncover a previously undefined MHC II-dependent regulation that tunes CD8 T-cell reactivity and may have implications for an improved understanding of CD8 T-cell homeostasis and functions.
Keywords: lymphocytes, proliferation, lymphopenia, self-peptide, proliferation
T-cell recognition of self-peptide–MHC complexes is a critical step that warrants the development of T cells with useful yet harmless antigen receptors. Whether TCR–MHC interactions are necessary for maintaining cell survival in the periphery remains a matter of debate (1–5); however, these “low-affinity” interactions can promote antigen sensitivity of CD4 T cells, in part by sustaining partial TCR-ζ chain phosphorylation (6). Under lymphopenic settings, naïve T cells undergo endogenous proliferation (7, 8) induced in part by recognition of self-peptide–MHC complexes, occurring in a MHC-restricted manner (9–11). Unlike naïve CD4 T cells that fail to proliferate in MHC II-deficient lymphopenic recipients, naïve CD8 T cells undergo substantial proliferation in MHC I-deficient lymphopenic conditions (12). This MHC I-independent proliferation is completely abolished in the absence of both MHC I and MHC II molecules, suggesting the possible involvement of a CD8 T-cell–MHC II molecule interaction in this proliferation (12). Previous studies indicated that some CD8 T cells gain unusual reactivity to MHC II-restricted epitopes, especially in CD4-deficient hosts, after bacterial or viral infection (13, 14). However, CD8 T-cell recognition of self-peptide–MHC II complexes and the biological significance of such interactions have not yet been formally explored.
Here we report that MHC II molecules negatively control naïve CD8 T-cell reactivity in vivo. Naïve CD8 T cells isolated from MHC II−/− donors exhibited enhanced proliferation and expansion when transferred to lymphopenic recipients. These CD8 T cells also responded vigorously under inflammatory conditions, inducing colitis after transfer into Rag−/− recipients and severe CD8-dependent contact hypersensitivity after hapten skin sensitization and challenge. Elevated production of proinflammatory cytokines was found after stimulation of MHC II−/− CD8 T cells. The hyperresponsiveness of MHC II−/− CD8 T cells was due primarily to the lack of MHC II expression rather than to the absence of CD4 T cells. Importantly, the hyperresponse of MHC II−/− CD8 T cells was not due to an anti-MHC II CD8 T-cell response. The expression of LAG-3, an activation-induced cell surface molecule that interacts with MHC II, was greater in MHC II−/− CD8 T cells after transfer into lymphopenic recipients, and administration of blocking anti-LAG-3 mAb increased the expansion. Most strikingly, naïve CD8 T cells isolated from H-2M−/− mice exhibited similar hyperproliferation as MHC II−/− CD8 T cells, suggesting that peptides loaded onto MHC II molecules are involved in regulating the CD8 T-cell responses. Taken together, our results suggest that interactions between self-peptide-MHC II complexes and CD8 T cells are likely to occur in vivo and may play a key role in controlling both proliferation and differentiation of CD8 T cells in response to homeostatic and inflammatory cues.
Results and Discussion
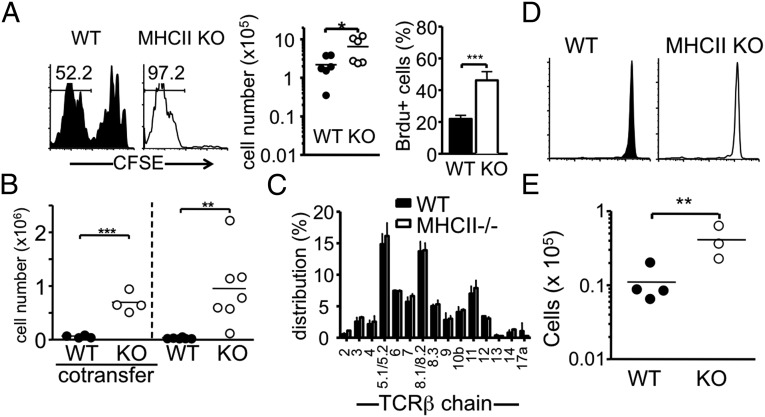
We previously reported that naïve CD8 T-cell proliferation under lymphopenic conditions is not influenced by the lack of MHC I, and that the MHC I-independent CD8 proliferation requires MHC II (12). CD8 T cells that either are restricted to or cross-recognize MHC II have been reported previously (13, 14), although the frequency of these cells is likely low. Aiming to explore the biology of MHC II-restricted CD8 T cells, we compared endogenous proliferation of naïve CD8 T cells isolated from WT and MHC II−/− donor mice, with the expectation that MHC II-restricted CD8 T cells might be absent in the MHC II−/− mice (13). When naïve (CD44low) CD8 T cells were transferred into Rag−/− recipients, MHC II−/− naïve CD8 T-cell proliferation was markedly pronounced compared with that of WT naïve CD8 T cells, based on carboxyfluorescein succinimidyl ester (CFSE) dilution and total cell recovery (Fig. 1A). Likewise, BrdU incorporation was significantly higher in MHC II−/− CD8 T cells than in WT cells (Fig. 1A). This unexpected behavior of MHC II−/− CD8 T cells was also seen when the cells were transferred into other lymphopenic (sublethally irradiated, TCR-β−/−, and TCR-βδ−/−) recipients (Fig. S1). When both WT and MHC II−/− CD8 T cells were cotransferred into the same recipients, MHC II−/− CD8 T cells still expanded better than WT cells (Fig. 1B), indicating that the enhanced proliferation of MHC II−/− CD8 T cells is a cell-intrinsic property.
Fig. 1.
Expansion of MHCII−/− CD8 T cells in different lymphopenic recipients. (A) CFSE-labeled 1 × 106 naive Thy1.1 WT or MHC II−/−CD8 T cells were transferred into groups of Rag1−/− recipients. CFSE profiles, absolute numbers, and BrdU incorporation of Thy1.1+ cells in the pLN were examined at 7 d after the transfer. The results shown are representative of six individually tested recipients. (B) FACS-sorted naïve Ly5.1 WT and Thy1.1 MHC II−/− CD8 T cells (5 × 105 cells per recipient) were cotransferred or transferred separately into TCR- β/δ−/− recipients. The absolute numbers of donor cells in the pLN were examined at 7 d after the transfer. Each symbol represents an individually tested recipient. (C) Distribution of TCR- Vβ expression was compared between WT and MHC II−/− naïve CD8 T cells. Data shown are the mean ± SD of individually tested mice (n = 3). (D) CFSE-labeled 1 × 106 naive Thy1.2 WT or Thy1.2 MHC II−/− CD8 T cells were transferred into Thy1.1 WT recipients. The CFSE profile was examined at 7 d after the transfer. (E) WT or MHCII−/− CD8 T cells were transferred into MHC II−/− Rag−/− recipients. The results shown are representative of three individually tested recipients. *P < 0.05; **P < 0.01; ***P < 0.001.
The TCR-Vβ distribution of naïve CD8 T cells was similar in the two strains of mice (Fig. 1C), and the TCR distribution remained similar after proliferation into TCR-β−/− recipients (Fig. S2), strongly suggesting that enhanced proliferation is not related to an oligoclonal expansion of a T-cell clone specific for undefined Ag expressed in the recipient mice. In support of this suggestion, when MHC II−/− CD8 T cells were transferred into lymphocyte-sufficient mice, the transferred cells remained undivided (Fig. 1D). Furthermore, naïve MHC II−/− CD8 T cells or proliferating CD8 T cells reisolated from TCR-β−/− recipients after transfer remained unstimulated by MHC II-expressing cells in vitro (Fig. S3A). These enhanced responses of MHC II−/− CD8 T cells were not seen after anti-CD3 stimulation in vitro (Fig. S3B). Moreover, the proliferative advantage of MHCII−/− CD8 T cells was also seen in Rag−/− recipients deficient in MHC II, again strongly suggesting that an anti-MHC II alloresponse is not a factor in the unexpected hyperexpansion of MHC II−/− CD8 T cells (Fig. 1E).
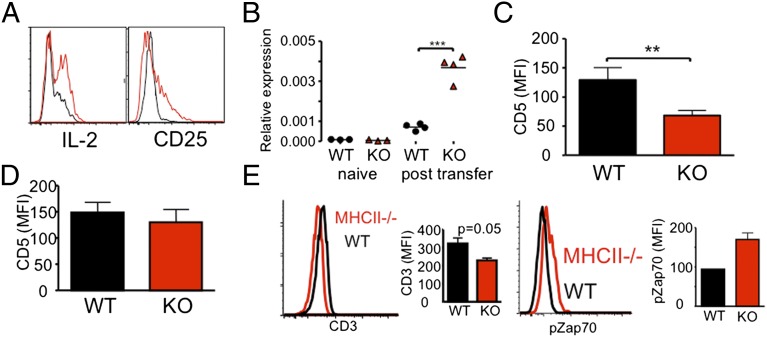
Enhanced in vivo responses of MHC II−/− CD8 T cells were further supported by the phenotypes acquired during proliferation. Frequencies of CD25+ and IL-2+ CD8 T cells were substantially higher in MHC II−/− CD8 T cells compared with WT CD8 T cells (Fig. 2A). Consistent with this finding, IL-2 mRNA expression was significantly increased in MHC II−/− CD8 T cells (Fig. 2B). Measurement of T-cell expansion at 2 wk posttransfer showed superior MHC II−/− CD8 T-cell expansion compared with WT cells; however, IL-2 and CD25 expression was then down-regulated (Fig. S4). This finding was expected, given the expected slowing of proliferation as the need for homeostatic expansion wanes over time. MHCII−/− CD8 T cells displayed a significant down-regulation in CD5 expression compared with WT CD8 T cells (Fig. 2C), although the expression on naïve CD8 T cells before transfer was similar in WT and MHC II−/− donors (Fig. 2D). In vivo CD3 down-regulation and Zap70 phosphorylation were more pronounced in MHC II−/− CD8 T cells compared with WT CD8 T cells (Fig. 2E). Collectively, these results suggest that the overall activation of naïve CD8 T cells in response to homeostatic signals is greatly enhanced when these cells are derived from MHC II−/− environments.
Fig. 2.
Activation phenotypes of MHC II−/− CD8 T cells in vivo. FACS-sorted naive Thy1.1 WT or MHC II−/− CD8 T cells were transferred into TCR-β−/− recipients. (A and B) CD25 and intracellular IL-2 expression (A) and CD8 T-cell IL-2 mRNA expression (B) were measured at 7 d after the transfer. Results shown are representative of between three and six individually tested recipients. (C and D) Surface CD5 expression was examined at 7 d posttransfer (C) or before transfer (D). Graphs show mean ± SD values of between four and six individually tested mice from two independent experiments. (E) Surface CD3ε expression on WT and MHC II−/− CD8 T cells was examined at 16 h posttransfer. The graph shows the mean ± SD of three individually tested mice. **P < 0.01; ***P < 0.001.
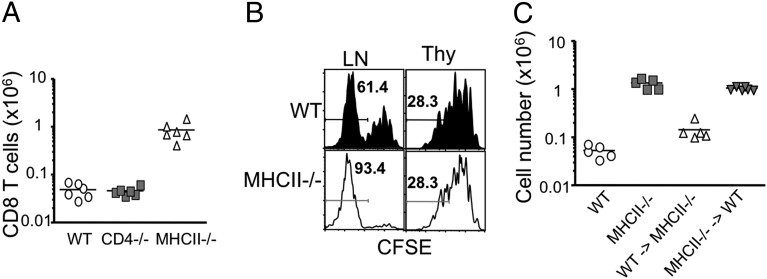
The foregoing hyperproliferation might possibly be attributed to the lack of CD25+ regulatory T cells in donor MHC II−/− mice (15). To examine this issue, we used naïve CD8 T cells isolated from CD4−/− donor mice lacking CD4 T cells, including regulatory T cells. Proliferation and expansion of CD4−/− CD8 T cells in Rag−/− recipients were similar to that in WT CD8 T cells (Fig. 3A). Likewise, naïve CD8 T cells isolated from WT mice treated for 3 wk with mAb to deplete CD25+ cells showed similar proliferated as WT CD8 T cells (Fig. S5). This finding indicates that MHC II deficiency confers hyperproliferation of MHC II−/− CD8 T cells in response to homeostatic signals. Importantly, enhanced proliferation was not observed when CD8 SP thymocytes isolated from MHC II−/− mice were used instead (Fig. 3B), suggesting that that the hyperresponse of MHC II−/− CD8 T cells is a feature of fully mature T cells. This finding also implies that MHC II expressed within the thymus is not directly involved in altering the behavior of developing CD8 T cells. To directly address this possibility, BM chimeras in which MHC II expression was compartmentalized were generated. Lethally irradiated MHC II−/− and WT mice were reconstituted with WT and MHC II−/− BM cells, respectively. Naïve CD8 T cells were then isolated from the LNs of MHC II−/− BM → WT or WT BM → MHC II−/− chimeric mice at 6 wk after reconstitution and transferred into Rag−/− recipients. Expansion of CD8 T cells derived from MHC II−/− BM → WT mice was markedly elevated, similar to CD8 T cells from MHC II−/− mice (Fig. 3C). In contrast, CD8 T cells from WT BM → MHC II−/− mice proliferated to similar levels as WT CD8 T cells. These results suggest that the enhanced proliferation of MHC II−/− CD8 T cells occurs only after they have matured and seeded the periphery, and that MHC II expressed in the periphery and/or MHC II+ cells of hematopoietic origin in the thymus control the hyperreactivity of CD8 T cells.
Fig. 3.
MHC II deficiency is responsible for the enhanced response of MHC II−/− CD8 T cells. (A) 1 × 106 naive Thy1.2 WT, CD4−/−, and MHC II−/− CD8 T cells were transferred into Rag−/− recipients. CFSE profiles and the absolute numbers of donor T cells in the pLN were examined at 7 d after the transfer. The results shown are representative of six individually tested recipients in two independent experiments. (B) Thy1.1 WT or MHC II−/− thymic CD8+CD4−CD44− or peripheral CD8+44− cells were transferred into Rag−/− recipients (1 × 106 per recipient). CFSE profiles of the donor cells in the pLN were examined at 7 d after transfer. Similar results were observed from two independent experiments. (C) Lethally irradiated WT and MHC II−/− mice received BM cells from MHC II−/− and WT mice, respectively. After 6 wk of BM reconstitution, naive CD8 T cells were sorted and transferred into TCR-β−/− recipients. Donor cell recovery in the pLN was analyzed at 7 d after the transfer. The graph shows the mean ± SD of two independent experiments.
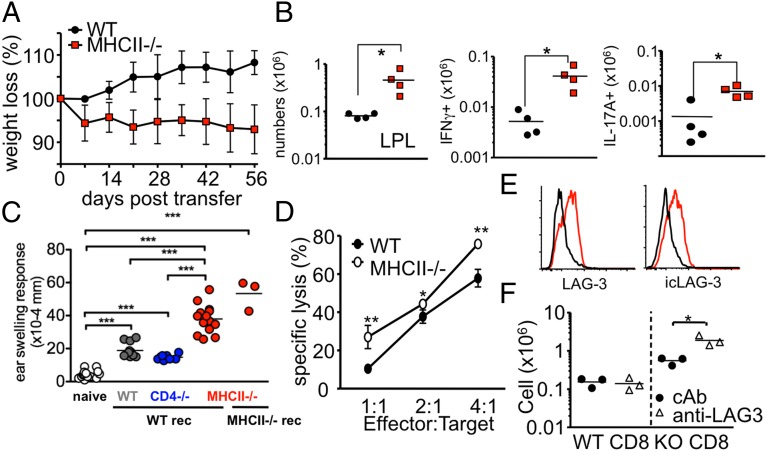
We next evaluated whether the hyperproliferative status of MHC II−/− CD8 T cells is also seen during CD8 T-cell–mediated immune responses. It was previously reported that CD8 T cells can differentiate into colitogenic IL-17A–producing cells in Rag−/− recipients in an IL-6–dependent manner (16). WT or MHC II−/− naïve CD8 T cells were transferred to Rag−/− mice, which were then monitored for the development of colitis. Unlike in the previous report (16), here the Rag−/− recipients of WT CD8 T cells continued to gain weight and had a normal histological appearance of the colon (Fig. 4A and Fig. S6A). In contrast, Rag−/− recipients of MHC II−/− CD8 T cells rapidly lost body weight and exhibited severely inflamed colon tissues (Fig. 4A and Fig. S6A). Consistent with this, donor CD8 T-cell accumulation in the mesenteric LNs (Fig. S6B) and lamina propria (Fig. 4B) was substantially greater in MHC II−/− T-cell recipients. More importantly, expression of proinflammatory cytokines IFN-γ and IL-17 was dramatically higher in MHC II−/− CD8 T cells than in WT CD8 T cells (Fig. 4B and Fig. S6B).
Fig. 4.
MHC II−/− CD8 T cells induced severe colitis and CHS. (A and B) 1 × 106 naive Thy1.1 WT and MHC II KO CD8 T cells were transferred into TCR-β−/− recipients. (A) Body weight was monitored weekly and is presented as percentage of the initial weight at day 0. (B) Lamina propria cells were isolated at 5 wk posttransfer, and cytokine expression was analyzed by intracellular cytokine staining. Total IFN-γ– and IL-17A–producing Thy1.1 CD8 T cells were enumerated. *P < 0.05. (C) WT, CD4−/−, and MHC II−/− mice were sensitized with DNFB as described in Materials and Methods. Draining LN CD8 T cells were isolated on day 5. Then 1 × 107 cells were transferred into naïve WT or MHC II−/− recipients, and the recipients were subsequently challenged on each side of both ears with DNFB. After 16 h, ear swelling was measured. Each symbol represents an individually tested mouse. (D) WT 2C or MHC II−/− 2C TCR Tg CD8 T cells were transferred into Rag−/− mice and then reisolated from the recipients at 7 d posttransfer. An ex vivo killing assay was performed using differentially CFSE-labeled target or control cells. (E) Surface and intracellular LAG-3 expression of WT (black) and MHC II−/− (red) was measured at 7 d posttransfer into Rag−/− mice. (F) WT and MHC II−/− CD8 T cells were transferred into TCR-βδ−/− mice that had been injected with rat IgG or anti–LAG-3 mAb.
Escalated responses of MHC II−/− CD8 T cells were also found in hapten-induced contact hypersensitivity (CHS), a response mediated by CD8 T cells producing IFN-γ and IL-17 (17). WT, CD4−/−, and MHC II−/− mice were sensitized with 2,4-dinitrofluorobenzene (DNFB). CD8 T cells were harvested from the draining LNs of the sensitized mice and then adoptively transferred into naïve recipients, which were immediately challenged on each side of both ears with DNFB to elicit the CHS response. Little or no ear swelling was seen in DNFB-challenged naïve mice (Fig. 4C). Significantly greater ear swelling was seen in challenged mice that had received sensitized WT or CD4−/− CD8 T cells. However, the mice that received sensitized MHC II−/− CD8 T cells demonstrated a significantly elevated CHS response compared with the other groups (Fig. 4C). Importantly, an enhanced CHS response of MHC II−/− T cells was also seen in MHC II−/− recipients (Fig. 4C), providing more evidence that anti-MHC II response is not an aspect of the hyperresponsiveness. Evaluation of cytokine secretion of hapten-specific CD8 T cells showed significantly higher numbers of hapten-specific CD8 T cells producing IL-17A in MHC II−/− donors, and higher numbers of hapten-specific CD8 T cells producing IFN-γ in mice that received either MHC II−/− or CD4−/− CD8 T cells (Fig. S7). On transfer of MHC II−/− 2C TCR Tg CD8 T cells were transferred into Rag−/− recipients, the expansion of MHC II−/− 2C cells exceeded that of WT 2C T cells (Fig. S8). Furthermore, MHC II−/− 2C cells displayed greater cytotoxicity (Fig. 4D). These results suggest that vigorous responses of MHC II−/− CD8 T cells are not limited to homeostasis, but are seen in inflammatory responses as well.
MHC II is known to interact with LAG-3, an activation-induced inhibitory molecule expressed on T cells (18). Our results raise the possibility that the absence of MHC II molecules may alter LAG-3 expression and/or function of MHC II−/− CD8 T cells, and thus account for their enhanced responsiveness. Although LAG-3 expression of naïve CD8 T cells was not detected in WT and MHC II−/− mice (Fig. S9A), both surface and intracellular LAG-3 proteins were significantly up-regulated in MHC II−/− CD8 T cells after transfer into Rag−/− recipients (Fig. 4E). Serum levels of soluble LAG-3 were also higher in Rag−/− recipients of MHC II−/− CD8 T cells (Fig. S9B). Consistent with the notion that LAG-3 plays an inhibitory role during T-cell proliferation in lymphopenic recipients (19), injection of anti–LAG-3 Ab further enhanced the expansion of MHC II−/− CD8 T cells (Fig. 4F). No such effect was seen in WT CD8 T cells, however, suggesting that the level of LAG-3 expression may determine the extent of inhibition induced by LAG-3.
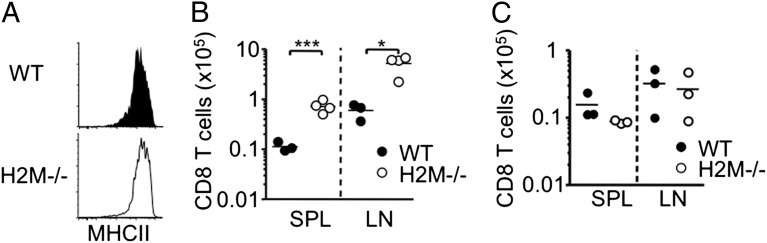
The finding that MHC II deficiency renders CD8 T cells hyperresponsive during immune responses raises an unexpected, yet interesting possibility that MHC II molecules control CD8 T-cell homeostasis by limiting the expansion of activated CD8 T cells. Whether peptides bound on the MHC II molecules play a role in this process is not known, however. To directly test this possibility, we used naïve CD8 T cells isolated from mice deficient in H-2M (20). MHC II expression was normal in the H-2M−/− mice (Fig. 5A); however, the peptides loaded on the MHC II molecules were predominantly class II-associated invariant chain peptides (20). Naïve H-2M−/− CD8 T-cell proliferation and subsequent expansion were greatly enhanced in Rag−/− recipients compared with WT cells, and the extent of expansion was similar to that seen in MHC II−/− CD8 T cells (Fig. 5B). Consistent with the results shown in Fig. 3, CD8 SP thymocytes from H-2M−/− mice did not exhibit hyperresponsiveness, again indicating that the hyperresponsive feature is achieved on maturation of these cells (Fig. 5C). Collectively, these results strongly suggest that the repertoire of peptides loaded in MHC II rather than the MHC II molecule itself plays a central role in setting CD8 T-cell reactivity.
Fig. 5.
H2M−/− naïve CD8 T cells expanded better than WT CD8 T cells. (A) Expression of MHC II molecule of WT and H2M−/− splenic CD11c+ dendritic cells was measured by FACS analysis. The results shown are representative of two or three individually tested recipients. (B) CFSE-labeled 1 × 106 naive Thy1.1 WT or Thy1.1 H2M−/− CD8 T cells were transferred into Rag1−/− recipients, and the absolute numbers of donor T cells were counted at 7 d after the transfer. The results shown are representative of three or four individually tested recipients. (C) CFSE-labeled CD8 SP thymocytes from H2M−/− mice were transferred into Rag−/− recipients. Absolute numbers in the pLN were examined at 7 d posttransfer. *P < 0.05; ***P < 0.001.
Our finding that naïve CD8 T cells raised in an MHC II−/− (or H-2M−/−) environment vigorously respond to both homeostatic and inflammatory signals uncovers an unexpected “inhibitory” role for self-peptide–MHC II complexes in limiting CD8 T-cell reactivity. This contrasts with naïve CD4 T cells that interact with MHC II complexes to maintain antigen sensitivity (6). The molecular mechanism underlying this discrepancy remains to be identified; however, a negative feedback mechanism involving the tyrosine phosphatase-1, SHP-1, and/or TCR desensitization via Lck kinase inactivation might play a role (21). As a result, TCR–MHC II interaction may generate a “tune down” signal, without which it induces a strong phosphorylation of ZAP70 as well as down-regulation of CD3 and CD5 and hyperactivation of CD8 T cells after activation. Consistent with this possibility, MHC II−/− CD8 T cells displayed phenotypes associated with stronger activation compared with those of WT CD8 T cells, including greater LAG-3 expression.
LAG-3 is known to play a negative role in T-cell activation. Blocking LAG-3 interaction with its ligand MHC II in vivo further enhanced the expansion of MHC II−/− CD8 T cells, indicating that LAG-3 may still be capable of limiting the expansion. Interestingly, however, MHC II−/− CD8 T cells expressing high levels of LAG-3 after in vivo proliferation expand better than WT CD8 T cells expressing low levels of LAG-3. Therefore, the level of LAG-3 expressed on activated MHCII−/− CD8 T cells does not appear to be sufficient to mediate the inhibitory role. The finding that blocking LAG-3 did not affect the expansion of WT CD8 T cells that express lower LAG-3 supports this notion. It also has been suggested that soluble LAG-3 molecules cleaved from activated T cells may bind to MHC II on antigen-presenting cells and result in activation of the antigen-presenting cells as well as T cells (22). However, this does not appear to be the case based on the results from the cotransfer experiment. Importantly, the fact that a dominant class II-associated invariant chain peptide bound on MHC II molecules is sufficient to allow CD8 T cells to become hyperresponsive strongly suggests that CD8 T-cell–MHC II interaction may involve TCR. Thus, CD8 T cells may “scan” self-peptide–MHC II complexes probably via TCR, which then generate a signal that tunes down CD8 T-cell activity. Given that LAG-3 is an activation-induced molecule, it will be important to investigate the relationship of the pathway that induces LAG-3 and that mediates LAG-3–dependent inhibition via LAG-3–MHC II. Collectively, CD8 T-cell sensitivity to antigen stimulation can be determined by a “nonconventional” pathway that uses MHC II–peptide complexes. Defining both cellular and molecular mechanisms will promote a better understanding of CD8 T-cell immunity.
Materials and Methods
Mice.
C57BL/6, B6 Ly5.1, B6 Thy1.1, B6 CD4−/−, B6 TCR-β−/−, B6 TCR-βδ−/−, B6 Rag1−/−, and B6 MHC II (H2dlAb1-Ea)−/− mice were purchased from Jackson Laboratory. B6 H-2M−/− mice were provided from Dr. Charles Surh (The Scripps Research Institute, La Jolla, CA). B6 2C TCR Tg and B6 MHC II−/− 2C TCR Tg mice were maintained in the laboratory. All animal procedures were conducted in accordance with the guidelines of the Lerner Research Institute Institutional Animal Care and Use Committee.
Cell Sorting and Adoptive Transfer.
LN naive T cells were obtained as follows. Peripheral LNs (pLNs; axillary, cervical, and inguinal LN) and mesenteric LNs (mLNs) were pooled and the total T cells were purified by negative selection. In brief, cells were stained with FITC-conjugated anti-B220 (RA3-6B2), anti-FcγR (clone 93), anti-NK1.1 (PK136), and anti-MHC II (M5/114) Abs (all purchased from eBioscience). FITC-labeled LN cells were subsequently incubated with anti-FITC microbeads (Miltenyi) and passed through a LS column (Miltenyi). CD44low naive T cells were further sorted using a FACSAria cell sorter (BD Bioscience). In some experiments, donor T cells were labeled with CFSE (Molecular Probes) and transferred i.v. into recipients. In some experiments, blocking rat anti–LAG-3 mAb (C9B7W) was injected (day 0, 100 μg; day 5, 50 μg) as described previously (23).
FACS Analysis.
Recipients were killed at the indicated time points after T-cell transfer. pLN, mLN, and the spleen cells were harvested, single cell suspension obtained, and stained with anti-CD3 (2C11), anti-CD5 (53-7.3), anti-CD8 (53-6.7), anti-CD25 (PC61), anti-CD122 (5H4), anti-CD127 (A7R34), anti-CD44 (IM7), anti-CD62L (MEL-14), anti-CD45.1 (A20), anti-MHC II (M5/114), anti–PD-1 (J43), anti-Thy1.1 (HIS51), and anti-Thy1.2 (30-H12) Abs. For intracellular staining, anti–IL-2 (PC61.5), anti–IFN-γ (XMG1.2), and anti–IL-17A (ebio17B7) Abs were used (all purchased from eBioscience). Cells were acquired with a BD Biosciences LSRII flow cytometer and analyzed with FlowJo software (TreeStar).
BM Reconstitution.
BM cells collected from the tibia of donor animals were transferred i.v. into lethally irradiated recipients. Gentamycin was injected (1 mg) into the recipients at day 0 and day 2 of BM transfer. Before experiments, BM cell reconstitution was confirmed by FACS analysis. Typically, reconstituted mice were used at 6–8 wk after BM transfer.
Lamina Propria Cell Isolation.
Colons were isolated and cleaned in HBSS. Colons were cut into small pieces, resuspended in HBSS containing 0.5 μM EDTA and 15 μg/mL of DTT, and shaken twice for 15 min at room temperature. Colons were then resuspended in complete RPMI with 400 μg/mL of DNase and 1 mg/mL of collagenase and shaken at 37 °C for 90 min. Supernatant and colon wwere passed through a 70-μm strainer and washed. Cells were resuspended in a 33% Percoll gradient and spun at room temperature for 20 min. The resulting pellet was collected, washed, and used for further experiments.
Ex Vivo Stimulation.
Cells harvested as described above were stimulated with PMA (10 ng/mL) and ionomycin (1 μM) for 4 h in the presence of 2 μM Monensin (Calbiochem) during the last 2 h. The cells were then immediately fixed with 4% paraformaldehyde, permeabilized, and stained with fluorescence conjugated antibodies.
Hapten Sensitization and Elicitation of CHS.
Mice were sensitized to DNFB by painting the shaved abdomen with 25 μL of 0.25% DNFB (Sigma-Aldrich) and each paw with 10 μL of 0.25% DNFB on days 0 and 1 (17). Then, 5 d later, CD8 T cells were isolated from draining LNs. A total of 1 × 107 cells were adoptively transferred into WT Thy1.1 mice that were subsequently challenged on each side of each ear with 10 μL of DNFB. After 16 h, ear thickness was measured using an engineer’s micrometer (Mitutoyo America). For enzyme-linked immunosorbent spot analysis, isolated CD8 T cells were stimulated with hapten-pulsed syngenic T-cell–depleted splenocytes, and cytokine-producing cells were enumerated.
Real-Time PCR.
Sorted CD8 T cells were disrupted using a TissueLyser II (Qiagen). Total RNA was extracted using an RNeasy column (Qiagen), and cDNA was obtained using SuperScript III reverse transcriptase (Invitrogen). Real-time PCR was performed using gene-specific primers and probe sets and an ABI 7500 PCR machine (Applied Biosystems).
Ex Vivo Cytotoxicity Assay.
In brief, T-cell–depleted splenocytes from BALB/c and B6 mice were differentially labeled with CFSE. Equal numbers of target (CFSElow) and control target (CFSEhigh) cells were mixed together and cocultured with WT or MHC II−/− 2C TCR Tg CD8 T cells reisolated from Rag−/− recipients that had received the cells 1 wk earlier. Target cells incubated alone were used as a negative control. The plate was briefly centrifuged (300 × g for 1 min) to gather cells into contact and incubated overnight at 37 °C. Specific lysis was calculated as follows: [1 − (ratio of targets only/ratio of target + T cells)] × 100.
Data Analysis.
Statistical significance was determined by the Student t test using the Prism 4 software (GraphPad). P < 0.05 was considered to indicate statistical significance.
Supplementary Material
Acknowledgments
We thank Katayoun Ayasoufi and Danielle Kish for technical assistance. This study was supported by National Institutes of Health (NIH) Grants AI074932 and NS064932 (to B.M.) and the Cleveland Clinic Foundation (B.M.). D.A.A.V was supported by NIH Grant AI39480, National Cancer Institute Comprehensive Cancer Center Support Grant CA21765, and American Lebanese Syrian Associated Charities.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1207219109/-/DCSupplemental.
References
- 1.Murali-Krishna K, et al. Persistence of memory CD8 T cells in MHC class I-deficient mice. Science. 1999;286:1377–1381. doi: 10.1126/science.286.5443.1377. [DOI] [PubMed] [Google Scholar]
- 2.Tanchot C, Lemonnier FA, Pérarnau B, Freitas AA, Rocha B. Differential requirements for survival and proliferation of CD8 naïve or memory T cells. Science. 1997;276:2057–2062. doi: 10.1126/science.276.5321.2057. [DOI] [PubMed] [Google Scholar]
- 3.Swain SL, Hu H, Huston G. Class II-independent generation of CD4 memory T cells from effectors. Science. 1999;286:1381–1383. doi: 10.1126/science.286.5443.1381. [DOI] [PubMed] [Google Scholar]
- 4.Dorfman JR, Stefanová I, Yasutomo K, Germain RN. CD4+ T cell survival is not directly linked to self-MHC–induced TCR signaling. Nat Immunol. 2000;1:329–335. doi: 10.1038/79783. [DOI] [PubMed] [Google Scholar]
- 5.Clarke SR, Rudensky AY. Survival and homeostatic proliferation of naive peripheral CD4+ T cells in the absence of self-peptide:MHC complexes. J Immunol. 2000;165:2458–2464. doi: 10.4049/jimmunol.165.5.2458. [DOI] [PubMed] [Google Scholar]
- 6.Stefanová I, Dorfman JR, Germain RN. Self-recognition promotes the foreign antigen sensitivity of naive T lymphocytes. Nature. 2002;420:429–434. doi: 10.1038/nature01146. [DOI] [PubMed] [Google Scholar]
- 7.Min B, Paul WE. Endogenous proliferation: burst-like CD4 T cell proliferation in lymphopenic settings. Semin Immunol. 2005;17:201–207. doi: 10.1016/j.smim.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 8.Do JS, et al. Both exogenous commensal and endogenous self antigens stimulate T cell proliferation under lymphopenic conditions. Cell Immunol. 2012;272:117–123. doi: 10.1016/j.cellimm.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moses CT, Thorstenson KM, Jameson SC, Khoruts A. Competition for self ligands restrains homeostatic proliferation of naive CD4 T cells. Proc Natl Acad Sci USA. 2003;100:1185–1190. doi: 10.1073/pnas.0334572100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gruber A, Brocker T. MHC class I-positive dendritic cells (DC) control CD8 T cell homeostasis in vivo: T cell lymphopenia as a prerequisite for DC-mediated homeostatic proliferation of naive CD8 T cells. J Immunol. 2005;175:201–206. doi: 10.4049/jimmunol.175.1.201. [DOI] [PubMed] [Google Scholar]
- 11.Tan JT, et al. Interleukin (IL)-15 and IL-7 jointly regulate homeostatic proliferation of memory phenotype CD8+ cells but are not required for memory phenotype CD4+ cells. J Exp Med. 2002;195:1523–1532. doi: 10.1084/jem.20020066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Do JS, Min B. Differential requirements of MHC and of DCs for endogenous proliferation of different T-cell subsets in vivo. Proc Natl Acad Sci USA. 2009;106:20394–20398. doi: 10.1073/pnas.0909954106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tyznik AJ, Sun JC, Bevan MJ. The CD8 population in CD4-deficient mice is heavily contaminated with MHC class II-restricted T cells. J Exp Med. 2004;199:559–565. doi: 10.1084/jem.20031961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pearce EL, Shedlock DJ, Shen H. Functional characterization of MHC class II-restricted CD8+CD4− and CD8−CD4− T cell responses to infection in CD4−/− mice. J Immunol. 2004;173:2494–2499. doi: 10.4049/jimmunol.173.4.2494. [DOI] [PubMed] [Google Scholar]
- 15.McNally A, Hill GR, Sparwasser T, Thomas R, Steptoe RJ. CD4+CD25+ regulatory T cells control CD8+ T-cell effector differentiation by modulating IL-2 homeostasis. Proc Natl Acad Sci USA. 2011;108:7529–7534. doi: 10.1073/pnas.1103782108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tajima M, et al. IL-6–dependent spontaneous proliferation is required for the induction of colitogenic IL-17–producing CD8+ T cells. J Exp Med. 2008;205:1019–1027. doi: 10.1084/jem.20071133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kish DD, Li X, Fairchild RL. CD8 T cells producing IL-17 and IFN-gamma initiate the innate immune response required for responses to antigen skin challenge. J Immunol. 2009;182:5949–5959. doi: 10.4049/jimmunol.0802830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Workman CJ, Dugger KJ, Vignali DA. Cutting edge: Molecular analysis of the negative regulatory function of lymphocyte activation gene-3. J Immunol. 2002;169:5392–5395. doi: 10.4049/jimmunol.169.10.5392. [DOI] [PubMed] [Google Scholar]
- 19.Workman CJ, Vignali DA. Negative regulation of T cell homeostasis by lymphocyte activation gene-3 (CD223) J Immunol. 2005;174:688–695. doi: 10.4049/jimmunol.174.2.688. [DOI] [PubMed] [Google Scholar]
- 20.Miyazaki T, et al. Mice lacking H2-M complexes, enigmatic elements of the MHC class II peptide-loading pathway. Cell. 1996;84:531–541. doi: 10.1016/s0092-8674(00)81029-6. [DOI] [PubMed] [Google Scholar]
- 21.Stefanová I, et al. TCR ligand discrimination is enforced by competing ERK positive and SHP-1 negative feedback pathways. Nat Immunol. 2003;4:248–254. doi: 10.1038/ni895. [DOI] [PubMed] [Google Scholar]
- 22.Buisson S, Triebel F. MHC class II engagement by its ligand LAG-3 (CD223) leads to a distinct pattern of chemokine and chemokine receptor expression by human dendritic cells. Vaccine. 2003;21:862–868. doi: 10.1016/s0264-410x(02)00533-9. [DOI] [PubMed] [Google Scholar]
- 23.Blackburn SD, et al. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol. 2009;10:29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.