Abstract
Increasing evidence highlights a role for the immune system in the pathogenesis of autism spectrum disorder (ASD), as immune dysregulation is observed in the brain, periphery, and gastrointestinal tract of ASD individuals. Furthermore, maternal infection (maternal immune activation, MIA) is a risk factor for ASD. Modeling this risk factor in mice yields offspring with the cardinal behavioral and neuropathological symptoms of human ASD. In this study, we find that offspring of immune-activated mothers display altered immune profiles and function, characterized by a systemic deficit in CD4+ TCRβ+ Foxp3+ CD25+ T regulatory cells, increased IL-6 and IL-17 production by CD4+ T cells, and elevated levels of peripheral Gr-1+ cells. In addition, hematopoietic stem cells from MIA offspring exhibit altered myeloid lineage potential and differentiation. Interestingly, repopulating irradiated control mice with bone marrow derived from MIA offspring does not confer MIA-related immunological deficits, implicating the peripheral environmental context in long-term programming of immune dysfunction. Furthermore, behaviorally abnormal MIA offspring that have been irradiated and transplanted with immunologically normal bone marrow from either MIA or control offspring no longer exhibit deficits in stereotyped/repetitive and anxiety-like behaviors, suggesting that immune abnormalities in MIA offspring can contribute to ASD-related behaviors. These studies support a link between cellular immune dysregulation and ASD-related behavioral deficits in a mouse model of an autism risk factor.
Keywords: immunity, neurodevelopment, prenatal programming, neuroimmunology
Autism is a complex neurodevelopmental disorder and a pressing medical concern, affecting over 1% of children in the United States (1). Although autism spectrum disorder (ASD) is characterized by stereotypic behaviors and language and social deficits, increasing evidence suggests a role for the immune system in ASD pathogenesis. Altered cytokine profiles in the postmortem brain, cerebrospinal fluid, and plasma are found in ASD, and several studies have demonstrated an elevated number and activation of microglia and astrocytes in the postmortem brain (2). There are also many reports of peripheral immune abnormalities in autistic individuals, including increased NK cell activity, differential monocyte responses to in vitro stimulation, and altered serum Ig levels (3, 4).
Abnormal activation of the immune system may also be involved in the etiology of autism. Several studies have associated ASD risk with immune-related susceptibility genes, such as those encoding MET receptor tyrosine kinase, PRKCB1, complement C4B, and specific HLA haplotypes (3, 4). In addition, antibrain antibodies are elevated in some ASD sera and in some mothers of autistic children (5–7). Family members of autistic children, particularly the mothers, show a higher incidence of allergy or autoimmune diseases (8, 9). Consistent with immune involvement are findings that maternal infection is a risk factor for autism (2). After the 1964 rubella pandemic, 8–13% of children born to infected mothers developed features of autism (10). In a recent study surveying all children born in Denmark from 1980 to 2005, a very significant association was found between autism and maternal viral infection during the first trimester of pregnancy (11). Moreover, elevation of IFN-γ, IL-4, or IL-5 in maternal serum is associated with increased risk for ASD in the offspring (12), as is elevation of monocyte chemoattractant protein-1 in the amniotic fluid (13).
Whether the immune abnormalities in ASD actually contribute to its behavioral symptoms or whether they are an epiphenomenon of primary neural dysfunction is an outstanding question. The immune system exhibits lifelong reciprocal interactions with the central nervous system, and the fact that the immune status can influence behavioral responses is exemplified by early studies demonstrating that responses to infection and inflammation are relayed to the brain, resulting in the induction of fever and sickness behavior (14). Moreover, administration of certain cytokines to human subjects frequently causes striking changes in mental state (15). Conversely, emotional and psychological state can influence immune function. Perhaps the strongest evidence to date is the finding that both short- and long-term stress lead to disruption of immune function (16). Immune dysregulation has also been implicated in the etiology of a variety of neurodegenerative, psychiatric, and neurodevelopmental disorders, including Parkinson, Huntington, and Alzheimer’s diseases, multiple sclerosis, major depression, schizophrenia, and addiction (17–20).
Here we ask whether a mouse model exhibiting many features of autism also displays altered immune function. We use the maternal immune activation (MIA) model, which is based on maternal infection as a key environmental risk factor for autism. Pregnant mice are injected with the synthetic, double-stranded RNA, poly(I:C), to initiate a proinflammatory antiviral response. This type of MIA yields offspring with the core behavioral and neuropathological symptoms of autism, including reduced social interaction, abnormal communication, stereotyped/repetitive behavior, and a spatially restricted deficit in Purkinje cells (21, 22).
In this study, we profile peripheral immune subtypes, assess the functional activities of major leukocyte lineages and evaluate the lineage potential of fetal and adult hematopoietic stem cells (HSCs) and progenitors. To explore the potential for prenatal programming of long-term immune dysfunction, we examine whether transferring HSCs from MIA offspring into non-MIA offspring can induce cell-autonomous immune abnormalities. To gain insight into whether immune abnormalities in MIA offspring contribute to the pathogenesis of ASD-related behaviors, we behaviorally assess poly(I:C) offspring repopulated with bone marrow (BM) from saline offspring. Our findings demonstrate that immune challenge during prenatal life leads to persistent immune alterations in the postnatal offspring, which can further impact the development or maintenance of abnormal behavior.
Results
MIA Offspring Exhibit Deficits in Regulatory T Cells and Elevated CD4+ T-Cell Responses.
Many studies of immune dysregulation in human ASD report a bias toward a proinflammatory phenotype (3, 4). We therefore investigated regulatory T cells (Tregs) as known suppressors of the innate and adaptive immune responses. Compared with controls, adult offspring of poly(I:C)-injected mothers display a ∼50% decrease in splenic CD4+ Foxp3+ CD25+ Tregs (Fig. 1A). This deficit is also reflected in significantly decreased levels of total CD4+ Foxp3+ cells and possible decreases (P = 0.0846) in CD4+ Foxp3+ CD25− T cells. Similar deficits in total CD4+ Foxp3+, Foxp3+ CD25−, and Foxp3+ CD25+ cells are also observed in mesenteric lymph nodes (MLNs) from adult poly(I:C) offspring (Fig. S1A). Despite these differences in Treg levels, there is no difference in the suppression of CD4+CD25− T-cell proliferation between Tregs from saline versus poly(I:C) offspring (Fig. S2). Taken together, these findings indicate a systemic deficit in the abundance of Tregs.
Fig. 1.
MIA leads to decreased levels of Tregs and hyperresponsiveness in CD4+ T cells from spleens of adult offspring. (A) Compared with controls, adult poly(I:C) offspring exhibit decreased levels of CD4+ Foxp3+ splenocytes and CD4+ Foxp3+ CD25+ Tregs (n = 5, where each sample represents a pool of three spleens). (B) Adult poly(I:C) offspring exhibit decreased levels of splenic CD4+ TCRβ+ Foxp3+ CD25+ Tregs, but no significant difference in splenic CD4+ TCRβ+ IFN-γ+ IL-17+ Th17+ Th1 cells or CD4+ TCRβ+ IFN-γ− IL-17+ Th17 cells (n = 4, where each sample represents a pool of three spleens). (C) CD4+ T cells from the spleens of adult offspring secrete elevated levels of IL-6 and IL-17 in response to PMA/ionomycin stimulation. In contrast, CD4+ T cells from spleens of adult poly(I:C) and saline offspring do not differ the level of TNF-α secreted (n = 11–16). *P < 0.05, **P < 0.01, ***P < 0.001; n.s., not significant. All panels show one representative experiment of at least two separate trials.
We further tested MIA offspring for levels of IL-17–producing CD4+ T (Th17) cells, given their proinflammatory nature and reported reciprocal relationship with Tregs. In assays done in parallel with those for Tregs, we find no significant difference from controls in levels of CD4+ TCRβ+ IL-17+ (Th17) cells, with or without IFN-γ expression (Fig. 1B). There is also no difference in the level of CD4+ TCRβ+ IL-17− IFN-γ+ (Th1) cells. To further examine CD4+ T cells, we measured their secretion of IL-6 and IL-17 in response to in vitro stimulation. Compared with controls, CD4+ cells from spleens of 15-wk-old poly(I:C) offspring release significantly more IL-6 and IL-17 after in vitro stimulation, with no difference in TNF-α secretion (Fig. 1C). This result is similarly observed with splenic CD4+ T cells derived from 3-wk-old offspring (Fig. S3A) and 1-y-old offspring (Fig. S3B), suggesting an early onset of persistent immune dysfunction. Interestingly, splenic CD4+ T cells from 3-wk-old mice produce much lower levels of IL-17 than do such cells from adult offspring (0–10 pg/mL compared with 50–400 pg/mL), reflecting the immunological immaturity reported in young versus adult mice and humans (23). CD4+ T cells from MLNs of poly(I:C) offspring are also hyperresponsive to in vitro stimulation, suggesting that this abnormality is common to secondary lymphoid organs (Fig. S1 C and D). Overall, MIA leads to lower Treg levels and elevated CD4+ T-cell responsiveness in spleens and MLNs from MIA offspring. This result can be characterized as a persistent, proinflammatory T-helper-cell phenotype.
MIA Offspring Display Increased Levels of Gr-1+ Cells and Skewed HSC Differentiation.
To determine whether MIA during fetal development alters the profile of other immune subtypes in the offspring, we assessed major leukocyte classes in spleens from poly(I:C) and saline offspring. Compared with controls, adult poly(I:C) offspring exhibit a 1.5-fold higher level of Gr-1+ cells and trending increase in CD11b+ cells (P = 0.1256) (Fig. 2A). In contrast, there is no difference from controls in the percentages of total B220+ B cells, NK1.1+ NK cells, CD4+ T cells, or CD8+ T cells. Moreover, no significant differences are detected for any of the primary leukocyte subtypes in the MLNs (Fig. S1B).
Fig. 2.
MIA leads to increased levels of splenic Gr-1+ cells in adult offspring and preferential differentiation of HSCs into granulocyte precursors in fetal and adult offspring. (A) Spleens from adult poly(I:C) offspring exhibit increased levels of Gr-1+ cells and no significant differences in other major lineages compared with controls (n = 4, where each sample represents a pool of three spleens). (B) Compared with BM HSCs from control offspring, BM HSCs from adult poly(I:C) offspring display increased differentiation into CFU-G precursors and decreased differentiation into early CFU-GM precursors (n = 4). Compared with controls, fetal liver HSCs from embryonic day (E) 13.5 (C, Left) and E15.5 (C, Right) poly(I:C) offspring also display increased differentiation into CFU-G and decreased differentiation into CFU-GM, in addition to decreased percentages of CFU-GEMM with E13.5 HSCs and increased percentages of CFU-E with E15.5 HSCs (n = 4, where each sample represents a pool of cells from six fetal livers from a single litter). *P < 0.05, **P < 0.01, ***P < 0.001; n.s., not significant. All panels represent one representative experiment of at least two separate trials.
Gr-1+ cells reflect a heterogeneous group of immune subtypes that includes neutrophils and monocytes, inflammatory cells, and suppressor cells, alike. To determine whether the elevated levels of Gr-1+ cells observed in poly(I:C) spleens could be attributed to particular Gr-1+ subtypes, we further characterized the splenic Gr-1+ population using CD11b, Ly6C, and Ly6G markers. Interestingly, poly(I:C) offspring exhibit mild increases in all three populations of Gr-1+ cells resolved: (i) Gr-1hi CD11b+ Ly6Cmid Ly6Ghi SSCmid, (ii) Gr-1mid CD11b+ Ly6Cmid Ly6Gmid SSCmid, and (iii) Gr-1mid CD11b+ Ly6Chi Ly6G- SSClo (Fig. S4). Gr-1+ subtypes i and ii are referred to as neutrophils, by histology and in line with their high granularity, and subtype iii is identified as a monocyte population (24). Overall, that poly(I:C) offspring display increases in all identified Gr-1+ populations suggests that skewing at the HSC or progenitor level may underlie the elevated Gr-1 phenotype observed in adult poly(I:C) offspring compared with controls.
To evaluate the origin of the increase in splenic Gr-1+ cells, we assessed the lineage potential and differentiation of BM cells from adult poly(I:C) versus saline offspring. Using a colony-forming assay to morphologically assess lineage differentiation, we find that HSCs and progenitors from poly(I:C) offspring BM exhibit increased differentiation into CFU-G (granulocyte) precursors and decreased differentiation into early CFU-GM (granulocyte-macrophage) precursors (Fig. 2B). Turning to the fetus, we find that similar differentiation is observed with fetal liver HSCs and progenitors from poly(I:C) offspring (Fig. 2C). Thus, MIA induces preferential differentiation of fetal as well as adult HSCs and progenitors into granulocyte precursors, which may account for the increased levels of mature Gr-1+ cells in spleens of poly(I:C) offspring compared with controls.
Immune Abnormalities Observed in MIA Offspring Are Not Transferred via BM Transplant.
Our data demonstrate that MIA leads to an altered profile of peripheral immune cells in the offspring, characterized by decreased levels of Tregs, hyperresponsive CD4+ T cells, and elevated levels of Gr-1+ cells. To explore whether these immune abnormalities (and the Gr-1 phenotype, in particular) can be attributed to cell-intrinsic developmental programming of HSCs, we transferred BM from the immunologically aberrant poly(I:C) offspring into irradiated saline and poly(I:C) offspring and assessed whether any MIA-associated immune abnormalities were reestablished.
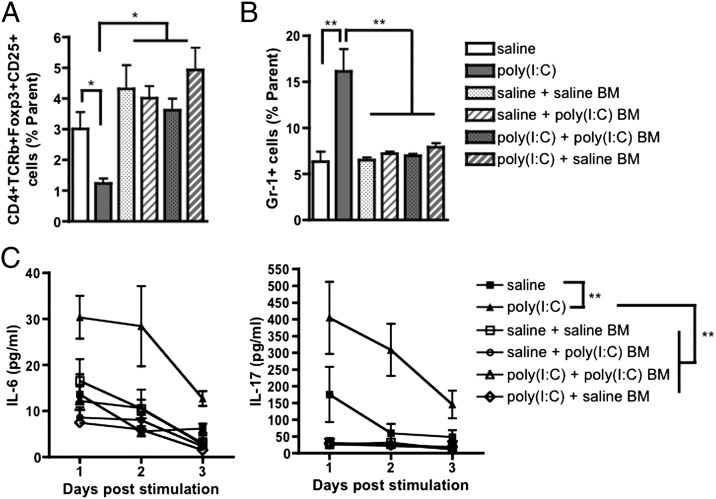
We find that saline and poly(I:C) offspring that were irradiated and reconstituted with poly(I:C) BM do not develop or retain any of the immune abnormalities exhibited by unmanipulated MIA offspring. All BM-transplanted groups exhibit levels of splenic CD4+ TCRβ+ CD25+ Foxp3+ Tregs and Gr-1+ cells that are comparable to those observed in untransplanted saline offspring (Fig. 3 A and B). There is also no difference between saline and BM-transplanted mice in distributions of progenitor subtypes after in vitro differentiation of BM HSCs (Fig. S5). Furthermore, CD4+ T cells isolated from spleens of any of the types of BM-transplanted mice secrete levels of IL-6 and IL-17 in response to phorbol 12-myristate 13-acetate (PMA)/ionomycin stimulation that are similar to those produced by CD4+ T cells isolated from spleens of untransplanted saline offspring (Fig. 3C). Importantly, the fact that saline mice transplanted with saline BM display equivalent levels of Tregs and Gr-1+ cells and similar CD4+ T-cell responsiveness to those observed in unmanipulated saline offspring suggests that the irradiation and BM-transplant procedure itself does not skew immune system profiles under these conditions. Overall, these experiments indicate that MIA-associated immune abnormalities are not transferred via transplant of poly(I:C) BM into irradiated saline or poly(I:C) offspring. This finding suggests either that the HSC microenvironment is important for maintaining altered HSC potential in MIA offspring, or that the particular MIA-associated immune abnormality is not programmed at the stem cell level.
Fig. 3.
Immune abnormalities observed in MIA offspring are not transferred by BM transplant into irradiated mice. (A) There is no difference in levels of splenic CD4+ TCRβ+ CD25+ Foxp3+ Tregs between saline or poly(I:C) offspring transplanted with BM from saline or poly(I:C) offspring. Levels of splenic Tregs between BM transplant groups are comparable to those observed in untransplanted saline offspring; untransplanted poly(I:C) offspring display a significant deficit in Treg percentages (n = 4–5). (B) BM-transplanted mice do not display MIA-associated increases in splenic Gr-1+ cells. Percentages of Gr-1+ cells are comparable between BM transplanted groups and untransplanted saline offspring (n = 4–5), but untransplanted poly(I:C) offspring exhibit significantly elevated levels of splenic Gr-1+ cells. (C) CD4+ T cells from BM-transplanted mice exhibit statistically equivalent levels of IL-6 (Left) and IL-17 (Right) in response to PMA/ionomycin stimulation in vitro. There are no differences between concentrations of IL-6 and IL-17 secreted by CD4+ cells from BM-transplanted groups and from untransplanted saline offspring, despite significant hyperresponsiveness of CD4+ T cells from poly(I:C) offspring [n = 11–16 for saline and poly(I:C) groups, 4–5 for BM transplant groups]. *P < 0.05, **P < 0.01. BM transplant data were acquired from one large experiment.
BM Transplant in MIA Offspring Normalizes Repetitive and Anxiety-Like Behavioral Abnormalities.
To investigate whether the immune abnormalities found in MIA offspring are an independent parallel pathology or if they actually contribute to the development or maintenance of ASD-like behaviors, we assessed behavioral performance in poly(I:C) offspring transplanted with saline BM. Poly(I:C) offspring were first confirmed to exhibit their expected behavioral phenotypes before the procedure. Compared with controls, poly(I:C) offspring display a deficit in prepulse inhibition (PPI) (Fig. S6A). PPI is used to measure sensorimotor gating of the startle reflex, and decreased PPI is frequently observed in autistic individuals (25). Poly(I:C) offspring also exhibit core behavioral symptoms of autism, including increased repetitive behavior as measured by higher levels of stereotypic marble burying and decreased social preference, as assessed by reduced duration spent and entries into a chamber housing a novel mouse versus a familiar mouse (Fig. S6 B and C). In addition, MIA offspring exhibit increased anxiety, as reflected by reduced duration spent, and entries into, the center arena of an open field, despite no significant difference in total distance traveled (Fig. S6D).
After this initial behavioral testing, the mice were irradiated and transplanted with donor BM harvested from either adult saline or poly(I:C) offspring. Notably, mouse heads were shielded during the procedure to limit known effects of irradiation on neurogenesis and glial activation, and to preclude any associated downstream influences on behavioral performance. Furthermore, MIA offspring do not exhibit global changes in levels of activated or total brain macrophages/microglia (Fig. S7), suggesting that microglial abnormalities do not contribute the persistence of ASD-related behaviors in these mice.
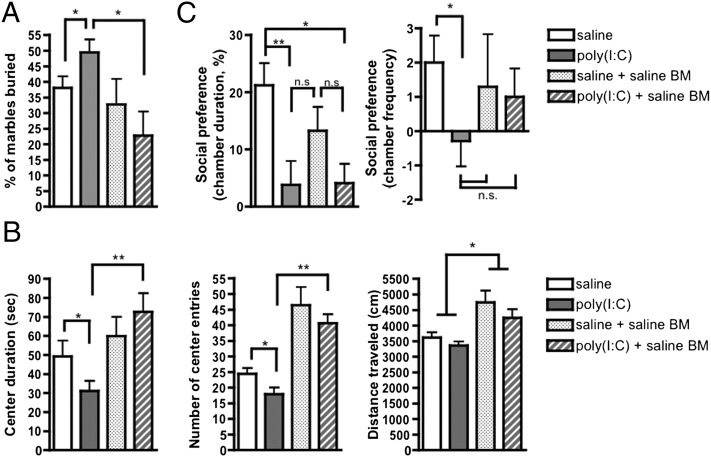
After irradiation and transplantation with saline BM, MIA offspring no longer exhibit behavioral abnormalities in several of these tests. Irradiation and saline BM transplant in poly(I:C) offspring restores repetitive marble burying to levels observed in saline controls (Fig. 4A). In addition, irradiated and BM-transplanted MIA offspring exhibit significantly decreased anxiety-like behavior, as measured by elevated duration of time spent and entries into the center arena of the open field (Fig. 4B). Interestingly, both saline and poly(I:C) BM-transplanted mice exhibit significantly increased number of center entries compared with untransplanted offspring. This finding is similarly reflected by a statistically significant increase in total distance traveled by transplanted offspring versus untransplanted controls. This finding may be a result of effects of the irradiation and transplant procedure on behavior rather than habituation of the mice to repeated testing, because we are able to replicate open field deficits of equivalent intensity in poly(I:C) offspring after retesting at different adult ages (Fig. S8). On the other hand, treatment of poly(I:C) offspring with saline BM has no significant effect on the deficit in social preference, as measured by duration and number of entries into the social chamber (Fig. 4C).
Fig. 4.
MIA offspring irradiated and transplanted with saline BM exhibit decreased repetitive and anxiety-like behavior. (A) Irradiation and transplantation of saline BM into MIA offspring restores repetitive marble-burying behavior to a level at or below that observed in controls. (B) Irradiation and transplantation of saline BM into MIA offspring decreases anxiety-like behavior as measured by significant increases in the number of center entries (Center) and duration in the center arena (Left), compared with those observed in untransplanted MIA mice. Both saline and poly(I:C) BM-transplant mice also exhibit increased overall activity in the open field, as measured by significantly increased total distance traveled compared with untransplanted mice (Right). (C) Transplanting saline offspring BM into poly(I:C) offspring has no significant effect on their social preference behavior, as measured by chamber duration (Left). There is, however, a trending improvement in social preference behavior as measured by chamber frequency in BM-transplanted MIA offspring (Right). *P < 0.05, **P < 0.01; n.s., not significant. BM-transplant data were acquired from one large experiment.
Although treatment of MIA offspring with irradiation and saline BM transplantation significantly restores normal behavior in several tests, we find that treatment of MIA offspring with irradiation and poly(I:C) BM transplantation also improves those behaviors. Notably, irradiated MIA offspring transplanted with poly(I:C) BM exhibit improved open field and marble-burying performance (Fig. S9). This result is consistent with our finding that transplant of poly(I:C) BM does not give rise to cell-autonomous immune abnormalities (Fig. 3). As such, both poly(I:C) BM and saline BM can be considered immunologically normal in these experiments. Taken together, these data demonstrate that MIA offspring that had previously exhibited abnormal behaviors fail to exhibit repetitive and anxiety-like behavior after exposure to irradiation and BM transplant.
Discussion
In the present study we identify differences in dynamic cellular immune responses in a mouse model of an autism risk factor. Offspring of immune-activated mothers develop altered immune profiles and function in the spleen and MLN, which are consistent with a proinflammatory phenotype. In addition, we demonstrate preferential myeloid differentiation of fetal HSCs and progenitors, which is also found in adult HSCs. This finding could form the basis for the altered immune distributions in secondary lymphoid organs from MIA offspring. However, BM transplant of HSCs derived from MIA offspring is not sufficient to recapitulate the elevated levels of Gr-1+ cells exhibited by untransplanted MIA offspring. This result highlights the importance of appropriate environmental cues for preserving this phenotype, as discussed below. We also used the irradiation-BM transplant approach to explore the role of peripheral immune dysfunction on behavioral performance. Interestingly, this procedure corrects some of the ASD-like behavioral symptoms in the MIA offspring.
MIA leads to permanently hyperresponsive CD4+ T cells, as well as decreased Tregs in the offspring, suggesting a chronic, proinflammatory phenotype. This finding is consistent with decreased numbers CD4+ CD25+ and CD3+GITR+ T cells observed in children with ASD (26, 27). Diminished Treg levels and associated decreases in immune regulation may reflect the finding that autistic individuals exhibit decreased levels of regulatory cytokines, such as TGF-β1, and increased levels of proinflammatory cytokines in serum, cerebrospinal fluid and postmortem brain (2). Furthermore, given that Tregs are critical for limiting immune activation and preventing self-reactivity, their deficiency may underlie the reports of a link between ASD and autoimmune disease (8, 28).
Our finding that CD4+ T cells from MIA offspring are hyperresponsive to in vitro stimulation further reflects diminished immune homeostasis. Increases in activated DR+ T cells are observed in human autism (29, 30). In addition, elevated levels of TNF-α– and IFN-γ–producing T cells are found in peripheral blood and gastrointestinal mucosa from autistic individuals (31). A number of other altered immune responses have been reported in ASD (3, 4), and we find that MIA offspring also exhibit some of these changes, including altered leukocyte subsets and CD4+ T-cell responses to stimulation. This overlap between findings of immune dysregulation in ASD and our current results lends support to MIA as a mouse model with construct and face validity for this disorder.
We further demonstrate that MIA during fetal development leads to significantly increased levels of peripheral Gr-1+ CD11b+ neutrophilic and monocytic cells in adult offspring. Granulocytosis is typically observed after acute inflammation (32, 33) and is also a feature of a number of chronic diseases (34–36). There have been no reports of altered neutrophil levels in ASD, however. In one study, children with ASD exhibited increased plasma monocyte counts, but no significant abnormalities in major granulocyte subtypes (37). In light of the present results, it will be of interest to extend these immunophenotypic studies to behavioral or symptomatic subpopulations of ASD individuals.
It is intriguing to consider that MIA effects on developing HSCs may underlie some of the persistent peripheral immune changes found in the offspring. We find that both adult and fetal HSCs and progenitor cells display preferential differentiation into CFU-G colonies, which provides an explanation for how increases in such short-lived cells could be maintained over the lifespan of MIA offspring. Maternal poly(I:C) injection is known to induce dramatic increases in proinflammatory cytokines in the placenta, a principal HSC niche during midgestation (38). Because HSCs and progenitors respond to inflammatory signals, such as cytokines and Toll-like receptor ligands, it will be interesting to explore whether MIA-induced changes in the HSC environment can skew hematopoietic lineage decisions and fate.
Despite the fact that HSCs and progenitors isolated from poly(I:C) offspring are developmentally skewed toward granulocyte precursors, transplantation of BM from poly(I:C) offspring into irradiated saline offspring or back into irradiated poly(I:C) offspring does not transfer the Gr-1 phenotype. This finding suggests that developmental programming of HSCs to skew lineage differentiation in MIA offspring is not governed solely by stably encoded cell-intrinsic factors, such as epigenetic modification. Rather, it is likely that an MIA-induced peripheral environment is necessary to supply the cues that guide preferential myeloid development. Changes in G-CSF, IL-5, and IL-3 levels, for example, are implicated in fine-tuning the transcription factor activity that governs lineage choice for granulocyte/macrophage progenitors (39). Indeed, we have found that MIA offspring exhibit dynamic and chronically altered peripheral blood and splenic cytokine profiles (40). It will be of interest to assess whether the BM microenvironment differs in poly(I:C) and saline offspring. Overall, it is intriguing that the Gr-1 phenotype observed in MIA offspring is not transferred via BM transplant, suggesting a lack of sufficient cell-intrinsic epigenetic programming and a key role for environmental factors in promoting this lineage choice.
We demonstrate that subjecting MIA offspring that have validated behavioral deficits to irradiation and reconstitution with immunologically normal BM alters their behavioral phenotype. Namely, these MIA offspring no longer exhibit their previous abnormalities in repetitive marble burying and open-field exploration. These findings imply that correcting immune function can correct some autism-related behavioral abnormalities. However, this experiment has a significant limitation. In the absence of a positive control (MIA offspring that retain behavioral deficits after irradiation and BM transplantation), we are unable to distinguish between the potential restorative effects of the BM itself (that is, the effects of restoring immune phenotype) versus the effect of confounding factors associated with the transplant procedure. The most obvious of these is the effect of irradiation on various aspects of recipient homeostasis, including metabolic function, gastrointestinal microbial composition, and oxidative stress, all of which may indirectly influence behavioral outcome. We are, however, inspired by recent work demonstrating that stereotyped grooming behavior in Hoxb8 mutant mice is, in fact, transferrable via BM transplant (41). This finding shows, at least in that experimental model, that mice can reconstitute an expected behavioral impairment via BM transplant despite prior irradiation. It will be important to explore other experimental approaches to treat immune dysfunction in MIA offspring and to further assess whether these can alleviate behavioral outcomes.
Nonetheless, our finding that behavioral abnormalities in the MIA offspring can be corrected by the irradiation-BM transplant procedure contributes to a growing number of studies reporting the efficacy of BM transplant on ameliorating symptoms of neurological disorders (41–43). Furthermore, several experiments using RAG1 KO, SCID, and athymic mice demonstrate that primary immune dysfunction can lead to behavioral impairment (44–47). Interestingly, work with germ-free mice links the absence of microbiota and associated alterations in the immune system to abnormal behavioral performance (48). Some immune alterations have been described in the maternal valproic acid model and the BTBR mouse strain that display behavioral features of autism (49, 50). In contrast, much remains to be learned about potential immune changes in mouse models of ASD candidate genes. One such gene is particularly attractive in this regard, MET, which encodes a tyrosine kinase receptor and is known to play a role in immune regulation (51).
Altered peripheral immune profiles and cellular activity are also associated with core behavioral impairments in human ASD. Increased plasma IL-4 levels correlate with greater deficits in communication, and increased plasma IL-8, IL-12p40, IL-6, and IL-1β are linked to stereotypy, hyperactivity, and lethargy scores from ASD children (52). Altered levels of other immune factors, including TGF, macrophage migration inhibitory factor (MIF), and CD31, have also been associated with the severity of ASD-related behaviors or pathophysiology (4). In addition, reduced levels of plasma IgG and IgM are associated with behavioral severity in ASD children (53).
It is striking that in a mouse model of an autism environmental risk factor that exhibits the cardinal behavioral and neuropathological symptoms of autism, there is also permanent peripheral immune dysregulation. This finding provides the opportunity to explore molecular mechanisms underlying the relationship between brain dysfunction and altered immunity in the manifestation of abnormal behavior. Furthermore, this finding provides a platform for investigating how prenatal challenges can program long-term postnatal immunity, health, and disease. Maternal insult-mediated epigenetic modification in HSC and progenitor cells is one possible mechanism for how effects may be established by transient environmental changes yet persist permanently into adulthood. However, the BM transplant results suggest that the peripheral environment of the MIA offspring is also critical for maintaining a permanently modified immune state.
Methods
Detailed methods are provided in SI Methods.
MIA.
Pregnant C57BL/6N mice were injected on E12.5 with saline or poly(I:C). For poly(I:C) injections, poly(I:C) potassium salt (Sigma Aldrich) was dissolved in saline at 4 mg/mL and administered intraperitoneally at 20 mg/kg [based on the weight of the poly(I:C) itself, not including the total weight of the potassium salt]. Control mice were injected with saline alone at 5 μL/g body weight.
CD4+ T-Cell in Vitro Stimulation.
106 CD4+ T cells were cultured in complete RPMI with PMA (50 ng/mL) and ionomycin (750 ng/mL) for 3 d at 37 °C with 5% (vol/vol) CO2. Each day, 0.5 mL supernatant was collected. ELISA to detect IL-6, IL-17, and TNF-α were performed according to the manufacturer’s instructions (eBioscience).
Flow Cytometry.
For subtyping of Gr-1+ splenocytes, cells were stained with Gr-1-APC, CD11b-PE, Ly6G-APC, Ly6C-FITC, and Ter119-PerCP-Cy5.5 (Biolegend). For detection of Th17 cells and Tregs, splenocytes were stimulated for 4 h with PMA/ionomycin in the presence of GolgiPLUG (BD Biosciences). Suspensions were blocked for Fc receptors and labeled with CD4-FITC, TCRb-PerCP-Cy5.5, and CD25-PE before labeling with IFN-γ–PE and Foxp3-APC (eBioscience). Samples were processed using the FACSCalibur cytometer (BD Biosciences). Data were analyzed using FlowJo software (TreeStar).
Methylcellulose Colony-Forming Assay.
2 × 105 fetal liver or BM cells were added to 3 mL of thawed methylcellulose supplemented with SCF, IL-3, IL-6, and Epo (StemCell). Total colony number was counted on day 5 and confirmed to be equivalent across groups. CFU-G, -M, -E (erythroid), -GM, and -GEMM colonies were blindly scored on day 12 according to standard protocols.
BM Transplantation.
Behaviorally tested, 9-wk-old mice were injected intraperitoneally with high-dose ketamine/xylazine (5.6 μL/1 g per mouse) and lethally irradiated (1,000 rads) with heads shielded by 4-mm lead. The effectiveness of the head shields was confirmed by selective graying of black coat color in the irradiated area. Recovered mice were anesthetized with isofluorane and injected retro-orbitally with 5 × 106 donor BM cells. Mice were retested in behavioral paradigms at 17–19 wk of age and killed for immunological assays at 19–20 wk of age.
Behavioral Testing.
At 6–8 wk of age, mice were behaviorally tested for PPI, open-field exploration, repetitive marble burying, and social preference (22, 38, 54, 55). Eight weeks after transplant, mice were similarly tested in all paradigms but PPI because PPI performance is highly sensitive to handling and prior testing experience (56).
Statistical Analysis.
Statistical analysis was performed with Prism software (Graphpad). Differences between two treatment groups were assessed using Student t test, and differences among multiple groups were assessed using one-way ANOVA and Bonferroni post hoc test. Two-way ANOVA and Bonferroni post hoc test was used for PPI, CD4+ T-cell stimulation and methylcellulose assay data.
Supplementary Material
Acknowledgments
The authors acknowledge the kind assistance of B. Deverman and G. Sharon for reviewing the manuscript, R. Sauza for caring for the animals, and M. Sadoshima for technical help. This work was supported by an Autism Speaks Weatherstone Fellowship (to E.Y.H.); National Institutes of Health Graduate Training Grant NIH/NRSA 5 T32 GM07737 (to E.Y.H. and J.C.); a Weston Havens Foundation grant (to S.K.M. and P.H.P.); a Caltech Innovation grant (to S.K.M. and P.H.P.); and a Congressionally Directed Medical Research Program Idea Development Award (to S.K.M. and P.H.P.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1202556109/-/DCSupplemental.
References
- 1.Autism and Developmental Disabilities Monitoring Network Surveillance Year 2008 Principal Investigators Centers for Disease Control and Prevention Prevalence of autism spectrum disorders—Autism and Developmental Disabilities Monitoring Network, 14 sites, United States, 2008. MMWR Surveill Summ. 2012;61:1–19. [PubMed] [Google Scholar]
- 2.Patterson PH. Maternal infection and immune involvement in autism. Trends Mol Med. 2011;17:389–394. doi: 10.1016/j.molmed.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goines P, Van de Water J. The immune system’s role in the biology of autism. Curr Opin Neurol. 2010;23:111–117. doi: 10.1097/WCO.0b013e3283373514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Onore C, Careaga M, Ashwood P. The role of immune dysfunction in the pathophysiology of autism. Brain Behav Immun. 2012;26:383–392. doi: 10.1016/j.bbi.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Croen LA, et al. Maternal mid-pregnancy autoantibodies to fetal brain protein: The early markers for autism study. Biol Psychiatry. 2008;64:583–588. doi: 10.1016/j.biopsych.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singer HS, et al. Antibodies against fetal brain in sera of mothers with autistic children. J Neuroimmunol. 2008;194:165–172. doi: 10.1016/j.jneuroim.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 7.Zimmerman AW, et al. Maternal antibrain antibodies in autism. Brain Behav Immun. 2007;21:351–357. doi: 10.1016/j.bbi.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 8.Atladóttir HO, et al. Association of family history of autoimmune diseases and autism spectrum disorders. Pediatrics. 2009;124:687–694. doi: 10.1542/peds.2008-2445. [DOI] [PubMed] [Google Scholar]
- 9.Comi AM, Zimmerman AW, Frye VH, Law PA, Peeden JN. Familial clustering of autoimmune disorders and evaluation of medical risk factors in autism. J Child Neurol. 1999;14:388–394. doi: 10.1177/088307389901400608. [DOI] [PubMed] [Google Scholar]
- 10.Chess S. Follow-up report on autism in congenital rubella. J Autism Child Schizophr. 1977;7:69–81. doi: 10.1007/BF01531116. [DOI] [PubMed] [Google Scholar]
- 11.Atladóttir HO, et al. Maternal infection requiring hospitalization during pregnancy and autism spectrum disorders. J Autism Dev Disord. 2010;40:1423–1430. doi: 10.1007/s10803-010-1006-y. [DOI] [PubMed] [Google Scholar]
- 12.Goines PE, et al. Increased midgestational IFN-γ, IL-4 and IL-5 in women bearing a child with autism: A case-control study. Mol Autism. 2011;2:13. doi: 10.1186/2040-2392-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abdallah MW, et al. Amniotic fluid chemokines and autism spectrum disorders: An exploratory study utilizing a Danish Historic Birth Cohort. Brain Behav Immun. 2012;26:170–176. doi: 10.1016/j.bbi.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 14.Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: When the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Capuron L, Miller AH. Immune system to brain signaling: neuropsychopharmacological implications. Pharmacol Ther. 2011;130:226–238. doi: 10.1016/j.pharmthera.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Segerstrom SC, Miller GE. Psychological stress and the human immune system: A meta-analytic study of 30 years of inquiry. Psychol Bull. 2004;130:601–630. doi: 10.1037/0033-2909.130.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amor S, Puentes F, Baker D, van der Valk P. Inflammation in neurodegenerative diseases. Immunology. 2010;129:154–169. doi: 10.1111/j.1365-2567.2009.03225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shie FS, Chen YH, Chen CH, Ho IK. Neuroimmune pharmacology of neurodegenerative and mental diseases. J Neuroimmune Pharmacol. 2011;6:28–40. doi: 10.1007/s11481-010-9241-8. [DOI] [PubMed] [Google Scholar]
- 19.Patterson PH. Neuroscience. Maternal effects on schizophrenia risk. Science. 2007;318:576–577. doi: 10.1126/science.1150196. [DOI] [PubMed] [Google Scholar]
- 20.Dantzer R, O’Connor JC, Lawson MA, Kelley KW. Inflammation-associated depression: From serotonin to kynurenine. Psychoneuroendocrinology. 2011;36:426–436. doi: 10.1016/j.psyneuen.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shi L, et al. Activation of the maternal immune system alters cerebellar development in the offspring. Brain Behav Immun. 2009;23:116–123. doi: 10.1016/j.bbi.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malkova NV, Yu CZ, Hsiao EY, Moore MJ, Patterson PH. Maternal immune activation yields offspring displaying mouse versions of the three core symptoms of autism. Brain Behav Immun. 2012;26:607–616. doi: 10.1016/j.bbi.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adkins B, Leclerc C, Marshall-Clarke S. Neonatal adaptive immunity comes of age. Nat Rev Immunol. 2004;4:553–564. doi: 10.1038/nri1394. [DOI] [PubMed] [Google Scholar]
- 24.Shi C, et al. Ly6G+ neutrophils are dispensable for defense against systemic Listeria monocytogenes infection. J Immunol. 2011;187:5293–5298. doi: 10.4049/jimmunol.1101721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perry W, Minassian A, Lopez B, Maron L, Lincoln A. Sensorimotor gating deficits in adults with autism. Biol Psychiatry. 2007;61:482–486. doi: 10.1016/j.biopsych.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 26.Ashwood P, et al. Altered T cell responses in children with autism. Brain Behav Immun. 2011;25:840–849. doi: 10.1016/j.bbi.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mostafa GA, Al Shehab A, Fouad NR. Frequency of CD4+CD25high regulatory T cells in the peripheral blood of Egyptian children with autism. J Child Neurol. 2010;25:328–335. doi: 10.1177/0883073809339393. [DOI] [PubMed] [Google Scholar]
- 28.Enstrom AM, Van de Water JA, Ashwood P. Autoimmunity in autism. Curr Opin Investig Drugs. 2009;10:463–473. [PMC free article] [PubMed] [Google Scholar]
- 29.Warren RP, Yonk J, Burger RW, Odell D, Warren WL. DR-positive T cells in autism: Association with decreased plasma levels of the complement C4B protein. Neuropsychobiology. 1995;31:53–57. doi: 10.1159/000119172. [DOI] [PubMed] [Google Scholar]
- 30.Plioplys AV, Greaves A, Kazemi K, Silverman E. Lymphocyte function in autism and Rett syndrome. Neuropsychobiology. 1994;29:12–16. doi: 10.1159/000119056. [DOI] [PubMed] [Google Scholar]
- 31.Ashwood P, Wakefield AJ. Immune activation of peripheral blood and mucosal CD3+ lymphocyte cytokine profiles in children with autism and gastrointestinal symptoms. J Neuroimmunol. 2006;173:126–134. doi: 10.1016/j.jneuroim.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 32.Ricevuti G, Mazzone A, Pasotti D, de Servi S, Specchia G. Role of granulocytes in endothelial injury in coronary heart disease in humans. Atherosclerosis. 1991;91:1–14. doi: 10.1016/0021-9150(91)90182-3. [DOI] [PubMed] [Google Scholar]
- 33.Hansen NE, Karle H, Andersen V, Malmquist J, Hoff GE. Neutrophilic granulocytes in acute bacterial infection. Sequential studies on lysozyme, myeloperoxidase and lactoferrin. Clin Exp Immunol. 1976;26:463–468. [PMC free article] [PubMed] [Google Scholar]
- 34.Passegué E, Jochum W, Schorpp-Kistner M, Möhle-Steinlein U, Wagner EF. Chronic myeloid leukemia with increased granulocyte progenitors in mice lacking junB expression in the myeloid lineage. Cell. 2001;104:21–32. doi: 10.1016/s0092-8674(01)00188-x. [DOI] [PubMed] [Google Scholar]
- 35.Courtney PA, et al. Increased apoptotic peripheral blood neutrophils in systemic lupus erythematosus: Relations with disease activity, antibodies to double stranded DNA, and neutropenia. Ann Rheum Dis. 1999;58:309–314. doi: 10.1136/ard.58.5.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cascão R, Rosário HS, Souto-Carneiro MM, Fonseca JE. Neutrophils in rheumatoid arthritis: More than simple final effectors. Autoimmun Rev. 2010;9:531–535. doi: 10.1016/j.autrev.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 37.Sweeten TL, Posey DJ, McDougle CJ. High blood monocyte counts and neopterin levels in children with autistic disorder. Am J Psychiatry. 2003;160:1691–1693. doi: 10.1176/appi.ajp.160.9.1691. [DOI] [PubMed] [Google Scholar]
- 38.Hsiao EY, Patterson PH. Activation of the maternal immune system induces endocrine changes in the placenta via IL-6. Brain Behav Immun. 2011;25:604–615. doi: 10.1016/j.bbi.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Robb L. Cytokine receptors and hematopoietic differentiation. Oncogene. 2007;26:6715–6723. doi: 10.1038/sj.onc.1210756. [DOI] [PubMed] [Google Scholar]
- 40.Garay PA, Hsiao EY, Patterson PH, McAllister AK. Maternal immune activation causes age- and region-specific changes in brain cytokines in offspring throughout development. Brain Behav Immun. 2012 doi: 10.1016/j.bbi.2012.07.008. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen SK, et al. Hematopoietic origin of pathological grooming in Hoxb8 mutant mice. Cell. 2010;141:775–785. doi: 10.1016/j.cell.2010.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kwan W, et al. Bone marrow transplantation confers modest benefits in mouse models of Huntington’s disease. J Neurosci. 2012;32:133–142. doi: 10.1523/JNEUROSCI.4846-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Derecki NC, et al. Wild-type microglia arrest pathology in a mouse model of Rett syndrome. Nature. 2012;484:105–109. doi: 10.1038/nature10907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brynskikh A, Warren T, Zhu J, Kipnis J. Adaptive immunity affects learning behavior in mice. Brain Behav Immun. 2008;22:861–869. doi: 10.1016/j.bbi.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 45.Cushman J, Lo J, Huang Z, Wasserfall C, Petitto JM. Neurobehavioral changes resulting from recombinase activation gene 1 deletion. Clin Diagn Lab Immunol. 2003;10:13–18. doi: 10.1128/CDLI.10.1.13-18.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kipnis J, Cohen H, Cardon M, Ziv Y, Schwartz M. T cell deficiency leads to cognitive dysfunction: Implications for therapeutic vaccination for schizophrenia and other psychiatric conditions. Proc Natl Acad Sci USA. 2004;101:8180–8185. doi: 10.1073/pnas.0402268101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mombaerts P, et al. Peripheral lymphoid development and function in TCR mutant mice. Int Immunol. 1994;6:1061–1070. doi: 10.1093/intimm/6.7.1061. [DOI] [PubMed] [Google Scholar]
- 48.Heijtz RD, et al. Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci USA. 2011;108:3047–3052. doi: 10.1073/pnas.1010529108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Heo Y, Zhang Y, Gao D, Miller VM, Lawrence DA. Aberrant immune responses in a mouse with behavioral disorders. PLoS ONE. 2011;6:e20912. doi: 10.1371/journal.pone.0020912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schneider T, et al. Gender-specific behavioral and immunological alterations in an animal model of autism induced by prenatal exposure to valproic acid. Psychoneuroendocrinology. 2008;33:728–740. doi: 10.1016/j.psyneuen.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 51.Campbell DB, Li C, Sutcliffe JS, Persico AM, Levitt P. Genetic evidence implicating multiple genes in the MET receptor tyrosine kinase pathway in autism spectrum disorder. Autism Res. 2008;1:159–168. doi: 10.1002/aur.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ashwood P, et al. Elevated plasma cytokines in autism spectrum disorders provide evidence of immune dysfunction and are associated with impaired behavioral outcome. Brain Behav Immun. 2011;25:40–45. doi: 10.1016/j.bbi.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Heuer L, et al. Reduced levels of immunoglobulin in children with autism correlates with behavioral symptoms. Autism Res. 2008;1:275–283. doi: 10.1002/aur.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Smith SE, Li J, Garbett K, Mirnics K, Patterson PH. Maternal immune activation alters fetal brain development through interleukin-6. J Neurosci. 2007;27:10695–10702. doi: 10.1523/JNEUROSCI.2178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Walf AA, Frye CA. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Protoc. 2007;2:322–328. doi: 10.1038/nprot.2007.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Plappert CF, Kuhn S, Schnitzler HU, Pilz PK. Experience increases the prepulse inhibition of the acoustic startle response in mice. Behav Neurosci. 2006;120:16–23. doi: 10.1037/0735-7044.120.1.16. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.