Abstract
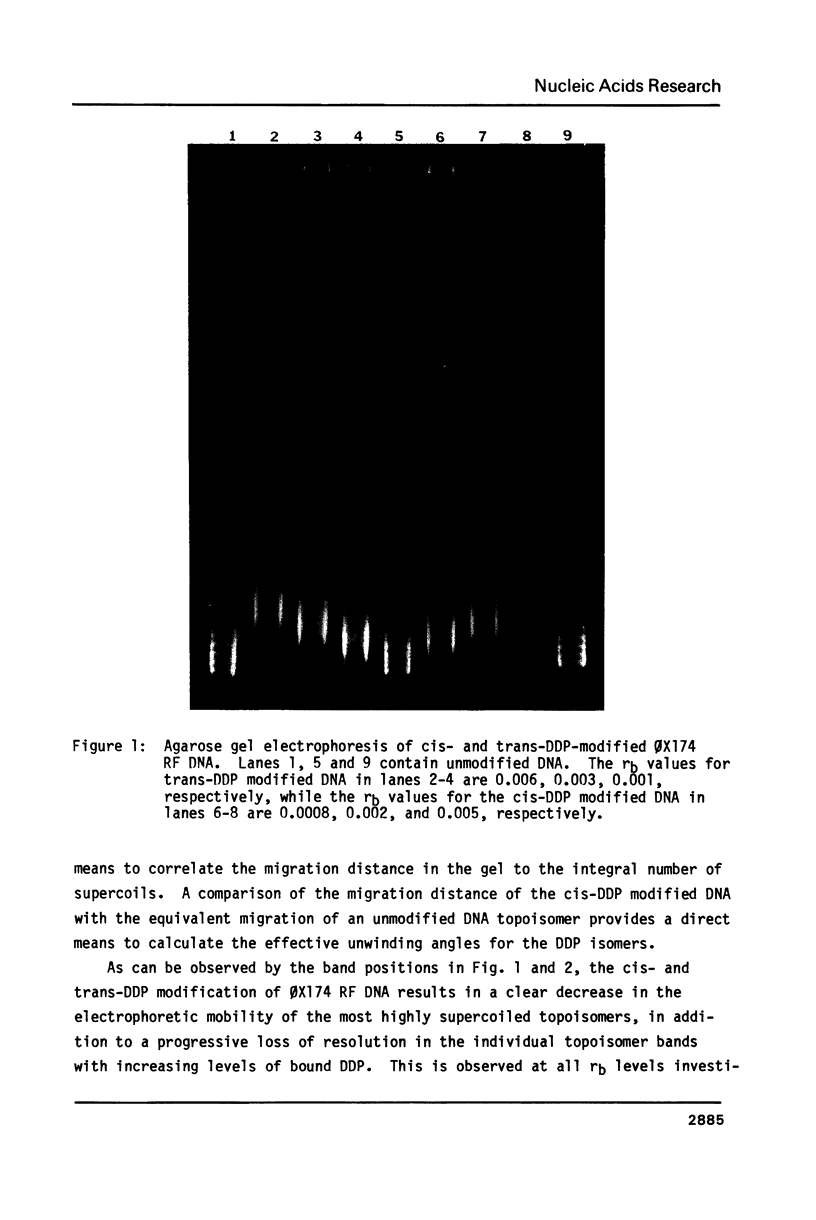
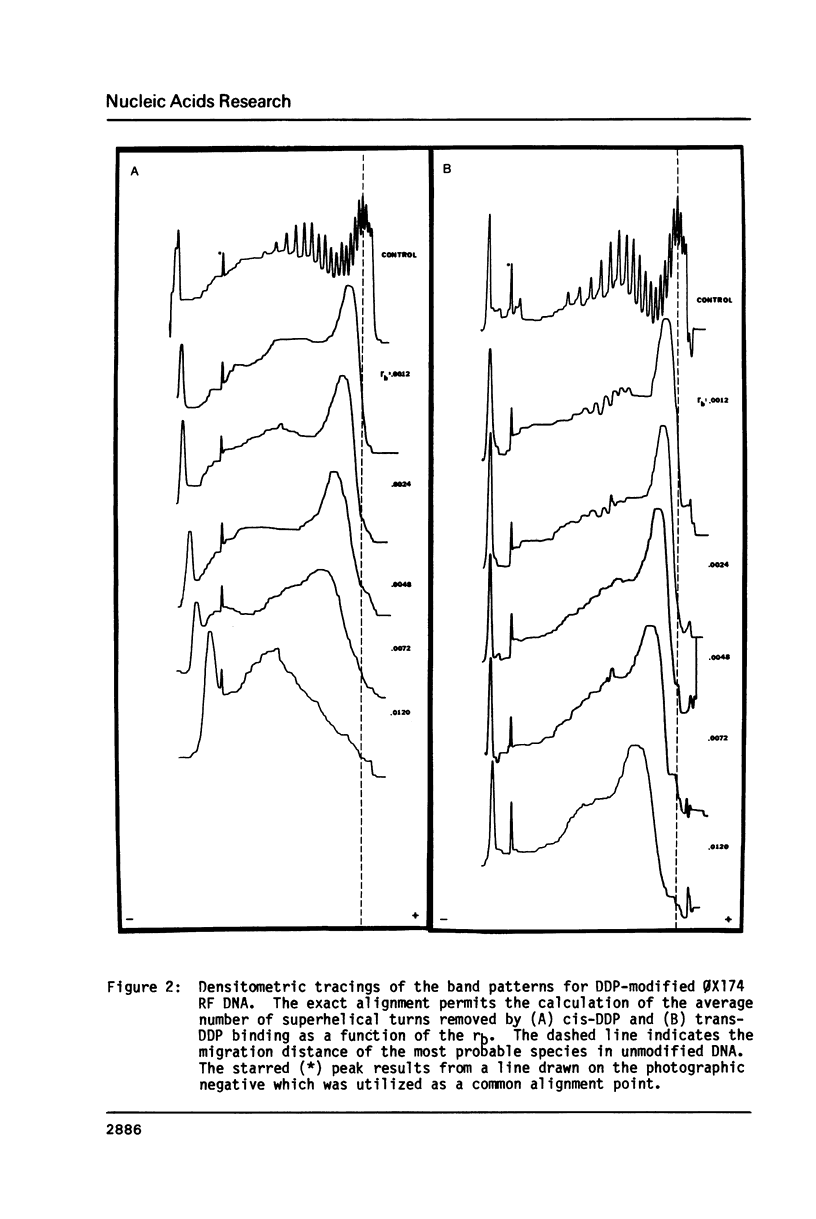
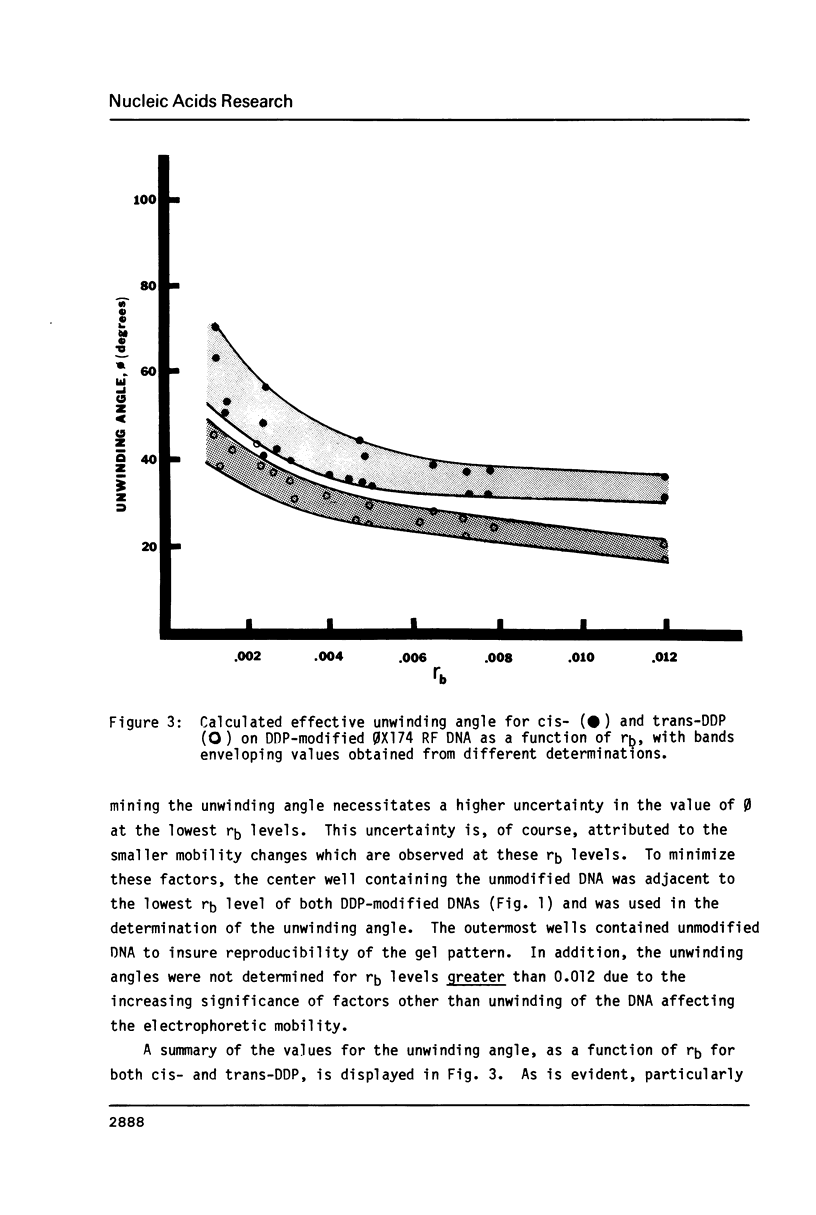
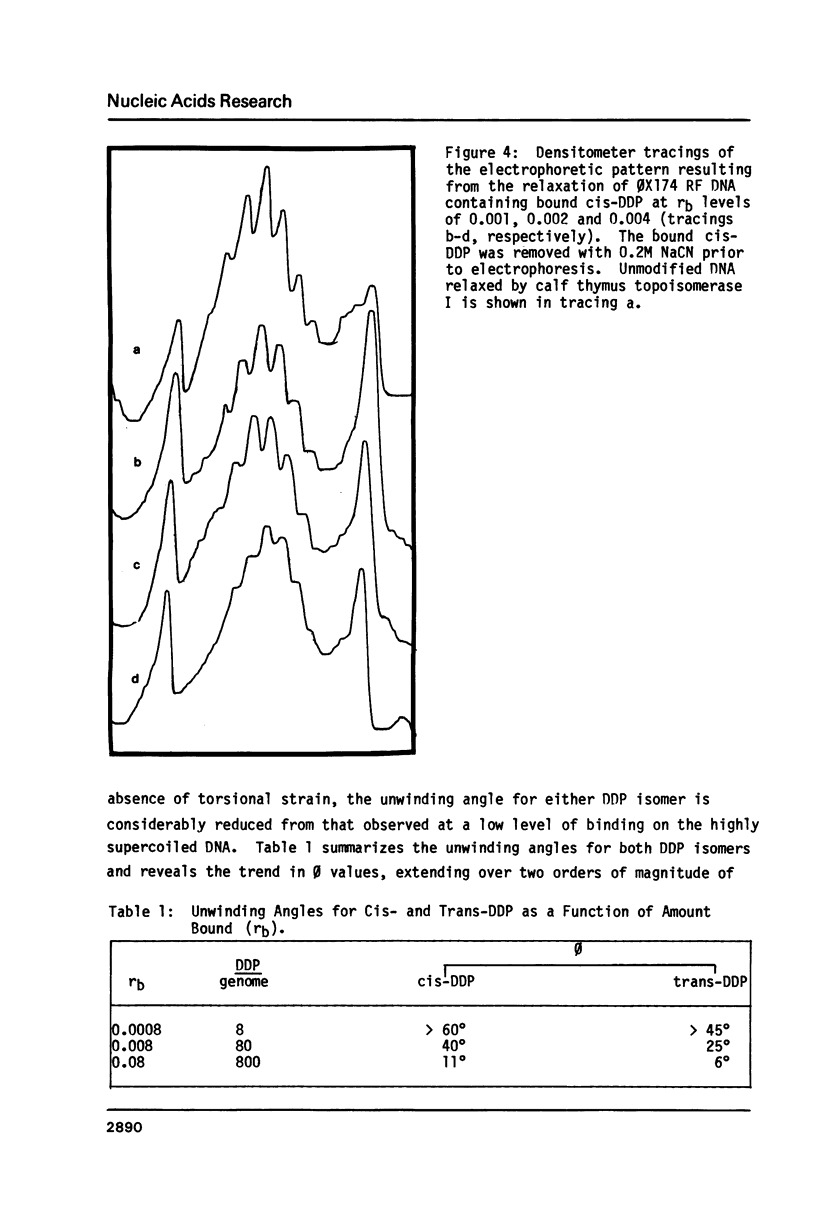
The effective unwinding angle, phi, for cis-diamminedichloroplatinum(II) (cis-DDP) and trans-DDP was determined by utilizing high resolution gel electrophoresis and supercoiled phi X174 RF DNA as a substrate. The effective unwinding angle was calculated by equating the reduction in mobility of the DDP-modified DNA to the removal of a number of superhelical turns. The value of the effective unwinding angle for both DDP isomers was greatest at the low levels of DDP bound and decreased with increasing amounts of unwinding agent. The cis-isomer is a better unwinding agent than is the trans-isomer, being nearly twice as effective in unwinding the supercoiled DNA at the DDP levels investigated. A comparison of the magnitude of phi below rb values of 0.005 and those at high levels of binding reveals that the extent of torsional strain in the supercoiled DNA influences the magnitude of the unwinding of the DNA by these complexes. When this method is used in the analysis of the unwinding angle for a covalently bound species on supercoiled DNA, it may provide a more reliable estimate of the magnitude of phi at high degrees of supercoiling and at low levels of modification.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauer W., Vinograd J. The interaction of closed circular DNA with intercalative dyes. I. The superhelix density of SV40 DNA in the presence and absence of dye. J Mol Biol. 1968 Apr 14;33(1):141–171. doi: 10.1016/0022-2836(68)90286-6. [DOI] [PubMed] [Google Scholar]
- Botchan P., Wang J. C., Echols H. Effect of circularity and superhelicity on transcription from bacteriophagelambda DNA. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3077–3081. doi: 10.1073/pnas.70.11.3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camerman N., Camerman A. Photodimer of thymine in ultraviolet-irradiated DNA: proof of structure by x-ray diffraction. Science. 1968 Jun 28;160(3835):1451–1452. doi: 10.1126/science.160.3835.1451. [DOI] [PubMed] [Google Scholar]
- Ciarrocchi G., Pedrini A. M. Determination of pyrimidine dimer unwinding angle by measurement of DNA electrophoretic mobility. J Mol Biol. 1982 Feb 25;155(2):177–183. doi: 10.1016/0022-2836(82)90445-4. [DOI] [PubMed] [Google Scholar]
- Cohen G. L., Bauer W. R., Barton J. K., Lippard S. J. Binding of cis- and trans-dichlorodiammineplatinum(II) to DNA: evidence for unwinding and shortening of the double helix. Science. 1979 Mar 9;203(4384):1014–1016. doi: 10.1126/science.370979. [DOI] [PubMed] [Google Scholar]
- Drinkwater N. R., Miller J. A., Miller E. C., Yang N. C. Covalent intercalative binding to DNA in relation to the mutagenicity of hydrocarbon epoxides and N-acetoxy-2-acetylaminofluorene. Cancer Res. 1978 Oct;38(10):3247–3255. [PubMed] [Google Scholar]
- Eastman A. Characterization of the adducts produced in DNA by cis-diamminedichloroplatinum(II) and cis-dichloro(ethylenediamine)platinum(II). Biochemistry. 1983 Aug 2;22(16):3927–3933. doi: 10.1021/bi00285a031. [DOI] [PubMed] [Google Scholar]
- Einhorn L. H. Testicular cancer as a model for a curable neoplasm: The Richard and Hinda Rosenthal Foundation Award Lecture. Cancer Res. 1981 Sep;41(9 Pt 1):3275–3280. [PubMed] [Google Scholar]
- Fritzsche H., Triebel H., Chaires J. B., Dattagupta N., Crothers D. M. Studies on interaction of anthracycline antibiotics and deoxyribonucleic acid: geometry of intercalation of iremycin and daunomycin. Biochemistry. 1982 Aug 17;21(17):3940–3946. doi: 10.1021/bi00260a006. [DOI] [PubMed] [Google Scholar]
- Gamper H. B., Hearst J. E. A topological model for transcription based on unwinding angle analysis of E. coli RNA polymerase binary, initiation and ternary complexes. Cell. 1982 May;29(1):81–90. doi: 10.1016/0092-8674(82)90092-7. [DOI] [PubMed] [Google Scholar]
- Gamper H. B., Straub K., Calvin M., Bartholomew J. C. DNA alkylation and unwinding induced by benzo[a]pyrene diol epoxide: modulation by ionic strength and superhelicity. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2000–2004. doi: 10.1073/pnas.77.4.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh T. S., Wang J. C. Thermodynamic properties of superhelical DNAs. Biochemistry. 1975 Feb 11;14(3):527–535. doi: 10.1021/bi00674a011. [DOI] [PubMed] [Google Scholar]
- Hyde J. E., Hearst J. E. Binding of psoralen derivatives to DNA and chromatin: influence of the ionic environment on dark binding and photoreactivity. Biochemistry. 1978 Apr 4;17(7):1251–1257. doi: 10.1021/bi00600a019. [DOI] [PubMed] [Google Scholar]
- Lilley D. M. The inverted repeat as a recognizable structural feature in supercoiled DNA molecules. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6468–6472. doi: 10.1073/pnas.77.11.6468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malfoy B., Hartmann B., Leng M. The B goes to Z transition of poly(dG-dC) . poly(dG-dC) modified by some platinum derivatives. Nucleic Acids Res. 1981 Nov 11;9(21):5659–5669. doi: 10.1093/nar/9.21.5659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinge J. M., Leng M. Reaction of cis-diamminedichloroplatinum (II) and DNA in B or Z conformation. EMBO J. 1984 Jun;3(6):1273–1279. doi: 10.1002/j.1460-2075.1984.tb01962.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusyk R., Sergeant A. A simple method for dialysis of small-volume samples. Anal Biochem. 1980 Jul 1;105(2):403–404. doi: 10.1016/0003-2697(80)90477-7. [DOI] [PubMed] [Google Scholar]
- Meehan T., Gamper H., Becker J. F. Characterization of reversible, physical binding of benzo[a]pyrene derivatives to DNA. J Biol Chem. 1982 Sep 10;257(17):10479–10485. [PubMed] [Google Scholar]
- Nordheim A., Hao W. M., Wogan G. N., Rich A. Salt-induced conversion of B-DNA to Z-DNA inhibited by aflatoxin B1. Science. 1983 Mar 25;219(4591):1434–1436. doi: 10.1126/science.6402818. [DOI] [PubMed] [Google Scholar]
- Nordheim A., Lafer E. M., Peck L. J., Wang J. C., Stollar B. D., Rich A. Negatively supercoiled plasmids contain left-handed Z-DNA segments as detected by specific antibody binding. Cell. 1982 Dec;31(2 Pt 1):309–318. doi: 10.1016/0092-8674(82)90124-6. [DOI] [PubMed] [Google Scholar]
- Panayotatos N., Wells R. D. Cruciform structures in supercoiled DNA. Nature. 1981 Feb 5;289(5797):466–470. doi: 10.1038/289466a0. [DOI] [PubMed] [Google Scholar]
- Peck L. J., Nordheim A., Rich A., Wang J. C. Flipping of cloned d(pCpG)n.d(pCpG)n DNA sequences from right- to left-handed helical structure by salt, Co(III), or negative supercoiling. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4560–4564. doi: 10.1073/pnas.79.15.4560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Povirk L. F., Hogan M., Dattagupta N. Binding of bleomycin to DNA: intercalation of the bithiazole rings. Biochemistry. 1979 Jan 9;18(1):96–101. doi: 10.1021/bi00568a015. [DOI] [PubMed] [Google Scholar]
- Royer-Pokora B., Gordon L. K., Haseltine W. A. Use of exonuclease III to determine the site of stable lesions in defined sequences of DNA: the cyclobutane pyrimidine dimer and cis and trans dichlorodiammine platinum II examples. Nucleic Acids Res. 1981 Sep 25;9(18):4595–4609. doi: 10.1093/nar/9.18.4595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scovell W. M., Capponi V. J. Cis-diamminedichloroplatinum(II) modified DNA stimulates far greater levels of S1 nuclease sensitive regions than does the modification produced by the trans- isomer. Biochem Biophys Res Commun. 1982 Aug;107(3):1138–1143. doi: 10.1016/0006-291x(82)90640-4. [DOI] [PubMed] [Google Scholar]
- Scovell W. M., Kroos L. R. Cis- and trans-diamminedichloroplatinum(II) binding products different tertiary structural changes on SV40 DNA. Biochem Biophys Res Commun. 1982 Feb 26;104(4):1597–1603. doi: 10.1016/0006-291x(82)91435-8. [DOI] [PubMed] [Google Scholar]
- Scovell W. M., Kroos L. R. Cis-diamminedichloroplatinum (II) modification of SV40 DNA occurs preferentially in (G+C) rich regions: implications into the mechanism of action. Biochem Biophys Res Commun. 1982 Sep 16;108(1):16–23. doi: 10.1016/0006-291x(82)91825-3. [DOI] [PubMed] [Google Scholar]
- Shure M., Vinograd J. The number of superhelical turns in native virion SV40 DNA and minicol DNA determined by the band counting method. Cell. 1976 Jun;8(2):215–226. doi: 10.1016/0092-8674(76)90005-2. [DOI] [PubMed] [Google Scholar]
- Singleton C. K., Klysik J., Stirdivant S. M., Wells R. D. Left-handed Z-DNA is induced by supercoiling in physiological ionic conditions. Nature. 1982 Sep 23;299(5881):312–316. doi: 10.1038/299312a0. [DOI] [PubMed] [Google Scholar]
- Ushay H. M., Santella R. M., Caradonna J. P., Grunberger D., Lippard S. J. Binding of [(dien)PtCl] Cl to poly(dG-dC)-poly(dG-dC) facilitates the B goes to Z conformational transition. Nucleic Acids Res. 1982 Jun 11;10(11):3573–3588. doi: 10.1093/nar/10.11.3573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. C. Interactions between twisted DNAs and enzymes: the effects of superhelical turns. J Mol Biol. 1974 Aug 25;87(4):797–816. doi: 10.1016/0022-2836(74)90085-0. [DOI] [PubMed] [Google Scholar]
- Wang J. C., Jacobsen J. H., Saucier J. M. Physiochemical studies on interactions between DNA and RNA polymerase. Unwinding of the DNA helix by Escherichia coli RNA polymerase. Nucleic Acids Res. 1977;4(5):1225–1241. doi: 10.1093/nar/4.5.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. C. The degree of unwinding of the DNA helix by ethidium. I. Titration of twisted PM2 DNA molecules in alkaline cesium chloride density gradients. J Mol Biol. 1974 Nov 15;89(4):783–801. doi: 10.1016/0022-2836(74)90053-9. [DOI] [PubMed] [Google Scholar]
- Weintraub H. A dominant role for DNA secondary structure in forming hypersensitive structures in chromatin. Cell. 1983 Apr;32(4):1191–1203. doi: 10.1016/0092-8674(83)90302-1. [DOI] [PubMed] [Google Scholar]
- Wells R. D., Miglietta J. J., Kłysik J., Larson J. E., Stirdivant S. M., Zacharias W. Spectroscopic studies on acetylaminofluorene-modified (dT-dG)n . (dC-dA)n suggest a left-handed conformation. J Biol Chem. 1982 Sep 10;257(17):10166–10171. [PubMed] [Google Scholar]
- Wiesehahn G., Hearst J. E. DNA unwinding induced by photoaddition of psoralen derivatives and determination of dark-binding equilibrium constants by gel electrophoresis. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2703–2707. doi: 10.1073/pnas.75.6.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwelling L. A., Kohn K. W., Ross W. E., Ewig R. A., Anderson T. Kinetics of formation and disappearance of a DNA cross-linking effect in mouse leukemia L1210 cells treated with cis- and trans-diamminedichloroplatinum(II). Cancer Res. 1978 Jun;38(6):1762–1768. [PubMed] [Google Scholar]
- den Hartog J. H., Altona C., Chottard J. C., Girault J. P., Lallemand J. Y., de Leeuw F. A., Marcelis A. T., Reedijk J. Conformational analysis of the adduct cis-[Pt(NH3)2 d(GpG)]+ in aqueous solution. A high field (500-300 MHz) nuclear magnetic resonance investigation. Nucleic Acids Res. 1982 Aug 11;10(15):4715–4730. doi: 10.1093/nar/10.15.4715. [DOI] [PMC free article] [PubMed] [Google Scholar]



