Abstract
Analyses of functional interactions between large-scale brain networks have identified two broad systems that operate in apparent competition or antagonism with each other. One system, termed the default mode network (DMN), is thought to support internally oriented processing. The other system acts as a generic external attention system (EAS) and mediates attention to exogenous stimuli. Reports that the DMN and EAS show anticorrelated activity across a range of experimental paradigms suggest that competition between these systems supports adaptive behavior. Here, we used functional MRI to characterize functional interactions between the DMN and different EAS components during performance of a recollection task known to coactivate regions of both networks. Using methods to isolate task-related, context-dependent changes in functional connectivity between these systems, we show that increased cooperation between the DMN and a specific right-lateralized frontoparietal component of the EAS is associated with more rapid memory recollection. We also show that these cooperative dynamics are facilitated by a dynamic reconfiguration of the functional architecture of the DMN into core and transitional modules, with the latter serving to enhance integration with frontoparietal regions. In particular, the right posterior cingulate cortex may act as a critical information-processing hub that provokes these context-dependent reconfigurations from an intrinsic or default state of antagonism. Our findings highlight the dynamic, context-dependent nature of large-scale brain dynamics and shed light on their contribution to individual differences in behavior.
Keywords: complex, graph, modularity, rest, connectome
Increasing evidence points to a fundamental distinction between two large-scale functional systems in the brain (1–4). One system, comprising regions of lateral prefrontal and parietal cortex, dorsal anterior cingulate, and anterior insula/frontoopercular regions, typically shows increased activation during performance of challenging cognitive tasks and has been implicated in attentional and cognitive control functions (5, 6). It may thus be generally referred to as an external attention system (EAS), but it has also been labeled the task-positive and extrinsic network (3, 4). The other system, often called the default mode network (DMN), is localized primarily to midline posterior and anterior cortical regions, the angular gyri, and medial and lateral temporal cortices (7, 8). It often shows decreased activity during tasks requiring attention to external stimuli (9, 10) and increased activity during unconstrained thought, introspection, and self-related processing (7, 11). The apparent antagonism between these two systems is mirrored in their spontaneous dynamics, which are often strongly anticorrelated (2). These competitive interactions are thought to promote adaptive and efficient alternation between DMN-dominated introspective thought and EAS-mediated processing of external stimuli (1–4).
Several lines of evidence support this bipartite model of brain function. First, DMN activity reductions during cognitively demanding tasks, termed deactivations, often scale in accordance with attentional demands (12). Second, reduced deactivation in such contexts has been associated with poorer task performance, putatively reflecting an interference of endogenous processes (e.g., mind wandering) with attention to the outside world (13, 14). Third, during memory task performance, greater DMN activity is associated with poor encoding (which requires attention to external stimuli) but successful memory retrieval (which requires attention to internal mental processes), whereas EAS regions show the opposite pattern (15). Fourth, activity in DMN and EAS regions is often anticorrelated (2), and individuals showing stronger anticorrelations display faster and less variable reaction times (RTs) during performance of cognitive control tasks (16, 17). Fifth, patients with psychiatric disorders associated with attentional disturbances show reduced anticorrelation between the DMN and EAS (18, 19). Finally, anticorrelated interactions emerge spontaneously in computational models of neural dynamics simulated on realistic anatomical architectures, suggesting that they reflect an intrinsic property of the brain’s dynamical behavior (20).
Recent work, however, indicates that this bipartite model may be somewhat simplistic. First, the DMN and EAS do not always operate as functionally homogeneous entities, often splitting into distinct subnetworks depending on task demands (5, 7, 21, 22). This finding is particularly true for the EAS, which has been functionally dissociated into a number of distinct subsystems (5, 6, 22–26), although similar observations have been noted for the DMN (7, 10, 21). Second, local field potentials recorded from putative DMN and EAS regions in felines are more often positively than negatively correlated with each other, suggesting that these regions often interact cooperatively (27). Third, there are multiple human functional MRI (fMRI) reports of coactivation or cooperation (positively correlated activity) between DMN and EAS regions during recollection (28), perception of near-threshold acoustic stimuli (29), working memory and attention (30, 31), goal-directed introspective processing (23, 32), and unattended mind wandering (11). The emergence of these cooperative interactions seems critically dependent on the task being performed by participants at the time of scanning; i.e., they are context-specific (17, 22, 23, 33).
The above findings suggest that certain task conditions may provoke a departure from an intrinsic or default state of competition between the DMN and EAS to enable cooperative interactions between the two systems. It is unclear, however, whether greater cooperation between the DMN and EAS actually facilitates better performance in such contexts and how these large-scale systems dynamically reconfigure themselves to support these context-dependent collaborative interactions.
In this study, we used fMRI to distinguish task-related, context-dependent functional interactions between the DMN and EAS from task-unrelated, spontaneous network dynamics in individuals performing a recollection task previously shown to coactivate regions of both networks (28). Our aims were threefold. First, we aimed to test the hypothesis that greater context-dependent cooperation between DMN and EAS regions would facilitate better recollection performance. Second, we aimed to map how changing task conditions provoke a dynamic reconfiguration of brain functional organization from a default state of antagonism to one of greater functional integration of DMN and EAS processes. Third, we aimed to characterize the role that each individual brain region plays in facilitating these shifts in functional network architecture.
Results
Group-Level Interactions Between the DMN and EAS Are Competitive.
Sixteen adult participants performed a task requiring them to recollect the context in which well-learned word pairs (e.g., bacon and eggs) were previously encountered (the second word was perceived or imagined by the participant or read aloud by either the participant or the experimenter). Blocks of recollection trials alternated with blocks of nonrecollection trials requiring simple semantic judgments about probe stimuli matched for basic perceptual features (SI Text, sections S.1 and S.2 and Fig. S1).
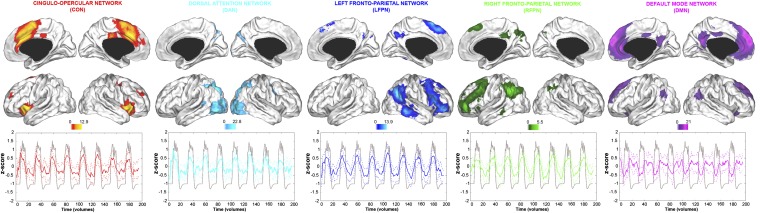
Task-related functional networks corresponding to the DMN and EAS were identified in an unbiased, data-driven manner using spatial independent component analysis (ICA) (34) (SI Text, section S.3). The method is well-suited for characterizing the spatial anatomy and temporal dynamics of each network, while accounting for possible functional heterogeneity within either the DMN and/or EAS (5, 7, 21, 22, 24) (SI Text, section S.3). The DMN was identified as a single component with a characteristic functional anatomy consistent with prior work (7, 10). The EAS was split into four distinct components: (i) a dorsal attention network (DAN) commonly associated with focusing attention on external stimuli (2, 6); (ii) a cinguloopercular network (CON) implicated in interoceptive awareness (35), salience processing of external stimuli (25), and maintenance of response set during cognitive task performance (5); and (iii and iv) left and (4) right frontoparietal networks (LFPN and RFPN, respectively) often implicated in top-down executive control processes (5, 24). ICA commonly identifies these left and right components as separate networks, and prior work supports functional dissociations between the two components (26). Our findings below support this functional distinction. The spatial anatomy and representative time courses of each of these five networks is presented in Fig. 1 (Table S1).
Fig. 1.
Connectivity Z maps and sample mean network time courses of each of the five networks of interest. Dotted lines represent SD. Time courses are overlaid on a task regressor modeling activity associated with recollection blocks and indicating the onset of these trials relative to the baseline condition (gray line, arbitrary units). The spatial maps display voxels showing significant functional connectivity at P < 0.05, familywise error corrected (cluster extent > 10 voxels). Left hemisphere presented on the right hand side of each panel.
Across participants, DMN activity was higher during the semantic baseline condition than recollection trials (t = −6.074, P < 0.001), whereas the opposite was true for the CON (t = 9.387, P < 0.001), DAN (t = 7.59, P < 0.001), LFPN (t = 11.618, P < 0.001), and RFPN (t = 9.661, P < 0.001) (Fig. 1). Accordingly, cross-correlation analysis of component time courses averaged across participants indicated that DMN activity was strongly anticorrelated with the CON (r = −0.78, P < 0.001), DAN (r = −0.79, P < 0.001), LFPN (r = −0.73, P < 0.001), and RFPN (r = −0.59, P < 0.001), whereas the activity of each of the four EAS networks was strongly positively correlated (0.70 < r < 0.88, all P < 0.001).
Context-Dependent Cooperation Between the DMN and RFPN Facilitates Rapid Recollection.
The group-level results indicate that, on average, the different EAS components showed strong cooperation with each other, while interacting competitively with the DMN. Analysis of individual differences suggested that there was considerable variability around this group-averaged behavior. To analyze these differences, we extracted activity time courses for each network and each participant and computed subject-specific estimates of both task-related and task-unrelated functional interactions between the DMN and each EAS component. This distinction was critical, because it allowed us to separate context-dependent (task-related) network interactions from putative spontaneous or intrinsic (task-unrelated) functional dynamics.
Spontaneous or task-unrelated network interactions, nis, were estimated by band-pass filtering of each network time course (0.008 < f < 0.08 Hz) and orthogonalizing it with respect to covariates modeling various noise sources and task-related variance in the data (36). Task-related network interactions (nit) were estimated using a correlational psychophysiological interaction (cPPI) approach that used partial correlations to isolate covariations in task-related modulations of network activity as distinct from task-unrelated connectivity, noise, and coactivation effects. For both types of analysis, interactions between the DMN and each of the four EAS components were estimated after partialing covariance with the remaining EAS components to ensure that only temporal correlations specific to each network pair were being analyzed (SI Text, section S.4 has additional details of these methods).
For both task-unrelated and task-related interactions, the degree to which cooperative or competitive functional interactions were expressed varied across individuals and network pairs (SI Text, section S.7 and Figs. S2 and S3). To determine whether these differences were associated with task performance, we correlated the subject-specific nis and nit estimates for each of the four DMN–EAS network pairs with measures of recollection accuracy and RT. We found a specific and significant negative correlation between recollection RT and task-related DMN–RFPN nit values (ρ = −0.671, P = 0.005) (Fig. S4B) but not task-unrelated nis values (ρ = −0.353, P = 0.176) (Fig. S4A). The former result survived Bonferroni correction for multiple comparisons. Moreover, the difference between the two correlations was significant (ZI* = −1.718, P = 0.043) (SI Text, section S.6). No other associations with behavior approached significance.
The results were replicated when the analysis was repeated after partialing covariance with all other 19 components identified by the ICA as representing distinct sources of signal and noise in the data. The correlation between RT and DMN–RFPN nit remained significant (ρ = −0.621, P = 0.008) (Fig. S4D); the association between RT and nis values was not significant (ρ = −0.056, P = 0.821) (Fig. S4C); and the difference between the two correlations was also significant (ZI* = −1.729, P = 0.042). This analysis provides a stringent test of the specificity of the association between RT and DMN–RFPN nit values and suggests that greater task-related cooperation between these two networks was associated with more rapid recollection.
Context-Dependent Reconfiguration of the DMN Supports Cooperative Interactions with the RFPN.
The specific association between higher DMN–RFPN nit values and faster RT suggests that greater functional integration between the DMN and RFPN supports rapid recollection performance. This network-level integration must be facilitated by a dynamic reconfiguration of pairwise functional connectivity between the constituent regions of the DMN and RFPN. We mapped this reconfiguration using graph analysis. Specifically, we extracted activity time courses from each of 34 regions comprising the DMN and RFPN (20 DMN and 14 RFPN regions) (Fig. 2A and Table S2) and separately computed task-related and task-unrelated functional connectivity between each pair of regions to yield two 34 × 34 functional connectivity matrices (one task-related and one task-unrelated) for each individual. These matrices were then decomposed into nonoverlapping sets of brain regions, termed modules, showing higher functional connectivity with each other than with other areas using a modularity decomposition algorithm suitable for unthresholded, weighted, and signed networks (37) (SI Text, section S.5).
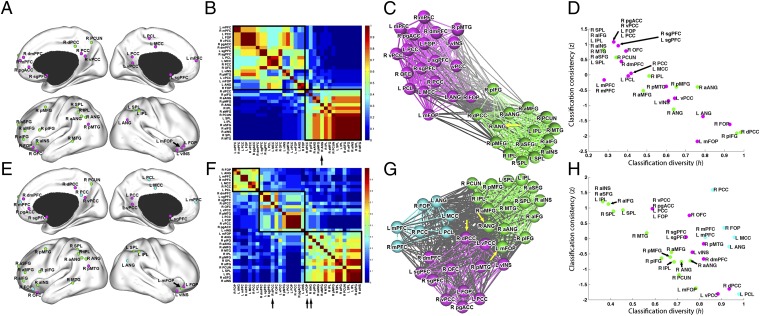
Fig. 2.
Illustration of node-specific functional roles mediating task-related and task-unrelated functional interactions between the DMN and RFPN. (A) Anatomical location of spherical regions of interest that comprise the DMN (magenta) and RFPN (green) modules, as identified by the modular decomposition of the task-unrelated functional connectivity data (maps colored according to the original assignments implied by the ICA can be seen in Fig. S5). (B) Task-unrelated group consistency coclassification matrices (SI Text, section S.5) reordered to emphasize the optimal modular structure for the sample. Solid black lines indicate boundaries between modules. Arrows highlight regions with module assignment that differed from the assignment implied by the initial ICA. (C) Fruchterman–Reingold force-directed projections showing intra- and intermodular connectivity in the task-unrelated network. Strongly connected nodes are placed in closer proximity to each other. Intramodule connections are colored according to the module identity of the nodes that they interconnect. Intermodular connections are colored black. Yellow arrows highlight regions with module assignments that differ from the assignments implied by the initial ICA (Fig. S5). (D) Scatterplots of classification consistency, z, and classification diversity, h, of each region in the task-unrelated data. Colors indicate the module to which each region belongs. (E) Location of regions belonging to the RFPN (green), DMNa (magenta), and DMNb (cyan) modules identified in the task-related functional connectivity analysis. (F–H) Group coclassification matrix, force-directed projection, and consistency-diversity scatterplot, respectively, for the task-related data. Table S2 explains the abbreviated node labels. R, right hemisphere; L, left hemisphere.
To account for individual variability in the number and composition of modules identified across participants, we adapted previously described methods (38) to identify the optimal modular architecture for the entire sample for both task-related and task-unrelated networks. Briefly, a coclassification matrix representing the frequency with which each pair of nodes was assigned to the same module across participants was constructed separately for the task-related (Fig. 2B) and task-unrelated data (Fig. 2F). These matrices were then subjected to a second-level modular decomposition (Fig. 2 C and G). By this procedure, two regions consistently coclassified in the same module across participants were assigned to the same module in the second-level partition (additional details in SI Text, section S.5).
For task-unrelated networks, the optimal decomposition identified two modules that almost perfectly replicated the ICA-based classification; i.e., all nodes derived from the RFPN component mapped onto a common module, whereas all DMN regions, except for the right angular gyrus, were also grouped together (Fig. 2 A–C, RFPN is shown in green, and DMN is shown in magenta). Thus, the initial ICA-based separation of DMN and RFPN regions was recapitulated by a modular decomposition of their pairwise spontaneous interactions. This consistency is noteworthy given the different processing and analysis techniques used to derive these partitions, and it supports the hypothesis that the default or intrinsic state of these two systems is one of functional segregation or antagonism.
In contrast, the optimal modular decomposition for task-related interactions identified three modules. All but one of the regions in the RFPN module were consistent with the analysis of task-unrelated data, but the DMN split into two smaller subgroups: a larger module comprising 12 nodes (DMNa module; magenta in Fig. 2 E and G) and a smaller group comprising seven regions (DMNb module; cyan in Fig. 2 E and G).
To understand the functional roles played by each module and their constituent nodes, we examined the consistency and diversity with which different regions were coclassified into the same module across participants. Classification consistency was estimated by computing the within-module strength, z, of each node separately in the task-related and task-unrelated group coclassification matrices. Classification diversity was computed using the diversity coefficient h (37, 39) (formal definitions are provided in SI Text, section S.5). Applied in this context, z quantified the degree to which each region was classified in the same module across participants relative to other nodes in the same module. Brain regions with high z values represent core components of their module and thus act as local connectivity hubs. The diversity coefficient, h, quantified the variability of each region’s modular assignment across participants. Regions with high h have a relatively equal probability of being classified into different modules across participants, because their connectivity is dispersed between modules from individual to individual. These regions, therefore, represent transitional nodes that facilitate functional integration between modules (37, 39) (additional details in SI Text, section S.5).
For task-unrelated networks, regional z and h were negatively correlated such that network nodes were characterized by either high classification consistency or high classification diversity (Fig. 2D). DMN regions with high z, representing core module elements, included known hubs such as posterior cingulate and dorsal and ventromedial prefrontal regions (7). Core RFPN elements included superior parietal and lateral prefrontal areas, consistent with prior work (24). Transitional nodes, characterized by high h, included areas that have previously been shown to promote functional integration between DMN and EAS regions in certain task contexts, such as dorsal posterior cingulate and right frontoopercular cortices (22, 30, 40).
A similar negative association between z and h values was evident for nodes in the DMNa and RFPN modules of task-related networks (magenta and green, respectively, in Fig. 2 E and G). In contrast, nodes in the DMNb module (cyan in Fig. 2 E and G) were primarily characterized by high h. This property suggests that these DMNb regions collectively formed a transitional module with connectivity dispersed across DMNa and RFPN regions. In other words, the DMNb module acted as a bridge facilitating functional integration within and between the DMN and RFPN. ANOVA confirmed that the mean diversity coefficient of DMNb regions (M = 0.90, SD = 0.062) was higher than for DMNa (M = 0.734, SD = 0.12) and RFPN (M = 0.569, SD = 0.160) regions [F(2, 31) = 15.512, P < 0.001]. Against this background, the right posterior cingulate cortex (PCC) stood out as a region showing both high h and high z, indicating that it was a core element of the transitional DMNb module that also retained diverse connectivity with DMNa and RFPN regions. This result suggests that the right PCC represents a putative information-processing bottleneck that acted as a connectivity hub within the transitional DMNb module, while concomitantly facilitating functional integration between DMNa and RFPN regions. Notably, however, individual differences in nodal connectivity measures did not correlate with recollection RT (SI Text, section S.6).
Discussion
Replicated reports of competitive or anticorrelated dynamics between large-scale functional brain systems have been interpreted as evidence for a fundamental distinction and antagonism between neural systems supporting introspective and extrospective processing (1, 2, 16). Our results suggest a revision of this view in light of (i) the diversity of interactions between the DMN and different components of the EAS; (ii) the context dependence of these interactions; and (iii) the substantial variability in the degree to which competitive or cooperative interactions between the DMN and different EAS components were expressed across individuals. In particular, our findings show that, under certain task conditions (such as the recollection paradigm studied here), greater cooperation between these systems is actually associated with better performance. This enhanced cooperation is facilitated by dynamic, context-dependent reconfiguration of the DMN into core and transitional modules, with the latter supporting greater functional integration between the DMN core and frontoparietal areas. In facilitating these dynamic shifts of functional network architecture, the right PCC seems to act as a critical information-processing bottleneck, representing a major hub of the transitional DMN module while also retaining high functional integration with other regions.
The association between faster recollection RT and stronger DMN–RFPN cooperation counters the hypothesis that greater antagonism between DMN and EAS regions invariably supports optimal task performance (1, 16). According to this view, the introspective processes mediated by the DMN can interfere with EAS-related processes that support attention to external stimuli. Consequently, greater separation of these functions, which are reflected in a stronger anticorrelation between their activation dynamics, should support adaptive and efficient behavior. This hypothesis has been supported by work using attentional or cognitive control tasks (14, 16, 17). Recent work using alternative experimental paradigms has, however, identified task contexts during which DMN and EAS regions coactivate or enhance functional connectivity (11, 22, 23, 29, 31–33, 41). Such findings indicate that competitive large-scale brain network dynamics are not an invariant property of brain function.
In our analyses, the need to isolate context-dependent network interactions was highlighted by the different findings that we obtained when considering task-unrelated dynamics. Indeed, our results indicate that cooperative interactions between DMN and EAS regions emerge as a context-dependent shift from an intrinsic or default state of antagonism as functional segregation between the DMN and different EAS components was generally more pronounced in task-unrelated functional connectivity measures (the major exception was the DMN–LFPN pair) (Fig. 1 and Fig. S2). Thus, methods that isolate task-related, context-dependent network interactions will provide a more sensitive characterization of circumstances under which cooperation between the DMN and EAS arises. Accordingly, many of the studies reporting evidence for cooperation between these systems have used such techniques (23, 31, 32, 41). In our analyses, the importance of isolating these interactions was underscored by the 33–38% greater covariance observed between recollection RT and DMN–RFPN nit values compared with nis measures.
Functional diversity within the EAS may also contribute to inconsistent reports of competitive or cooperative interactions with the DMN across studies. The EAS is loosely defined as a collection of brain regions often showing increased activation during cognitively demanding tasks, and it has been labeled with a variety of names, including the task positive network (3, 15), extrinsic network (4), external awareness network (42), and cognitive control network (30). Part of this confusion stems from differences in the methods used to define the system, which have included activation patterns (5), seed-based correlation analyses (24), anticorrelation with the DMN (2), and ICA (3, 16, 30). Each of these methods can result in different network definitions. For example, the work by Vincent et al. (24) used seed-based correlation analysis of resting-state fMRI data to identify a broad frontoparietal control system, comprising regions of the CON, LFPN, and RFPN as defined in our study. They argued that this control system was anatomically interposed between the DMN and anticorrelated DAN, and was thus well-positioned to facilitate functional integration between the two. Support for this view comes from evidence that regions of the frontoparietal control system flexibly couple with either the DMN or DAN, depending on the task at hand (23, 33). However, this view does not account for metaanalytic evidence supporting functional dissociations between cinguloopercular and frontoparietal components of the broader control system described by Vincent et al. (5, 24, 26) as well as evidence that the CON may initiate switches between competitive processing modes dominated by the DMN and frontoparietal regions (22).
Our results support a functional distinction between the CON and frontoparietal systems as well as between left- and right-lateralized components of the frontoparietal network. Specifically, we found that the CON, LFPN, and RFPN each showed diverse modes of interaction with the DMN (Fig. S2); that recollection RT correlated specifically RFPN–DMN interactions; and that it was the context-dependent interactions between these networks that most strongly dissociated the RFPN from other components of the EAS. The last conclusion is supported by our analyses of group-averaged and task-related data, which pointed to strong functional segregation between the DMN and all EAS components (Figs. 1 and 2). This result again highlights the importance of considering the context-dependent character of large-scale brain dynamics.
The identification of DMN–RFPN interactions as being specifically associated with recollection RT is notable given the proposed roles of these networks in recollection. The DMN is commonly activated during successful memory retrieval and less active during recollection errors (15). Accordingly, it is thought to play a critical role in the storage and/or recollection of contextual associations (43). In contrast, activation specifically of right-lateralized frontal and parietal regions has been associated with controlled memory retrieval (44) and heuristic evaluation processes (45), the monitoring of retrieved memoranda (46), and more general retrieval-related decision-making (47). Together, these findings indicate that the DMN supports rapid, effortless retrieval of contextual associations, whereas the RFPN may support more strategic searches through memory and/or the monitoring of retrieved information. Our findings indicate that cooperative interactions between these two networks, as reflected in greater positive functional connectivity, are associated with more rapid recollection performance. Thus, one hypothesis to emerge from these data is that enhanced functional coupling of DMN and right-lateralized frontoparietal regions reflects greater access of RFPN-related search and monitoring processes to well-learned contextual associations mediated by the DMN, resulting in relatively effortless and rapid retrieval. This view predicts that memory recalled with greater confidence should be associated with greater DMN–RFPN cooperation. To test this hypothesis, we ran secondary analyses testing for associations between DMN–RFPN nit values and behavioral measures of recollection confidence acquired during our task (SI Text, section S.1). We found that strong positive functional connectivity between the DMN and RFPN was indeed associated with greater memory confidence (ρ = 0.544, P = 0.027). This result supports the hypothesis that enhanced RFPN–DMN coupling is associated with rapid, effortless recollection.
Greater cooperation between the DMN and RFPN was facilitated by a context-dependent reconfiguration of the DMN into two distinct components: a core DMNa module and a smaller DMNb transitional module. The high mean classification diversity of nodes in the transitional module indicated that it acted as a bridge supporting functional integration between the DMNa and RFPN modules. In particular, the right PCC stood out as a putative information-processing bottleneck, representing a core hub of the transitional module while also retaining high connectivity with the other two modules. It may thus act as a catalyst that provokes context-dependent departures from a default state of DMN–RFPN antagonism to support greater functional integration during recollection. In this regard, although there was no significant correlation between recollection RT and individual differences in PCC function (or between RT and functional measures computed for any other area) (SI Text, section S.6), the region played a critical role in facilitating the context-dependent shifts of large-scale network organization that did support rapid recollection (i.e., greater task-related collaboration between DMN and RFPN regions). The centrality of the PCC to these network interactions is also supported by recent evidence that the region plays an important role in recollection (43), that it is a major connectivity hub in the brain (48) with functional properties that are under strong genetic influence (49, 50), and that it represents a core region that flexibly interacts with different DMN components depending on the task being performed at any given time (30).
In summary, our findings highlight the context dependence of large-scale functional network interactions in the brain. In particular, they indicate that competitive interactions between DMN and EAS regions are not an invariant property of adaptive behavior and that these systems can interact cooperatively in certain circumstances to support optimal task performance. A greater appreciation of the diversity of functional interactions in the brain, their context sensitivity, and the roles that individual brain regions play in facilitating these interactions will yield a more accurate characterization of the behavioral significance of large-scale brain network dynamics.
Methods
Experimental Design.
Sixteen healthy, right-handed participants (seven male; mean age = 24.3 y, range = 19–36 y) underwent five study and five test phases of a contextual recollection task in the scanner, although only test phases were scanned (additional details in SI Text, section S.1 and details on image acquisition, processing, and general linear model in SI Text, section S.2). All participants gave written, informed consent. The study was approved by the Cambridge Local Research Ethics Committee.
Network Analyses.
We identified spatially independent, temporally coherent networks of voxels using spatial ICA, which was implemented in the Group ICA for fMRI Toolbox (GIFT; http://mialab.mrn.org/software/gift/) (SI Text, section S.3). Task-related and task-unrelated functional interactions between these large-scale networks were computed using partial correlation of representative component time courses. Task-related network interactions were estimated using a correlational psychophysiological interaction (cPPI) analysis developed specifically for this purpose using freely available code (http://www.psychiatry.unimelb.edu.au/centres-units/mnc/research/connectivity_software.html) (SI Text, section S.4). Task-unrelated functional interactions were estimated after task-related variance was removed from each network’s time course using a Gramm–Schmidt orthogonalization procedure, consistent with previous studies (36) (SI Text, section S.4). We chose this method over a pure resting-state design, because we wanted to examine putative spontaneous processes during performance of the actual task and not during a completely different experimental context, which can influence estimates of spontaneous functional interactions (51–53) (SI Text, section S.4). Modularity analyses were performed using freely available software (https://sites.google.com/a/brain-connectivity-toolbox.net/bct/) (SI Text, section S.5). Associations with behavior were tested using Spearman’s rank correlation coefficient combined with permutation testing and Bonferroni correction for multiple comparisons (SI Text, section S.6).
Supplementary Material
Acknowledgments
We thank Mika Rubinov, Aaron Alexander-Bloch, Ed Bullmore, and John Suckling for various contributions to this manuscript. A.F. was supported by National Health & Medical Research Council (NHMRC) Grant ID 454797, A.Z. by Australian Research Council Grant ID DP0986320, B.H. by NHMRC Grant ID 628509, and J.S.S. by Biotechnology & Biological Sciences Research Council Grant ID BB/G014795/1.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1204185109/-/DCSupplemental.
References
- 1.Sonuga-Barke EJ, Castellanos FX. Spontaneous attentional fluctuations in impaired states and pathological conditions: A neurobiological hypothesis. Neurosci Biobehav Rev. 2007;31:977–986. doi: 10.1016/j.neubiorev.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 2.Fox MD, et al. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fransson P. How default is the default mode of brain function? Further evidence from intrinsic BOLD signal fluctuations. Neuropsychologia. 2006;44:2836–2845. doi: 10.1016/j.neuropsychologia.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 4.Golland Y, Golland P, Bentin S, Malach R. Data-driven clustering reveals a fundamental subdivision of the human cortex into two global systems. Neuropsychologia. 2008;46:540–553. doi: 10.1016/j.neuropsychologia.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dosenbach NU, et al. A core system for the implementation of task sets. Neuron. 2006;50:799–812. doi: 10.1016/j.neuron.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: From environment to theory of mind. Neuron. 2008;58:306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: Anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 8.Gusnard DA, Raichle ME, Raichle ME. Searching for a baseline: Functional imaging and the resting human brain. Nat Rev Neurosci. 2001;2:685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- 9.Shulman GL, et al. Common blood flow changes across visual tasks: II. Decreases in cerebral cortex. J Cogn Neurosci. 1997;9:648–663. doi: 10.1162/jocn.1997.9.5.648. [DOI] [PubMed] [Google Scholar]
- 10.Harrison BJ, et al. Consistency and functional specialization in the default mode brain network. Proc Natl Acad Sci USA. 2008;105:9781–9786. doi: 10.1073/pnas.0711791105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Christoff K, Gordon AM, Smallwood J, Smith R, Schooler JW. Experience sampling during fMRI reveals default network and executive system contributions to mind wandering. Proc Natl Acad Sci USA. 2009;106:8719–8724. doi: 10.1073/pnas.0900234106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McKiernan KA, Kaufman JN, Kucera-Thompson J, Binder JR. A parametric manipulation of factors affecting task-induced deactivation in functional neuroimaging. J Cogn Neurosci. 2003;15:394–408. doi: 10.1162/089892903321593117. [DOI] [PubMed] [Google Scholar]
- 13.Weissman DH, Roberts KC, Visscher KM, Woldorff MG. The neural bases of momentary lapses in attention. Nat Neurosci. 2006;9:971–978. doi: 10.1038/nn1727. [DOI] [PubMed] [Google Scholar]
- 14.Eichele T, et al. Prediction of human errors by maladaptive changes in event-related brain networks. Proc Natl Acad Sci USA. 2008;105:6173–6178. doi: 10.1073/pnas.0708965105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim H, Daselaar SM, Cabeza R. Overlapping brain activity between episodic memory encoding and retrieval: Roles of the task-positive and task-negative networks. Neuroimage. 2010;49:1045–1054. doi: 10.1016/j.neuroimage.2009.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Competition between functional brain networks mediates behavioral variability. Neuroimage. 2008;39:527–537. doi: 10.1016/j.neuroimage.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 17.De Pisapia N, Turatto M, Lin P, Jovicich J, Caramazza A. Unconscious priming instructions modulate activity in default and executive networks of the human brain. Cereb Cortex. 2012;22:639–649. doi: 10.1093/cercor/bhr146. [DOI] [PubMed] [Google Scholar]
- 18.Whitfield-Gabrieli S, et al. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc Natl Acad Sci USA. 2009;106:1279–1284. doi: 10.1073/pnas.0809141106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Castellanos FX, et al. Cingulate-precuneus interactions: A new locus of dysfunction in adult attention-deficit/hyperactivity disorder. Biol Psychiatry. 2008;63:332–337. doi: 10.1016/j.biopsych.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Honey CJ, Kötter R, Breakspear M, Sporns O. Network structure of cerebral cortex shapes functional connectivity on multiple time scales. Proc Natl Acad Sci USA. 2007;104:10240–10245. doi: 10.1073/pnas.0701519104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL. Functional-anatomic fractionation of the brain’s default network. Neuron. 2010;65:550–562. doi: 10.1016/j.neuron.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci USA. 2008;105:12569–12574. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spreng RN, Stevens WD, Chamberlain JP, Gilmore AW, Schacter DL. Default network activity, coupled with the frontoparietal control network, supports goal-directed cognition. Neuroimage. 2010;53:303–317. doi: 10.1016/j.neuroimage.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J Neurophysiol. 2008;100:3328–3342. doi: 10.1152/jn.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seeley WW, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith SM, et al. Correspondence of the brain’s functional architecture during activation and rest. Proc Natl Acad Sci USA. 2009;106:13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Popa D, Popescu AT, Paré D. Contrasting activity profile of two distributed cortical networks as a function of attentional demands. J Neurosci. 2009;29:1191–1201. doi: 10.1523/JNEUROSCI.4867-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simons JS, Henson RN, Gilbert SJ, Fletcher PC. Separable forms of reality monitoring supported by anterior prefrontal cortex. J Cogn Neurosci. 2008;20:447–457. doi: 10.1162/jocn.2008.20036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sadaghiani S, Hesselmann G, Kleinschmidt A. Distributed and antagonistic contributions of ongoing activity fluctuations to auditory stimulus detection. J Neurosci. 2009;29:13410–13417. doi: 10.1523/JNEUROSCI.2592-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leech R, Kamourieh S, Beckmann CF, Sharp DJ. Fractionating the default mode network: Distinct contributions of the ventral and dorsal posterior cingulate cortex to cognitive control. J Neurosci. 2011;31:3217–3224. doi: 10.1523/JNEUROSCI.5626-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bluhm RL, et al. Default network connectivity during a working memory task. Hum Brain Mapp. 2011;32:1029–1035. doi: 10.1002/hbm.21090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gerlach KD, Spreng RN, Gilmore AW, Schacter DL. Solving future problems: Default network and executive activity associated with goal-directed mental simulations. Neuroimage. 2011;55:1816–1824. doi: 10.1016/j.neuroimage.2011.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gao W, Lin W. Frontal parietal control network regulates the anti-correlated default and dorsal attention networks. Hum Brain Mapp. 2012;33:192–202. doi: 10.1002/hbm.21204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Calhoun VD, Adali T, Pekar JJ. A method for comparing group fMRI data using independent component analysis: Application to visual, motor and visuomotor tasks. Magn Reson Imaging. 2004;22:1181–1191. doi: 10.1016/j.mri.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 35.Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nat Neurosci. 2004;7:189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- 36.Fair DA, et al. A method for using blocked and event-related fMRI data to study “resting state” functional connectivity. Neuroimage. 2007;35:396–405. doi: 10.1016/j.neuroimage.2006.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rubinov M, Sporns O. Weight-conserving characterization of complex functional brain networks. Neuroimage. 2011;56:2068–2079. doi: 10.1016/j.neuroimage.2011.03.069. [DOI] [PubMed] [Google Scholar]
- 38.van den Heuvel M, Mandl R, Hulshoff Pol H. Normalized cut group clustering of resting-state FMRI data. PloS One. 2008;3:e2001. doi: 10.1371/journal.pone.0002001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guimerà R, Nunes Amaral LA. Functional cartography of complex metabolic networks. Nature. 2005;433:895–900. doi: 10.1038/nature03288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leech R, Braga R, Sharp DJ. Echoes of the brain within the posterior cingulate cortex. J Neurosci. 2012;32:215–222. doi: 10.1523/JNEUROSCI.3689-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Prado J, Weissman DH. Heightened interactions between a key default-mode region and a key task-positive region are linked to suboptimal current performance but to enhanced future performance. Neuroimage. 2011;56:2276–2282. doi: 10.1016/j.neuroimage.2011.03.048. [DOI] [PubMed] [Google Scholar]
- 42.Boly M, et al. Intrinsic brain activity in altered states of consciousness: How conscious is the default mode of brain function? Ann N Y Acad Sci. 2008;1129:119–129. doi: 10.1196/annals.1417.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bar M. The proactive brain: Using analogies and associations to generate predictions. Trends Cogn Sci. 2007;11:280–289. doi: 10.1016/j.tics.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 44.Velanova K, et al. Functional-anatomic correlates of sustained and transient processing components engaged during controlled retrieval. J Neurosci. 2003;23:8460–8470. doi: 10.1523/JNEUROSCI.23-24-08460.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dobbins IG, Simons JS, Schacter DL. fMRI evidence for separable and lateralized prefrontal memory monitoring processes. J Cogn Neurosci. 2004;16:908–920. doi: 10.1162/0898929041502751. [DOI] [PubMed] [Google Scholar]
- 46.Henson RN, Rugg MD, Shallice T, Dolan RJ. Confidence in recognition memory for words: Dissociating right prefrontal roles in episodic retrieval. J Cogn Neurosci. 2000;12:913–923. doi: 10.1162/08989290051137468. [DOI] [PubMed] [Google Scholar]
- 47.Hayama HR, Rugg MD. Right dorsolateral prefrontal cortex is engaged during post-retrieval processing of both episodic and semantic information. Neuropsychologia. 2009;47:2409–2416. doi: 10.1016/j.neuropsychologia.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hagmann P, et al. Mapping the structural core of human cerebral cortex. PLoS Biol. 2008;6:e159. doi: 10.1371/journal.pbio.0060159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fornito A, et al. Genetic influences on cost-efficient organization of human cortical functional networks. J Neurosci. 2011;31:3261–3270. doi: 10.1523/JNEUROSCI.4858-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Glahn DC, et al. Genetic control over the resting brain. Proc Natl Acad Sci USA. 2010;107:1223–1228. doi: 10.1073/pnas.0909969107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Barnes A, Bullmore ET, Suckling J. Endogenous human brain dynamics recover slowly following cognitive effort. PLoS One. 2009;4:e6626. doi: 10.1371/journal.pone.0006626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Harrison BJ, et al. Modulation of brain resting-state networks by sad mood induction. PLoS One. 2008;3:e1794. doi: 10.1371/journal.pone.0001794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fornito A, Bullmore ET. What can spontaneous fluctuations of the blood oxygenation-level-dependent signal tell us about psychiatric disorders? Curr Opin Psychiatry. 2010;23:239–249. doi: 10.1097/YCO.0b013e328337d78d. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.