Abstract
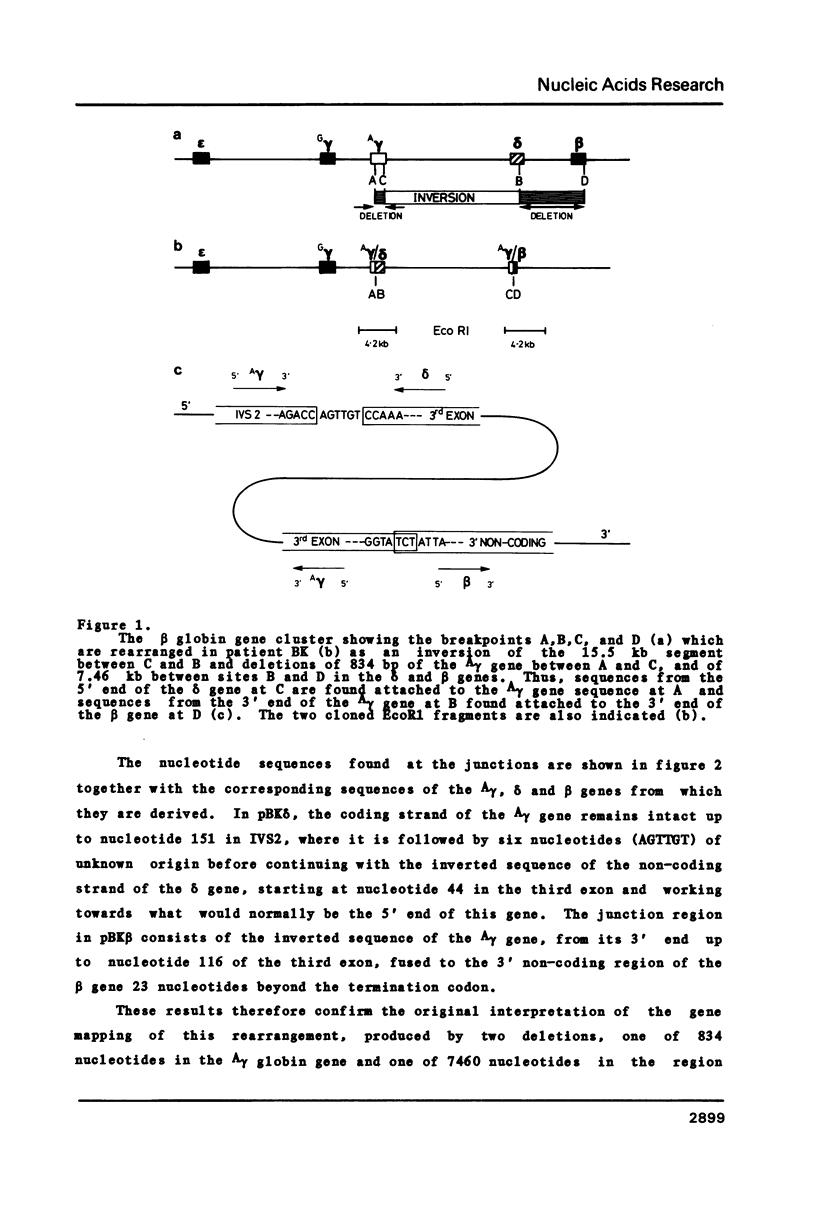
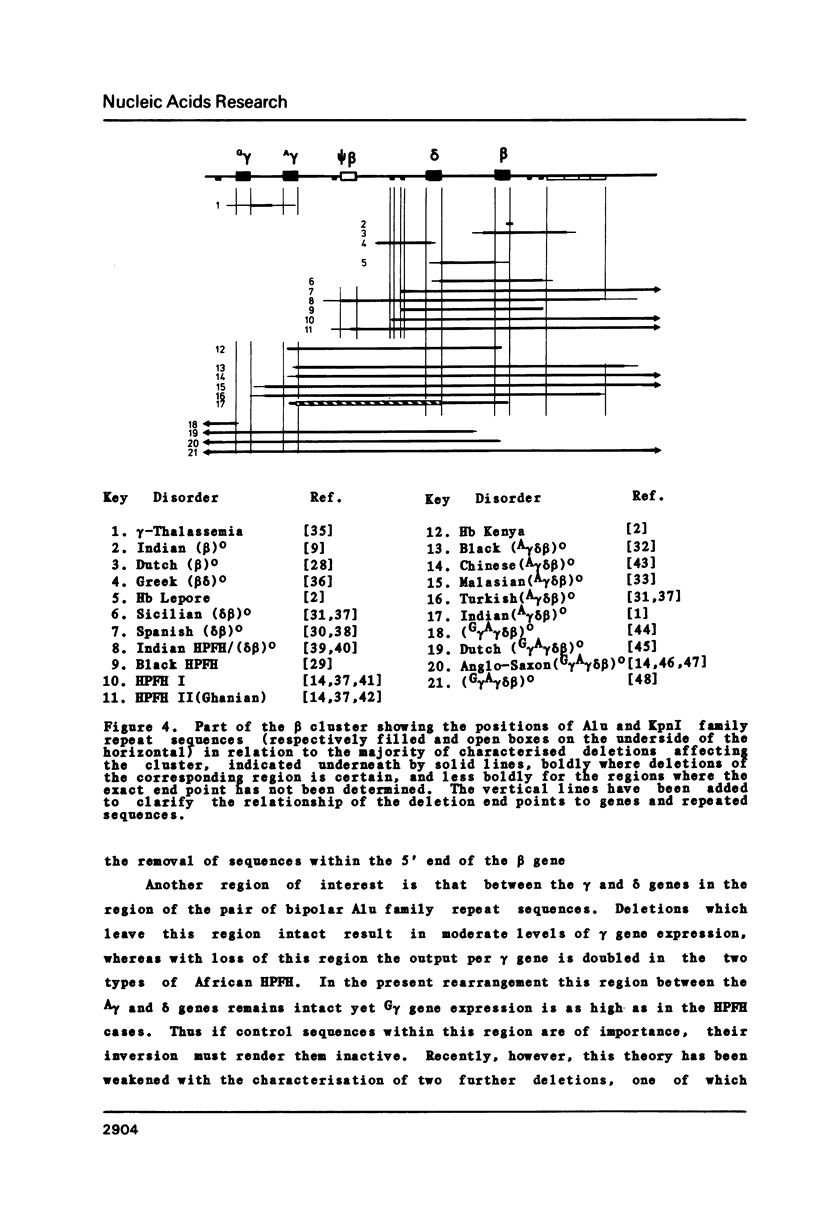
We have cloned and sequenced the DNA from two regions of the defective beta-globin gene cluster from a patient with Indian A gamma delta beta thalassaemia, and confirmed the complex and unusual pattern of rearrangement involving two separate deletions (0.8 kb and 7.5 kb) the inversion of the 15.5 kb segment separating them, as previously proposed from gene mapping studies [1]. All four breakpoints occur within the transcribed region of the globin genes and at one junction are found six nucleotides of unknown origin. This unique rearrangement results in enhanced expression of the upstream fetal gene, and is therefore is pertinent to the localisation of any putative control region involved in the coordinate expression of fetal and adult genes.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amin A. B., Pandya N. L., Diwin P. P., Darbre P. D., Kattamis C., Metaxatou-Mavromati A., White J. M., Wood W. G., Clegg J. B., Weatherall D. J. A comparison of the homozygous states for G gamma and G gamma A gamma delta beta thalassaemia. Br J Haematol. 1979 Dec;43(4):537–548. doi: 10.1111/j.1365-2141.1979.tb03786.x. [DOI] [PubMed] [Google Scholar]
- Anderson R. A., Kato S., Camerini-Otero R. D. A pattern of partially homologous recombination in mouse L cells. Proc Natl Acad Sci U S A. 1984 Jan;81(1):206–210. doi: 10.1073/pnas.81.1.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernards R., Flavell R. A. Physical mapping of the globin gene deletion in hereditary persistence of foetal haemoglobin (HPFH). Nucleic Acids Res. 1980 Apr 11;8(7):1521–1534. doi: 10.1093/nar/8.7.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botchan M., Stringer J., Mitchison T., Sambrook J. Integration and excision of SV40 DNA from the chromosome of a transformed cell. Cell. 1980 May;20(1):143–152. doi: 10.1016/0092-8674(80)90242-1. [DOI] [PubMed] [Google Scholar]
- Davison A. J., Wilkie N. M. Inversion of the two segments of the herpes simplex virus genome in intertypic recombinants. J Gen Virol. 1983 Jan;64(Pt 1):1–18. doi: 10.1099/0022-1317-64-1-1. [DOI] [PubMed] [Google Scholar]
- Fritsch E. F., Lawn R. M., Maniatis T. Molecular cloning and characterization of the human beta-like globin gene cluster. Cell. 1980 Apr;19(4):959–972. doi: 10.1016/0092-8674(80)90087-2. [DOI] [PubMed] [Google Scholar]
- Gilman J. G., Huisman T. H., Abels J. Dutch beta 0-thalassaemia: a 10 kilobase DNA deletion associated with significant gamma-chain production. Br J Haematol. 1984 Feb;56(2):339–348. doi: 10.1111/j.1365-2141.1984.tb03961.x. [DOI] [PubMed] [Google Scholar]
- Höchtl J., Zachau H. G. A novel type of aberrant recombination in immunoglobulin genes and its implications for V-J joining mechanism. Nature. 1983 Mar 17;302(5905):260–263. doi: 10.1038/302260a0. [DOI] [PubMed] [Google Scholar]
- Johnson A. D., Barkan A., Mertz J. E. Nucleotide sequence analysis of the recombinant joints in 16 naturally arising deletion mutants of simian virus 40. Virology. 1982 Dec;123(2):464–469. doi: 10.1016/0042-6822(82)90281-1. [DOI] [PubMed] [Google Scholar]
- Jones R. W., Old J. M., Trent R. J., Clegg J. B., Weatherall D. J. Major rearrangement in the human beta-globin gene cluster. Nature. 1981 May 7;291(5810):39–44. doi: 10.1038/291039a0. [DOI] [PubMed] [Google Scholar]
- Jones R. W., Old J. M., Trent R. J., Clegg J. B., Weatherall D. J. Restriction mapping of a new deletion responsible for G gamma (delta beta)o thalassemia. Nucleic Acids Res. 1981 Dec 21;9(24):6813–6825. doi: 10.1093/nar/9.24.6813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan Y. W., Forget B. G., Nathan D. G. Gamma-beta thalassemia: a cause of hemolytic disease of the newborn. N Engl J Med. 1972 Jan 20;286(3):129–134. doi: 10.1056/NEJM197201202860304. [DOI] [PubMed] [Google Scholar]
- Kataoka T., Kondo S., Nishi M., Kodaira M., Honjo T. Isolation and characterization of endonuclease J: a sequence-specific endonuclease cleaving immunoglobulin genes. Nucleic Acids Res. 1984 Aug 10;12(15):5995–6010. doi: 10.1093/nar/12.15.5995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutlar A., Gardiner M. B., Headlee M. G., Reese A. L., Cleek M. P., Nagle S., Sukumaran P. K., Huisman T. H. Heterogeneity in the molecular basis of three types of hereditary persistence of fetal hemoglobin and the relative synthesis of the G gamma and A gamma types of gamma chain. Biochem Genet. 1984 Feb;22(1-2):21–35. doi: 10.1007/BF00499284. [DOI] [PubMed] [Google Scholar]
- Leder P., Max E. E., Seidman J. G., Kwan S. P., Scharff M., Nau M., Norman B. Recombination events that activate, diversify, and delete immunoglobulin genes. Cold Spring Harb Symp Quant Biol. 1981;45(Pt 2):859–865. doi: 10.1101/sqb.1981.045.01.103. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Orkin S. H., Goff S. C., Nathan D. G. Heterogeneity of DNA deletion in gamma delta beta-thalassemia. J Clin Invest. 1981 Mar;67(3):878–884. doi: 10.1172/JCI110105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottolenghi S., Giglioni B. The deletion in a type of delta 0-beta 0-thalassaemia begins in an inverted AluI repeat. Nature. 1982 Dec 23;300(5894):770–771. doi: 10.1038/300770a0. [DOI] [PubMed] [Google Scholar]
- Plasterk R. H., Kanaar R., van de Putte P. A genetic switch in vitro: DNA inversion by Gin protein of phage Mu. Proc Natl Acad Sci U S A. 1984 May;81(9):2689–2692. doi: 10.1073/pnas.81.9.2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poncz M., Schwartz E., Ballantine M., Surrey S. Nucleotide sequence analysis of the delta beta-globin gene region in humans. J Biol Chem. 1983 Oct 10;258(19):11599–11609. [PubMed] [Google Scholar]
- Ruley H. E., Fried M. Clustered illegitimate recombination events in mammalian cells involving very short sequence homologies. Nature. 1983 Jul 14;304(5922):181–184. doi: 10.1038/304181a0. [DOI] [PubMed] [Google Scholar]
- Seidman J. G., Leder P. A mutant immunoglobulin light chain is formed by aberrant DNA- and RNA-splicing events. Nature. 1980 Aug 21;286(5775):779–783. doi: 10.1038/286779a0. [DOI] [PubMed] [Google Scholar]
- Shen S. H., Slightom J. L., Smithies O. A history of the human fetal globin gene duplication. Cell. 1981 Oct;26(2 Pt 2):191–203. doi: 10.1016/0092-8674(81)90302-0. [DOI] [PubMed] [Google Scholar]
- Slightom J. L., Blechl A. E., Smithies O. Human fetal G gamma- and A gamma-globin genes: complete nucleotide sequences suggest that DNA can be exchanged between these duplicated genes. Cell. 1980 Oct;21(3):627–638. doi: 10.1016/0092-8674(80)90426-2. [DOI] [PubMed] [Google Scholar]
- Spritz R. A., Orkin S. H. Duplication followed by deletion accounts for the structure of an Indian deletion beta (0)-thalassemia gene. Nucleic Acids Res. 1982 Dec 20;10(24):8025–8029. doi: 10.1093/nar/10.24.8025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staden R. Graphic methods to determine the function of nucleic acid sequences. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 2):521–538. doi: 10.1093/nar/12.1part2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukumaran P. K., Nakatsuji T., Gardiner M. B., Reese A. L., Gilman J. G., Huisman T. H. Gamma thalassemia resulting from the deletion of a gamma-globin gene. Nucleic Acids Res. 1983 Jul 11;11(13):4635–4643. [PMC free article] [PubMed] [Google Scholar]
- Tonegawa S. Somatic generation of antibody diversity. Nature. 1983 Apr 14;302(5909):575–581. doi: 10.1038/302575a0. [DOI] [PubMed] [Google Scholar]
- Tuan D., Feingold E., Newman M., Weissman S. M., Forget B. G. Different 3' end points of deletions causing delta beta-thalassemia and hereditary persistence of fetal hemoglobin: implications for the control of gamma-globin gene expression in man. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6937–6941. doi: 10.1073/pnas.80.22.6937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Ploeg L. H., Konings A., Oort M., Roos D., Bernini L., Flavell R. A. gamma-beta-Thalassaemia studies showing that deletion of the gamma- and delta-genes influences beta-globin gene expression in man. Nature. 1980 Feb 14;283(5748):637–642. doi: 10.1038/283637a0. [DOI] [PubMed] [Google Scholar]
- Vanin E. F., Henthorn P. S., Kioussis D., Grosveld F., Smithies O. Unexpected relationships between four large deletions in the human beta-globin gene cluster. Cell. 1983 Dec;35(3 Pt 2):701–709. doi: 10.1016/0092-8674(83)90103-4. [DOI] [PubMed] [Google Scholar]
- Vitelli L., Weinberg E. S. An inverted sea urchin histone gene sequence with breakpoints between TATA boxes and mRNA cap sites. Nucleic Acids Res. 1983 Apr 11;11(7):2135–2153. doi: 10.1093/nar/11.7.2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S. W., Robins A. J., d'Andrea R., Wells J. R. Inverted duplication of histone genes in chicken and disposition of regulatory sequences. Nucleic Acids Res. 1985 Feb 25;13(4):1369–1387. doi: 10.1093/nar/13.4.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]


