Abstract
Survival of free-living and symbiotic dinoflagellates (Symbiodinium spp.) in coral reefs is critical to the maintenance of a healthy coral community. Most coral reefs exist in oligotrophic waters, and their survival strategy in such nutrient-depleted waters remains largely unknown. In this study, we found that two strains of Symbiodinium spp. cultured from the environment and acquired from the tissues of the coral Alveopora japonica had the ability to feed heterotrophically. Symbiodinium spp. fed on heterotrophic bacteria, cyanobacteria (Synechococcus spp.), and small microalgae in both nutrient-replete and nutrient-depleted conditions. Cultured free-living Symbiodinium spp. displayed no autotrophic growth under nitrogen-depleted conditions, but grew when provided with prey. Our results indicate that Symbiodinium spp.’s mixotrophic activity greatly increases their chance of survival and their population growth under nitrogen-depleted conditions, which tend to prevail in coral habitats. In particular, free-living Symbiodinium cells acquired considerable nitrogen from algal prey, comparable to or greater than the direct uptake of ammonium, nitrate, nitrite, or urea. In addition, free-living Symbiodinium spp. can be a sink for planktonic cyanobacteria (Synechococcus spp.) and remove substantial portions of Synechococcus populations from coral reef waters. Our discovery of Symbiodinium’s feeding alters our conventional views of the survival strategies of photosynthetic Symbiodinium and corals.
Keywords: mixotrophy, zooxanthella, coral bleaching, food web, Heterosigma
Corals in the ocean have drawn much attention from scientists, tourists, fishermen, and government officials (1–16). Free-living and symbiotic dinoflagellates in the genus Symbiodinium (also known as zooxanthellae) were once considered exclusively photosynthetic, except for the uptake of dissolved organic materials (17). Corals protect Symbiodinium in hospite and provide inorganic nutrients and dissolved organic materials to their partner, whereas Symbiodinium provide the products of photosynthesis to corals (18). Surprisingly, corals acquire up to 90% of their nutrition from Symbiodinium (19). Thus, Symbiodinium is a critical partner of zooxanthellate corals. In sexual reproduction, adults or larvae of corals must acquire Symbiodinium cells from environmental pools (i.e., horizontal transmission) or from their parents (i.e., vertical transmission) (18, 20). In addition, viable Symbiodinium cells released from a coral can infect other corals or larvae to create a new association by horizontal transmission (18). Therefore, the survival of free-living and symbiotic Symbiodinium cells in coral reefs is crucial to the maintenance of a healthy coral community.
Concentrations of inorganic nutrients in coral reef waters are typically low (8, 21, 22). High nutrient input, or eutrophication, is known to cause macroalgae to overgrow and often kill corals (23, 24). Thus, Symbiodinium cells in the water column (i.e., free-living strains or motile symbiotic cells leaving corals) are likely to experience low inorganic nutrient conditions that are unfavorable for photosynthesis. Some corals reportedly take up dissolved organic materials, such as free amino acids and urea, as sources of nitrogen (25, 26). Some critical questions arise. Do free-living or symbiotic Symbiodinium survive only by conducting photosynthesis? If not, do they have any alternative nutritional strategy? For example, do they obtain materials through feeding? If so, what are the prey items? Furthermore, does ingestion of prey cells enable Symbiodinium to survive in nutrient-depleted conditions? In addition, do Symbiodinium acquire nitrogen from prey as much as from inorganic or dissolved organic nitrogen? Can feeding by Symbiodinium be an important source of mortality for prey? To answer these questions, we explored feeding by two strains of Symbiodinium spp. (clade E), one cultured from the environment and the other acquired from the tissues of the coral Alveopora japonica, originating from the coastal waters off Jeju Island, Korea.
Results and Discussion
Feeding of Cultured Free-Living Symbiodinium sp.
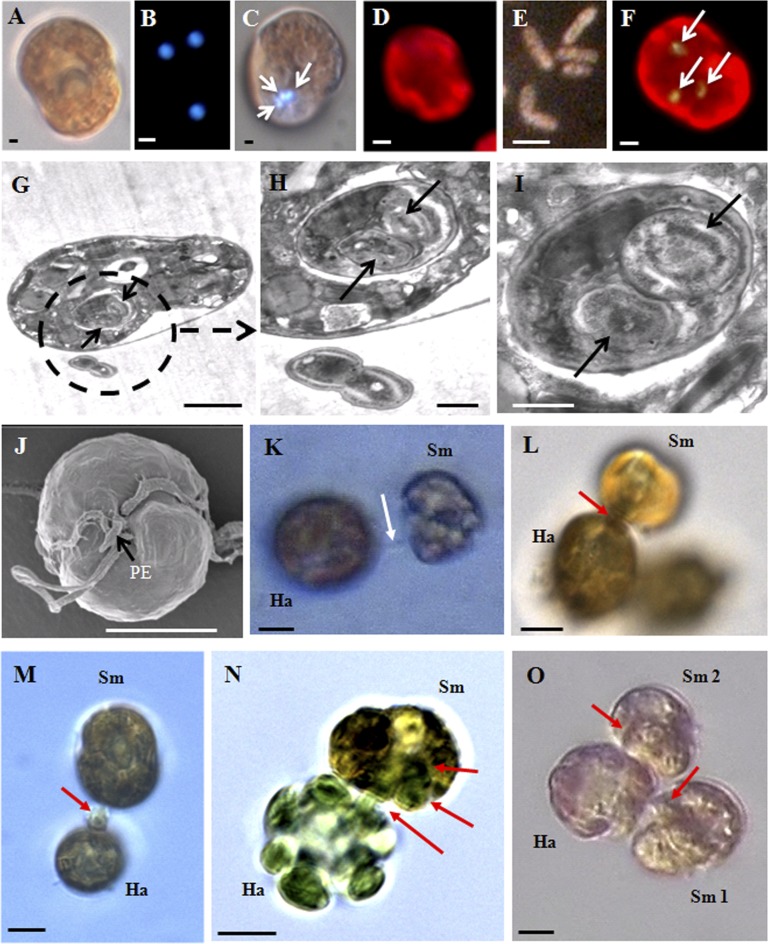
We found that free-living Symbiodinium sp. cells ingested 0.5- to 2-μm microbeads, heterotrophic bacteria, fluorescently labeled bacteria, and Synechococcus cells (Fig. 1 A–I and Table S1). Among the algal prey provided, free-living Symbiodinium ingested small algal species (equivalent spherical diameters of ≤11.5 μm) except the diatom Skeletonema costatum using a peduncle (feeding tube) (Fig. 1 J–O, Fig. S1 A–E, and Table S1). Symbiodinium sp. deployed a tow filament to anchor the raphidophyte Heterosigma akashiwo cell (Fig. 1K) and then attached a peduncle to the prey cell. Symbiodinium then sucked the contents out of the H. akashiwo cell through the peduncle (Fig. 1 L–N). Predator cells first sucked the liquid materials, followed by the chloroplasts (Fig. 1 L and M), and several prey chloroplasts were observed inside the protoplasm of predator cells (Fig. 1N). Occasionally, two or three Symbiodinium cells fed on a prey cell simultaneously (Fig. 1O). The bacteria and small algal prey are always present in coral reefs (27, 28); thus, Symbiodinium in coral reefs likely encounter prey cells on a continuous basis.
Fig. 1.
Images of free-living Symbiodinium cells feeding on diverse prey. (A–C) Micrographs of Symbiodinium sp. cells without added prey observed under a light microscope (A) and bacterium-sized fluorescent beads (1 μm in diameter) (B) and three beads (arrows) inside the protoplasm of Symbiodinium cells observed under an epifluorescent microscope (C). (D–F) Micrographs of Symbiodinium sp. cells without added prey (D), fluorescently labeled bacteria (E), and three fluorescently labeled bacteria (arrows) inside the protoplasm of Symbiodinium cells observed under an epifluorescent microscope (F). (G–I) Transmission electron microscope images showing Symbiodinium sp. with ingested Synechococcus sp. cells (arrows or inside a circle). Uningested Synechococcus cells were observed outside the predator cell (G and H). H is enlarged from G. (J–O) Images of feeding by Symbiodinium cells on H. akashiwo prey. (J) Scanning electron micrograph of a Symbiodinium cell showing a peduncle (PE; feeding tube, arrow). (K) Light microscope image showing a Symbiodinium cell (Sm) deploying a tow filament and anchored on an H. akashiwo (Ha) (arrow). (L) A Symbiodinium cell (Sm) sucking materials from an H. akashiwo (Ha) cell through a peduncle (arrow). (M) A chloroplast of H. akashiwo (Ha) sucked by Symbiodinium cell (Sm) through a peduncle (arrow). (N) Several chloroplasts of the prey cell (greenish) were observed inside the protoplasm of the predator cell. (O) Two Symbiodinium cells (Sm 1 and Sm 2) simultaneously sucking materials (arrows) from an H. akashiwo (Ha) cell. (Scale bars: 5 μm for J–O, 2 μm for D–G, 1 μm for A–C, and 0.5 μm for H and I.)
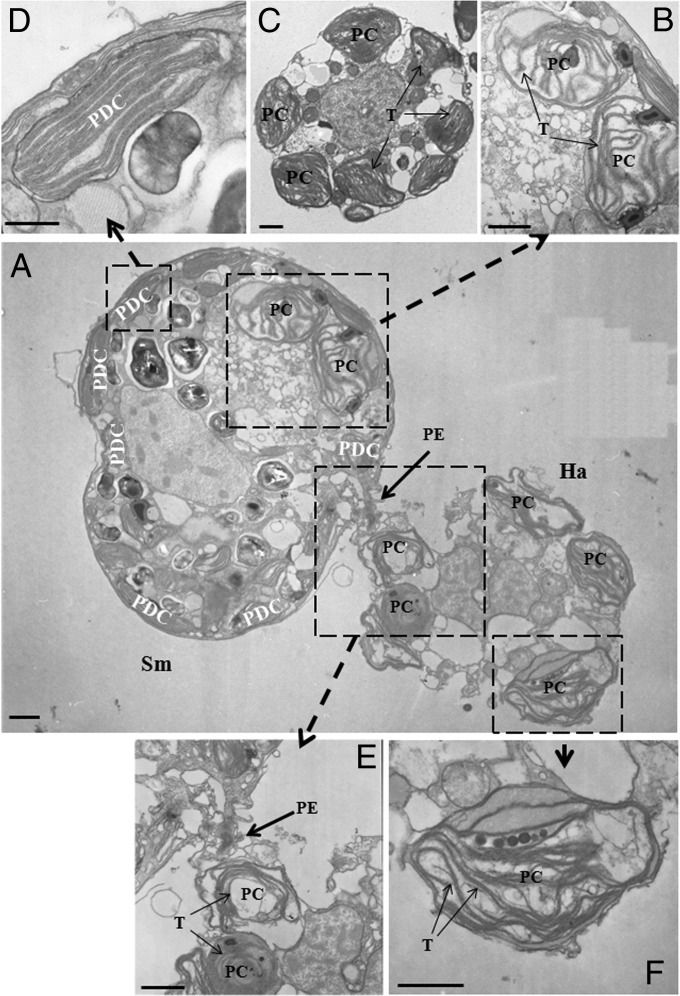
Transmission electron micrographs clearly showed a free-living Symbiodinium cell feeding on a H. akashiwo prey cell using a peduncle (Fig. 2A). The Symbiodinium cell contained ingested prey chloroplasts that had less-dense thylakoids (most likely being semidigested compared with dense thylakoids of intact prey chloroplasts) and were clearly different from the predator chloroplasts (Fig. 2). Some of the remaining chloroplasts inside the protoplasm of the prey cell also had less-dense thylakoids, suggesting that digestive enzymes of predator cells may be introduced to the protoplasm of prey cells.
Fig. 2.
Transmission electron micrographs of free-living Symbiodinium feeding on H. akashiwo prey. (A) Micrograph showing a Symbiodinium cell (Sm) sucking materials from H. akashiwo (Ha) through a peduncle (PE). (B and D–F) Images enlarged from A. The predator cell contains ingested prey chloroplasts (PCs) (B), which have less-dense thylakoids (T), most likely owing to semidigestion, compared with intact prey chloroplasts (C) and different from the chloroplasts of the predator (PDC) (D). (E) A prey chloroplast moving through a peduncle. (F) Enlarged image showing prey thylakoids (T) inside a prey chloroplast (PC). (Scale bars: 1 μm.)
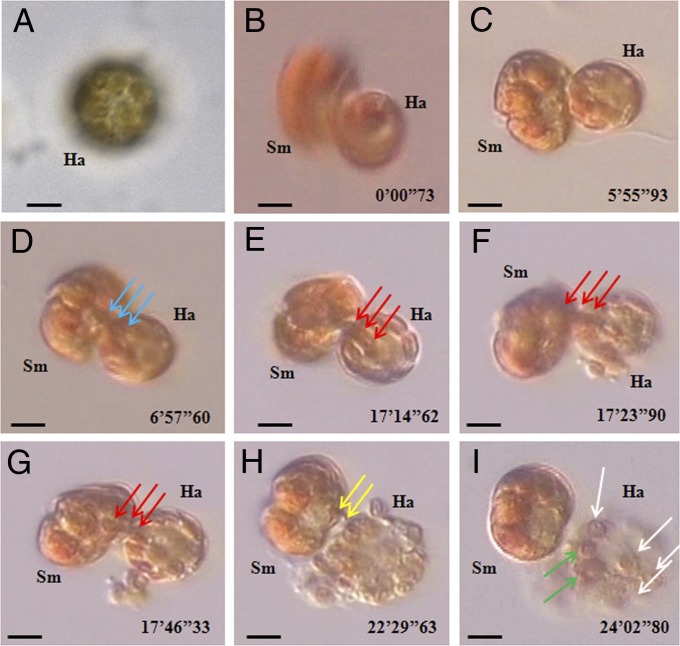
The entire feeding process of free-living Symbiodinium cells on H. akashiwo prey was recorded by video microscopy (Fig. 3). The H. akashiwo cells contained 20–35 chloroplasts per cell (Fig. 3A). Symbiodinium cells anchored to the H. akashiwo cells using tow filaments, after which the predator cells deployed and attached peduncles to the prey (stage 1; Fig. 1 K–M). The time lag between the deployment of tow filaments and peduncles was 2–4 s (Table S2). Stage 2 was associated with spinning motions in the feeding Symbiodinium cells accompanied by use of a peduncle to suck chloroplasts (>10) from the algal prey (Fig. 3 B–D). This stage took 540–1,140 s. In stage 3, the Symbiodinium cells stopped spinning but continued to suck additional chloroplasts from the H. akashiwo cells. Subsequently, the prey cells collapsed and burst (Fig. 3 E–G). Approximately 8–10 chloroplasts remained within each burst prey cell. The time interval between the cessation of predator cell spinning and the bursting of prey cells was 50–470 s. In the final stage (stage 4), the Symbiodinium cells sucked additional chloroplasts from the burst H. akashiwo cells, leaving only one or two remaining chloroplasts by the end of the feeding process (Fig. 3 H and I). Stage 4 continued for 185–520 s. Other Symbiodinium cells fed similarly on H. akashiwo prey. The pattern shown in Fig. 3 is representative of the regular feeding process of free-living Symbiodinium cells on H. akashiwo prey.
Fig. 3.
Feeding processes of free-living Symbiodinium cells on H. akashiwo prey, observed under an inverted microscope and recorded by video microscopy. (A) An unfed H. akashiwo (Ha) cell containing many chloroplasts (20–35 chloroplasts per cell). A Symbiodinium cell (Sm) feeding on an H. akashiwo (Ha) prey after deploying a tow filament and then a peduncle (stage 1), as shown in Fig. 1 K–M. (B–D) A Symbiodinium cell (Sm) feeding on an H. akashiwo (Ha) prey with spinning (stage 2). In stage 2, several prey chloroplasts were sucked by the predator. (E–G) A Symbiodinium cell (Sm) feeding on an H. akashiwo prey (Ha) after it had stopped rotating (stage 3). In stage 3, additional prey chloroplasts (blue and red arrows indicate different chloroplasts) were sucked by the predator again, after which the prey chloroplasts collapsed and burst, most likely owing to large empty spaces where prey chloroplasts occurred previously. Approximately 8–10 chloroplasts were observed within the burst prey cell. (H and I) Additional prey chloroplasts (yellow arrows) were sucked again by the predator from the burst prey cell (stage 4). At the end of stage 4, several empty (i.e., completely digested, white arrows) and one or two thylakoid-retained (green arrows) chloroplasts were observed inside the prey cell (I). (Scale bars: 5 μm.)
Feeding of Cultured Symbiodinium Acquired from Tissues of A. japonica.
Cells of Symbiodinium isolated from A. japonica became motile (Fig. S2 A–D). Similar to free-living Symbiodinium cells, these Symbiodinium cells were able to feed on 1-μm microbeads, heterotrophic bacteria, fluorescently labeled bacteria, Synechococcus cells, and H. akashiwo cells (Fig. S2 E–O). This evidence suggests that Symbiodinium cells leaving (or expelled from) hosts become motile and are able to feed. It is likely that swimming Symbiodinium cells acquire carbon, nitrogen, and phosphorus from bacteria and/or small algal prey while planktonic.
Growth and/or Ingestion Rate of Free-Living Symbiodinium Feeding on Prey.
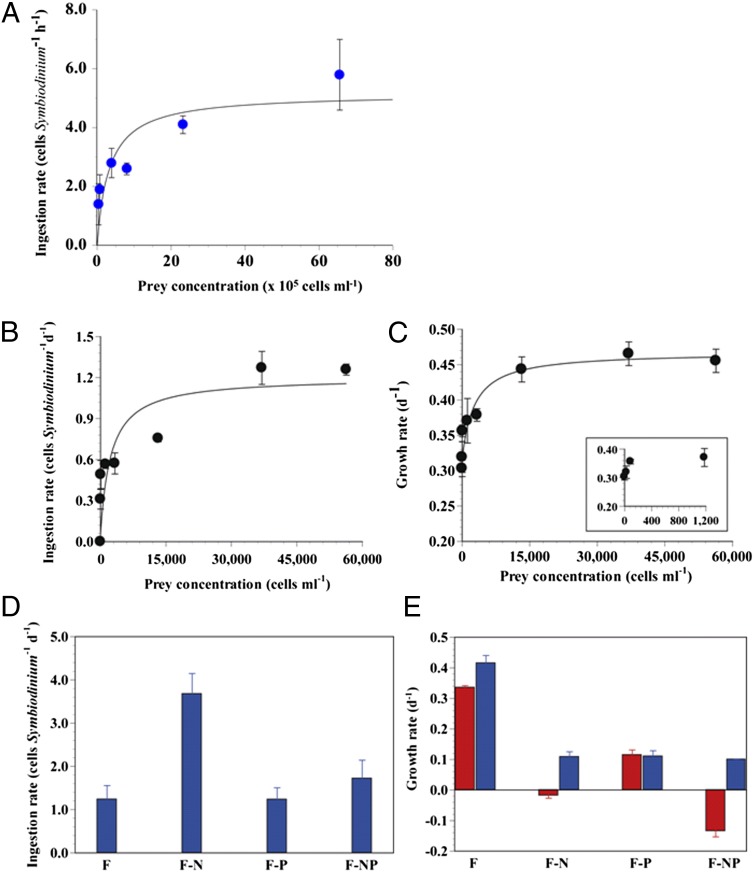
With increasing Synechococcus concentrations, the ingestion rate of free-living Symbiodinium on Synechococcus sp. under nutrient-replete conditions increased but eventually reached saturation (Fig. 4A). The maximum ingestion rate was 5.3 cells Symbiodinium−1 h−1. Assuming the nitrogen content per Synechococcus and Symbiodinium cell is 0.05 pg N and 21 pg N, respectively (29, 30). Symbiodinium is able to acquire up to 6.4 pg N from Synechococcus daily. This amount is equivalent to a Symbiodinium sp. acquiring 30% of its body nitrogen in a day (Tables S3 and S4). Thus, if Symbiodinium feeds on Synechococcus, it could acquire sufficient N from prey cells in nitrogen-depleted waters. This value was comparable to that for nitrate, nitrite, or ammonium (1.1–24.2 pg N Symbiodinium−1d−1, or 6–115% of body nitrogen in a day) but greater than that from urea (0.034 pg N Symbiodinium−1d−1, or 0.2% of its body nitrogen in a day) (26, 30–32). This indicates that Synechococcus spp. can serve as an important nitrogen source for Symbiodinium.
Fig. 4.
Feeding rates of free-living Symbiodinium sp. on Synechococcus sp. and H.akashiwo prey. (A) Ingestion rate of Symbiodinium on Synechococcus as a function of the initial prey concentration (x, cells mL−1). Symbols represent treatment mean ±1 SE. The curves are fitted to the Michaelis–Menten equations using all of the treatments. Ingestion rate: (cells Symbiodinium−1 h−1) = 5.3 [x/(3.9 × 105 + x)], R2 = 0.581. (B and C) Ingestion (B) and growth rates (C) of Symbiodinium sp. on H. akashiwo as a function of the mean prey concentration (x, cells mL−1). Ingestion rate: (cells Symbiodinium−1 d−1) = 1.2 [x/(2,280 + x)]; R2 = 0.838. Growth rate: (d−1) = 0.467 [(x + 1,720)/(880 + (x + 1,720))]; R2 = 0.608. (D and E) Effects of inorganic nutrients on feeding rates of free-living Symbiodinium sp. Ingestion (D) and growth rates (E) of Symbiodinium sp. on H. akashiwo under different nutrient conditions; Red bars indicate no added prey; blue bars, mean prey concentrations = 15,200–19,600 cells mL−1. F, f/2 medium (see Materials and Methods for concentrations); F-N, N-depleted f/2 medium; F-P, P-depleted f/2 medium; F-NP, N- and P- depleted f/2 medium. Symbols represent treatment mean ±1 SE.
We estimated the portion of Synechococcus cells removed by Symbiodinium spp. in 1 h relative to the initial prey density by combining field data on the densities of Symbiodinium spp. and Synechococcus spp. (33, 34) with the ingestion rates of the predator on Synechococcus spp. obtained in the present study (Table S5). The estimated hourly removal rates of Synechococcus spp. attributable to Symbiodinium spp. in Lizard Island, Australia were 0.04–3% h−1 using a matrix of the densities of Symbiodinium spp. and Synechococcus spp. (Table S5). Thus, free-living Symbiodinium spp. may consume large numbers of Synechococcus cells through heterotrophic feeding in coral reefs.
With increasing concentrations of the microalga H. akashiwo, the ingestion rate of free-living Symbiodinium on H. akashiwo under nutrient-replete conditions increased but soon reached saturation (Fig. 4B). Each Symbiodinium cell was able to ingest a maximum of 1.2 H. akashiwo cells per day (Fig. 4B). Feeding on H. akashiwo increased the growth rate of Symbiodinium by up to 57% (mixotrophic growth rate, 0.47 d−1) compared with the growth rate without added prey (i.e., autotrophic growth rate, 0.30 d−1) (Fig. 4C). This finding indicates that free-living Symbiodinium can increase their populations through by a combination of feeding and photosynthesis.
Effects of Nutrient Conditions on Feeding of Free-Living Symbiodinium sp.
We found that the ingestion rates of free-living Symbiodinium sp. on H. akashiwo under nitrate-depleted (F-N) conditions were significantly higher than those under nutrient-enriched (F) or phosphate-depleted conditions (F-P) for similar mean prey concentrations (3.7 cells Symbiodinium−1d−1 vs. 1.2 cells Symbiodinium−1d−1 under both conditions; P < 0.01, one-tailed t test) (Fig. 4D). Thus, nitrate depletion stimulates feeding by Symbiodinium on H. akashiwo cells. In addition, under F-N or F-NP conditions, the growth rates of Symbiodinium without added prey were negative (−0.02 d−1 or −0.13 d−1), whereas the growth rates of those fed on H. akashiwo were positive (0.11 d−1 for both conditions) (Fig. 4E). Assuming N content of 13 pg per H. akashiwo cell and 21 pg per Symbiodinium cell (30), Symbiodinium sp. can acquire 229% and 105% of its body nitrogen from H. akashiwo in 1 d under F-N and F-NP conditions, respectively (Table S3). Our findings indicate that feeding may enable free-living Symbiodinium to increase its population in the nitrogen-depleted environments typical of coral reefs (Fig. 4D). The high density of free-living Symbiodinium cells on reefs may aid the maintenance of healthy coral populations, because newly settled polyps of many corals need to acquire Symbiodinium symbionts to prosper (35).
Our results can be used to resolve the paradox of how Symbiodinium cells can survive or even prosper in nitrogen-deficient coral reefs (14). Even under nutrient-replete conditions, the growth rate of Symbiodinium sp. that fed on prey was considerably higher than that of unfed cells. Thus, feeding can be a critical survival strategy for Symbiodinium spp. in all nutrient conditions. In addition to previously known inorganic nutrients and dissolved organic materials, prey (i.e., particulate organic materials) should be included as major carbon and nitrogen sources for Symbiodinium cells.
There are many strains in many clades of Symbiodinium (20), not all of which behave the same way. Thus, evaluating the mixotrophic ability of each strain is warranted. In addition to corals, other marine life are known to contain Symbiodinium spp., including sponges, anemones, jellyfish, nudibranchs, clams, and protists such as ciliates and foraminifera (36, 37). Our results may stimulate exploration of the heterotrophic feeding ability of Symbiodinium spp. related to these diverse planktonic and benthic organisms and protists.
Materials and Methods
Preparation of Organisms.
A. japonica, a scleractinian coral, was collected by divers off Jeju Island, Korea in August 2011. The water temperature and salinity at the time of collection were 26.7 °C and 31.9, respectively. Symbiodinium obtained from inside the detached tentacles of two different polyps of the corals were transferred to six-well tissue culture plates, and three clonal cultures were established by two serial single-cell isolations. These cultures were maintained at an illumination of 20 μE m−2 s−1 at 20 °C with a 14-h light/10-h dark cycle. The small and/or large subunit rDNA sequences of these three symbiotic strains (GenBank accession no. HE653239) were almost identical to that of Symbiodinium californium or Symbiodinium varians belonging to the Symbiodinium clade E (38). Using DNA sequence specific primers and quantitative PCR, we confirmed the presence of Symbiodinium clade E inside A. japonica polyps, which were collected by divers off Jeju Island in early June 2012. These coral polyps also contained Symbiodinium clade F.
Free-living Symbiodinium spp. were isolated from water samples collected off Jeju Island in May 2008 at a water temperature of 18.6 °C and salinity of 31.2. Five clonal cultures were established. The small and large subunit rDNA sequences of these free-living strains (GenBank accession no. HE653238) were identical to those of the symbiotic Symbiodinium strains.
Feeding of Cultured Free-Living Symbiodinium.
In experiment 1, we investigated the feeding habits of free-living Symbiodinium sp. on bacteria and microalgal species (Table S1). We examined the inclusion of these prey species within Symbiodinium sp. food vacuoles using a procedure similar to the methods described by Jeong et al. (39). For single-cell transmission electron microscopy, free-living Symbiodinium cells feeding on H. akashiwo cells were isolated under a dissecting microscope and transferred to cold 2.5% (wt/vol) glutaraldehyde in distilled water and fixed for 1 h at 4 °C. Glutaraldehyde-fixed cells were washed three times in 0.2 M cacodylate buffer at pH 7.4. Before postfixation in 1% (vol/vol) osmium tetroxide, the cells were embedded in 1% (wt/vol) agarose. The specimens were then processed according to the methods of Jeong et al. (39, 40).
In experiment 2, we analyzed the feeding mechanisms of Symbiodinium sp. when provided with an edible unialgal prey, H. akashiwo. Video microscopy was used to observe and document feeding mechanisms using methods similar to those described by Jeong et al. (40).
Feeding of Cultured Symbiodinium Acquired from Tissues of A. japonica.
In experiment 3, we investigated the feeding occurrence and mechanisms of cultured Symbiodinium sp. isolated from A. japonica on bacteria and H. akashiwo using the same methods as for experiments 1 and 2.
Ingestion and Growth Rates of Free-Living Symbiodinium on Prey.
Experiment 4 was designed to determine the ingestion rate of Symbiodinium sp. feeding on Synechococcus sp. as a function of the prey concentration. The rates were measured using the “prey-inclusion method” described by Jeong et al. (41). Experiment 5 was designed to measure the growth and ingestion rates of Symbiodinium sp. on H. akashiwo. The rates were measured as described by Jeong et al. (39).
Nutrient Effects.
Experiment 6 was designed to investigate the effects of inorganic nutrients on ingestion and growth rates of free-living Symbiodinium sp. feeding on H. akashiwo. Dense cultures of photosynthetically growing Symbiodinium sp. were transferred to 1-L polycarbonate bottles containing only filtered seawater [nitrate plus nitrite (N), <1 μM; phosphate (P), <0.1 μM] and placed on a shelf under illumination and temperature conditions similar to those described for experiment 1. After 2 wk, the dense layer of cells in the upper third of the bottle was gently removed and distributed among four 1-L bottles containing 500 mL of the algal growth medium f/2 (F), f/2 medium without N (F-N), f/2 medium without P (F-P), or f/2 medium without N and P (F-NP). The dense layer of cells was also transferred to 1-L bottles containing only seawater and incubated for 2 wk.
Each day thereafter, subsamples were taken from each bottle and gently filtered through glass fiber filters (GF/Fs), and the N and P concentrations were measured using a Seal Analytical QuAAtro AutoAnalyzer. After 6 d, the N and P concentrations were reduced to undetectable levels (F-NP bottle: N, 0.90 μM; P, 0.01–0.09 μM; F-N bottle: N, not detected; P, 12–38 μM; F-P bottle: N, 102–247 μM; P, not detected; F bottle: N, 137–243 μM; P, 12–32 μM). The N and P concentrations in the bottles containing H. akashiwo cells in seawater also dropped, to <1 μM and <0.1 μM, respectively, within 6 d.
In this experiment, aliquots (2–3 mL) from each bottle containing acclimated cells of Symbiodinium sp. were transferred to twelve 42-mL polycarbonate bottles containing H. akashiwo cultures (ca. 6 mL) with very low N and P concentrations and 16 mL of matching target nutrients or seawater. Six of the 12 bottles were the experimental bottles (predator plus prey), 3 were the prey control bottles (only prey), and 3 were the predator control bottles (only predator). Three of the six experimental bottles were used for measuring the concentrations of N and P at the beginning of the experiment. These bottles were incubated for 2 d, and the growth and ingestion rates of Symbiodinium sp. feeding on H. akashiwo were measured as described by Jeong et al. (39).
Supplementary Material
Acknowledgments
We thank Dr. Diane Stoecker for valuable comments, Y. Ko for collecting corals, and E. Y. Yoon, Y. J. Hwang, and E. Potvin for technical support. This work was supported by National Research Foundation Grant NRF C1ABA001-2010-0020700, the Ecological Disturbance Research Program, the Long-Term Change of Structure and Function in Marine Ecosystems of Korea program (Korea Institute of Marine Science and Technology Promotion / Ministry of Land, Transport, and Maritime Affairs award, to H.J.J. and K.L.), and the Infrastructuring Grant for Marine Biotechnology Program (KIMST award to W.Y.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database (accession nos. HE653238 and HE653239).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1204302109/-/DCSupplemental.
References
- 1.Hughes TP, et al. Climate change, human impacts, and the resilience of coral reefs. Science. 2003;301:929–933. doi: 10.1126/science.1085046. [DOI] [PubMed] [Google Scholar]
- 2.Tang YZ, Koch F, Gobler CJ. Most harmful algal bloom species are vitamin B1 and B12 auxotrophs. Proc Natl Acad Sci USA. 2010;107:20756–20761. doi: 10.1073/pnas.1009566107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mumby PJ, Hastings A, Edwards HJ. Thresholds and the resilience of Caribbean coral reefs. Nature. 2007;450:98–101. doi: 10.1038/nature06252. [DOI] [PubMed] [Google Scholar]
- 4.Pennisi E. Reefs in trouble: Spawning for a better life. Science. 2007;318:1712–1717. doi: 10.1126/science.318.5857.1712. [DOI] [PubMed] [Google Scholar]
- 5.Maina J, McClanahan TR, Venus V, Ateweberhan M, Madin J. Global gradients of coral exposure to environmental stresses and implications for local management. PLoS ONE. 2011;6:e23064. doi: 10.1371/journal.pone.0023064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bellwood DR, Hughes TP, Folke C, Nyström M. Confronting the coral reef crisis. Nature. 2004;429:827–833. doi: 10.1038/nature02691. [DOI] [PubMed] [Google Scholar]
- 7.Lewis CL, Coffroth MA. The acquisition of exogenous algal symbionts by an octocoral after bleaching. Science. 2004;304:1490–1492. doi: 10.1126/science.1097323. [DOI] [PubMed] [Google Scholar]
- 8.Silverman J, Lazar B, Erez J. Community metabolism of a coral reef exposed to naturally varying dissolved inorganic nutrient loads. Biogeochemistry. 2007;84:67–82. [Google Scholar]
- 9.Shinzato C, et al. Using the Acropora digitifera genome to understand coral responses to environmental change. Nature. 2011;476:320–323. doi: 10.1038/nature10249. [DOI] [PubMed] [Google Scholar]
- 10.Levy O, et al. Complex diel cycles of gene expression in coral-algal symbiosis. Science. 2011;331:175. doi: 10.1126/science.1196419. [DOI] [PubMed] [Google Scholar]
- 11.Osinga R, et al. The biology and economics of coral growth. Mar Biotechnol (NY) 2011;13:658–671. doi: 10.1007/s10126-011-9382-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gattuso J-P, Reynaud-Vaganay S, Furla P, Romaine-Lioud S, Jaubert J. Calcification does not stimulate photosynthesis in the zooxanthellate scleractinian coral Stylophora pistillata. Limnol Oceanogr. 2000;45:246–250. [Google Scholar]
- 13.Tchernov D, et al. Apoptosis and the selective survival of host animals following thermal bleaching in zooxanthellate corals. Proc Natl Acad Sci USA. 2011;108:9905–9909. doi: 10.1073/pnas.1106924108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iglesias-Prieto R, Matta JL, Robins WA, Trench RK. Photosynthetic response to elevated temperature in the symbiotic dinoflagellate Symbiodinium microadriaticum in culture. Proc Natl Acad Sci USA. 1992;89:10302–10305. doi: 10.1073/pnas.89.21.10302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baker AC, Glynn PW, Riegl B. Climate change and coral reef bleaching: An ecological assessment of long-term impacts, recovery trends and future outlook. Estuar Coast Shelf Sci. 2008;80:435–471. [Google Scholar]
- 16.Falkowski PG, Dubinsky Z, Muscatine L, McCloskey L. Population control in symbiotic corals. Bioscience. 1993;43:606–611. [Google Scholar]
- 17.Stanley GD., Jr Ecology: Photosymbiosis and the evolution of modern coral reefs. Science. 2006;312:857–858. doi: 10.1126/science.1123701. [DOI] [PubMed] [Google Scholar]
- 18.Stambler N. Zooxanthellae: The yellow symbionts inside animals. In: Dubinsky Z, Stambler N, editors. Coral Reefs: An Ecosystem in Transition. New York: Springer; 2011. pp. 87–106. [Google Scholar]
- 19.Johnson MD. The acquisition of phototrophy: Adaptive strategies of hosting endosymbionts and organelles. Photosynth Res. 2011;107:117–132. doi: 10.1007/s11120-010-9546-8. [DOI] [PubMed] [Google Scholar]
- 20.Coffroth MA, Santos SR. Genetic diversity of symbiotic dinoflagellates in the genus Symbiodinium. Protist. 2005;156:19–34. doi: 10.1016/j.protis.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 21.Adey WH, Goertemiller T. Coral reef algal turfs: Master producers in nutrient poor seas. Phycologia. 1987;26:374–386. [Google Scholar]
- 22.Kleypas JA, McManus JW, Meñez LAB. Environmental limits to coral reef development: Where do we draw the line? Am Zool. 1999;39:146–159. [Google Scholar]
- 23.Littler MM, Littler DS, Brooks BL. Harmful algae on tropical coral reefs: Bottom-up eutrophication and top-down herbivory. Harmful Algae. 2006;5:565–585. [Google Scholar]
- 24.Bell PR, Lapointe BE, Elmetri I. Reevaluation of ENCORE: Support for the eutrophication threshold model for coral reefs. Ambio. 2007;36:416–424. doi: 10.1579/0044-7447(2007)36[416:roesft]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 25.Hoegh-Guldberg O, Williamson J. Availability of two forms of dissolved nitrogen to the coral Pocillopora damicornis and its symbiotic zooxanthellae. Mar Biol. 1999;133:561–570. [Google Scholar]
- 26.Grover R, Maguer J-F, Allemand D, Ferrier-Pagès C. Urea uptake by the sceractinian coral Stylophora pistillata. J Exp Mar Biol Ecol. 2006;332:216–225. [Google Scholar]
- 27.Sakka A, Legendre L, Gosselin M, Niquil N, Delesalle B. Carbon budget of the planktonic food web in an atoll lagoon (Takapoto, French Polynesia) J Plankton Res. 2004;24:301–320. [Google Scholar]
- 28.Grami B, et al. The plankton food web of the Bizerte Lagoon (south-western Mediterranean), II: Carbon steady-state modeling using inverse analysis. Estuar Coast Shelf Sci. 2008;79:101–113. [Google Scholar]
- 29.Bertilsson S, Berglund O, Karl DM, Chisholm SW. Elemental composition of marine Prochlorococcus and Synechococcus: Implications for the ecological stoichiometry of the sea. Limnol Oceanogr. 2003;48:1721–1731. [Google Scholar]
- 30.Domotor SL, D’Elia CF. Nutrient uptake kinetics and growth of zooxanthellae maintained in laboratory culture. Mar Biol. 1984;80:93–101. [Google Scholar]
- 31.D’Elia CF, Domotor SL, Webb KL. Nutrient uptake kinetics of freshly isolated zooxanthellae. Mar Biol. 1983;75:157–167. [Google Scholar]
- 32.McAuley PJ, Smith VJ. Effect of diel photoperiod on nitrogen metabolism of cultured and symbiotic zooxanthellae. Mar Biol. 1995;123:145–152. [Google Scholar]
- 33.Moriarty DJW, Pollard PC, Hunt WG. Temporal and spatial variation in bacterial production in the water column over a coral reef. Mar Biol. 1985;85:285–292. [Google Scholar]
- 34.Littman RA, van Oppen MJH, Willis BL. Methods for sampling free-living Symbiodinium (zooxanthellae) and their distribution and abundance at Lizard Island (Great Barrier Reef) J Exp Mar Biol Ecol. 2008;364:48–53. [Google Scholar]
- 35.Coffroth MA, Santos SR, Goulet TL. Early ontogenetic expression of specificity in a cnidarian-algal symbiosis. Mar Ecol Prog Ser. 2001;222:85–96. [Google Scholar]
- 36.Fay SA, Weber MX, Lipps JH. The distribution of Symbiodinium diversity within individual host foraminifera. Coral Reefs. 2009;28:717–726. [Google Scholar]
- 37.Hill M, Allenby A, Ramsby B, Schönberg C, Hill A. Symbiodinium diversity among host clionaid sponges from Caribbean and Pacific reefs: Evidence of heteroplasmy and putative host-specific symbiont lineages. Mol Phylogenet Evol. 2011;59:81–88. doi: 10.1016/j.ympev.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 38.LaJeunesse TC. Investigating the biodiversity, ecology, and phylogeny of endosymbiotic dinoflagellates in the genus Symbiodinium using the internal transcribed spacer region: In search of a “species”-level marker. J Phycol. 2001;37:866–880. [Google Scholar]
- 39.Jeong HJ, et al. Ecology of Gymnodinium aureolum, I: Feeding in western Korean waters. Aquat Microb Ecol. 2010;59:239–255. [Google Scholar]
- 40.Jeong HJ, et al. Feeding and grazing impact by small marine heterotrophic dinoflagellates on heterotrophic bacteria. J Eukaryot Microbiol. 2008;55:271–288. doi: 10.1111/j.1550-7408.2008.00336.x. [DOI] [PubMed] [Google Scholar]
- 41.Jeong HJ, et al. Feeding by the red-tide dinoflagellates on the cyanobacterium Synechococcus. Aquat Microb Ecol. 2005;41:131–143. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.