Abstract
The signaling molecule RGS9-2 is a potent modulator of G-protein-coupled receptor function in striatum. Our earlier work revealed a critical role for RGS9-2 in the actions of the μ-opioid receptor (MOR) agonist morphine. In this study, we demonstrate that RGS9-2 may act as a positive or negative modulator of MOR-mediated behavioral responses in mice depending on the agonist administered. Paralleling these findings we use coimmunoprecipitation assays to show that the signaling complexes formed between RGS9-2 and Gα subunits in striatum are determined by the MOR agonist, and we identify RGS9-2 containing complexes associated with analgesic tolerance. In striatum, MOR activation promotes the formation of complexes between RGS9-2 and several Gα subunits, but morphine uniquely promotes an association between RGS9-2 and Gαi3. In contrast, RGS9-2/Gαq complexes assemble after acute application of several MOR agonists but not after morphine application. Repeated morphine administration leads to the formation of distinct complexes, which contain RGS9-2, Gβ5, and Gαq. Finally, we use simple pharmacological manipulations to disrupt RGS9-2 complexes formed during repeated MOR activation to delay the development of analgesic tolerance to morphine. Our data provide a better understanding of the brain-region-specific signaling events associated with opiate analgesia and tolerance and point to pharmacological approaches that can be readily tested for improving chronic analgesic responsiveness.
Introduction
The 76 kDa protein RGS9-2 plays a potent modulatory role in striatal function by controlling responsiveness of several G-protein-coupled receptors (GPCRs) (Rahman et al., 1999, 2003; Cabrera-Vera et al., 2004; Kovoor et al., 2005; Terzi et al., 2009; Traynor et al., 2009). RGS9-2 is the brain-specific splice variant of the RGS9 gene and member of the R7 group of the regulator of G-protein signaling (RGS) family, abundantly expressed in all types of striatal neurons (Gold et al., 1997; Rahman et al., 2003; Cabrera-Vera et al., 2004). In vitro work has proved that RGS9-2 interacts with activated Gα subunits to promote their GTPase activity (Dohlman and Thorner, 1997; Berman and Gilman, 1998; Terzi et al., 2009), but the Gα subunit selectivity for RGS9-2 in the striatum remains unknown. In addition to the conserved RGS region, RGS9-2 contains an N-terminal DEP domain that mediates association with cell membrane adaptor proteins (Martemyanov et al., 2003, 2005; Ballon et al., 2006; Jayaraman et al., 2009), a GGL (G gamma like) domain that promotes stability by binding to Gβ5 protein (He et al., 2000; Chen et al., 2003), and a C-terminal phosphodiesterase γ-like domain (Rahman et al., 1999). All these domains play important roles in RGS9-2 actions by controlling the protein stability, localization, or its interactions with other proteins. Because recent studies reveal that manipulations of RGS9-2 levels in the striatum may potently modulate pharmacological responses (Rahman et al., 2003; Zachariou et al., 2003; Kovoor et al., 2005; Traynor and Neubig, 2005; Gold et al., 2007), it is essential to understand the mechanism via which RGS9-2 modulates different GPCRs in this brain region. Opiate analgesics, including morphine, fentanyl, and methadone, exert their actions via activation of the G-protein-coupled μ-opioid receptor (MOR) (Contet et al., 2004). Morphine is a very efficient analgesic, but clinicians limit its use because of numerous side effects, the development of analgesic tolerance, and its significant abuse potential (Kreek, 2001). Our previous work demonstrated that RGS9-2 is a negative modulator of the analgesic and rewarding actions of morphine (Zachariou et al., 2003). Using cell culture models, we have shown that RGS9-2 associates with MOR and affects several events that follow MOR activation, including the phosphorylation of extracellular signal-regulated kinase (ERK) and the rate of receptor internalization (Psifogeorgou et al., 2007). Here, we use behavioral and biochemical assays to investigate the role of RGS9-2 in MOR signaling in striatum. At the behavioral level, RGS9-2 is a negative modulator of morphine actions but acts as a positive modulator of the analgesic actions of fentanyl and methadone. Our biochemical findings suggest that this difference results from the formation of distinct complexes in striatum between RGS9-2, Gα subunits, and other signal transduction elements, after activation of MOR by different agonists. Our coimmunoprecipitation data reveal that changes in the composition of these complexes after chronic morphine administration correlate with the development of analgesic tolerance. Finally, we developed simple pharmacological manipulations to prevent the formation of stable RGS9-2-containing complexes to delay analgesic tolerance to morphine.
Materials and Methods
Mouse breedings and treatments.
RGS9 mutant mice used in this study (Zachariou et al., 2003) were generated from breedings of heterozygous RGS9 knock-out (KO) mice (backcrossed for 16 generations onto C57BL/6 background). For all behavioral assays, we used naive 2-month-old male KO mice and their wild-type (WT) littermates. For immunoprecipitation assays, striata were extracted 30 min after saline, fentanyl, methadone, or morphine injections (Charlton et al., 2008). For immunoblotting analysis, tissue from 2-month-old C57BL/6 mice was extracted 10 and 20 min after saline or drug treatment as described (Zachariou et al., 2003). Animals were housed in a 12 h dark/light cycle room according to the animal care and use committees of Mount Sinai Medical Center and the University of Crete. Chronic treatments for immunoprecipitation assays involved subcutaneous injections of increasing morphine (from 15 to 80 mg/kg) or fentanyl (from 0.04 to 0.32 mg/kg) doses given twice per day for 4 d and the morning of the fifth day. The afternoon of the fifth day, striata were extracted 30 min after morphine (20 mg/kg) or fentanyl (0.125 mg/kg) injection.
Coimmunoprecipitation and Western blot assays.
Western blot analysis studies were preformed as described previously (Charlton et al., 2008). For immunoprecipitation (IP) assays, striatal tissues of mice treated with saline, morphine, or fentanyl for 30 min were rapidly dissected as describer previously (Charlton et al., 2008). Briefly, samples were centrifuged for 30 min at 13,000 rpm, and cell lysates were subjected to a second centrifugation for 20 more min [lysis buffer: 20 mm Tris-HCl, pH 7.5, 150 mm NaCl, 0.1% Triton X-100, 1 mm EDTA, 1× protease inhibitor cocktail (P8430; Sigma), and 0.2 mm sodium orthovanadate (Sigma)]. Lysates were precleared with 20 μl of G agarose beads (Roche) by 1–2 h incubation at 4°C. Immunoprecipitation was performed by incubating the supernatants with primary antibody of interest overnight (Charlton et al., 2008). The following antibodies were used for IP and Western blot assays: rabbit protein A-purified anti-RGS9 antibody (1:10,000) (Psifogeorgou et al., 2007) (supplemental Fig. 1d, available at www.jneurosci.org as supplemental material) and a rabbit anti-Gβ5 (C terminus) antibody (1:10,000; W. Simonds, National Institute of Diabetes, Digestive, and Kidney Diseases, Bethesda, MD), rabbit anti-MOR (1:1000; Immunostar) (Arvidsson et al., 1995; Haberstock Debic et al., 2005; Jin et al., 2010), rabbit anti-Gαi1, rabbit anti-Gαi2, rabbit anti-Gαi3, and rabbit anti-Gαs (1:1000; all provided by S. Mumby, University of Texas Southwestern Medical Center, Dallas, TX) (Mumby and Gilman 1991), rabbit anti-Gαq (1:1000; Paul Sternweis, University of Texas Southwestern Medical Center), rabbit anti-GRK2 (1:1000; Millipore), rabbit anti-phosphorylated phospholipase Cβ3 (pPLCβ3) or anti-PLCβ3 (1:400; Cell Signaling Technology), and rabbit anti-β-arrestin-2 (1:1000; J. Benovic, Thomas Jefferson University, Philadelphia, PA) (Mundell et al., 1999). For ERK phosphorylation assays, we used a mouse pERK antiserum and a purified rabbit ERK antiserum (1:1000; Sigma).
Analgesia and tolerance studies.
Analgesia was measured using the 52°C hotplate test, as described previously (Zachariou et al., 2003). For analgesic tolerance assays, mice were monitored at baseline and 30 min after drug administration for 4 consecutive days. All hotplate data are expressed as percentage maximal possible effect [MPE = (latency − baseline)/(cutoff − latency)]. A cutoff time of 40 s has been used in all hotplate experiments.
Results
Ligand-dependent effects of RGS9-2 on behavioral responses
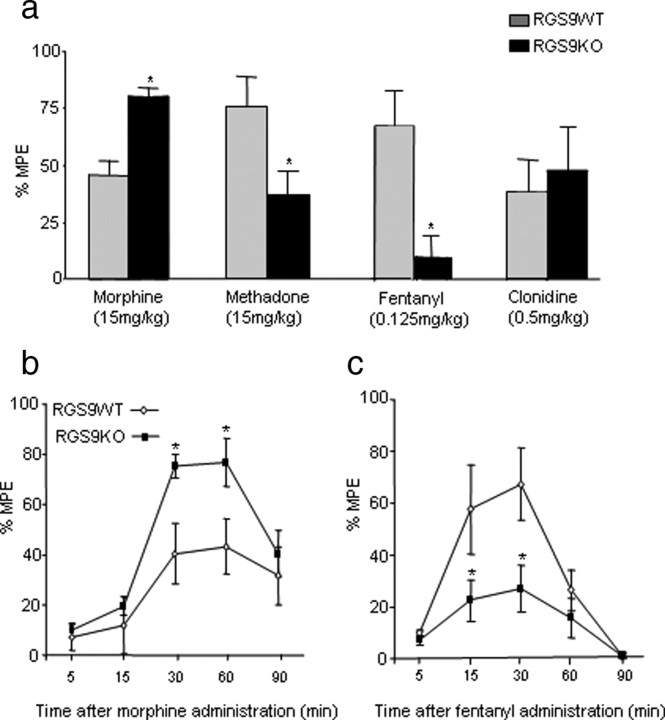
Our previous behavioral studies demonstrated that functional deletion of the RGS9 gene increases sensitivity to morphine reward and analgesia and delays the development of analgesic tolerance (Zachariou et al., 2003, Psifogeorgou et al., 2007). To assess whether the effects of RGS9-2 deletion generalized to other MOR agonists, we used the 52°C hotplate assay and compared responses of RGS9 wild-type (RGS9 WT) and RGS9 knock-out (RGS9KO) mice to several opioid analgesics. Consistent with previous findings, RGS9KO mice show a greater analgesic response to morphine (15 mg/kg, s.c.) in the hotplate assay (Fig. 1a). In contrast, mice lacking RGS9 show reduced analgesic responses to methadone (15 mg/kg, s.c.) and fentanyl (0.125 mg/kg, s.c.) compared with their wild-type littermate controls. At higher doses (0.2 mg/kg), fentanyl produces a significant analgesic response that is still lower than that observed in their wild-type littermates (%MPE for RGS9WT = 80 ± 8 and RGS9KO = 53 ± 13). The effects of RGS9-2 deletion in analgesia are specific to MOR agonists, because no phenotype is observed when the α2 adrenergic receptor agonist clonidine is used (Fig. 1a). Notably, the responses of RGS9KO mice to morphine and fentanyl are not related to differences in the onset or duration of analgesia between genotypes (Fig. 1b,c).
Figure 1.
Differential regulation of the analgesic actions of morphine, methadone, and fentanyl by RGS9. a, Mice lacking the RGS9 gene show increased analgesic response to morphine (15 mg/kg, s.c.) in the hotplate test. Conversely, RGS9KO mice show a reduced response to the opioid analgesics methadone (15 mg/kg, s.c.) and fentanyl (0.125 mg/kg, s.c.) compared with their wild-type littermates. Administration of the α2-adrenergic receptor agonist clonidine (0.4 mg/kg) did not reveal any significant difference in the analgesic response between genotypes. In the hotplate assay, knockout of RGS9 leads to increased response to morphine (10 mg/kg) without affecting the onset or duration of analgesia (b). c shows that the analgesic response to fentanyl (0.125 mg/kg) in RGS9KO mice are lower than that of their wild-type littermates, at different time points after drug administration. Data are expressed as means ± SEM. *p < 0.05 for genotype versus treatment, two-way ANOVA followed by Bonferroni's post hoc test.
Agonist-dependent formation of MOR signaling complexes in the striatum
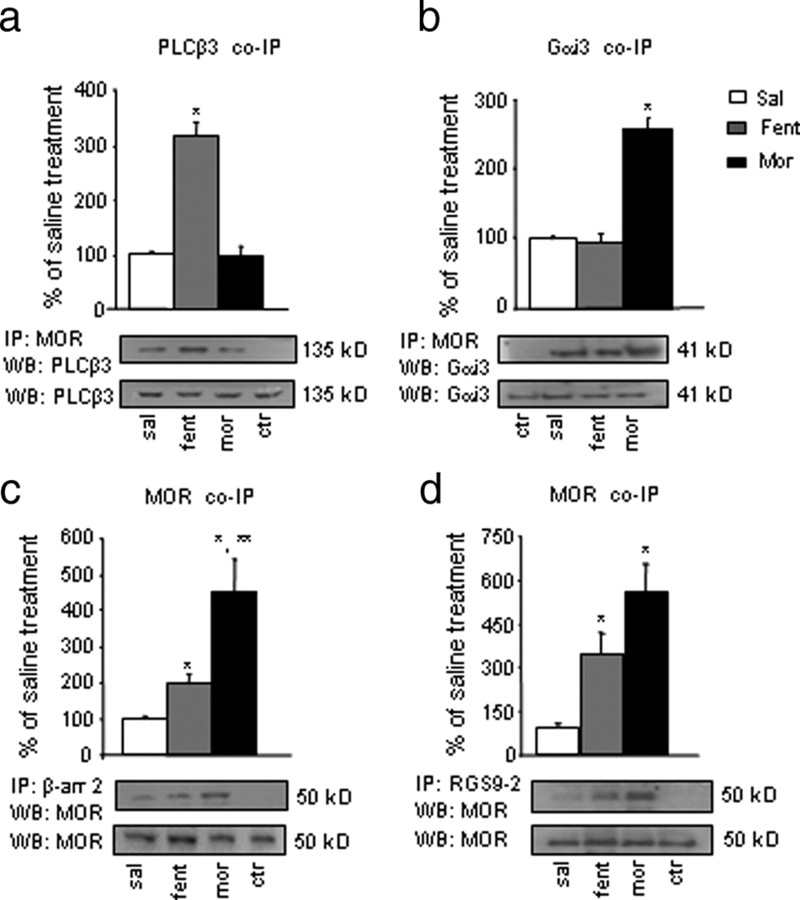
In an effort to mechanistically understand the agonist-selective phenotype of RGS9KO mice, we used co-IP assays to examine the composition of RGS9-2 complexes that are formed in striatum after fentanyl or morphine application. For all immunoprecipitation assays, we selected doses of fentanyl (0.125 mg/kg) and morphine (20 mg/kg) that produce maximal analgesia in C57BL/6 mice in the hotplate assay. The time point of 30 min was selected as the earliest time point after drug application that changes in RGS9-2 interactions were detected. First, we examined the interactions between MOR and Gα subunits after application of fentanyl or morphine. As shown in Table 1, activation of MOR promotes the formation of complexes with several Gα subunits in striatum, but some of these interactions are associated with particular agonists. We recently showed that MOR activation by fentanyl (but not morphine) promotes association of the receptor with Gαq proteins (Han et al., 2010). Here, we demonstrate that fentanyl application promotes the formation of complexes between MOR and the Gαq effector PLCβ3 (Fig. 2a). Conversely, MOR/Gαi3 complexes are observed only after morphine administration (Fig. 2b). The next set of IPs investigated the interactions between MOR and β-arrestin-2, an essential protein for receptor desensitization. Consistent with previous findings, activation of MOR by morphine leads to an increased association with β-arrestin-2 in striatum (454 ± 88%) relative to saline controls (Fig. 2c). Fentanyl also promotes the formation of complexes between MOR and β-arrestin-2, but this effect is smaller than that of morphine (197 ± 27%). Finally, we show that RGS9-2 is part of these signaling complexes because, 30 min after activation of MOR by fentanyl or morphine, there is an increased interaction between the receptor and RGS9-2 in striatum (Fig. 2d) (348 ± 70% increase after fentanyl application and 560 ± 97% increase after morphine, both relative to saline application).
Table 1.
Interactions of MOR with Gα subunits
| Basal | Fentanyl | Morphine | |
|---|---|---|---|
| MOR/Gαi1 | + | ++ | ++ |
| MOR/Gαi2 | + | ++ | +++ |
| MOR/Gαi3 | + | + | +++ |
| MOR/Gαq | + | +++ | + |
| MOR/Gαo | + | ++ | ++ |
Mice were treated with saline, morphine (20 mg/kg), or fentanyl (0.125 mg/kg) for 30 min. Striatal extracts were immunoprecipitated with an anti-MOR antibody, and the immunoprecipitate was immunoblotted for Gαi1, Gαi2, Gαi3, Gαq, or Gαo. Each experiment was performed at least three times.
Figure 2.
Morphine and fentanyl administration lead to distinct MOR signaling complexes in striatum. a–d, Mice were treated with saline (sal), morphine (mor; 20 mg/kg), or fentanyl (fent; 0.125 mg/kg) for 30 min. Striatal extracts were IP with anti-MOR (a, b), β-arrestin-2 (c), or RGS9-2 (d) antibodies, and the immunoprecipitate was immunoblotted (WB) with the indicated antibodies. a, PLCβ3 was coimmunoprecipitated with MOR after fentanyl administration. b, Gαi3 was uniquely coimmunoprecipitated with MOR after morphine and showed low levels of interaction with MOR under baseline conditions or after fentanyl treatment. Data are expressed as means ± SEM. *p < 0.01 between treatments, one-way ANOVA followed by Dunnett's post hoc test. In c, striatal extracts were immunoprecipitated (IP) with an anti-β-arrestin-2 antibody, and the immunoprecipitate was immunoblotted (WB) for MOR. Activation of MOR by fentanyl or morphine increases its association with β-arrestin-2 (b; *p < 0.001 for morphine and fentanyl vs saline, **p < 0.05 for morphine vs fentanyl treatment, one-way ANOVA followed by Dunnett's post hoc test). Finally, d shows that MOR is immunoprecipitated with RGS9-2, and this interaction is strengthened after fentanyl or morphine application. Data are expressed as means ± SEM. *p < 0.01 between treatments, one-way ANOVA followed by Dunnett's post hoc test. For all experiments, n = 4–5 per treatment group. Ctr (control), Striata from morphine-treated mice immunoprecipitated with an anti-flag antiserum. WB for protein levels in total lysates are shown below each IP blot.
Because our immunoprecipitation assays demonstrate that MOR interactions with both Gα subunits and β-arrestin-2 are agonist dependent and our behavioral assays show that fentanyl and morphine produce opposite phenotypes in RGS9KO mice in the hotplate assay, we next examined the role of RGS9-2 in signal transduction complexes formed after MOR activation by these agonists. Paralleling our co-IP findings with MOR, morphine administration promotes the formation of complexes between RGS9-2 and β-arrestin-2,.whereas fentanyl promotes a smaller but still significant increase in this complex compared with control conditions (Fig. 3a). RGS9-2 appears to follow the same pattern as MOR regarding agonist-dependent interactions with Gα subunits after fentanyl or morphine exposure. Specifically, although RGS9-2 forms complexes with both Gαi1 and Gαi2 after MOR activation by either fentanyl or morphine, morphine administration also promotes the formation of complexes between RGS9-2 and Gαi3 in striatum (Fig. 3b), whereas fentanyl administration promotes the association of RGS9-2 with Gαq and with its downstream effector molecule PLCβ3 (Fig. 3c,d). Fentanyl treatment also promotes the association of RGS9-2 with GRK2 in the striatum (274 ± 14% compared with saline-treated control). Another striking difference we observed between fentanyl and morphine is that, although both drugs promote the association of RGS9-2 to MOR, only morphine promotes the formation of RGS9-2/Gβ5 complexes (Fig. 3e). This is a key finding because Gβ5 protein is a required binding partner for RGS9-2 stability and, in the absence of Gβ5, RGS9-2 has a very short half-life (Chen et al., 2003; Witherow et al., 2003). Consistent with our behavioral findings, methadone application promotes the formation of signal transduction complexes similar to those observed with fentanyl (supplemental Fig. 1, available at www.jneurosci.org as supplemental material).
Figure 3.
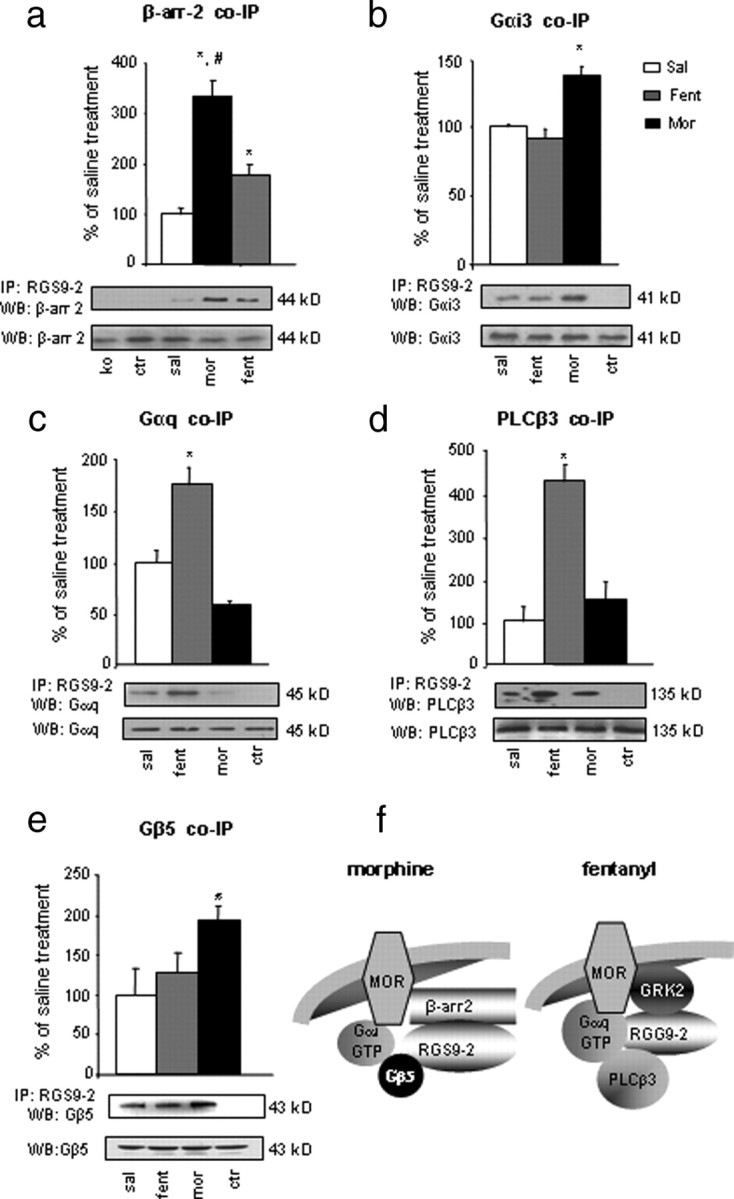
RGS9-2 forms distinct signaling complexes in striatum after morphine and fentanyl administration. a, Mice were treated with saline (sal), morphine (mor; 20 mg/kg), or fentanyl (fent; 0.125 mg/kg) for 30 min, as in the previous figure. Striatal extracts were immunoprecipitated (IP) with an anti-RGS9-2 antibody, and the immunoprecipitate was immunoblotted (WB) for β-arrestin-2 (β-arr-2). Activation of MOR leads to an increase in RGS9-2/β-arrestin-2 complex in striatum, an effect that is more prominent during morphine administration (*p < 0.05 between fentanyl and saline or morphine and #p < 0.001 between morphine and saline). b, Activation of MOR by morphine (but not fentanyl) increases the association between RGS9-2 and Gαi3. c, d, Conversely, fentanyl application promotes the formation of signaling complexes between RGS9-2 and Gαq (c) and between RGS9-2 and PLCβ3 (d). Finally, morphine but not fentanyl application drives the formation of complexes between RGS9-2 and Gβ5 (e). For all experiments, n = 3–6 per treatment group. KO, Striata from morphine treated RGS9KO mice; Ctr (control), striata from morphine-treated mice immunoprecipitated with an anti-flag antiserum. WB for protein levels in total lysates are shown below each IP blot. Data are expressed as means ± SEM. *p < 0.01 between treatments, one-way ANOVA followed by Dunnett's post hoc test. f shows a schematic representation of RGS9-2 complexes formed in striatum after activation of MOR by morphine or fentanyl.
Regulation of ERK and PLCβ3 phosphorylation by RGS9-2
Because our coimmunoprecipitation results demonstrate that RGS9-2 may form complexes containing Gαq or Gαi subunits in striatum, we hypothesized that deletion of the RGS9 gene will affect the activity of their respective effector molecules. In particular, we examined the role of RGS9-2 in MOR-induced phosphorylation of ERK and PLCβ3. Previous studies in cell culture models showed that RGS9-2 overexpression prevents induction of pERK levels by MOR agonists (Psifogeorgou et al., 2007). In the brain, morphine may increase or decrease ERK phosphorylation, depending on the neural circuit and drug dose (Eitan et al., 2003; Muller and Unterwald, 2004). Figure 4a shows that, in the nucleus accumbens (NAc), systemic administration of a low morphine dose leads to a decrease in pERK levels. This reduction is more pronounced in RGS9KO mice. Fentanyl administration also reduces ERK phosphorylation in the NAc. However, and in contrast to morphine, fentanyl-induced reduction of pERK is not observed in brains of RGS9KO mice (Fig. 4b).
Figure 4.
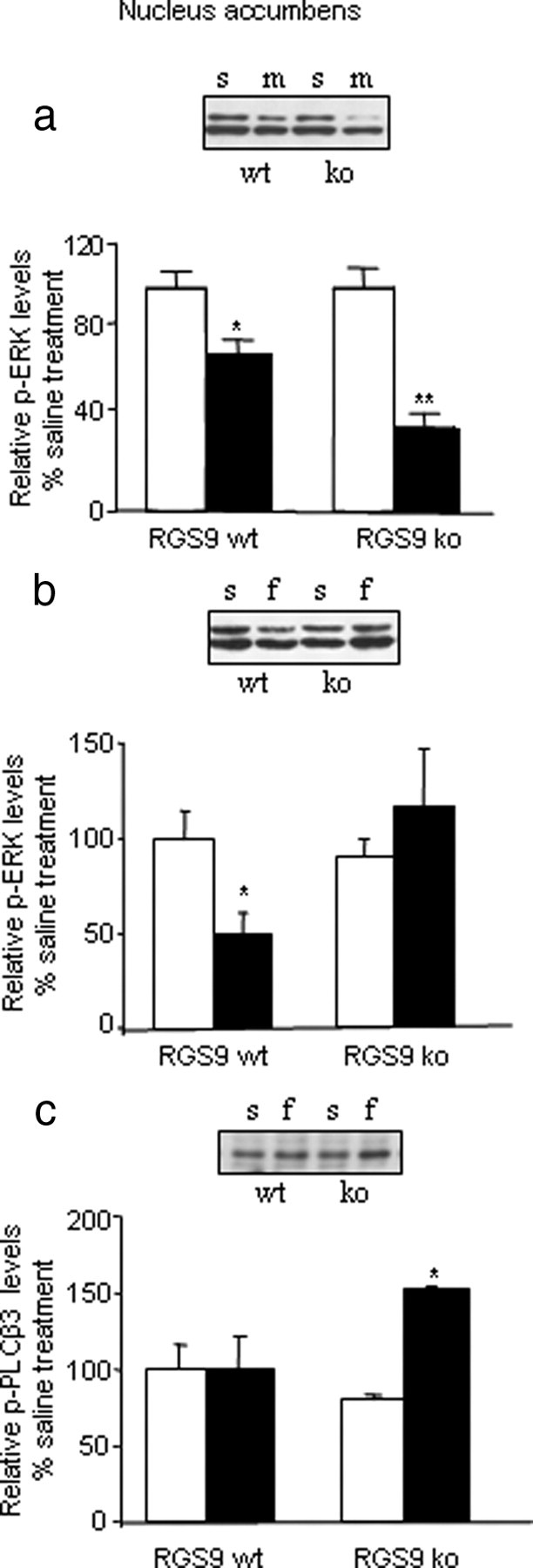
Modulation of ERK and PLCβ3 phosphorylation by RGS9-2. RGS9 knockout affects the pattern of ERK phosphorylation by opioids in the NAc. a, Immunoblot analysis of pERK levels in NAc of RGS9WT and RGS9KO mice 20 min after saline (s) or morphine (m) (10 mg/kg) administration (n = 3 per group). Morphine decreases pERK levels in the NAc of wild-type animals, and this effect is more pronounced when the RGS9 gene is deleted. Immunoblot analysis of pERK levels in NAc of RGS9KO mice and their wild-type controls 10 min after an injection of saline or fentanyl (f) (0.125 mg/kg, s.c.) (b; n = 4–5 per group). As indicated by the graphs, fentanyl decreases pERK levels in the NAc of RGS9WT mice, but this effect is abolished in RGS9KOs. c demonstrates changes in pPLCβ3 levels in the NAc, 10 min after fentanyl injections, in RGS9WT and RGS9KO mice. Knockout of the RGS9 gene leads to increased pPLCβ3 levels after fentanyl application. Data are expressed as means ± SEM. n = 4 per group; *p < 0.05 and **p < 0.01 for genotype versus treatment, two-way ANOVA followed by Bonferroni's post hoc test.
In addition to ERK, opioids modulate the activity of PLCβ proteins, which are known downstream effectors of Gαq and PKC signaling (Chakrabarti et al., 2003). We hypothesized that given the promotion of the association of both MOR and RGS9-2 with Gαq by fentanyl, RGS9-2 deletion effectively removes a competitor of Gαq/PLCβ3 interactions. Thus, RGS9 deletion should increase Gαq signaling by enhancing the binding and activation of Gαq to its effector molecule PLCβ3. To test our hypothesis, we measured pPLCβ3 levels in the NAc 10 min after fentanyl injection. Figure 4c shows that deletion of the RGS9 gene results in increased PLCβ3 phosphorylation in the NAc after fentanyl application. These same doses show no effect on PLCβ3 phosphorylation in samples harvested from wild-type NAc. This finding correlates with the behavioral data, which show that RGS9 deletion leads to reduced analgesic responses to fentanyl and indicate that RGS9-2 antagonizes the actions of the Gαq effector PLCβ3 in striatum. Notably, knockout of RGS9 did not affect morphine-induced PLCβ3 phosphorylation in the NAc. In all our pPLCβ3 studies, RGS9 deletion did not cause changes in total PLCβ3 abundance (data not shown).
Chronic morphine-induced changes in RGS9-2 signaling complexes correlates with tolerance
Because the key clinical challenge is the development of tolerance after chronic morphine treatment, our next goal was to identify possible differences in the composition of RGS9-2-containing complexes between acute and chronic MOR activation. Because morphine produces analgesic tolerance more quickly than fentanyl, we examined the composition of RGS9-2 complexes in striatum of mice repeatedly treated with fentanyl or morphine. Our preliminary studies indicated that pretreatment with a low fentanyl dose may prevent analgesic tolerance to morphine. For that reason, we included an additional group of animals that received a low fentanyl dose 30 min before each morphine injection. In particular, chronic morphine studies contained four groups of animals: mice chronically treated with (1) saline, (2) fentanyl, (3) morphine, and (4) a low fentanyl dose (0.04 mg/kg, s.c.), 30 min before each morphine injection. On day 5, striata were harvested 30 min after administration of saline, fentanyl (0.125 mg/kg), or morphine (20 mg/kg). As shown in Figure 5a–c, after repeated morphine exposure, activation of MOR promotes associations of RGS9-2 with MOR (Fig. 5a) (363.6 ± 92%), Gαq (Fig. 5b) (216 ± 39%), and Gβ5 (Fig. 5c) (171 ± 21%) compared with striata from vehicle-treated mice. Such interactions are not observed after chronic fentanyl treatment. The same blots show that pretreatment with a low fentanyl dose (fent/mor group) prevents the association between RGS9-2 and MOR or between RGS9-2 and Gβ5 (Fig. 5c). Increased coimmunoprecipitation of RGS9-2 with β-arrestin-2 is observed in the chronic fentanyl or fent/mor groups but not after chronic morphine administration (Fig. 5d). Notably, although chronic morphine promotes the interactions between RGS9-2 and Gαq, it does not promote the formation of RGS9-2/PLCβ3 complexes. Chronic morphine induces the same changes in the composition of MOR-containing complexes as it does to RGS9-2-containing complexes. Consistent with the RGS9-2 co-IP findings, β-arrestin-2/MOR complexes are not observed in chronic morphine-treated animals, but they are observed in the fentanyl and fent/mor group. Chronic morphine promotes the formation of complexes between MOR and Gβ5, as well as between MOR and Gαq (supplemental Fig. 2, available at www.jneurosci.org as supplemental material). Similar to the co-IP assays using RGS9 antibody, pretreatment with a low fentanyl dose prevents the formation of MOR coimmunoprecipitation with either Gβ5 or Gαq (supplemental Fig. 2, available at www.jneurosci.org as supplemental material). Knockout of the RGS9 gene does not affect the composition of MOR/Gα subunit complexes (supplemental Fig. 3a,d, available at www.jneurosci.org as supplemental material). Thus, acute and chronic morphine promote the same MOR/Gα subunit complexes observed in wild-type animals, but in mutant mice these complexes are no more regulated by RGS9-2. MOR and β-arrestin-2 complexes observed after acute morphine and fentanyl administration are also observed in RGS9KO mice (supplemental Fig. 3b, available at www.jneurosci.org as supplemental material). As expected, although in wild-type mice chronic morphine promotes the formation of complexes between MOR and the RGS9 binding partner Gβ5, these complexes are no longer observed in RGS9KO mice (supplemental Fig. 3c, available at www.jneurosci.org as supplemental material).
Figure 5.
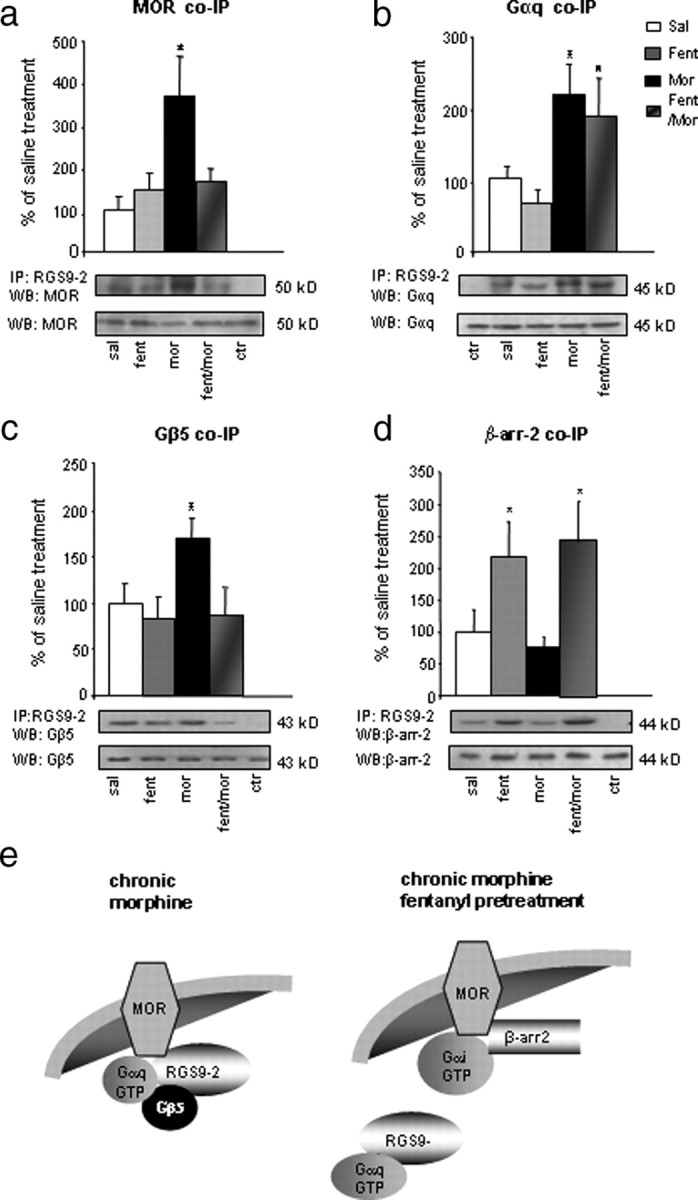
Signaling complexes containing RGS9-2 in striata of mice chronically treated with fentanyl or morphine. a, Mice received chronic injections of saline, fentanyl, morphine, or a chronic morphine treatment in which low fentanyl dose was administered 30 min before each morphine injection (fent/mor). Striatal extracts were immunoprecipitated (IP) with an anti-RGS9-2 antibody, and the immunoprecipitate was immunoblotted (WB) for MOR. WB for protein levels in total lysates are shown below each IP blot. Morphine promotes signaling complexes between RGS9-2 and MOR. *p < 0.01 for morphine versus saline and fentanyl, one-way ANOVA followed by Dunnett's post hoc test. Striatal tissue from mice treated as in a were also immunoprecipitated with anti-RGS9-2 antibody, and the immunoprecipitate was blotted for Gαq, Gβ5, and β-arrestin-2. As shown in b, chronic morphine promotes the association between RGS9-2 and Gαq, an effect that is not significantly affected by fentanyl pretreatment. *p < 0.01 for morphine or fent/mor versus saline and fentanyl, one-way ANOVA followed by Dunnett's post hoc test. As shown in c, chronic morphine promotes the formation of signaling complexes between RGS9-2 and Gβ5, an effect that is prevented by fentanyl pretreatment. d shows that complexes between RGS9-2 and β-arrestin-2 are only formed after chronic fentanyl of fent/mor treatments. Data are expressed as means ± SEM. n = 4–6 per group; *p < 0.01 between treatments, one-way ANOVA followed by Dunnett's post hoc test. e shows a schematic representation of RGS9-2 complexes formed in striatum after chronic morphine application or chronic morphine application when morphine administration is preceded by an injection a low fentanyl dose.
Manipulation of RGS9-2 complexes prevents morphine tolerance
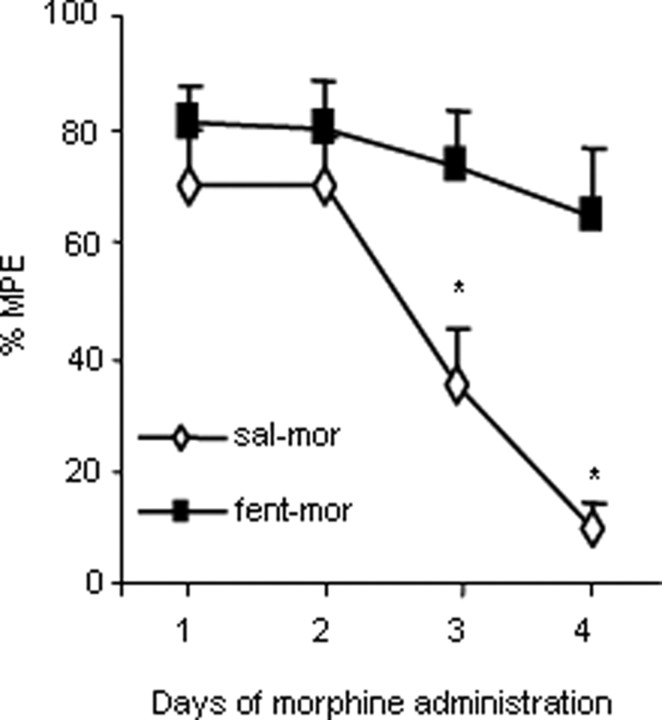
Finally, to test whether the chronic agonist-induced signaling complexes may subserve changes in the behavioral responses to MOR agonists, we examined whether treatments that prevent the formation of stable RGS9-2 complexes with Gαq and Gβ5 can prevent morphine tolerance. For that purpose, we used the hotplate analgesia assay and examined whether administration of a low fentanyl dose 30 min before morphine injection prevents the development of morphine tolerance. In particular, C57BL/6 mice were administered a low fentanyl dose (0.04 mg/kg, s.c.) or saline 30 min before each morphine injection, and analgesic responses were assessed for 4 consecutive days. Fentanyl pretreated mice show no tolerance development compared with the control group (Fig. 6). As expected, fentanyl pretreatment has no additional effect on the analgesic responses of RGS9KO mice (data not shown).
Figure 6.
Pretreatment with a low fentanyl dose prevents the development of analgesic tolerance to morphine. Injection of a low fentanyl (fent) dose (0.04 mg/kg) 30 min before morphine (mor) administration delays the development of analgesic tolerance in the hotplate assay. Analgesic responses were taken at baseline and 30 min after morphine injection. Control animals received saline (sal) instead of fentanyl 30 min before morphine injection. Responses are expressed as percentage maximal possible effect [%MPE = (latency − baseline)/(cutoff − latency)]. Data are expressed as means ± SEM. n = 6–8 per group; *p < 0.05, two-way ANOVA followed by Bonferroni's test.
Discussion
Our study provides evidence for a novel modulatory role of RGS9-2 on MOR signaling after acute and chronic activation. Our findings reveal brain region- and agonist-specific signal transduction events associated with MOR activation and point to RGS9-2 as a major determinant of the acute and chronic actions of opioids. This is the first evidence that a striatal protein may act as a positive or negative modulator of opiate analgesia, depending on the agonist administered. Our studies suggest that MOR couples to several Gα subunits in striatum, including Gαq. Furthermore, RGS9-2 differentially modulates Gαi or Gαq signaling in a ligand-dependent manner. Finally, we demonstrate that some of the behavioral consequences of chronic morphine administration are associated with changes in the composition of RGS9-2-containing complexes. Specifically, the formation of stable MOR/RGS9-2/Gαq/Gβ5 complexes after chronic morphine administration have detrimental effects on MOR signaling and desensitization. Based on the information we gained from coimmunoprecipitation assays on the composition of RGS9-2 complexes after acute fentanyl or morphine application, we developed simple pharmacological manipulations to prevent RGS9-2 interactions associated with analgesic tolerance to morphine. We show that, when administration of a low fentanyl dose precedes morphine administration, chronic morphine treatment does not produce analgesic tolerance. Notably, fentanyl pretreatment affects the development of morphine tolerance but not the acute analgesic actions of morphine, which do not involve Gαq subunits (for 15 mg/kg morphine, %MPEs are as follows: fentanyl pretreated = 39.8 ± 13; saline pretreated = 42.2 ± 12).
Several groups have demonstrated that an RGS protein potentially associates with more than one type of Gα subunits. For example, RGS4 associates with Gαi or Gαq subunits (Huang et al., 1997; Posner et al., 1999; Tesmer et al., 2005). We show that, in striatum, after MOR activation, RGS9-2 may associate with several Gαi subunits and with Gαq. These findings are important, because they provide information on the mechanism of RGS9-2 actions in a particular brain region and also because they contribute to our understanding of the molecular mechanisms underlying opiate actions. Another RGS member, RGS4, does not play a role in morphine analgesia or tolerance but has a small effect as a positive modulator of fentanyl actions by dissociating from MOR after fentanyl application (Han et al., 2010).
In vitro studies have shown that MOR signals via different Gα subunits, but there is little information on MOR/Gα subunit interactions in specific neural networks (Piros et al., 1996; Tesmer et al., 2005). Our coimmunoprecipitation assays demonstrate that, in striatum, activation of MOR by different ligands promotes the formation of RGS9-2 complexes that vary in the Gα subunit composition and that may or may not contain Gβ5. Although RGS9-2 forms complexes with several Gα subunits after MOR activation, some of these complexes are only formed after activation of the receptor by particular ligands. Specifically, complexes between RGS9-2 and Gαi3 are only observed after morphine administration. Fentanyl and methadone promote the formation of complexes containing RGS9-2 and Gαq but not Gβ5. These are likely to be less stable complexes because they do not contain Gβ5, an important determinant of RGS9-2 stability (Sondek and Siderovski, 2001). Fentanyl activation also leads to formation of complexes between RGS9-2 and PLCβ3. Increasing evidence implicates the PLCβ pathway in morphine analgesia and dependence. Behavioral analysis of PLCβ3 knock-out mice indicates that this protein acts as a negative modulator of opiate actions (Xie et al., 1999), whereas studies in guinea pig myenteric plexus tissue show adaptations in the phosphorylation of PLCβ1 and PLCβ3 isoforms to chronic morphine (Chakrabarti et al., 2003). It is thus possible that RGS9-2 acts as an effector antagonist for Gαq because loss of the RGS9 gene leads to increased PLCβ3 phosphorylation after fentanyl administration. This hypothesis is in accordance with previous studies (Hepler et al., 1997) that have demonstrated that RGS proteins may act as effector antagonists for Gα subunits. The observed increase in PLCβ3 activity correlates with reduced analgesic response to fentanyl in the hotplate test.
Numerous reports document differences in the analgesic potency, duration of action, abuse potential, and rate of tolerance development between MOR agonists used as analgesics (Shen et al., 2000; Koch et al., 2005; Narita et al., 2006). Although morphine is a potent analgesic for several types of chronic pain, a major limit for its long-term use has to do with the development of analgesic tolerance, an effect that is observed much earlier than other opiate analgesics (Bohn et al., 1999; Inturrisi, 2002). This early tolerance development is thought to be a result of adaptive changes in MOR signaling and desensitization. Most of the information on MOR signal transduction derives from in vitro studies. Clearly, analgesia and tolerance involve complex mechanisms and several brain networks. The striatum is not typically thought to be involved in pain perception or analgesia, and most studies focus on other CNS regions, such as the dorsal horn of the spinal cord, the periaqueductal gray, and the thalamus. Recent evidence supports a role of the NAc in chronic pain responses (Baliki et al., 2010). Our previous work (Zachariou et al., 2006; Han et al., 2010) demonstrated that molecular adaptations in the NAc affect morphine analgesia and tolerance. We focused on RGS9-2 complexes in the striatum because of our interest on tolerance mechanisms in brain regions mediating addiction. We hypothesized that striatal proteins that dynamically modulate morphine reward and dependence also modulate opiate analgesic responsiveness and the development of tolerance. Our next aim is to investigate RGS9-2 interactions in more complex behaviors, such as locomotor sensitization to MOR agonists.
Genetic mouse models have provided an important tool for the identification of key molecules implicated in opiate analgesia and tolerance (Bohn et al., 1999; Nestler, 2001; Chakrabarti et al., 2003; Charlton et al., 2008). Because the function of several signal transduction proteins is highly determined by the complexes they form, it is essential to understand the adaptive changes in the composition of such complexes in different brain regions. Our studies suggest that, after chronic morphine treatment, the strong association between RGS9-2, Gαq, Gβ5, and MOR in striatal neurons prevents the actions of several molecules involved in signal transduction and receptor desensitization and contributes to the development of analgesic tolerance. These complexes containing Gβ5 would be expected to be particularly stable and thereby have a more prominent effect on receptor responsiveness (Sondek and Siderovski, 2001; Cheever et al., 2008). Although RGS9-2 is expressed in much lower levels outside the striatum, it is possible that the complexes we describe in this study modulate opiate analgesia in other brain regions as well.
Because several in vitro studies report that opioid receptors form heterodimers (Rozenfeld and Devi, 2007), the possibility that adaptive responses in signal transduction events after MOR activation involve receptor dimerization should also be considered. It is possible that the different RGS9-2/Gα subunit complexes formed after morphine or fentanyl administration or the changes in signaling complexes formed after chronic morphine result from the formation of MOR heterodimers with δ-opioid receptors (DORs) or other GPCRs. A number of recent studies implicate MOR/DOR heterodimers in opiate actions (Xie et al., 2009; Gupta et al., 2010). Although preliminary studies with the RGS9KO line show no involvement of RGS9-2 in behavioral responses to DOR agonists, we cannot exclude the possibility that RGS9-2 modulates MOR/DOR dimers in the striatum. Future studies will explore this possibility. The differential association between RGS9-2 and Gα subunits may also be explained by the existence of more than one μ-opioid receptor subtypes. There is pharmacological evidence for multiple MOR splice variants that function via distinct signaling mechanisms (Pan et al., 2005), but their expression and function in striatum has not been investigated. Future studies aim to explore the exact cell types mediating the diverse effects of opioids in striatum and to further understand the receptor and protein–protein interactions involved in acute and chronic opiate actions in this brain region.
Our biochemical and behavioral data suggest that administration of a low fentanyl dose 30 min before morphine treatment prevents the formation of RGS9-2/Gαq/Gβ5 complexes and delays the development of analgesic tolerance. Several reports in the past suggested that coadministration of morphine with other opioids prevents cellular events associated with tolerance. Because delayed MOR internalization/recycling leads to the development of morphine tolerance, administration of DOR agonists along with morphine in rodents prevents tolerance by promoting β-arrestin-2 function and MOR internalization (Whistler and von Zastrow, 1998). In accord with these studies, our results suggest that MOR responsiveness is highly affected by the RGS9-2/Gα subunit interactions, and interventions in these interactions may improve the function of several signal transduction molecules, including β-arrestin-2. Recent studies indicate that targeting RGS9-2 complexes may provide a novel approach toward the improvement of drug responses or prevention of undesired drug actions. Recent studies in primates demonstrated that overexpressing RGS9-2 in striatum may prevent some of the undesired effects of levodopa, such as dyskinesia, by limiting dopamine D2 receptor signaling (Gold et al., 2007). In the same line, the present findings demonstrate that interventions in the formation of RGS9-2 complexes comprise an efficient strategy to prevent a side effect of morphine (analgesic tolerance) without affecting the therapeutic effect (analgesia).
In summary, we investigated differences in the composition of RGS9-2-containing complexes formed during acute or chronic MOR activation by various ligands and demonstrated that RGS9-2 may act as a positive or negative regulator of opiate analgesic actions. Using information from our coimmunoprecipitation assays, we developed simple pharmacological treatments to prevent the formation of stable RGS9-2 complexes that inhibit MOR signaling. Thus, our study proposes drug administration strategies that target RGS9-2 and can be immediately applied to delay the development of morphine tolerance.
Footnotes
This work was supported by Greek General Secretariat for Research and Technology Grant PENED03-860.
References
- Arvidsson U, Riedl M, Chakrabarti S, Lee JH, Nakano AH, Dado RJ, Loh HH, Law PY, Wessendorf MW, Elde R. Distribution and targeting of a mu-opioid receptor (MOR1) in brain and spinal cord. J Neurosci. 1995;15:3328–3341. doi: 10.1523/JNEUROSCI.15-05-03328.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baliki MN, Geha PY, Fields HL, Apkarian AV. Predicting value of pain and analgesia: nucleus accumbens response to noxious stimuli changes in the presence of chronic pain. Neuron. 2010;66:149–160. doi: 10.1016/j.neuron.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballon DR, Flanary PL, Gladue DP, Konopka JB, Dohlman HG, Thorner J. DEP-domain-mediated regulation of GPCR signaling responses. Cell. 2006;126:1079–1093. doi: 10.1016/j.cell.2006.07.030. [DOI] [PubMed] [Google Scholar]
- Berman DM, Gilman AG. Mammalian RGS proteins: barbarians at the gates. J Biol Chem. 1998;273:1269–1272. doi: 10.1074/jbc.273.3.1269. [DOI] [PubMed] [Google Scholar]
- Bohn LM, Lefkowitz RJ, Gainetdinov RR, Peppel K, Caron MG, Lin FT. Enhanced morphine analgesia in mice lacking β-arrestin 2. Science. 1999;286:2495–2498. doi: 10.1126/science.286.5449.2495. [DOI] [PubMed] [Google Scholar]
- Cabrera-Vera TM, Hernandez S, Earls LR, Medkova M, Sundgren-Andersson AK, Surmeier DJ, Hamm HE. RGS9-2 modulates D2 dopamine receptor-mediated Ca2+ channel inhibition in rat striatal cholinergic interneurons. Proc Natl Acad Sci U S A. 2004;101:16339–16344. doi: 10.1073/pnas.0407416101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti S, Liu NJ, Gintzler AR. Reciprocal modulation of phospholipase C beta isoforms: adaptation to chronic morphine. Proc Natl Acad Sci U S A. 2003;100:13686–13691. doi: 10.1073/pnas.2335885100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlton JJ, Allen PB, Psifogeorgou K, Chakravarty S, Gomes I, Neve RL, Devi LA, Greengard P, Nestler EJ, Zachariou V. Multiple actions of spinophilin modulate mu opioid receptor function. Neuron. 2008;58:238–247. doi: 10.1016/j.neuron.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheever ML, Snyder JT, Gershburg S, Siderovski DP, Harden TK, Sondek J. Crystal structure of the multifunctional RGS9-2-Gb5 complex. Nat Struct Mol Biol. 2008;15:155–162. doi: 10.1038/nsmb.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CK, Eversole-Cire P, Zhang H, Mancino V, Chen YJ, He W, Wensel TG, Simon MI. Instability of GGL domain containing RGS proteins in mice lacking the G protein beta subunit Gβ5. Proc Natl Acad Sci U S A. 2003;100:6604–6609. doi: 10.1073/pnas.0631825100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contet C, Kieffer BL, Befort K. Mu opioid receptor: a gateway to drug addiction. Curr Opin Neurobiol. 2004;14:370–378. doi: 10.1016/j.conb.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Dohlman HG, Thorner J. RGS proteins and signaling by heterotrimeric G proteins. J Biol Chem. 1997;272:3871–3874. doi: 10.1074/jbc.272.7.3871. [DOI] [PubMed] [Google Scholar]
- Eitan S, Bryant CD, Saliminejad N, Yang YC, Vojdani E, Keith D, Jr, Polakiewicz R, Evans CJ. Brain region-specific mechanisms for acute morphinmitogen-activated protein kinase modulation and distinct patterns of activation during analgesic tolerance and locomotor sensitization. J Neurosci. 2003;23:8360–8369. doi: 10.1523/JNEUROSCI.23-23-08360.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold SJ, Ni YG, Dohlman HG, Nestler EJ. Regulators of G-protein signaling (RGS) proteins: region-specific expression of nine subtypes in rat brain. J Neurosci. 1997;17:8024–8037. doi: 10.1523/JNEUROSCI.17-20-08024.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold SJ, Hoang CV, Potts BW, Porras G, Pioli E, Kim KW, Nadjar A, Qin C, LaHoste GJ, Li Q, Bioulac BH, Waugh JL, Gurevich E, Neve RL, Bezard E. RGS9-2 negatively modulates l-3,4-dihydroxyphenylalanine-induced dyskinesia in experimental Parkinson's disease. J Neurosci. 2007;27:14338–14348. doi: 10.1523/JNEUROSCI.4223-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A, Mulder J, Gomes I, Rozenfeld R, Bushlin I, Ong E, Lim M, Maillet E, Junek M, Cahill CM, Harkany T, Devi LA. Increased abundance of opioid receptor heteromers after chronic morphine administration. Sci Signal. 2010;3:ra54. doi: 10.1126/scisignal.2000807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberstock-Debic H, Kim KA, Yu YJ, von Zastrow M. Morphine promotes rapid, arrestin-dependent endocytosis of μ-opioid receptors in striatal neurons. J Neurosci. 2005;25:7847–7857. doi: 10.1523/JNEUROSCI.5045-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han MH, Renthal W, Ring RH, Rahman Z, Psifogeorgou K, Howland D, Birnbaum S, Young K, Neve R, Nestler EJ, Zachariou V. Brain region specific actions of regulator of G protein signaling 4 oppose morphine reward and dependence but promote analgesia. Biol Psychiatry. 2010;67:761–769. doi: 10.1016/j.biopsych.2009.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He W, Lu L, Zhang X, El-Hodiri HM, Chen CK, Slep KC, Simon MI, Jamrich M, Wensel TG. Modules in the photoreceptor RGS9–1.Gbeta 5L GTPase-accelerating protein complex control effector coupling, GTPase acceleration, protein folding, and stability. J Biol Chem. 2000;275:37093–37100. doi: 10.1074/jbc.M006982200. [DOI] [PubMed] [Google Scholar]
- Hepler JR, Berman DM, Gilman AG, Kozasa T. RGS and GAIP are GTPase activating proteins for Gq alpha and block activation of phospholipase C beta by gamma-thio-GTP-Gq-alpha. Proc Natl Acad Sci U S A. 1997;94:428–432. doi: 10.1073/pnas.94.2.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Hepler JR, Gilman AG, Mumby SM. Attenuation of Gi- and Gq-mediated signaling by expression of RGS4 or GAIP in mammalian cells. Proc Natl Acad Sci U S A. 1997;94:6159–6163. doi: 10.1073/pnas.94.12.6159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inturrisi CE. Clinical pharmacology of opioids for pain. Clin J Pain. 2002;18(Suppl 4):S3–13. doi: 10.1097/00002508-200207001-00002. [DOI] [PubMed] [Google Scholar]
- Jayaraman M, Zhou H, Jia L, Cain MD, Blumer KJ. R9AP and R7BP: traffic cops for the RGS7 family in phototransduction and neuronal GPCR signaling. Trends Pharmacol Sci. 2009;30:17–24. doi: 10.1016/j.tips.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J, Kittanakom S, Wong V, Reyes BA, Van Bockstaele EJ, Stagljar I, Berrettini W, Levenson R. Interaction of the mu-opioid receptor with GPR177 (Wntless) inhibits Wnt secretion: potential implications for opioid dependence. BMC Neurosci. 2010;11:33–37. doi: 10.1186/1471-2202-11-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch T, Widera A, Bartzsch K, Schulz S, Brandenburg LO, Wundrack N, Beyer A, Grecksch G, Höllt V. Receptor endocytosis counteracts the development of opioid tolerance. Mol Pharmacol. 2005;67:280–287. doi: 10.1124/mol.104.004994. [DOI] [PubMed] [Google Scholar]
- Kovoor A, Seyffarth P, Ebert J, Barghshoon S, Chen CK, Schwarz S, Axelrod JD, Cheyette BN, Simon MI, Lester HA, Schwarz J. D2 dopamine receptors colocalize regulator of G protein signalling 9-2 via the RGS9 DEP domain, and RGS9 knockout mice develop dyskinesias associated with dopamine pathways. J Neurosci. 2005;25:2157–2165. doi: 10.1523/JNEUROSCI.2840-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreek MJ. Drug addictions. Molecular and cellular endpoints. Ann NY Acad Sci. 2001;937:27–49. [PubMed] [Google Scholar]
- Martemyanov KA, Lishko PV, Calero N, Keresztes G, Sokolov M, Strissel KJ, Leskov IB, Hopp JA, Kolesnikov AV, Chen CK, Lem J, Heller S, Burns ME, Arshavsky VY. The DEP domain determines subcellular targeting of the GTPase activating protein RGS9 in vivo. J Neurosci. 2003;23:10175–10181. doi: 10.1523/JNEUROSCI.23-32-10175.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martemyanov KA, Yoo PJ, Skiba NP, Arshavsky VY. R7BP, a novel neuronal protein interacting with RGS proteins and the R7 subfamily. J Biol Chem. 2005;280:5133–5136. doi: 10.1074/jbc.C400596200. [DOI] [PubMed] [Google Scholar]
- Muller DL, Unterwald EM. In vivo regulation of extracellular signal-regulated protein kinase (ERK) and protein kinase B (Akt) phosphorylation by acute and chronic morphine. J Pharmacol Exp Ther. 2004;310:774–782. doi: 10.1124/jpet.104.066548. [DOI] [PubMed] [Google Scholar]
- Mumby SM, Gilman AG. Synthetic peptide antisera with determined specificity for G protein alpha or beta subunits. Methods Enzymol. 1991;195:215–233. doi: 10.1016/0076-6879(91)95168-j. [DOI] [PubMed] [Google Scholar]
- Mundell SJ, Loudon RP, Benovic JL. Characterization of G protein-coupled receptor regulation in antisense mRNA-expressing cells with reduced arrestin levels. Biochemistry. 1999;38:8723–8732. doi: 10.1021/bi990361v. [DOI] [PubMed] [Google Scholar]
- Narita M, Suzuki M, Narita M, Niikura K, Nakamura A, Miyatake M, Yajima Y, Suzuki T. mu-Opioid receptor internalization-dependent and -independent mechanisms of the development of tolerance to mu-opioid receptor agonists: comparison between etorphine and morphine. Neuroscience. 2006;138:609–619. doi: 10.1016/j.neuroscience.2005.11.046. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Molecular basis of long-term underlying addiction. Nat Rev Neurosci. 2001;2:119–128. doi: 10.1038/35053570. [DOI] [PubMed] [Google Scholar]
- Pan YX, Xu J, Bolan E, Moskowitz HS, Xu M, Pasternak GW. Identification of four novel exon 5 splice variants of the mouse mu-opioid receptor gene: functional consequences of C-terminal splicing. Mol Pharmacol. 2005;68:866–875. doi: 10.1124/mol.105.011858. [DOI] [PubMed] [Google Scholar]
- Piros ET, Hales TG, Evans CJ. Functional analysis of cloned opioid receptors in transfected cell lines. Neurochem Res. 1996;21:1277–1285. doi: 10.1007/BF02532368. [DOI] [PubMed] [Google Scholar]
- Posner BA, Mukhopadhyay S, Tesmer JJ, Gilman AG, Ross EM. Modulation of the affinity and selectivity of RGS protein interaction with G alpha subunits by a conserved asparagine/serine residue. Biochemistry. 1999;38:7773–7779. doi: 10.1021/bi9906367. [DOI] [PubMed] [Google Scholar]
- Psifogeorgou K, Papakosta P, Russo SJ, Neve RL, Kardassis D, Gold SJ, Zachariou V. RGS9-2 is a negative modulator of mu opioid receptor function. J Neurochem. 2007;103:617–625. doi: 10.1111/j.1471-4159.2007.04812.x. [DOI] [PubMed] [Google Scholar]
- Rahman Z, Gold SJ, Potenza MN, Cowan CW, Ni YG, He W, Wensel TG, Nestler EJ. Cloning and characterization of RGS9-2, a striatal enriched alternatively spliced product of the RGS9 gene. J Neurosci. 1999;19:2016–2026. doi: 10.1523/JNEUROSCI.19-06-02016.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman Z, Schwarz J, Gold SJ, Zachariou V, Wein MN, Choi KH, Kovoor A, Chen CK, DiLeone RJ, Schwarz SC, Selley DE, Sim-Selley LJ, Barrot M, Luedtke RR, Self D, Neve RL, Lester HA, Simon MI, Nestler EJ. RGS9 modulates dopamine signaling in the basal ganglia. Neuron. 2003;38:941–952. doi: 10.1016/s0896-6273(03)00321-0. [DOI] [PubMed] [Google Scholar]
- Rozenfeld R, Devi LA. Receptor heterodimerization leads to a switch in signaling: beta-arrestin2-mediated ERK activation by mu-delta opioid receptor heterodimers. FASEB J. 2007;21:2455–2465. doi: 10.1096/fj.06-7793com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Benedict Gomes A, Gallagher A, Stafford K, Yoburn BC. Role of cAMP-dependent protein kinase (PKA) in opioid agonist-induced mu-opioid receptor downregulation and tolerance in mice. Synapse. 2000;38:322–327. doi: 10.1002/1098-2396(20001201)38:3<322::AID-SYN11>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Sondek J, Siderovski DP. G-γ like domains: new frontiers in G protein signalling and β-propeller scaffolding. Biochem Pharmacol. 2001;61:1329–1337. doi: 10.1016/s0006-2952(01)00633-5. [DOI] [PubMed] [Google Scholar]
- Terzi D, Stergiou E, King SL, Zachariou V. RGS proteins in neuropsychiatric disorders. Prog Mol Biol Translational Sci Vol. 2009;86C:299–333. doi: 10.1016/S1877-1173(09)86010-9. [DOI] [PubMed] [Google Scholar]
- Tesmer VM, Kawano T, Shankaranarayanan A, Kozasa T, Tesmer JJ. Snapshot of activated G proteins at the membrane: the Galphaq-GRK2-Gbetagamma complex. Science. 2005;310:1686–1690. doi: 10.1126/science.1118890. [DOI] [PubMed] [Google Scholar]
- Traynor JR, Neubig RR. Regulators of G protein signaling and drugs of abuse. Mol Interv. 2005;5:30–41. doi: 10.1124/mi.5.1.7. [DOI] [PubMed] [Google Scholar]
- Traynor JR, Terzi D, Caldarone BJ, Zachariou V. RGS9-2: probing an intracellular modulator of behaviour as a drug target. Trends Pharmacol Sci. 2009;30:105–111. doi: 10.1016/j.tips.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whistler JL, von Zastrow M. Morphine-activated opioid receptors elude desensitization by beta-arrestin. Proc Natl Acad Sci U S A. 1998;95:9914–9919. doi: 10.1073/pnas.95.17.9914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witherow DS, Tovey SC, Wang Q, Willars GB, Slepak VZ. G beta 5.RGS7 inhibits Galpha q-mediated signaling via a direct protein-protein interaction. J Biol Chem. 2003;278:21307–21313. doi: 10.1074/jbc.M212884200. [DOI] [PubMed] [Google Scholar]
- Xie W, Samoriski GM, McLaughlin JP, Romoser VA, Smrcka A, Hinkle PM, Bidlack JM, Gross RA, Jiang H, Wu D. Genetic alterations in phospholipase C beta3 expression modulates behavioral and cellular responses to opioids. Proc Natl Acad Sci U S A. 1999;96:10385–10390. doi: 10.1073/pnas.96.18.10385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie WY, He Y, Yang YR, Li YF, Kang K, Xing BM, Wang Y. Disruption of Cdk5 associated phosphorylation of residue threonine-161 of the δ-opioid receptor: impaired receptor function and attenuated morphine antinociceptive tolerance. J Neurosci. 2009;29:3551–35564. doi: 10.1523/JNEUROSCI.0415-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachariou V, Georgescu D, Sanchez N, Rahman Z, DiLeone R, Berton O, Neve RL, Sim-Selley LJ, Selley DE, Gold SJ, Nestler EJ. Essential role for RGS9 in opiate action. Proc Natl Acad Sci. 2003;100:13656–13661. doi: 10.1073/pnas.2232594100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachariou V, Bolanos CA, Selley DE, Theobald D, Cassidy MP, Kelz MB, Shaw-Lutchman T, Berton O, Sim-Selley LJ, Dileone RJ, Kumar A, Nestler EJ. An essential role for DeltaFosB in the nucleus accumbens in morphine action. Nat Neurosci. 2006;9:205–211. doi: 10.1038/nn1636. [DOI] [PubMed] [Google Scholar]