Abstract
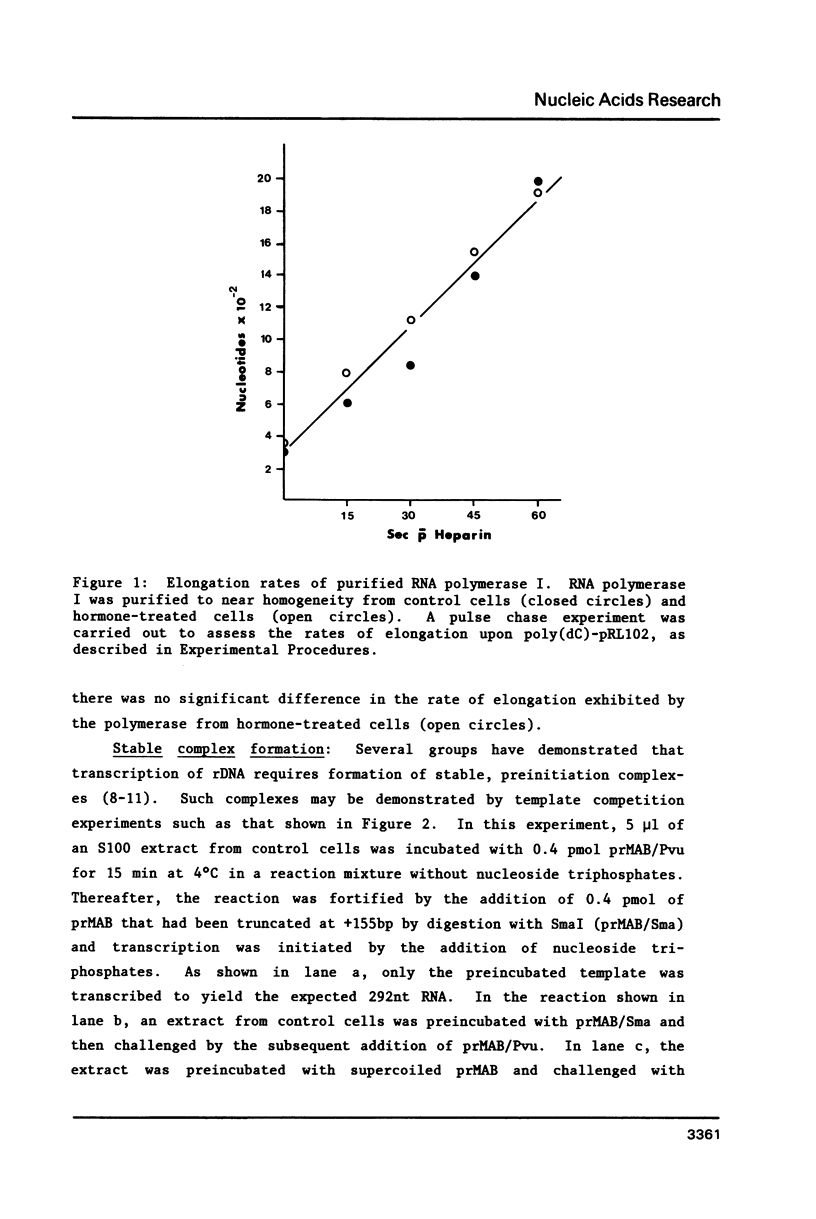
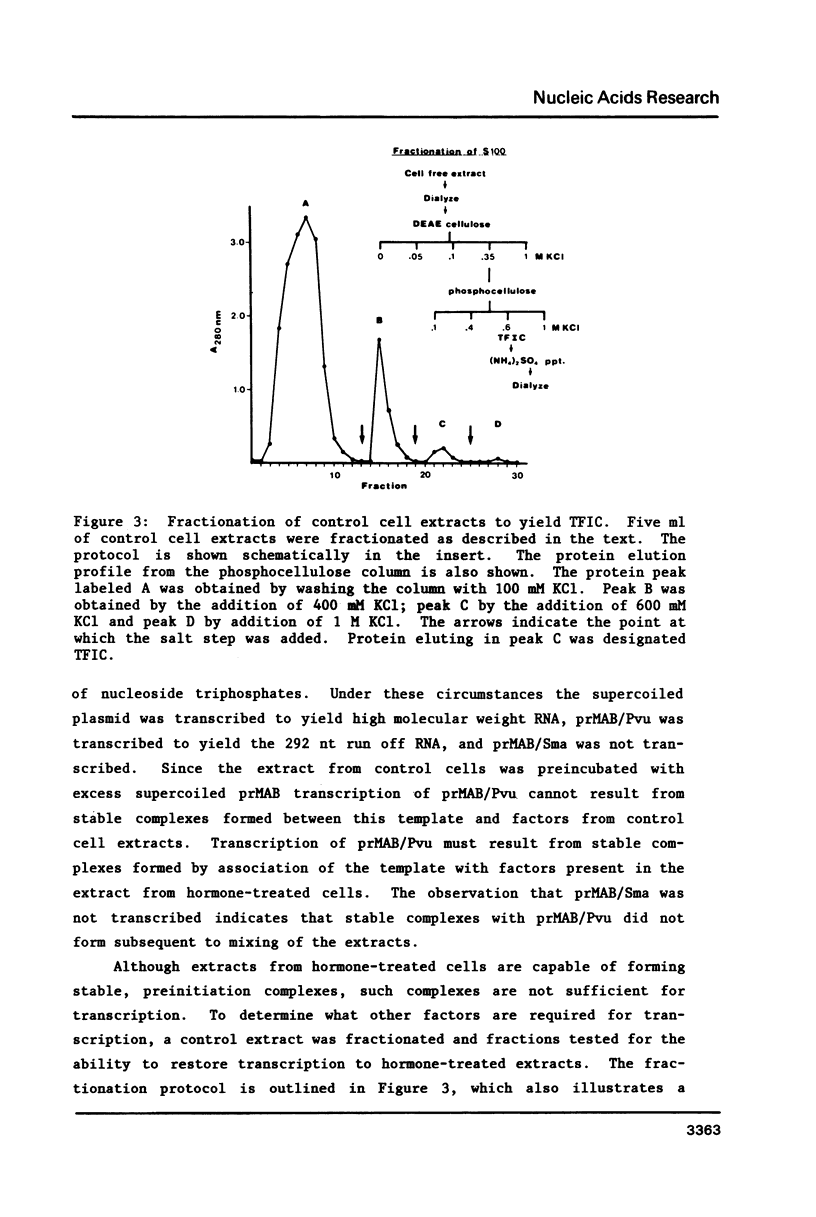
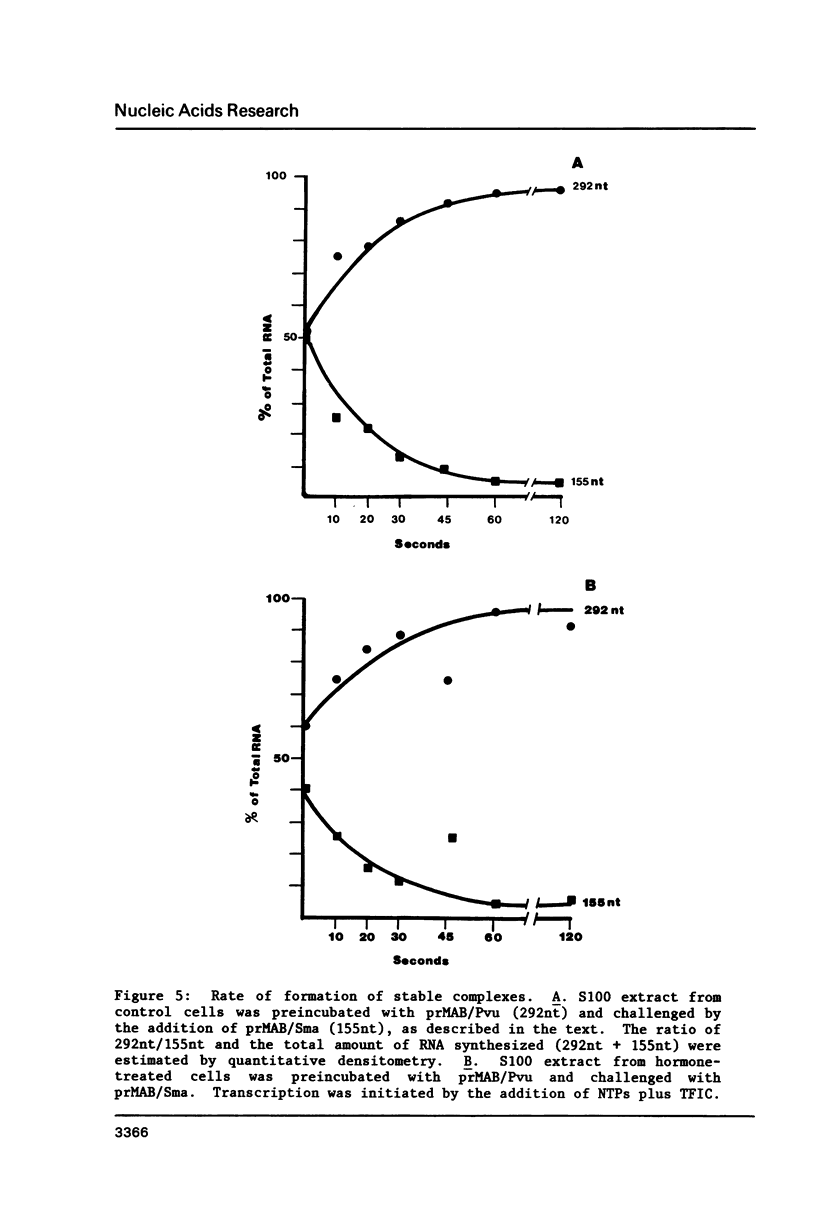
Various parameters of transcription of cloned mouse rDNA have been examined using extracts from control P1798 cells and from cells treated 24 h with 0.1 microM dexamethasone. Highly purified RNA polymerase I from either source catalyzes nucleotidyl transfer (elongation) at a rate of approximately 30 nucleotides/sec. Extracts from hormone-treated cells are capable of forming stable, preinitiation complexes. The rates of stable complex formation are the same in extracts from control and hormone-treated cells. Nevertheless, initiation of transcription does not occur in extracts from hormone-treated cells. Initiation in such extracts may be restored by the addition of a partially purified RNA polymerase I initiation factor, designated TFIC. The data indicate that initiation by RNA polymerase I is a multi-step process. The first step involves the formation of stable, preinitiation complexes, as demonstrated by a number of groups. Initiation, per se, requires an additional protein, TFIC. Glucocorticoids and perhaps other mitogenic agents regulate transcription of rDNA by influencing the amount or activity of TFIC.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cavanaugh A. H., Gokal P. K., Lawther R. P., Thompson E. A., Jr Glucocorticoid inhibition of initiation of transcription of the DNA encoding rRNA (rDNA) in lymphosarcoma P1798 cells. Proc Natl Acad Sci U S A. 1984 Feb;81(3):718–721. doi: 10.1073/pnas.81.3.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavannaugh A. H., Thompson E. A., Jr Hormonal regulation of transcription of rDNA. Inhibition of transcription during glucocorticoid-mediated inhibition of proliferation of lymphosarcoma P1798 cells in culture. J Biol Chem. 1983 Aug 25;258(16):9768–9773. [PubMed] [Google Scholar]
- Cizewski V., Sollner-Webb B. A stable transcription complex directs mouse ribosomal RNA synthesis by RNA polymerase I. Nucleic Acids Res. 1983 Oct 25;11(20):7043–7056. doi: 10.1093/nar/11.20.7043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duceman B. W., Jacob S. T. Transcriptionally active RNA polymerases from Morris hepatomas and rat liver. Elucidation of the mechanism for the preferential increase in the tumour RNA polymerase I. Biochem J. 1980 Sep 15;190(3):781–789. doi: 10.1042/bj1900781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duceman B. W., Rose K. M., Jacob S. T. Activation of purified hepatoma RNA polymerase I by homologous protein kinase NII. J Biol Chem. 1981 Nov 10;256(21):10755–10758. [PubMed] [Google Scholar]
- Franze-Fernández M. T., Pogo A. O. Regulation of the nucleolar DNA-dependent RNA polymerase by amino acids in Ehrlich ascites tumor cells. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3040–3044. doi: 10.1073/pnas.68.12.3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grummt F., Paul D., Grummt I. Regulation of ATP pools, rRNA and DNA synthesis in 3T3 cells in response to serum or hypoxanthine. Eur J Biochem. 1977 Jun 1;76(1):7–12. doi: 10.1111/j.1432-1033.1977.tb11564.x. [DOI] [PubMed] [Google Scholar]
- Grummt I. Nucleotide sequence requirements for specific initiation of transcription by RNA polymerase I. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6908–6911. doi: 10.1073/pnas.79.22.6908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grummt I., Smith V. A., Grummt F. Amino acid starvation affects the initiation frequency of nucleolar RNA polymerase. Cell. 1976 Mar;7(3):439–445. doi: 10.1016/0092-8674(76)90174-4. [DOI] [PubMed] [Google Scholar]
- Kadesch T. R., Chamberlin M. J. Studies of in vitro transcription by calf thymus RNA polymerase II using a novel duplex DNA template. J Biol Chem. 1982 May 10;257(9):5286–5295. [PubMed] [Google Scholar]
- Lawther R. P., Nichols B., Zurawski G., Hatfield G. W. The nucleotide sequence preceding and including the beginning of the ilvE gene of the ilvGEDA operon of Escherichia coli K12. Nucleic Acids Res. 1979 Dec 20;7(8):2289–2301. doi: 10.1093/nar/7.8.2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miesfeld R., Arnheim N. Species-specific rDNA transcription is due to promoter-specific binding factors. Mol Cell Biol. 1984 Feb;4(2):221–227. doi: 10.1128/mcb.4.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miesfeld R., Arnheim N. Species-specific rDNA transcription is due to promoter-specific binding factors. Mol Cell Biol. 1984 Feb;4(2):221–227. doi: 10.1128/mcb.4.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishima Y., Financsek I., Kominami R., Muramatsu M. Fractionation and reconstitution of factors required for accurate transcription of mammalian ribosomal RNA genes: identification of a species-dependent initiation factor. Nucleic Acids Res. 1982 Nov 11;10(21):6659–6670. doi: 10.1093/nar/10.21.6659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz L. B., Roeder R. G. Purification and subunit structure of deoxyribonucleic acid-dependent ribonucleic acid polymerase I from the mouse myeloma, MOPC 315. J Biol Chem. 1974 Sep 25;249(18):5898–5906. [PubMed] [Google Scholar]
- Thompson E. A., Jr Properties of a cell-culture line derived from lymphosarcoma P1798. Mol Cell Endocrinol. 1980 Feb;17(2):95–102. doi: 10.1016/0303-7207(80)90121-5. [DOI] [PubMed] [Google Scholar]
- Wandelt C., Grummt I. Formation of stable preinitiation complexes is a prerequisite for ribosomal DNA transcription in vitro. Nucleic Acids Res. 1983 Jun 11;11(11):3795–3809. doi: 10.1093/nar/11.11.3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z. Y., Thompson E. A., Stallcup M. R. Hormonal regulation of transcription of rDNA: use of nucleoside thiotriphosphates to measure initiation in isolated nuclei. Nucleic Acids Res. 1984 Nov 12;12(21):8115–8128. doi: 10.1093/nar/12.21.8115. [DOI] [PMC free article] [PubMed] [Google Scholar]




