Abstract
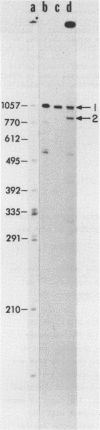
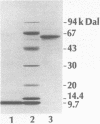
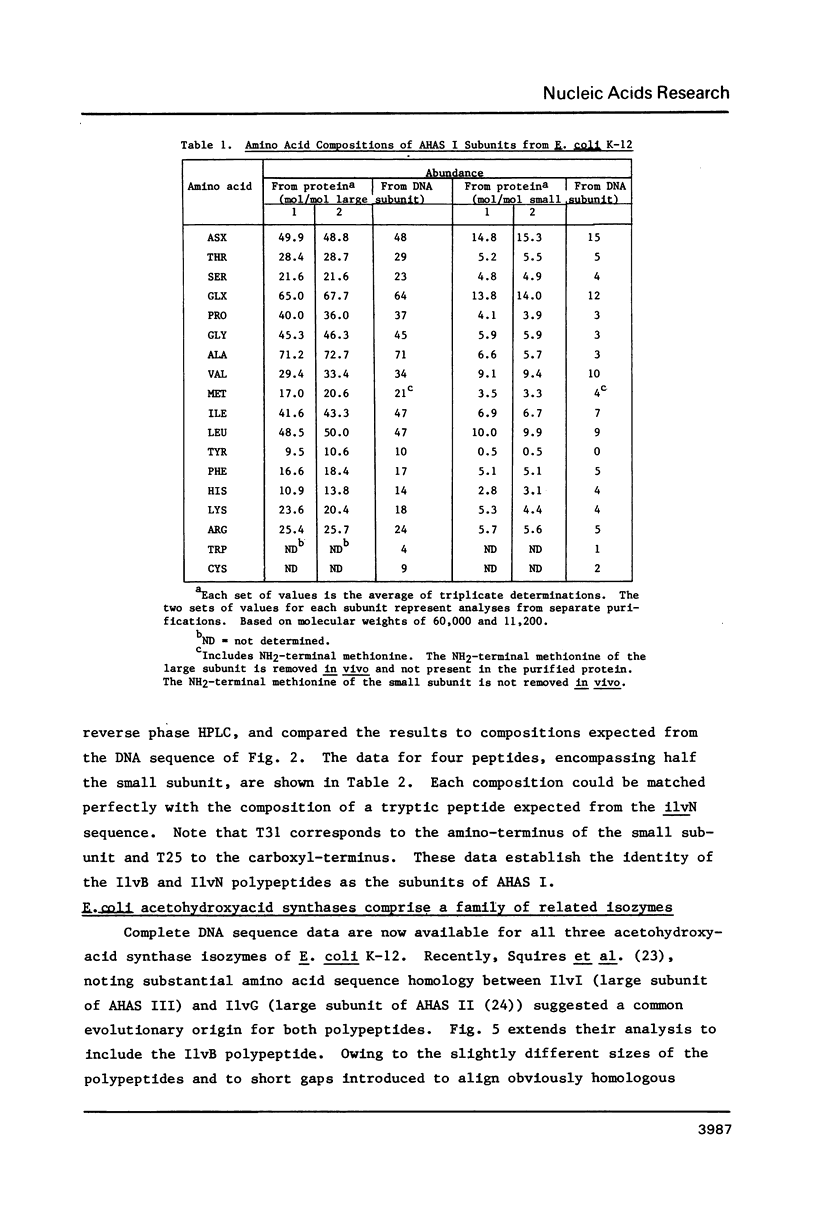
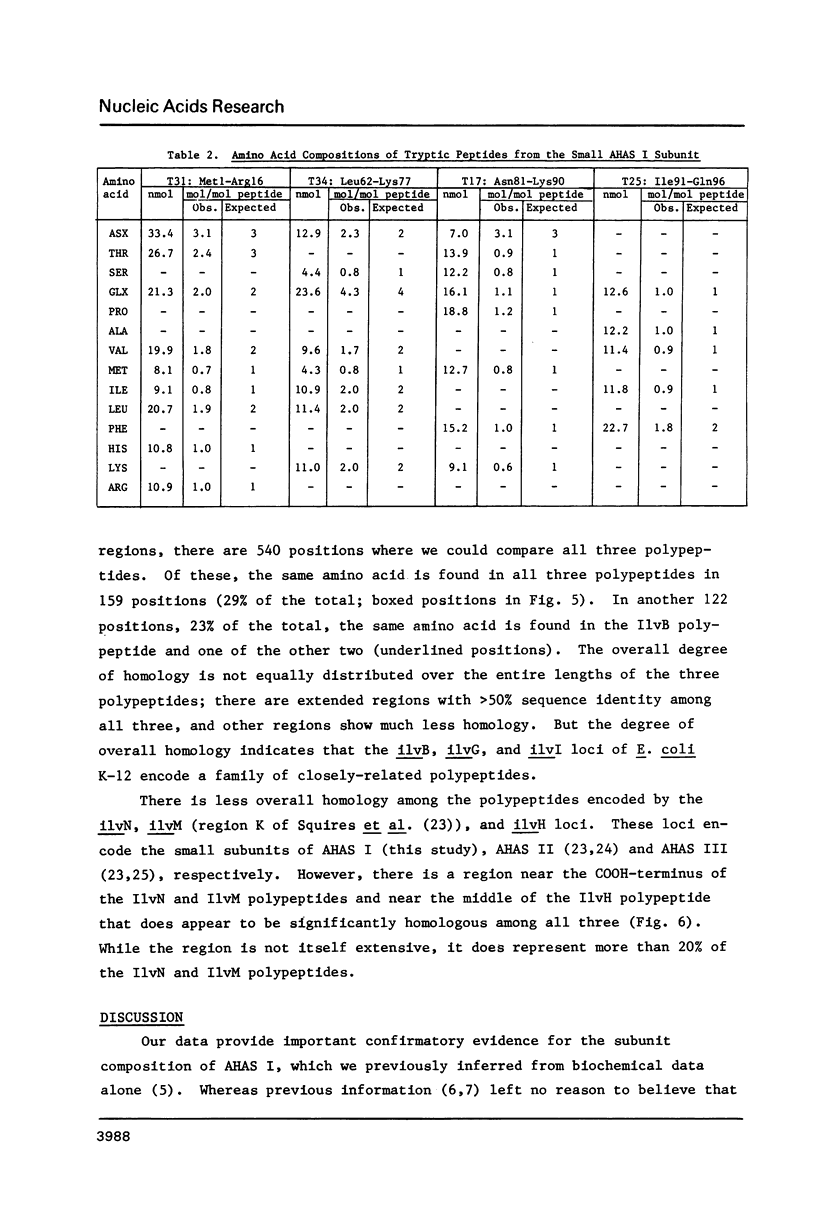
The ilvB locus of Escherichia coli K-12 encloses two open reading frames defining polypeptides of 60,000 and 11,200 molecular weight. The entire locus, about 2.3 kb, is co-transcribed as an operon. The molecular weights and amino acid compositions of the presumptive operon polypeptides agree with those of the large and small subunit polypeptides of acetohydroxyacid synthase (AHAS) I, for which ilvB is the structural locus. We reserve the designation ilvB for the promoter proximal (longer) cistron and designate the promoter distal cistron ilvN. The molecular weight and amino acid sequence of the ilvB polypeptide are strikingly similar to those of the I1vI (larger subunit of AHAS III) and I1vG (larger subunit of AHAS II) polypeptides. There is less size uniformity among the I1vN, I1vH (smaller subunit of AHAS III), and I1vM (smaller subunit of AHAS II) polypeptides. Nevertheless, there is significant amino acid sequence homology among the three small subunit polypeptides. Thus, all three AHAS isozymes of E. coli K-12 probably have a common evolutionary origin.
Full text
PDF





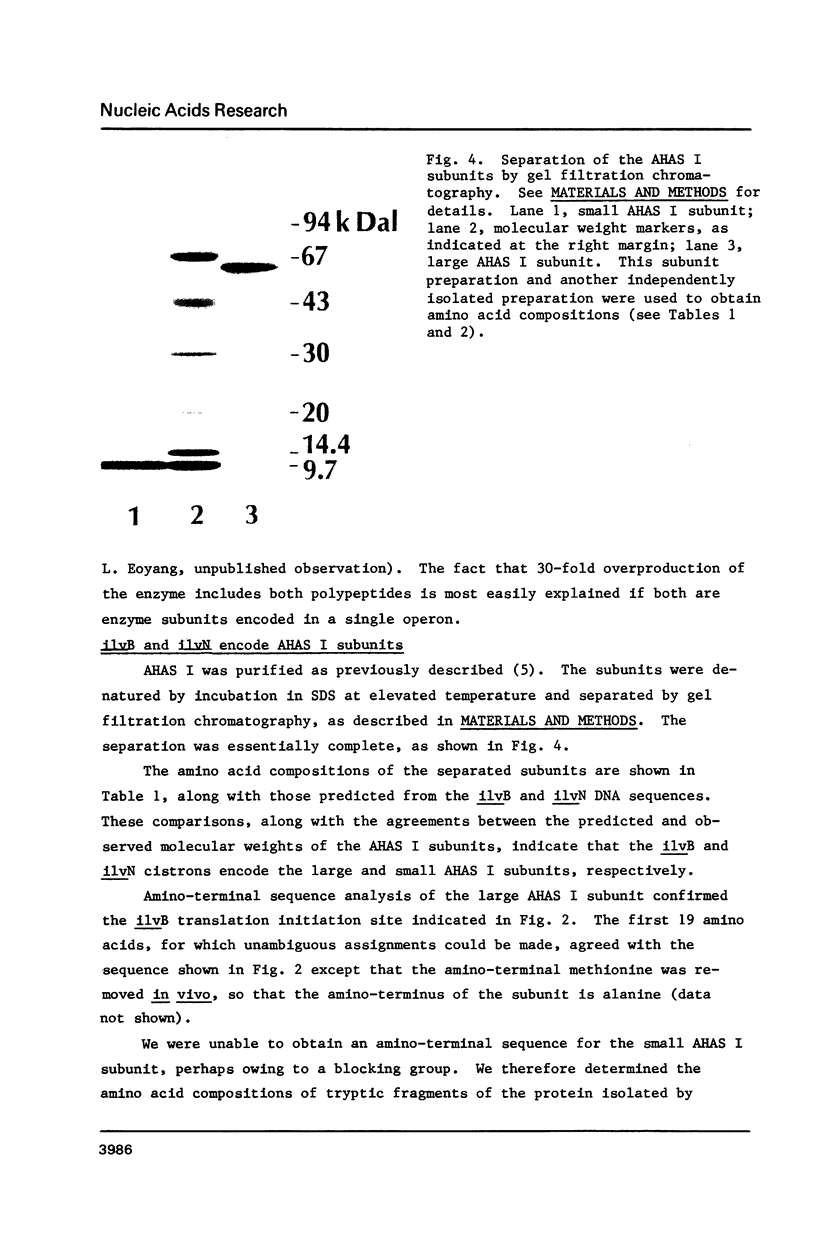





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arfin S. M., Koziell D. A. Acetolactate synthase of Pseudomonas aeruginosa. II. Evidence for the presence of two nonidentical subunits. Biochim Biophys Acta. 1973 Sep 15;321(1):356–360. doi: 10.1016/0005-2744(73)90090-9. [DOI] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Botsford J. L. Cyclic nucleotides in procaryotes. Microbiol Rev. 1981 Dec;45(4):620–642. doi: 10.1128/mr.45.4.620-642.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman J., Green P. J., Inouye M. The use of RNAs complementary to specific mRNAs to regulate the expression of individual bacterial genes. Cell. 1984 Jun;37(2):429–436. doi: 10.1016/0092-8674(84)90373-8. [DOI] [PubMed] [Google Scholar]
- De Felice M., Guardiola J., Esposito B., Iaccarino M. Structural genes for a newly recognized acetolactate synthase in Escherichia coli K-12. J Bacteriol. 1974 Dec;120(3):1068–1077. doi: 10.1128/jb.120.3.1068-1077.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eoyang L., Silverman P. M. Purification and subunit composition of acetohydroxyacid synthase I from Escherichia coli K-12. J Bacteriol. 1984 Jan;157(1):184–189. doi: 10.1128/jb.157.1.184-189.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favaloro J., Treisman R., Kamen R. Transcription maps of polyoma virus-specific RNA: analysis by two-dimensional nuclease S1 gel mapping. Methods Enzymol. 1980;65(1):718–749. doi: 10.1016/s0076-6879(80)65070-8. [DOI] [PubMed] [Google Scholar]
- Friden P., Newman T., Freundlich M. Nucleotide sequence of the ilvB promoter-regulatory region: a biosynthetic operon controlled by attenuation and cyclic AMP. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6156–6160. doi: 10.1073/pnas.79.20.6156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friden P., Tsui P., Okamoto K., Freundlich M. Interaction of cyclic AMP receptor protein with the ilvB biosynthetic operon in E. coli. Nucleic Acids Res. 1984 Nov 12;12(21):8145–8160. doi: 10.1093/nar/12.21.8145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimminger H., Umbarger H. E. Acetohydroxy acid synthase I of Escherichia coli: purification and properties. J Bacteriol. 1979 Feb;137(2):846–853. doi: 10.1128/jb.137.2.846-853.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guardiola J., De Felice M., Lamberti A., Iaccarino M. The acetolactate synthase isoenzymes of Escherichia coli K-12. Mol Gen Genet. 1977 Nov 4;156(1):17–25. doi: 10.1007/BF00272247. [DOI] [PubMed] [Google Scholar]
- Hauser C. A., Hatfield G. W. Attenuation of the ilvB operon by amino acids reflecting substrates or products of the ilvB gene product. Proc Natl Acad Sci U S A. 1984 Jan;81(1):76–79. doi: 10.1073/pnas.81.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser C. A., Hatfield G. W. Nucleotide sequence of the ilvB multivalent attenuator region of Escherichia coli K12. Nucleic Acids Res. 1983 Jan 11;11(1):127–139. doi: 10.1093/nar/11.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRossa R. A., Smulski D. R. ilvB-encoded acetolactate synthase is resistant to the herbicide sulfometuron methyl. J Bacteriol. 1984 Oct;160(1):391–394. doi: 10.1128/jb.160.1.391-394.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawther R. P., Calhoun D. H., Adams C. W., Hauser C. A., Gray J., Hatfield G. W. Molecular basis of valine resistance in Escherichia coli K-12. Proc Natl Acad Sci U S A. 1981 Feb;78(2):922–925. doi: 10.1073/pnas.78.2.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McEwen J., Silverman P. Mutations in genes cpxA and cpxB of Escherichia coli K-12 cause a defect in isoleucine and valine syntheses. J Bacteriol. 1980 Oct;144(1):68–73. doi: 10.1128/jb.144.1.68-73.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill B. M., Williams K. R., Chase J. W., Konigsberg W. H. Photochemical cross-linking of the Escherichia coli single-stranded DNA-binding protein to oligodeoxynucleotides. Identification of phenylalanine 60 as the site of cross-linking. J Biol Chem. 1984 Sep 10;259(17):10850–10856. [PubMed] [Google Scholar]
- Newman T. C., Levinthal M. A new map location for the ilvB locus of Escherichia coli. Genetics. 1980 Sep;96(1):59–77. doi: 10.1093/genetics/96.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman T., Friden P., Sutton A., Freundlich M. Cloning and expression of the ilvB gene of Escherichia coli K-12. Mol Gen Genet. 1982;186(3):378–384. doi: 10.1007/BF00729457. [DOI] [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Squires C. H., De Felice M., Devereux J., Calvo J. M. Molecular structure of ilvIH and its evolutionary relationship to ilvG in Escherichia coli K12. Nucleic Acids Res. 1983 Aug 11;11(15):5299–5313. doi: 10.1093/nar/11.15.5299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squires C. H., De Felice M., Lago C. T., Calvo J. M. IlvHI locus of Salmonella typhimurium. J Bacteriol. 1983 Jun;154(3):1054–1063. doi: 10.1128/jb.154.3.1054-1063.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton A., Freundlich M. Regulation of cyclic AMP of the ilvB-encoded biosynthetic acetohydroxy acid synthase in Escherichia coli K-12. Mol Gen Genet. 1980 Apr;178(1):179–183. doi: 10.1007/BF00267227. [DOI] [PubMed] [Google Scholar]
- Sutton A., Newman T., McEwen J., Silverman P. M., Freundlich M. Mutations in genes cpxA and cpxB of Escherichia coli K-12 cause a defect in acetohydroxyacid synthase I function in vivo. J Bacteriol. 1982 Aug;151(2):976–982. doi: 10.1128/jb.151.2.976-982.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umbarger H. E. Amino acid biosynthesis and its regulation. Annu Rev Biochem. 1978;47:532–606. doi: 10.1146/annurev.bi.47.070178.002533. [DOI] [PubMed] [Google Scholar]
- Weinberg R. A., Burns R. O. Regulation of expression of the ilvB operon in Salmonella typhimurium. J Bacteriol. 1984 Dec;160(3):833–841. doi: 10.1128/jb.160.3.833-841.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams K. R., Murphy J. B., Chase J. W. Characterization of the structural and functional defect in the Escherichia coli single-stranded DNA binding protein encoded by the ssb-1 mutant gene. Expression of the ssb-1 gene under lambda pL regulation. J Biol Chem. 1984 Oct 10;259(19):11804–11811. [PubMed] [Google Scholar]
- Yousaf S. I., Carroll A. R., Clarke B. E. A new and improved method for 3'-end labelling DNA using [alpha-32P]ddATP. Gene. 1984 Mar;27(3):309–313. doi: 10.1016/0378-1119(84)90075-1. [DOI] [PubMed] [Google Scholar]