Abstract
Dermal exposure can represent a significant health risk in settings involving potential contact with soil contaminated with polycyclic aromatic hydrocarbons (PAHs). However, there is limited work on the ability of PAHs in contaminated soil to reach the skin surface via desorption from the soil. We evaluated PAH desorption from a field-contaminated soil to a two-dimensional hydrophobic surface (C18 extraction disk) as a measure of potential dermal exposure as a function of soil loading (5 to 100 mg dry soil/cm2), temperature (20 °C to 40 °C), and soil moisture content (2% to 40%) over periods up to 16 d. The efficacy of bioremediation in removing the most readily desorbable PAH fractions was also evaluated. Desorption kinetics were described well by an empirical two-compartment kinetic model. PAH mass desorbed to the C18 disk kept increasing at soil loadings well above the estimated monolayer coverage, suggesting mechanisms for PAH transport to the surface other than by direct contact. Such mechanisms were reinforced by observations that desorption occurred even with dry or moist glass microfiber filters placed between the C18 disk and the soil. Desorption of all PAHs was substantially reduced at a soil moisture content corresponding to field capacity, suggesting that transport through pore air contributed to PAH transport to the C18 disk. The lower molecular weight PAHs had greater potential to desorb from soil than higher molecular weight PAHs. Biological treatment of the soil in a slurry-phase bioreactor completely eliminated PAH desorption to the C18 disks.
Keywords: PAHs, desorption, soil, dermal exposure, bioremediation
1. Introduction
Polycyclic aromatic hydrocarbons (PAHs) are of great concern because of their known or suspected genotoxicity, mutagenicity and carcinogenicity (Santodonato, 1997; Bostrom et al., 2002). Dermal exposure can represent a significant health risk in settings involving potential contact with complex materials containing PAHs, including PAH-contaminated soil or sediment (Boffetta et al., 1997; Sobus et al., 2009). Most previous work has been concerned with integrated uptake of chemicals through the skin and not with how a contaminant reaches the skin surface in the first place. However, only a contaminant that reaches the skin surface is available for dermal absorption (Roy et al., 1998; Shatkin et al., 2002). Desorption properties, such as dynamic conditions by which soil contacts the skin, interactions of the soil with the skin surface and chemical interaction with the soil, have been identified to influence dermal uptake of chemicals (McKone and Howd, 1992; Spalt et al., 2009). Therefore, it is important to understand desorption of PAHs from contaminated soil or sediment to the skin surface.
To account for the association of hydrophobic contaminants such as PAHs with compartments of varying sorptive strength in soil (Alexander, 1995; Xing and Pignatello, 1997; Cornelissen et al., 2005), a so-called two-compartment desorption model assumes a simplified situation in which a fraction of the contaminant is released relatively rapidly and the remainder is released relatively slowly (Cornelissen et al., 1998; Hawthorne et al., 2001; Zhu et al., 2008). By incorporating two-compartment desorption kinetics into a fugacity model, Shatkin et al. (2002) illustrated that a greater rapid-desorbing fraction of a chemical would result in greater dermal uptake. In most previous experimental work on dermal uptake of contaminants from soil, an individual contaminant was introduced into the soil through a solvent that subsequently evaporated (Spalt et al., 2009). However, exposure to a spiked chemical does not account for the effect of contaminant aging that would have occurred in field-contaminated soil (Roy et al., 1998; Stroo et al., 2000; Spalt et al., 2009), which is well known to decrease its bioavailability (Alexander, 2000).
The objective of this study was to evaluate desorption of PAHs from field-contaminated soil from a former MGP site to a two-dimensional hydrophobic surface (Empore™ C18 extraction disk) as a measure of potential dermal exposure. Various factors affecting desorption were investigated, including soil loading, temperature, soil moisture content (SMC), and exposure time. We also compared desorption to the C18 disk to a conventional method of evaluating potential contaminant bioavailability in soil, desorption to Tenax® beads in a well-mixed aqueous slurry (Loehr et al., 2003). The efficacy of bioremediation (in a slurry-phase bioreactor) in removing the most readily desorbable PAH fractions was evaluated with both methods.
2. Materials and methods
2.1. Materials
Source soil used in this study was collected from a former MGP site in Salisbury, North Carolina, USA. Samples were air-dried, sieved (250 μm mesh) and maintained at 4 °C prior to use. The total organic matter fraction (foc) was 0.16 (dry mass basis, wt/wt), SMC was 2.0% (wt/wt), field capacity was 40% (wt/wt), and soil particle density was 2.57 g/cm3 (methods are identified in Table S1, Supplementary Material). The total concentration of 14 target PAHs (the 16 priority PAHs, excluding acenaphthylene and indeno[1,2,3-cd]pyrene) was 780 ± 10 mg/kg (dry mass basis, wt/wt; individual PAH concentrations are shown in Table S2); the most abundant PAHs were phenanthrene (PHE, 322 ± 5.1 mg/kg) and pyrene (PYR, 121 ± 0.05 mg/kg). Soil samples were mixed with de-ionized water to reach desired SMC levels prior to desorption experiments. Treated soil was the slurry from a continuously stirred, semi-continuous (draw and fill), laboratory-scale aerobic bioreactor (Zhu et al., 2008) treating the source soil. The treated soil had a total PAH concentration of 121 ± 8 mg/kg (individual PAH concentrations are shown in Table S2).
Empore™ C18 extraction disks (25 mm diameter, 0.5 mm thickness) were obtained from 3M (St. Paul, MN, USA) and cleaned by acetone extraction overnight and air-dried before use. Tenax® TA beads (60/80 mesh) were purchased from Alltech (Deerfield, IL, USA) and cleaned by Soxhlet extraction in acetone: hexane (50:50, v/v) mixture overnight and air-dried before use. PAH standards (EPA 610 PAHs Mixture) were obtained from Sigma-Aldrich (St. Louis, MO, USA). Anthracene-D10 was obtained from Cambridge Isotope Laboratories (Andover, MA, USA). Solvents were high-pressure liquid chromatography (HPLC) grade and were obtained from Fisher Scientific (Pittsburgh, PA, USA).
The C18 extraction disks were used to evaluate variables that might influence the transfer of PAHs from soil to a static hydrophobic interface. This method is analogous to the Tenax beads method for evaluating PAH desorption kinetics in slurry systems (Loehr et al., 2003), in that both C18 extraction disks and Tenax beads serve as an infinite sink; however, we believe that the C18 disk is more relevant to the application of soil to skin in a dermal exposure scenario.
2.2. Desorption experiments
Desorption of PAHs from soil samples to C18 disks was determined at three different temperatures (20 °C, 30 °C and 40 °C), four SMC levels (2%, 8%, 20% and 40%) and seven soil loadings (5 to 100 mg dry soil/cm2) over periods of 6 d, when total PAHs desorbed from soil to a C18 disk reached an apparent equilibrium (Fig. S1). Kinetics for desorption of PAHs from soil to C18 disks were investigated over periods of 16 d. The sorption capacity of C18 disks was evaluated by repeated soil loading of the same disk; results indicated that the sorption capacity greatly exceeded the amount of PAHs desorbed in any given experiment (Table S3). Soil with a specified SMC level was spread as evenly as possible (under microscopic observation) onto the C18 disk, which was then transferred with an aluminum spatula onto an aluminum weighing dish. Soil weight was determined by weight difference of the C18 disk before and after soil loading. The aluminum weighing dish was then transferred into a sealed container and kept in the dark in a constant-temperature room set to the desired temperature. After each desired time interval, disks were removed and rinsed with de-ionized water three times for subsequent PAH extraction. To investigate possible mechanisms of PAH transport from soil to the hydrophobic surface, dry or moist Whatman glass microfiber filters (pore size 0.7μm, pre-baked at 400°C for 4h) (Fisher Scientific, Pittsburgh, PA, USA) were placed between the C18 disk and the soil. Triplicates of procedure blanks (no soil) were included. Total PAH recovery over all experiments was 94 ± 6% (individual PAH recoveries are shown in Table S4), calculated by comparing the initial PAH mass in the soil with the PAH mass desorbed to the C18 disk and the PAH mass remaining in the soil after desorption.
Desorption of PAHs from soil to Tenax beads was carried out at 20 °C. Approximately 3 g of soil (dry wt.) and 0.2 g Tenax beads were suspended in 20 mL phosphate buffer (pH 7.5) amended with 4.15 g/L NaN3 in a 30-mL glass serum vial with a PTFE-lined septum and screw cap. The vials were placed on a wrist-action shaker at 240 rpm in the dark. After 1, 2, 4, 8 and 16d, the vials were centrifuged at 3500 rpm for 15 min, Tenax beads were removed from the vials for subsequent extraction as described by Zhu et al. (2008), and the supernatant was discarded. For all but the 16-d time point, 20 mL fresh medium was added along with 0.2 g fresh Tenax beads into the vials. The mass recovery of Tenax beads over all time points was 97 ± 2%. Total PAH recovery was 92 ± 10% for combined experiments with source soil and treated soil (individual PAH recoveries are in Table S4).
2.3. PAH extraction and analysis
C18 disks and Tenax beads were extracted with 10 mL acetone and 10 mL methanol, respectively, in a 20-mL test tube with a PTFE-lined septum and screw cap; each tube was amended with 20 μL anthracene-D10 (100 μg/L) as recovery surrogate. The tubes were placed on a wrist-action shaker at 240 rpm in the dark for 24 h. A 1-mL aliquot of extract from the C18 disk was then removed for HPLC analysis. The extract from Tenax beads was filtered through a Millipore (Billerica, MA, USA) nylon membrane (pore size 0.20 μm) to remove the beads and subsequently analyzed by HPLC. Soil samples were extracted overnight twice each with a mixture of 10 mL acetone and 10 mL dichloromethane as described elsewhere (Zhu et al., 2008). All extracts were analyzed by HPLC (Zhu et al., 2008).
2.4. Data analysis
SPSS® (v16.0, SPSS Inc.) was applied for data analysis. One-way ANOVA followed by Tukey’s test was employed to test for differences among multiple groups. The maximum soil loading required to provide monolayer coverage was estimated according to Eq. 1 (Duff and Kissel, 1996) assuming solid spherical soil particles and face-centered packing:
| (1) |
where SLmonolayer is the soil loading representing a monolayer (mg/cm2);ρparticle is the soil particle density (g/cm3); d is the soil particle diameter (μm). Desorption kinetics data were evaluated with the commonly used two-compartment kinetic model, Eq. 2 (Cornelissen et al., 1998; Hawthorne et al., 2001; Zhu et al., 2008):
| (2) |
where S0 is the initial soil concentration of a given PAH (μg/g); St is the soil concentration at time t (μg/g); fr and fs are the fractions of the PAH that desorb rapidly and slowly, respectively; kr and ks are the rate constants for rapid and slow desorption, respectively (d−1); and t is the desorption time (d). All model parameters with their standard errors and coefficients of determination were determined using nonlinear regression.
3. Results
3.1.Effects of soil loading, temperature and SMC on desorption to C18 disks
The mass of PAHs desorbed from soil to a C18 disk increased with increasing soil loading, although the percentage desorbed decreased as loading increased (Fig. 1). According to Eq. 1, the maximum soil loading required to provide monolayer coverage was estimated as no more than 34 mg/cm2 with soil particle density of 2.57 g/cm3 and soil particle diameter less than 250 μm (soil was sieved through 250 μm mesh). It is obvious from Fig. 1 that the total PAH mass desorbed to the C18 disk kept increasing at soil loadings well above the estimated monolayer coverage.
Fig. 1.
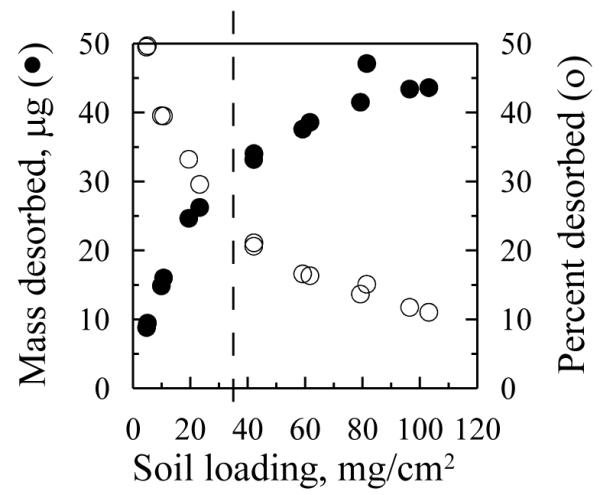
Total PAHs desorbed from source soil to C18 extraction disks as a function of soil loading. Soil moisture content was 2%, temperature 20 °C and contact time 6 d. Dashed vertical lines indicate the estimated maximum monolayer coverage of 34 mg/cm2.
Soil loading effects on PAH desorption to C18 disks was influenced by temperature, but the influences depended on the specific PAH (Fig. S2). For naphthalene (NAP), the mass desorbed was constant for all soil loadings at each of the three temperatures evaluated (20, 30 and 40 °C). For acenaphthene (ACE) and fluorene (FLU), the mass desorbed was proportional to soil loading at each temperature. For phenanthrene (PHN), anthracene (ANT), fluoranthene (FLA) and pyrene (PYR), the mass desorbed asymptotically approached a maximum that appeared to have been reached for each compound at 20 °C and 30 °C over soil loadings up to 100 mg/cm2. The asymptote generally tended to be approached at higher soil loadings as temperature increased. In separate experiments, we verified that the apparent maximum mass of PAH desorbed did not approach the sorption capacity of the C18 disk (Table S3).
Increased desorption of PAHs to the C18 disk at soil loadings well above monolayer coverage suggested that PAHs were transferred to the disk by mechanisms other than direct soil contact. Accordingly, with a fixed soil loading above monolayer coverage (40 mg/cm2), we evaluated the extent to which placing a barrier to direct contact between the soil and the disk would affect desorption. The desorption of PAHs from soil to the C18 disk still occurred even with a dry or moist glass microfiber filter placed between the hydrophobic surface and the soil (Fig. 2). For NAP, ACE and FLU, there were no significant differences between desorption with a dry or a moist filter and that without a filter. However, for PHN, ANT, FLA and PYR, desorption from the soil to the C18 disk was significantly lower with a dry or a moist filter than without a filter; in addition, desorption was significantly lower in the presence of a moist filter than in the presence of a dry filter. Desorption in the presence of two dry or two moist filters was only slightly less than in the presence of only one dry or one moist filter for all PAHs (data not shown). No PAH was detected in the dry or moist filter that was not in direct contact with the soil, suggesting that PAHs were not sorbed by the filters.
Fig. 2.
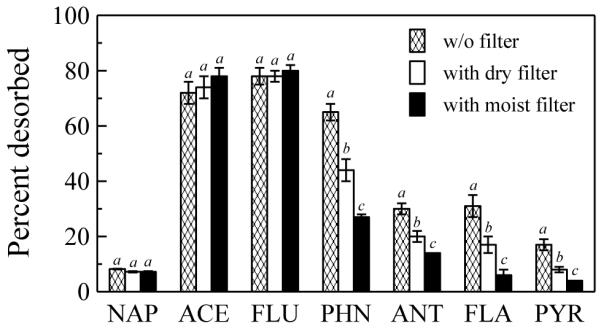
Desorption of PAHs to C18 disks from source soil with or without a glass microfiber filter placed between the C18 disk and the soil. Soil moisture content was 2%, soil loading 40 mg/cm2, temperature 20 °C, and contact time 6 d. Desorption was less than 2% for BaA, CHR, BbF, BkF, BaP, DBA and BgP under all three conditions. The same letter is assigned to conditions for which there was no significant difference (p > 0.05).
The desorption of all PAHs to the C18 disk with a fixed soil loading well above monolayer coverage (50 mg/cm2) was substantially reduced at an SMC of 40%, which corresponded to approximate field capacity of the soil (Fig. 3). Desorption of total PAHs from soil at an SMC of 40% was only one-third of that at an SMC of 2%. For NAP, ACE, FLU and PHN, there were no significant differences between desorption from soil at SMC from 2% to 20%. For ANT, FLA and PYR, there was no significant difference between desorption from soil at SMC of 2% and 8%; at an SMC of 20% there was a statistically significant, but modest, decrease in desorption.
Fig. 3.
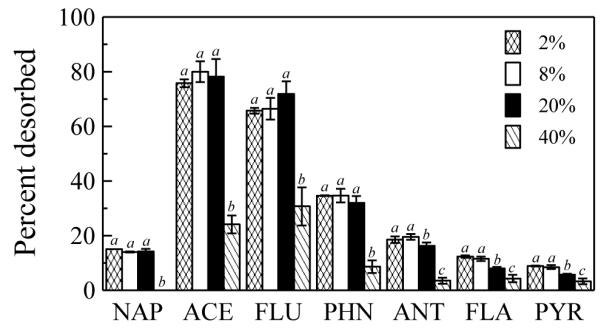
Effect of soil moisture content on desorption of PAHs to C18 disks from source soil. Soil loading was 50 mg/cm2, temperature 20 °C, and contact time 6 d. Desorption was less than 2% for BaA, CHR, BbF, BkF, BaP, DBA and BgP under all four conditions. The same letter is assigned to conditions for which there was no significant difference (p > 0.05).
3.2. Effects of bioremediation on desorption to C18 disks and to Tenax beads
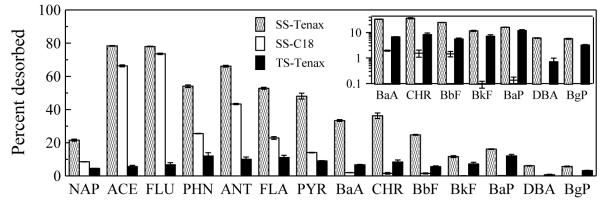
Desorption of PAHs to C18 disks from both the source soil and the biologically treated soil was compared to desorption to Tenax beads in a vigorously mixed aqueous slurry (Fig. 4). For the source soil, only 62 % of the total PAHs desorbed to Tenax beads was desorbed to C18 disks. Lower molecular weight PAHs had greater potential to desorb from soil than higher molecular weight PAHs; the percentage desorption both to Tenax beads and to C18 disks decreased as PAH molecular weight increased. For the biologically treated soil, desorption to C18 disks was not observed for any PAH (data not shown), suggesting that biological treatment of the soil in an aerobic, slurry-phase bioreactor removed the fraction of each PAH that was capable of desorbing to the C18 disk. The percentage desorption of all PAHs from biologically treated soil to Tenax beads was much lower than that from the source soil, reinforcing that the most bioavailable fractions of the PAHs had been removed by biological treatment.
Fig. 4.
Desorption of individual PAHs from source soil to Tenax beads (SS-Tenax) or to C18 disks (SS-C18) or from biologically treated soil to Tenax beads (TS-Tenax). The inset enlarges the results for higher molecular weight PAHs. All measurements were at 20 °C at a 16-d contact time. For desorption to C18 disks, soil moisture content was 2% and soil loading 50 mg/cm2. Desorption to C18 disks from biologically treated soil was not detectable.
Desorption kinetics of each individual PAH from source soil to both C18 disks and Tenax beads were described well by the commonly used two-compartment kinetic model (Eq. 2), as illustrated in Fig. S3. Fitted parameter values are summarized in Table S5. The rapidly desorbing fraction (fr) for desorption from the source soil to Tenax beads was higher than that for desorption to the C18 disk. For desorption of PAHs from the biologically treated soil to Tenax beads, fr was not significantly different from 0 for any PAH. The rate constant for the slowly desorbing fraction (ks) for biologically treated soil was lower than that for source soil in the Tenax-bead system.
4. Discussion
Current guidance from the US Environmental Protection Agency (USEPA) for assessment of dermal exposure to contaminants in soil (USEPA, 2004) stipulates a fixed percentage absorption (13%) for dermal uptake of benzo[a]pyrene and other PAHs based on measurements from a single study (Wester et al., 1990). Although this single value simplifies human health risk calculation, it doesn’t account for the effects of soil loading configuration on dermal absorption. Bunge and Parks (1998) proposed two distinct situations based on a mathematical model describing dermal absorption of organic chemicals from contaminated soils for a given exposure time. When soil loading is less than monolayer coverage, the percentage absorption remains constant as soil loading increases while the mass absorbed per unit skin area dramatically increases; when soil loading exceeds monolayer coverage, the percentage absorption decreases significantly as soil loading increases while the mass absorbed per unit skin area remains constant. Several investigators have confirmed these results experimentally (Duff and Kissel, 1996; Roy and Singh, 2001; Touraille et al., 2005). However, such predictions are based on the assumption that the contaminant concentration in the soil remains constant (i.e., percentage absorption less than 10% of applied dose) during dermal absorption (Bunge and Parks, 1998). When the contaminant is depleted from the soil, the percentage absorption would decrease with increasing soil loading above monolayer coverage while the mass absorbed would increase disproportionately (Bunge and Parks, 1998). This prediction is consistent with our observation that the mass of PAHs desorbed from soil to the hydrophobic surface disproportionately increased with increasing soil loading well above monolayer coverage while the percentage desorption was more than 10% of the applied dose (Fig. 1). Touraille et al. (2005) also observed the phenomenon that the mass of 4-cyanophenol (CP) absorbed increased with increasing soil loading above monolayer coverage for an exposure time of 24 h.
The increasing mass of PAH desorbed at soil loadings beyond monolayer coverage demonstrated that not only the contaminants in a monolayer of soil particles in direct contact with the hydrophobic surface were desorbed. One explanation for this result is the depletion of PAHs in the soil, thus establishing concentration gradients away from the surface (Bunge and Parks, 1998). Since the soil was unstirred, the concentration of a desorbable chemical in the soil will depend on the distance from the hydrophobic surface. As chemicals in soil layers closer to the hydrophobic surface become depleted, the concentration gradient provides a larger driving force for chemicals in upper soil layers to diffuse toward the hydrophobic surface. However, it is difficult to experimentally confirm concentration gradients through the soil depth.
We propose that contaminants can move from the soil to the hydrophobic surface through a combination of three processes: direct contact transfer from soil solids, diffusion through soil pore air, and diffusion through soil pore water. The pore air and pore water transport pathways would be most important at soil loadings beyond monolayer coverage of the hydrophobic surface. Transport through pore air is likely to be more significant because diffusivity in air is far greater than that in water (Schwarzenbach et al., 2003), and air would be the predominant fluid phase in pores for soil moisture contents below field capacity. Also, soil-to-air diffusion of PAHs and other volatile and semi-volatile organic pollutants is well-documented (Meijer et al., 2003; Ribes et al., 2003; Yang and Holmen, 2008). This hypothesis is supported by our finding that the mass of PAHs desorbed from the soil to the hydrophobic surface was considerable even when dry or moist glass microfiber filters were placed between the hydrophobic surface and the soil (Fig. 2). These filters prohibited transfer of PAHs from soil solids to the hydrophobic surface by direct contact. Transport by mechanisms other than direct contact was most significant for the lower-molecular weight PAHs (NAP, ACE, and FLU; Fig. 2) that also have the highest vapor pressures (summarized in Table S6). Although soil moisture contents below field capacity had a limited effect on PAH desorption from the soil, the significant reduction of PAH desorption to the C18 disk at SMC corresponding to field capacity (Fig. 3) also suggests that PAH diffusion in pore air was a predominant mechanism of transport to the C18 disk.
Besides soil loading configuration, other site-specific properties may also influence desorption of PAHs from soil to a two-dimensional hydrophobic surface. Temperature is one of these factors, as diffusion coefficients are positively correlated with temperature (Schwarzenbach et al., 2003). We observed inconsistent effects of temperature on desorption to the C18 disks. For several compounds (ACE, FLU, and ANT), the effect of temperature was limited (Fig. S2). For the remaining compounds (NAP, PHN, FLA, and PYR), the effect of temperature on desorption was substantial (Fig. S2). Several other investigators have also observed a positive relation between temperatures and desorption of semi-volatile compounds from soil to air (Hippelein and McLachlan, 2000; He et al., 2009). Overall, the effect of temperature in our work would not be easy to predict, as temperature affects not only the soil-air partitioning equilibrium and PAH diffusivity, but the PAH-C18 sorption equilibrium as well; these effects of temperature could be counter-acting for a given PAH.
Bioremediation can be an attractive remediation approach for PAH-contaminated systems (Aitken and Long, 2004). In this study, treatment of the source soil in an aerobic bioreactor reduced total PAH concentration by approximately 80% (Table S2) and seemed to eliminate the most readily desorbable fraction of all PAHs (Fig. 4). Similar decreases in the rapidly desorbed fractions of PAHs after bioremediation have also been observed in previous research (Cornelissen et al., 1998; Hawthorne et al., 2001; Richardson and Aitken, 2011). For the treated soil, no PAH desorption to C18 disks was observed, and there was no rapidly desorbing fraction in the slurry-based Tenax bead desorption system. It therefore appeared that biological treatment eliminated the PAHs that could desorb to a two-dimensional surface, and thus might substantially decrease the dermal bioavailability of PAHs in contaminated soil.
The default assumption for exposure time in dermal exposure assessments for contaminated soil is 24 h (USEPA, 2004). Although we carried out most of our experiments over a six-day period, the majority of the desorbed PAH mass desorbed within 24 h (Fig. S1). The experiments we performed are easily modified to 24-h duration if desired for an actual exposure analysis. We also recognize that skin is a complex matrix containing both hydrophobic and hydrophilic compartments as well as metabolic enzymes. A uniformly hydrophobic surface might overestimate the flux of PAHs from soil to the skin surface and/or neglect the metabolism of PAH by skin. The more important point is that dermal exposure assessment from soil should consider site-specific conditions that influence the bioavailability of hydrophobic contaminants to skin. The effects of remediation on potential dermal exposure should consider not only the reduction in contaminant concentration but also the reduction in contaminant bioavailability.
Supplementary Material
Highlights.
- We evaluated PAH desorption from field-contaminated soil to a hydrophobic surface.
- PAHs desorbed at soil loadings in excess of monolayer coverage.
- Transport of PAHs through the vapor phase is an important mechanism.
- Bioremediation eliminates PAHs capable of desorbing to the hydrophobic surface.
Acknowledgements
This work was supported by the National Institute of Environmental Health Sciences Superfund Research Program (grant 5 P42 ES005948). We thank Stephen Richardson for assistance with the PAH analyses and Chad Roper for design of the method to measure desorption to the C18 disk.
Footnotes
Appendix A. Supplementary material
jinghu@live.unc.edu(J. Hu); mike_aitken@unc.edu (M. D. Aitken)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aitken MD, Long TC. Biotransformation, biodegradation and bioremediation of polycyclic aromatic hydrocarbons. In: Singh A, Ward OP, editors. Soil Biology. Volume 2: iodegradation and Bioremediation. Springer-Verlag; Heidelberg, Germany: 2004. pp. 83–124. [Google Scholar]
- Alexander M. How toxic are toxic chemicals in soil. Environmental Science & Technology. 1995;29:2713–2717. doi: 10.1021/es00011a003. [DOI] [PubMed] [Google Scholar]
- Alexander M. Aging, bioavailability, and overestimation of risk from environmental pollutants. Environmental Science & Technology. 2000;34:4259–4265. [Google Scholar]
- Boffetta P, Jourenkova N, Gustavsson P. Cancer risk from occupational and environmental exposure to polycyclic aromatic hydrocarbons. Cancer Causes & Control. 1997;8:444–472. doi: 10.1023/a:1018465507029. [DOI] [PubMed] [Google Scholar]
- Bostrom CE, Gerde P, Hanberg A, Jernstrom B, Johansson C, Kyrklund T, Rannug A, Tornqvist M, Victorin K, Westerholm R. Cancer risk assessment, indicators, and guidelines for polycyclic aromatic hydrocarbons in the ambient air. Environmental Health Perspectives. 2002;110:451–488. doi: 10.1289/ehp.110-1241197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge AL, Parks JM. Soil contamination: Theoretical description. In: Roberts MS, Walters KA, editors. Dermal Absorption and Toxicity Assessment. Marcel Decker, Inc.; New York: 1998. pp. 669–696. [Google Scholar]
- Cornelissen G, Gustafsson O, Bucheli TD, Jonker MTO, Koelmans AA, Van Noort PCM. Extensive sorption of organic compounds to black carbon, coal, and kerogen in sediments and soils: Mechanisms and consequences for distribution, bioaccumulation, and biodegradation. Environmental Science & Technology. 2005;39:6881–6895. doi: 10.1021/es050191b. [DOI] [PubMed] [Google Scholar]
- Cornelissen G, Rigterink H, Ferdinandy MMA, Van Noort PCM. Rapidly desorbing fractions of PAHs in contaminated sediments as a predictor of the extent of bioremediation. Environmental Science & Technology. 1998;32:966–970. [Google Scholar]
- Duff RM, Kissel JC. Effect of soil loading on dermal absorption efficiency from contaminated soils. Journal of Toxicology and Environmental Health. 1996;48:93–106. doi: 10.1080/009841096161492. [DOI] [PubMed] [Google Scholar]
- Hawthorne SB, Poppendieck DG, Grabanski CB, Loehr RC. PAH release during water desorption, supercritical carbon dioxide extraction, and field bioremediation. Environmental Science & Technology. 2001;35:4577–4583. doi: 10.1021/es010771i. [DOI] [PubMed] [Google Scholar]
- He X, Chen S, Quan X, Zhao YZ, Zhao HM. Temperature-dependence of soil/air partition coefficients for selected polycyclic aromatic hydrocarbons and organochlorine pesticides over a temperature range of-30 to+30 degrees C. Chemosphere. 2009;76:465–471. doi: 10.1016/j.chemosphere.2009.03.038. [DOI] [PubMed] [Google Scholar]
- Hippelein M, McLachlan MS. Soil/air partitioning of semivolatile organic compounds. 2. Influence of temperature and relative humidity. Environmental Science & Technology. 2000;34:3521–3526. [Google Scholar]
- Loehr RC, Lamar MR, Poppendieck DG. A protocol to estimate the release of anthropogenic hydrocarbons from contaminated soils. Environmental Toxicology and Chemistry. 2003;22:2202–2208. doi: 10.1897/02-463. [DOI] [PubMed] [Google Scholar]
- McKone TE, Howd RA. Estimating dermal uptake of nonionic organic chemicals from water and soil. 1. Unified fugacity-based models for risk assessments. Risk Analysis. 1992;12:543–557. doi: 10.1111/j.1539-6924.1992.tb00711.x. [DOI] [PubMed] [Google Scholar]
- Meijer SN, Shoeib M, Jantunen LMM, Jones KC, Harner T. Air-soil exchange of organochlorine pesticides in agricultural soils. 1. Field measurements using a novel in situ sampling device. Environmental Science & Technology. 2003;37:1292–1299. [Google Scholar]
- Ribes S, Van Drooge B, Dachs J, Gustafsson O, Grimalt JO. Influence of soot carbon on the soil-air partitioning of polycyclic aromatic hydrocarbons. Environmental Science & Technology. 2003;37:2675–2680. doi: 10.1021/es0201449. [DOI] [PubMed] [Google Scholar]
- Richardson SD, Aitken MD. Desorption and bioavailability of PAHs in contaminated soil subjected to long-term in situ biostimulation. Environmental Toxicology and Chemistry. 2011;30:2674–2681. doi: 10.1002/etc.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy TA, Krueger AJ, Taylor BB, Mauro DM, Goldstein LS. Studies estimating the dermal bioavailability of polynuclear aromatic hydrocarbons from manufactured gas plant tar-contaminated soils. Environmental Science & Technology. 1998;32:3113–3117. [Google Scholar]
- Roy TA, Singh R. Effect of soil loading and soil sequestration on dermal bioavailability of polynuclear aromatic hydrocarbons. Bulletin of Environmental Contamination and Toxicology. 2001;67:324–331. doi: 10.1007/s001280128. [DOI] [PubMed] [Google Scholar]
- Santodonato J. Review of the estrogenic and antiestrogenic activity of polycyclic aromatic hydrocarbons: Relationship to carcinogenicity. Chemosphere. 1997;34:835–848. doi: 10.1016/s0045-6535(97)00012-x. [DOI] [PubMed] [Google Scholar]
- Schwarzenbach RP, Gschwend PM, Imboden DM. Environmental Organic Chemistry. Wiley-Interscience; Hoboken, NJ, U.S.A.: 2003. [Google Scholar]
- Shatkin JA, Wagle M, Kent S, Menzie CA. Development of a biokinetic model to evaluate dermal absorption of polycyclic aromatic hydrocarbons from soil. Human and Ecological Risk Assessment. 2002;8:713–734. [Google Scholar]
- Sobus JR, McClean MD, Herrick RF, Waidyanatha S, Nylander-French LA, Kupper LL, Rappaport SM. Comparing urinary biomarkers of airborne and dermal exposure to polycyclic aromatic compounds in asphalt-exposed workers. Annals of Occupational Hygiene. 2009;53:561–571. doi: 10.1093/annhyg/mep042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalt EW, Kissel JC, Shirai JH, Bunge AL. Dermal absorption of environmental contaminants from soil and sediment: a critical review. Journal of Exposure Science and Environmental Epidemiology. 2009;19:119–148. doi: 10.1038/jes.2008.57. [DOI] [PubMed] [Google Scholar]
- Stroo HF, Jensen R, Loehr RC, Nakles DV, Fairbrother A, Liban CB. Environmentally acceptable endpoints for PAHs at a manufactured gas plant site. Environmental Science & Technology. 2000;34:3831–3836. [Google Scholar]
- Touraille GD, McCarley KD, Bunge AL, Marty JP, Guy RH. Percutaneous absorption of 4-cyanophenol from freshly contaminated soil in vitro: Effects of soil loading and contamination concentration. Environmental Science & Technology. 2005;39:3723–3731. doi: 10.1021/es0494454. [DOI] [PubMed] [Google Scholar]
- USEPA . Risk Assessment Guidance for Superfund Volume I: Human Health Evaluation Manual (Part E, Supplemental Guidance for Dermal Risk Assessment) Washington, DC: 2004. [Google Scholar]
- Wester RC, Maibach HI, Bucks DAW, Sedik L, Melendres J, Liao C, Dizio S. Percutaneous-absorption of [C-14] DDT and [C-14] benzo[a]pyrene from soil. Fundamental and Applied Toxicology. 1990;15:510–516. [PubMed] [Google Scholar]
- Xing BS, Pignatello JJ. Dual-mode sorption of low-polarity compounds in glassy poly(vinyl chloride) and soil organic matter. Environmental Science & Technology. 1997;31:792–799. [Google Scholar]
- Yang WL, Holmen BA. Relative effects of surfactants and humidity on soil/air desorption of chloroacetanilide and dinitroaniline herbicides. Environmental Science & Technology. 2008;42:6843–6848. doi: 10.1021/es800684h. [DOI] [PubMed] [Google Scholar]
- Zhu HB, Roper JC, Pfaender FK, Aitken MD. Effects of anaerobic incubation on the desorption of polycyclic aromatic hydrocarbons from contaminated soils. Environmental Toxicology and Chemistry. 2008;27:837–844. doi: 10.1897/07-166.1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.