Abstract
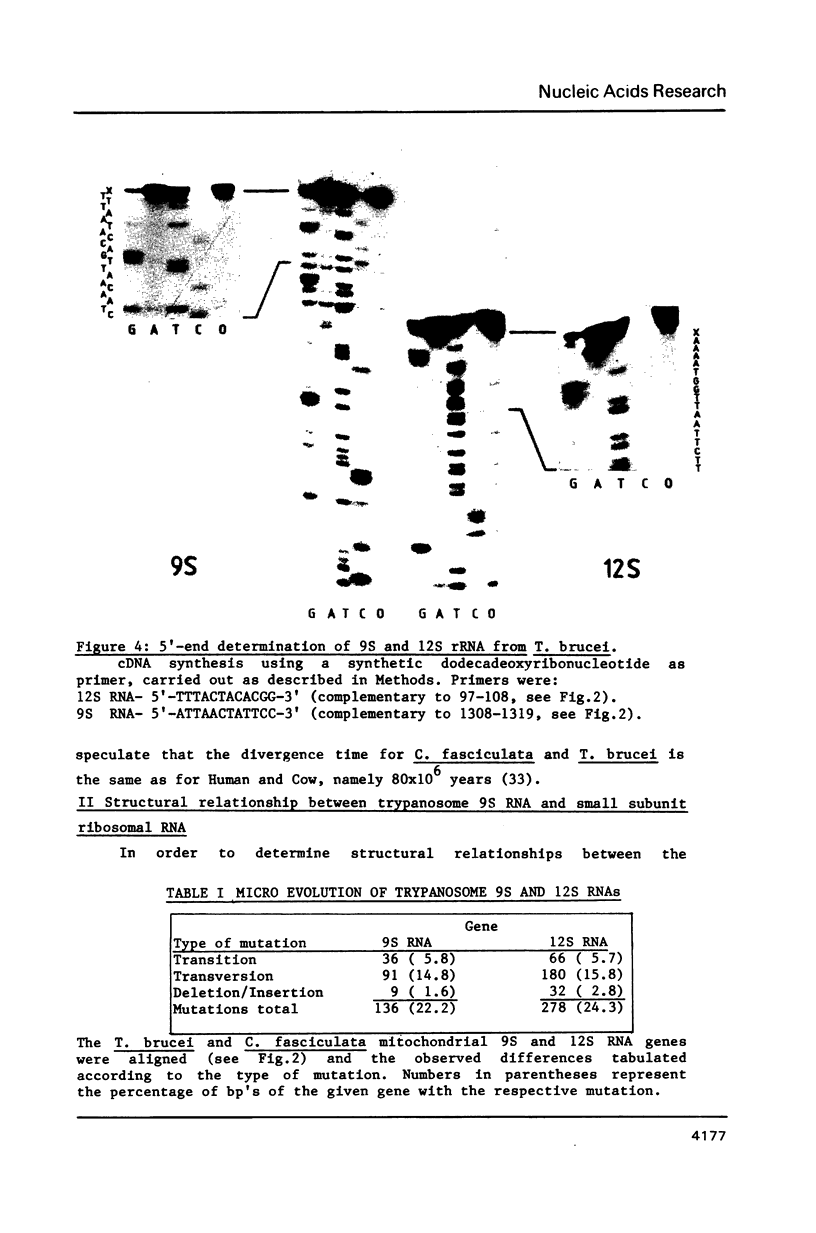
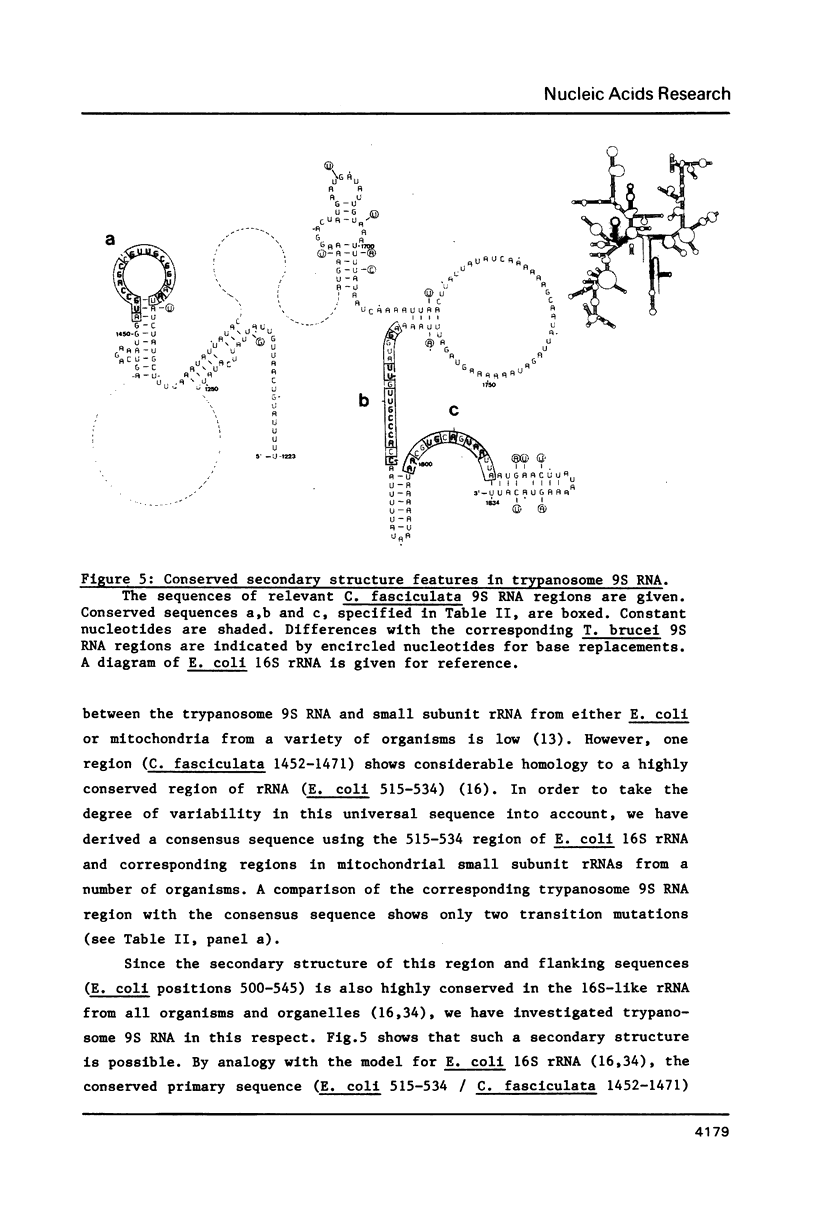
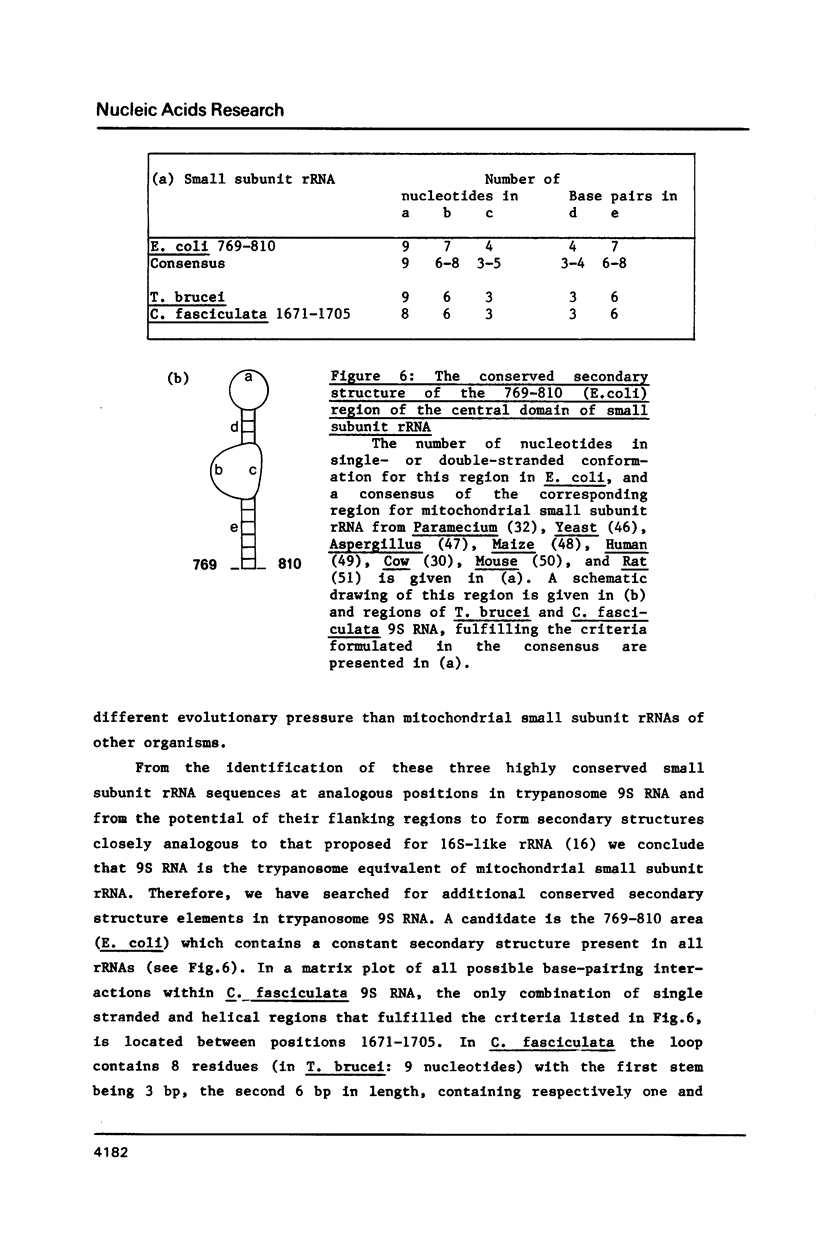
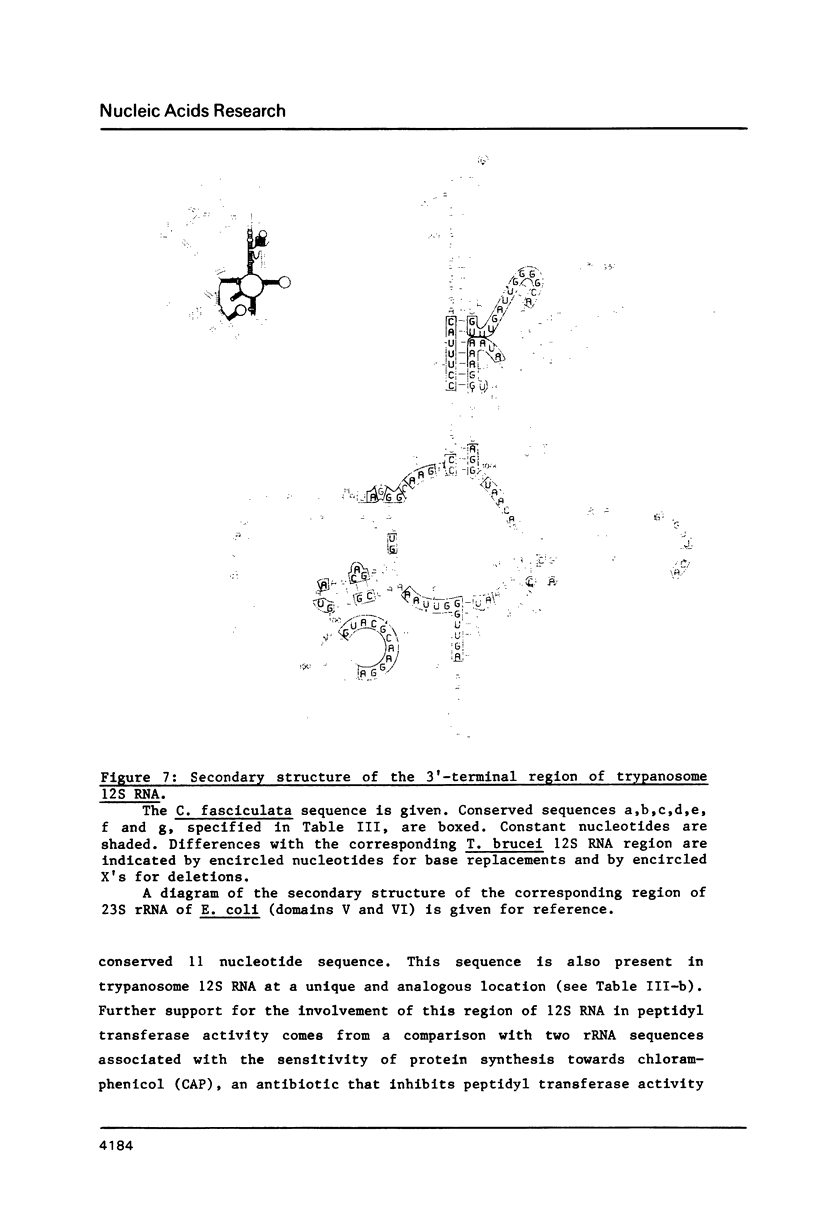
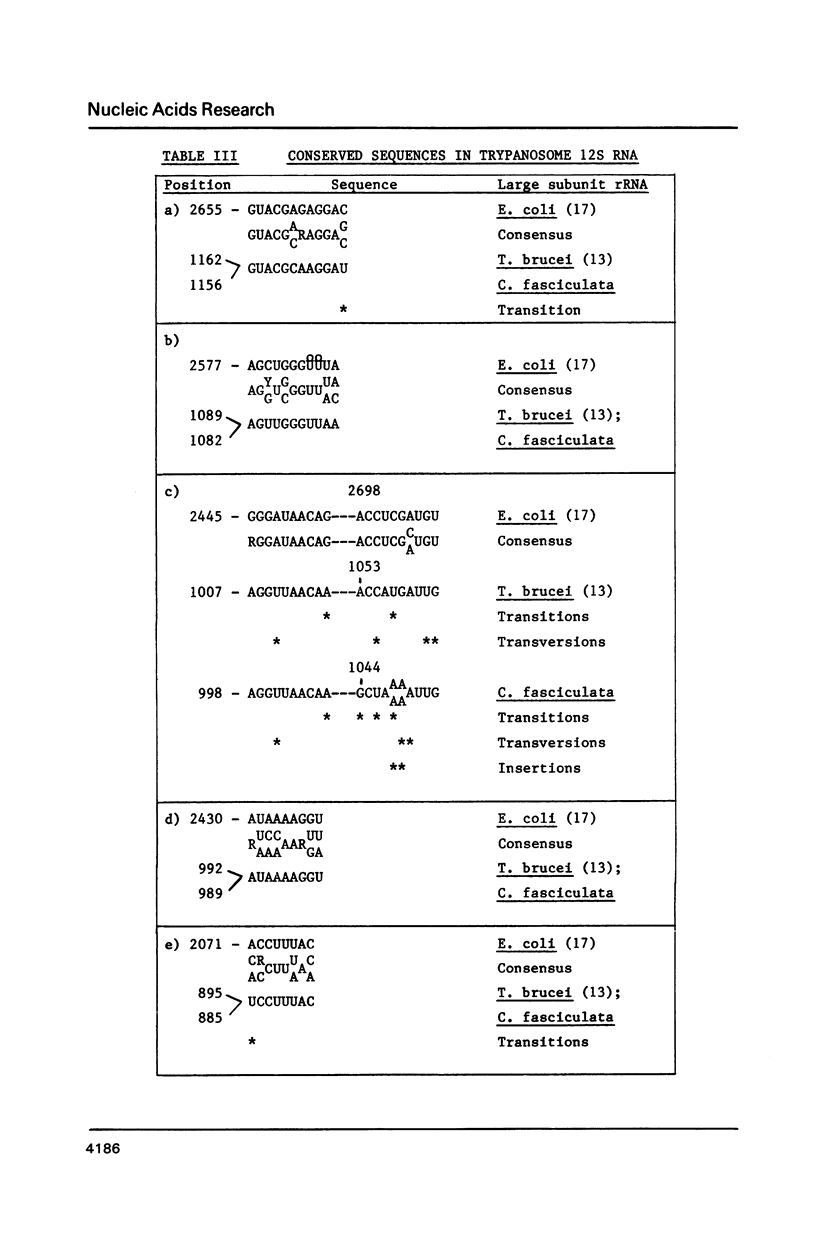
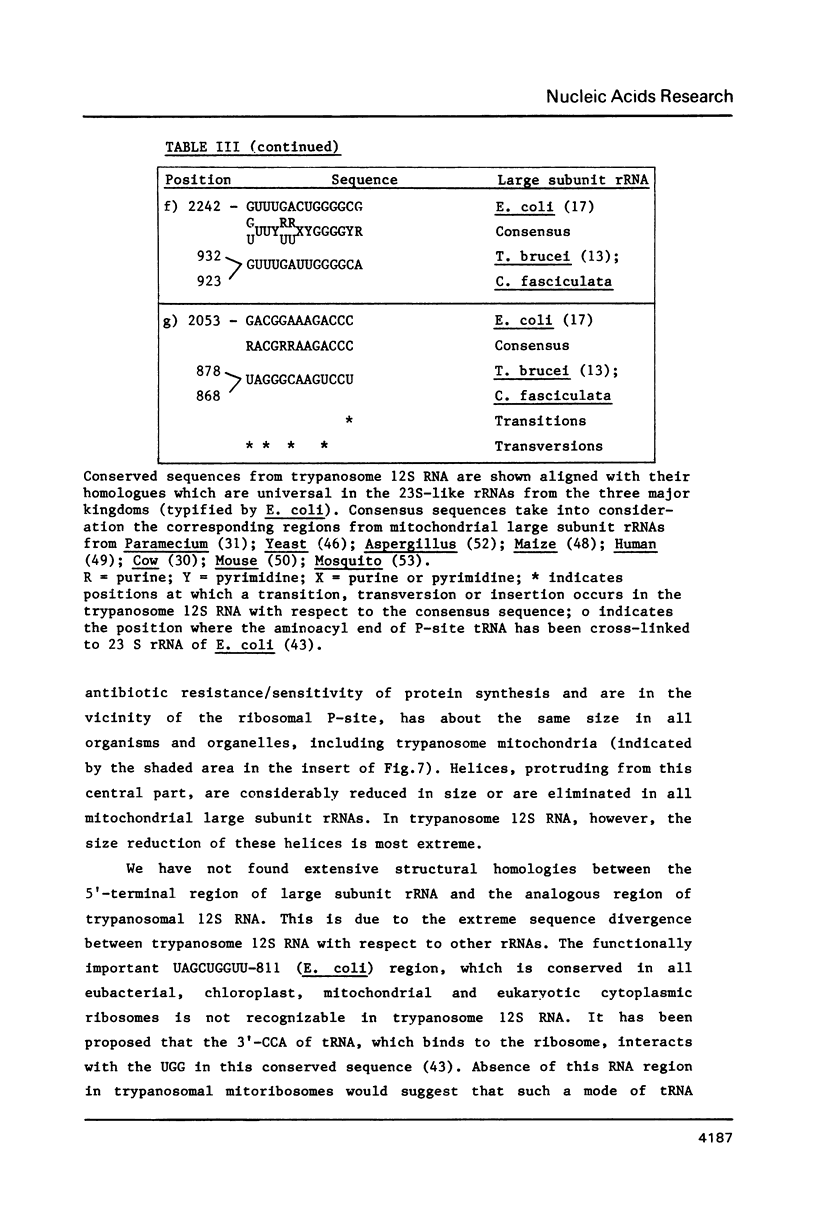
We have determined the nucleotide sequence of a maxi-circle segment from the insect trypanosome Crithidia fasciculata mitochondrial DNA, on which the genes for the major maxicircle transcripts of 9S and 12S are localized. The 5'-terminal sequences of these RNAs were determined by wandering spot analysis. The map coordinates of the 9S and 12S RNAs from Trypanosoma brucei were adjusted with respect to a previous report with the aid of primer extension analysis with reverse transcriptase. This approach allowed us to align the corresponding genes from both organisms which show an overall sequence homology of 77%. The 9S and 12S RNA genes from the two trypanosome species contain sequences, closely related to some of the regions that are universally conserved among ribosomal RNAs from members of the three primary kingdoms and their organelles, even though the overall level of sequence homology is extremely low. These universal sequences occur at positions in the 9S and 12S RNAs that are analogous to those occupied by their counterparts in authentic ribosomal RNAs. The characteristic secondary structure elements flanking these universal sequences in genuine ribosomal RNAs can also be formed in the trypanosomal 9S and 12S RNAs. These results provide unequivocal evidence for a ribosomal function of the 9S and 12S RNAs of trypanosomal mitochondria, notwithstanding their extremely small size (estimated to be 612 and 1141 nucleotides in C. fasciculata, 611 and 1150 nucleotides in T. brucei) and their unusual base composition (83% A+U).
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S., de Bruijn M. H., Coulson A. R., Eperon I. C., Sanger F., Young I. G. Complete sequence of bovine mitochondrial DNA. Conserved features of the mammalian mitochondrial genome. J Mol Biol. 1982 Apr 25;156(4):683–717. doi: 10.1016/0022-2836(82)90137-1. [DOI] [PubMed] [Google Scholar]
- Auffray C., Rougeon F. Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. Eur J Biochem. 1980 Jun;107(2):303–314. doi: 10.1111/j.1432-1033.1980.tb06030.x. [DOI] [PubMed] [Google Scholar]
- Barta A., Steiner G., Brosius J., Noller H. F., Kuechler E. Identification of a site on 23S ribosomal RNA located at the peptidyl transferase center. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3607–3611. doi: 10.1073/pnas.81.12.3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benne R., De Vries B. F., Van den Burg J., Klaver B. The nucleotide sequence of a segment of Trypanosoma brucei mitochondrial maxi-circle DNA that contains the gene for apocytochrome b and some unusual unassigned reading frames. Nucleic Acids Res. 1983 Oct 25;11(20):6925–6941. doi: 10.1093/nar/11.20.6925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borst P., Hoeijmakers J. H. Kinetoplast DNA. Plasmid. 1979 Jan;2(1):20–40. doi: 10.1016/0147-619x(79)90003-9. [DOI] [PubMed] [Google Scholar]
- Brown W. M., Prager E. M., Wang A., Wilson A. C. Mitochondrial DNA sequences of primates: tempo and mode of evolution. J Mol Evol. 1982;18(4):225–239. doi: 10.1007/BF01734101. [DOI] [PubMed] [Google Scholar]
- Chan Y. L., Endo Y., Wool I. G. The sequence of the nucleotides at the alpha-sarcin cleavage site in rat 28 S ribosomal ribonucleic acid. J Biol Chem. 1983 Nov 10;258(21):12768–12770. [PubMed] [Google Scholar]
- Chan Y. L., Gutell R., Noller H. F., Wool I. G. The nucleotide sequence of a rat 18 S ribosomal ribonucleic acid gene and a proposal for the secondary structure of 18 S ribosomal ribonucleic acid. J Biol Chem. 1984 Jan 10;259(1):224–230. [PubMed] [Google Scholar]
- Chao S., Sederoff R., Levings C. S., 3rd Nucleotide sequence and evolution of the 18S ribosomal RNA gene in maize mitochondria. Nucleic Acids Res. 1984 Aug 24;12(16):6629–6644. doi: 10.1093/nar/12.16.6629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donis-Keller H., Maxam A. M., Gilbert W. Mapping adenines, guanines, and pyrimidines in RNA. Nucleic Acids Res. 1977 Aug;4(8):2527–2538. doi: 10.1093/nar/4.8.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douthwaite S., Christensen A., Garrett R. A. Higher order structure in the 3'-minor domain of small subunit ribosomal RNAs from a gram negative bacterium, a gram positive bacterium and a eukaryote. J Mol Biol. 1983 Sep 5;169(1):249–279. doi: 10.1016/s0022-2836(83)80183-1. [DOI] [PubMed] [Google Scholar]
- Dubin D. T., HsuChen C. C. The 3'-terminal region of mosquito mitochondrial small ribosomal subunit RNA: sequence and localization of methylated residues. Plasmid. 1983 May;9(3):307–320. doi: 10.1016/0147-619x(83)90008-2. [DOI] [PubMed] [Google Scholar]
- Eperon I. C., Anderson S., Nierlich D. P. Distinctive sequence of human mitochondrial ribosomal RNA genes. Nature. 1980 Jul 31;286(5772):460–467. doi: 10.1038/286460a0. [DOI] [PubMed] [Google Scholar]
- Eperon I. C., Janssen J. W., Hoeijmakers J. H., Borst P. The major transcripts of the kinetoplast DNA of Trypanosoma brucei are very small ribosomal RNAs. Nucleic Acids Res. 1983 Jan 11;11(1):105–125. doi: 10.1093/nar/11.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett R. A., Christensen A., Douthwaite S. Higher-order structure in the 3'-terminal domain VI of the 23 S ribosomal RNAs from Escherichia coli and Bacillus stearothermophilus. J Mol Biol. 1984 Nov 15;179(4):689–712. doi: 10.1016/0022-2836(84)90162-1. [DOI] [PubMed] [Google Scholar]
- Gray M. A., Cunningham I., Gardiner P. R., Taylor A. M., Luckins A. G. Cultivation of infective forms of Trypanosoma congolense from trypanosomes in the proboscis of Glossina morsitans. Parasitology. 1981 Feb;82(1):81–95. doi: 10.1017/s0031182000041883. [DOI] [PubMed] [Google Scholar]
- Hensgens L. A., Brakenhoff J., De Vries B. F., Sloof P., Tromp M. C., Van Boom J. H., Benne R. The sequence of the gene for cytochrome c oxidase subunit I, a frameshift containing gene for cytochrome c oxidase subunit II and seven unassigned reading frames in Trypanosoma brucei mitochondrial maxi-circle DNA. Nucleic Acids Res. 1984 Oct 11;12(19):7327–7344. doi: 10.1093/nar/12.19.7327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeijmakers J. H., Borst P. RNA from the insect trypanosome Crithidia luciliae contains transcripts of the maxi-circle and not of the mini-circle component of kinetoplast DNA. Biochim Biophys Acta. 1978 Nov 21;521(1):407–411. doi: 10.1016/0005-2787(78)90282-4. [DOI] [PubMed] [Google Scholar]
- Hoeijmakers J. H., Schoutsen B., Borst P. Kinetoplast DNA in the insect trypanosomes Crithidia luciliae and Crithidia fasciculata. I. Sequence evolution and transcription of the maxicircle. Plasmid. 1982 May;7(3):199–209. doi: 10.1016/0147-619x(82)90001-4. [DOI] [PubMed] [Google Scholar]
- Hoeijmakers J. H., Snijders A., Janssen J. W., Borst P. Transcription of kinetoplast DNA in Trypanosoma brucei bloodstream and culture forms. Plasmid. 1981 May;5(3):329–350. doi: 10.1016/0147-619x(81)90009-3. [DOI] [PubMed] [Google Scholar]
- HsuChen C. C., Kotin R. M., Dubin D. T. Sequences of the coding and flanking regions of the large ribosomal subunit RNA gene of mosquito mitochondria. Nucleic Acids Res. 1984 Oct 25;12(20):7771–7785. doi: 10.1093/nar/12.20.7771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huysmans E., Dams E., Vandenberghe A., De Wachter R. The nucleotide sequences of the 5S rRNAs of four mushrooms and their use in studying the phylogenetic position of basidiomycetes among the eukaryotes. Nucleic Acids Res. 1983 May 11;11(9):2871–2880. doi: 10.1093/nar/11.9.2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson B. J., Hill G. C., Donelson J. E. The maxicircle of Trypanosoma brucei kinetoplast DNA encodes apocytochrome b. Mol Biochem Parasitol. 1984 Oct;13(2):135–146. doi: 10.1016/0166-6851(84)90108-7. [DOI] [PubMed] [Google Scholar]
- Kobayashi M., Seki T., Yaginuma K., Koike K. Nucleotide sequences of small ribosomal RNA and adjacent transfer RNA genes in rat mitochondrial DNA. Gene. 1981 Dec;16(1-3):297–307. doi: 10.1016/0378-1119(81)90085-8. [DOI] [PubMed] [Google Scholar]
- Köchel H. G., Küntzel H. Mitochondrial L-rRNA from Aspergillus nidulans: potential secondary structure and evolution. Nucleic Acids Res. 1982 Aug 11;10(15):4795–4801. doi: 10.1093/nar/10.15.4795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köchel H. G., Küntzel H. Nucleotide sequence of the Aspergillus nidulans mitochondrial gene coding for the small ribosomal subunit RNA: homology to E. coli 16S rRNA. Nucleic Acids Res. 1981 Nov 11;9(21):5689–5696. doi: 10.1093/nar/9.21.5689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., Tzagoloff A., Underbrink-Lyon K., Martin N. C. Identification of the paromomycin-resistance mutation in the 15 S rRNA gene of yeast mitochondria. J Biol Chem. 1982 May 25;257(10):5921–5928. [PubMed] [Google Scholar]
- Maly P., Brimacombe R. Refined secondary structure models for the 16S and 23S ribosomal RNA of Escherichia coli. Nucleic Acids Res. 1983 Nov 11;11(21):7263–7286. doi: 10.1093/nar/11.21.7263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monro R. E., Staehelin T., Celma M. L., Vazquez D. The peptidyl transferase activity of ribosomes. Cold Spring Harb Symp Quant Biol. 1969;34:357–368. doi: 10.1101/sqb.1969.034.01.042. [DOI] [PubMed] [Google Scholar]
- Noller H. F., Kop J., Wheaton V., Brosius J., Gutell R. R., Kopylov A. M., Dohme F., Herr W., Stahl D. A., Gupta R. Secondary structure model for 23S ribosomal RNA. Nucleic Acids Res. 1981 Nov 25;9(22):6167–6189. doi: 10.1093/nar/9.22.6167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noller H. F. Structure of ribosomal RNA. Annu Rev Biochem. 1984;53:119–162. doi: 10.1146/annurev.bi.53.070184.001003. [DOI] [PubMed] [Google Scholar]
- Poncz M., Solowiejczyk D., Ballantine M., Schwartz E., Surrey S. "Nonrandom" DNA sequence analysis in bacteriophage M13 by the dideoxy chain-termination method. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4298–4302. doi: 10.1073/pnas.79.14.4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince J. B., Taylor B. H., Thurlow D. L., Ofengand J., Zimmermann R. A. Covalent crosslinking of tRNA1Val to 16S RNA at the ribosomal P site: identification of crosslinked residues. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5450–5454. doi: 10.1073/pnas.79.18.5450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler D. G., Davies J. E. Specific cleavage of ribosomal RNA caused by alpha sarcin. Nucleic Acids Res. 1977 Apr;4(4):1097–1110. doi: 10.1093/nar/4.4.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seilhamer J. J., Gutell R. R., Cummings D. J. Paramecium mitochondrial genes. II. Large subunit rRNA gene sequence and microevolution. J Biol Chem. 1984 Apr 25;259(8):5173–5181. [PubMed] [Google Scholar]
- Seilhamer J. J., Olsen G. J., Cummings D. J. Paramecium mitochondrial genes. I. Small subunit rRNA gene sequence and microevolution. J Biol Chem. 1984 Apr 25;259(8):5167–5172. [PubMed] [Google Scholar]
- Silberklang M., Prochiantz A., Haenni A. L., Rajbhandary U. L. Studies on the sequence of the 3'-terminal region of turnip-yellow-mosaic-virus RNA. Eur J Biochem. 1977 Feb;72(3):465–478. doi: 10.1111/j.1432-1033.1977.tb11270.x. [DOI] [PubMed] [Google Scholar]
- Simpson A. M., Simpson L. Kinetoplast DNA and RNA of Trypanosoma brucei. Mol Biochem Parasitol. 1980 Dec;2(2):93–108. doi: 10.1016/0166-6851(80)90034-1. [DOI] [PubMed] [Google Scholar]
- Simpson A. M., Simpson L., Livingston L. Transcription of the maxicircle kinetoplast DNA of Leishmania tarentolae. Mol Biochem Parasitol. 1982 Oct;6(4):237–252. doi: 10.1016/0166-6851(82)90057-3. [DOI] [PubMed] [Google Scholar]
- Simpson L., Simpson A. G. Kinetoplast RNA of Leishmania tarentolae. Cell. 1978 May;14(1):169–178. doi: 10.1016/0092-8674(78)90311-2. [DOI] [PubMed] [Google Scholar]
- Sor F., Fukuhara H. Identification of two erythromycin resistance mutations in the mitochondrial gene coding for the large ribosomal RNA in yeast. Nucleic Acids Res. 1982 Nov 11;10(21):6571–6577. doi: 10.1093/nar/10.21.6571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart K. D., Gelvin S. B. Localization of kinetoplast DNA maxicircle transcripts in bloodstream and procyclic form Trypanosoma brucei. Mol Cell Biol. 1982 Jul;2(7):845–852. doi: 10.1128/mcb.2.7.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Etten R. A., Walberg M. W., Clayton D. A. Precise localization and nucleotide sequence of the two mouse mitochondrial rRNA genes and three immediately adjacent novel tRNA genes. Cell. 1980 Nov;22(1 Pt 1):157–170. doi: 10.1016/0092-8674(80)90164-6. [DOI] [PubMed] [Google Scholar]
- Van Knippenberg P. H., Van Kimmenade J. M., Heus H. A. Phylogeny of the conserved 3' terminal structure of the RNA of small ribosomal subunits. Nucleic Acids Res. 1984 Mar 26;12(6):2595–2604. doi: 10.1093/nar/12.6.2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Ploeg L. H., Liu A. Y., Michels P. A., De Lange T., Borst P., Majumder H. K., Weber H., Veeneman G. H., Van Boom J. RNA splicing is required to make the messenger RNA for a variant surface antigen in trypanosomes. Nucleic Acids Res. 1982 Jun 25;10(12):3591–3604. doi: 10.1093/nar/10.12.3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woese C. R., Gutell R., Gupta R., Noller H. F. Detailed analysis of the higher-order structure of 16S-like ribosomal ribonucleic acids. Microbiol Rev. 1983 Dec;47(4):621–669. doi: 10.1128/mr.47.4.621-669.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]