Abstract
Peroxisome proliferator-activated receptor γ (PPAR-γ) is a key regulator of fatty acid metabolism, promoting its storage in adipose tissue and reducing circulating concentrations of free fatty acids. Activation of PPAR-γ has favorable effects on measures of adipocyte function, insulin sensitivity, lipoprotein metabolism, and vascular structure and function. Despite these effects, clinical trials of thiazolidinedione PPAR-γ activators have not provided conclusive evidence that they reduce cardiovascular morbidity and mortality. The apparent disparity between effects on laboratory measurements and clinical outcomes may be related to limitations of clinical trials, adverse effects of PPAR-γ activation, or off-target effects of thiazolidinedione agents. This review addresses these issues from a clinician's perspective and highlights several ongoing clinical trials that may help to clarify the therapeutic role of PPAR-γ activators in cardiovascular disease.
Keywords: peroxisome proliferator-activated receptor gamma, diabetes, insulin resistance, fatty acids, atherosclerosis, randomized clinical trials
The nuclear transcription factor peroxisome proliferator-activated receptor γ (PPAR-γ) is highly expressed in adipose tissue and plays a central role in adipocyte function, fat storage, and lipid metabolism (1–4). PPAR-γ activators are commonly used to treat patients with type 2 diabetes who share metabolic abnormalities that include excessive and inflamed adipose tissue (5), particularly in visceral depots (6); elevated circulating concentrations of nonesterified fatty acids (NEFA), triglycerides, glucose, insulin, and inflammatory mediators; and reduced concentrations of adiponectin and high-density lipoprotein cholesterol (HDL-C). Each of these abnormalities is associated with and may contribute to increased cardiovascular risk (7–9).
PPAR-γ promotes adipogenesis in subcutaneous fat, stimulating the differentiation of preadipocytes into adipocytes with capacity for incremental lipid filling (1, 2). Consequently, treatment with a PPAR-γ activator shifts fat stores toward subcutaneous adipose tissue and away from visceral adipose tissue (Fig. 1), liver, and skeletal muscle cells (10–14). The resulting metabolic effects, discussed below, include enhanced insulin sensitivity; improved glycemic control; decreased plasma NEFA, triglycerides, and inflammatory markers; and increased plasma adiponectin and HDL-C (15–17). Moreover, both experimental and clinical evidence indicate that PPAR-γ mobilizes endothelial progenitor cells, promotes vascular endothelial repair, improves endothelium-mediated vasodilator function, decreases vascular smooth muscle proliferation, lowers blood pressure, retards the progression of atherosclerosis, and mitigates ischemia/reperfusion injury (18–21).
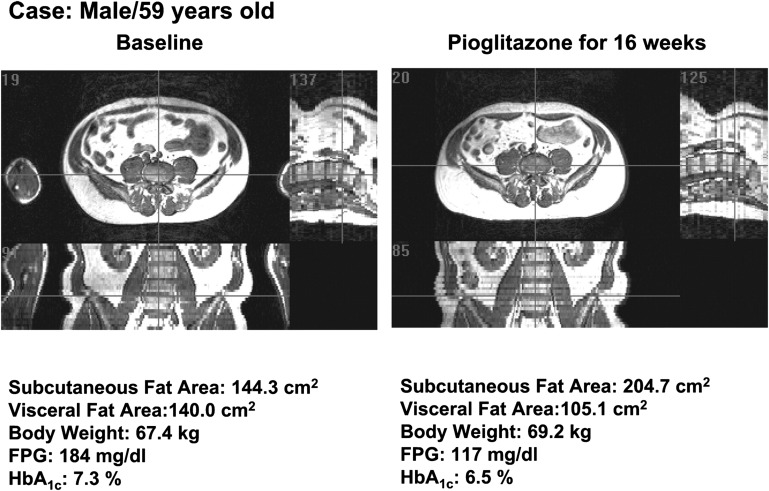
Fig. 1.
Changes in body fat distribution induced by short-term treatment with thiazolidinedione. MRI images demonstrating changes in visceral and subcutaneous abdominal fat depots with 16 weeks of treatment with pioglitazone in a 59-year-old individual with type 2 diabetes. Transverse (left upper), sagittal (right upper), and coronal (left lower) views show that pioglitazone increased subcutaneous fat and decreased visceral fat, with increased total body weight. Reproduced from Ref. 13 with permission.
This portfolio of metabolic and vascular effects would lead to a reasonable expectation that treatment with a PPAR-γ activator would have substantial, favorable effects on cardiovascular morbidity and mortality. However, evidence from clinical trials conducted to date has not substantiated this prediction, possibly because other, undesirable cardiovascular actions of PPAR-γ mitigate its beneficial effects. For example, renal salt and water retention may cause intravascular and extracellular volume expansion, edema, and risk of congestive heart failure (22); increased adipose tissue mass contributes to weight gain (13); some PPAR-γ activators may increase concentrations of low density lipoprotein cholesterol (LDL-C) (23); and in vitro and animal studies suggest that thiazolidinedione (TZD) PPAR-γ activators may interact with cardiac ion channels to promote arrhythmias (24–26). While some studies have shown that PPAR-γ activation retards atherosclerosis (18), others have indicated a greater propensity for plaque necrosis (27, 28). Thus, the net balance of favorable and unfavorable effects of PPAR-γ activation on cardiovascular outcomes remains uncertain (Table 1).
TABLE 1.
Tissue-specific actions of PPAR-γ with potential to influence cardiovascular outcomes
| Tissue | Favorable Effects (Refs.) | Evidence | Unfavorable Effects (Refs.) | Evidence |
| Arterial wall | Decrease arterial wall inflammation, retard progression of atherosclerosis (18, 123–125, 128–139) | E, C | Plaque hemorrhage and necrosis (27, 28) | E |
| Mobilize endothelial progenitor cells, promote endothelial repair, improve endohelial function (20, 102–110, 112, 113) | E, C | |||
| Lower blood pressure (92–95) | C | |||
| Prevent vascular smooth muscle proliferation, prevent restenosis after angioplasty (115–121) | E, C | |||
| Adipose | Redistribute mass from visceral to subcutaneous depots (1–4, 12, 13) | E, C | Weight gain (13, 92, 190, 191, 193) | E, C |
| Anti-inflammatory (80–83) | E, C | |||
| Increase adiponectin secretion (17, 73–75) | E, C | |||
| Enhance insulin sensitivity (29, 30, 32) | E, C | |||
| Circulating lipids and lipoproteins | Raise HDL-C, reduce triglycerides (23, 43, 84, 92) | C | Increase LDL-C (rosiglitazone) (23, 91, 193) | C |
| Reduce NEFA (60, 62–65, 67) | C | |||
| Liver and muscle | Reduce steatosis, enhance local and systemic insulin sensitivity (10, 11, 14, 29, 31–36) | E, C | ||
| Kidney | Sodium and fluid retention (163, 166–171) | E, C | ||
| Heart | Mitigate ischemia/reperfusion injury (140–150) | E | Congestive heart failure (22, 92, 94, 191, 193).Block cardiac ion channels, promote ischemic arrhythmias (24–26, 180) | CE |
| Brain | Reduce infarct size (156–161) | E |
C, clinical evidence; E, experimental evidence.
This article reviews metabolic and cardiovascular effects of PPAR-γ activation that might influence cardiovascular outcomes, in juxtaposition to the results of major completed clinical trials and observational studies of TZD drugs. Key questions are posed for ongoing and future clinical investigation of PPAR-γ as a therapeutic target in cardiovascular disease.
BLOOD GLUCOSE AND INSULIN SENSITIVITY
Experimental and clinical data from transgenic animal models and studies in patients with dominant negative PPAR-γ polymorphisms indicate that the action of PPAR-γ is crucial to insulin sensitivity of adipose tissue, liver, and skeletal muscle (29–32). In clinical therapy of type 2 diabetes, the use of a TZD PPAR-γ agonist as monotherapy or add-on therapy improves insulin sensitivity, manifest by reduced fasting plasma glucose and insulin concentrations, lowering of hemoglobin A1c (HbA1c) by 0.5–1.5%, and improvement in indices of insulin sensitivity determined by euglycemic hyperinsulinemic clamp, glucose tolerance testing, or homeostasis model (33–36).
A large body of experimental evidence implicates insulin resistance as a driver of atherosclerosis (37). Insulin-resistant states, including type 2 diabetes, impaired glucose tolerance, and/or metabolic syndrome substantially increase the incident risk of cardiovascular disease and worsen the prognosis of established cardiovascular disease (38–41). The severity of hyperglycemia, marked by average HbA1c levels, correlates with the risk of macrovascular atherosclerotic events (42). However, a critical but unanswered question is whether pharmacologic treatment that increases insulin sensitivity thereby reduces cardiovascular risk. In the Prospective Pioglitazone Clinical Trial in Macrovascular Events (PROACTIVE) trial, the effect of pioglitazone on cardiovascular endpoints was not related to its effect on HbA1c (43). Several large trials in patients with type 2 diabetes found no significant difference in cardiovascular morbidity and mortality among patients treated with more intensive or less intensive glycemic control regimens (44–46). The frequency of use and/or dosage of TZDs (predominantly rosiglitazone) was higher in the intensive control arms of these trials (45, 46). However, because the trials did not employ a uniform treatment regimen in each arm, but rather adjusted treatment for each patient according to HbA1c levels, the data do not elucidate the relations among TZD use, insulin sensitivity, and cardiovascular outcomes.
The BARI-2D trial took an additional step to address the hypothesis that an insulin-sensitizing treatment strategy would provide greater cardiovascular benefit than an insulin-providing strategy. It included 2,368 patients with type 2 diabetes and coronary heart disease (CHD) (47). The insulin-providing strategy utilized insulin (61% of patients) and/or sulfonylurea (52% of patients). The insulin-sensitizing strategy involved metformin (75% of patients) and/or TZD (62% of patients). In both arms, a target of HbA1c less than 7% was set. In attempting to achieve this target, there was substantial crossover use of insulin and sulfonylurea in the insulin-sensitizing arm and metformin in the insulin-providing arm. The insulin-sensitizing arm, compared with the insulin-providing arm, achieved lower mean HbA1c (7 versus 7.5%) and median fasting insulin levels (6 versus 10 μU/ml), suggesting greater insulin sensitivity, and it resulted in fewer hypoglycemic episodes. Nonetheless, after mean follow-up of 5 years, the occurrence of death, myocardial infarction (MI), or stroke was nearly identical in both arms. The results of BARI-2D do not support the conclusion that insulin sensitization reduces cardiovascular risk in type 2 diabetes. However, as with the trials discussed above, the use of multiple glycemic control agents in a nonstandardized fashion in both arms of the trial precludes inferences regarding the efficacy of TZDs or any other specific drug class.
NEFA
High plasma NEFA concentrations may be deleterious to vascular function, intermediary metabolism, and cardiovascular outcomes. At high concentrations, NEFA are toxic to endothelial cells. High circulating NEFA can cause oxidant stress and inflammatory responses in endothelium and are associated clinically with hypertension and endothelial dysfunction (48, 49). Moreover, endothelial dysfunction can be induced in normal subjects by acute elevation of circulating NEFA from infusion of triglyceride emulsion and heparin (50, 51). High NEFA levels can cause or exacerbate β cell dysfunction (52) and are central in the pathogenesis of skeletal muscle and liver insulin resistance (53) and hepatic steatosis (54). A positive feedback loop can develop whereby insulin resistance causes NEFA levels to rise, and elevated NEFA in turn worsens insulin resistance. High levels of NEFA, particularly saturated fatty acids, may alter electrophysiologic properties of cardiac myocytes, predisposing to ventricular arrhythmias (55). Prospective studies have related high NEFA levels to risk of sudden cardiac death (56).
Plasma NEFA concentrations are elevated in patients with diabetes, insulin resistance, or metabolic syndrome. In normal subjects, fasting NEFA concentration averages 300–400 μmol/l. In patients with type 2 diabetes, NEFA concentrations are typically 50–100% higher, in the 500–800 μmol/l range (57, 58). Similar elevations are observed in nondiabetic, insulin-resistant patients (59). TZD PPAR-γ activators are among the most effective available pharmacologic agents to reduce plasma NEFA concentrations, with effects similar in magnitude to therapeutic doses of insulin (60). Decreases in NEFA are apparent as soon as 4 weeks after initiation of treatment (61). TZD drugs reduce both fasting and postprandial NEFA levels by 20–40% in both type 2 diabetic and nondiabetic, insulin-resistant subjects (11, 62, 63). Reductions in postprandial NEFA presumably reflect an improvement in adipose tissue insulin sensitivity, allowing greater insulin suppression of lipolysis. Reductions produced by rosiglitazone and pioglitazone are similar (64). Moreover, using [1-14C]palmitate as a tracer, rosiglitazone was shown to reduce NEFA turnover rate by 15% in type 2 diabetic patients (62). Pioglitazone and rosiglitazone each produce 20–25% reductions in NEFA when added to sulfonylurea and/or metformin therapy in type 2 diabetes (60, 65). The effect of TZD treatment on fasting NEFA is strongly correlated with the effect of treatment on glycemic control, marked by reduction of hemoglobin A1c (66). TZDs reduce NEFA to a greater extent than metformin, sulfonylurea agents, statins, or fibrates (66–69).
ADIPONECTIN
Adipose tissue serves important autocrine, paracrine, and endocrine functions. Among its secretory products, adiponectin plays a key role in maintaining insulin sensitivity and may exert anti-inflammatory and cardioprotective effects (70). Low adiponectin levels are associated with traditional cardiovascular risk factors, including diabetes, hypertension, and dyslipidemia. Experimental administration of adiponectin improves insulin sensitivity, glycemic control and lipoprotein profile (71, 72). TZD PPAR-γ activators are the most effective class of approved drugs to raise circulating adiponectin concentrations. In patients with type 2 diabetes or other insulin-resistant conditions, TZD treatment typically produces a doubling of plasma adiponectin concentration (17, 73–75). In contrast, other antidiabetic or lipid-modifying drugs produce much smaller or no changes in adiponectin (76–79). In parallel with increased adiponectin production by adipose tissue, activation of PPAR-γ in adipocytes or in mononuclear inflammatory cells resident in adipose tissue reduces local inflammation and inhibits release of inflammatory mediators from adipose tissue (80–83).
CIRCULATING LIPOPROTEINS
TZD PPAR-γ activators may exert favorable effects on plasma lipoproteins, with increased HDL-C and decreased triglyceride concentration. Lipoprotein changes are more favorable with pioglitazone than with rosiglitazone (23, 84), possibly due to the additional action of the former as a weak PPAR-α activator (85–87). Although a relationship between circulating triglyceride concentration and cardiovascular risk remains uncertain, a large body of observational data relates higher HDL-C to lower cardiovascular risk. Among patients treated with statins, the risk of adverse cardiovascular events diminishes by 1–1.5% with each 1 mg/dl increment in HDL-C (88). If that relation applies when HDL-C is raised pharmacologically, assuming a baseline HDL-C concentration of 40 mg/dl and a 10% increase with TZD treatment, one might expect TZDs to reduce cardiovascular risk by approximately 5% on the basis of increased HDL-C alone. Indeed, in the PROACTIVE study, a beneficial effect of pioglitazone on the risk of death, MI, or stroke was related to increases in HDL-C, but not decreases in HbA1c (43), and favorable effects of pioglitazone on progression of carotid intima-media thickness (cIMT) and coronary atherosclerosis have also been related to the drug's effects on HDL-C or HDL-C/triglyceride ratio (89, 90). Pioglitazone and rosiglitazone have similar effects on HDL-C, and both agents increase LDL particle size (23); however, the compounds differ in their effects on LDL-C and apolipoprotein B concentration: Pioglitazone generally has minimal effect (23), whereas rosiglitazone can produce increases of 10–20% (23, 91). It remains uncertain whether differential effects of pioglitazone and rosiglitazone on lipoproteins account for differences in clinical efficacy of these agents.
BLOOD PRESSURE
Large clinical trials of TZDs demonstrate small but consistent reductions in blood pressure. For example, in the PROACTIVE study, in which 75% of subjects had a history of hypertension, pioglitazone reduced systolic blood pressure by a mean of 3 mm Hg, compared with placebo (92). In a Diabetes Outcome Progression Trial (ADOPT), in which 78% of patients had a history of hypertension, monotherapy with rosiglitazone, compared with metformin or glyburide, was associated with 1–2 mm Hg lower systolic and diastolic blood pressure after 4 years of treatment (93). In the open-label Rosiglitazone Evaluated for Cardiovascular Outcomes in Oral Agent Combination Therapy (RECORD) trial, there was no difference in office blood pressure when rosiglitazone was added to sulfonylurea or metformin, compared with the combination of sulfonylurea and metformin (94). However, in a subset of 759 patients (85% with a history of hypertension) who underwent ambulatory blood pressure monitoring, 24 h mean systolic and diastolic blood pressure were 2–3 mm Hg lower in the rosiglitazone arm after 1 year of treatment (95). A reduction of blood pressure by 2–3 mm Hg, if sustained over the long term, might be expected to significantly reduce the risk of adverse renal and cardiovascular events, particularly congestive heart failure (96). However, in clinical trials with a relatively brief duration of observation, early effects of PPAR-γ activators to increase plasma volume and promote heart failure might dominate before any long-term beneficial effect to reduce heart failure through blood pressure reduction became evident.
ENDOTHELIAL FUNCTION, VASCULAR SMOOTH MUSCLE CELL PROLIFERATION, AND RESTENOSIS
PPAR-γ is expressed in human vascular endothelial cells (97). Vascular endothelial dysfunction usually accompanies type 2 diabetes and metabolic syndrome. Both animal models and clinical studies of these conditions are characterized by reduced number and functionality of endothelial progenitor cells (EPC) (98–100). In turn, reduced EPC number has been related to the degree of impairment of flow-mediated vasodilation in type 2 diabetes (101). In experimental animal models and clinical studies, TZD drugs have been shown to stimulate production of EPCs and promote endothelial repair after vascular injury (102–106).
Activation of PPAR-γ with TZDs increases endothelial nitric oxide bioavailability by direct actions on endothelium, including suppression of inflammatory gene expression or activation of endothelial AMP-activated protein kinase, or indirect mechanisms related to increased adiponectin and decreased circulating NEFA concentrations (20, 107–109). In patients with type 2 diabetes or other insulin-resistant states, TZD treatment improves brachial artery endothelial function, as marked by greater flow or acetylcholine-mediated vasodilation (75, 110–112). Improvements in coronary endothelium-dependent vasodilation have been demonstrated with pioglitazone (113, 114).
PPAR-γ is expressed in vascular smooth muscle, where its activation may inhibit proliferation and migration (115–118). These effects, along with those to promote endothelial repair, have raised interest in PPAR-γ activators as a potential strategy to prevent restenosis after percutaneous coronary revascularization. Several small, randomized trials have been performed in diabetic and nondiabetic patients after bare metal stent implantation, with clinical and angiographic endpoints. Meta-analyses of these trials suggest that TZDs may reduce late lumen loss and need for further target vessel revascularization, with more convincing effects in diabetics and with pioglitazone than in nondiabetics or with rosiglitazone (119–121). It has also been hypothesized that salutary endothelial effects of PPAR-γ activation might reduce the risk of stent thrombosis after drug-eluting stent implantation (122), but to date there are no data to either corroborate or refute this.
DEVELOPMENT AND PROGRESSION OF ATHEROSCLEROSIS
Because PPAR-γ is expressed in vascular tissues, including endothelium and smooth muscle, as well as in macrophages that reside in atherosclerotic lesions, a role of PPAR-γ in modulating the development and progression of atherosclerosis has been postulated. Most, but not all, experimental evidence indicates an antiatherogenic effect. Most animal studies have shown that PPAR-γ ligands retard progression of atherosclerosis (18), whereas genetic deletion of PPAR-γ from macrophages accelerates atherosclerosis (123, 124). In rabbits with atherosclerosis induced by high-cholesterol diet and balloon arterial injury, 3 months of treatment with pioglitazone reduced lesion inflammation, as gauged noninvasively by 18F-deoxyglucose positron emission tomography and dynamic contrast-enhanced magnetic resonance imaging and pathologically by lesion macrophage density and neovascularization (125). However, a study of advanced atherosclerotic lesions in LDL-receptor knockout mice showed that TZD drugs promoted plaque necrosis (27), and a recent study determined that atheromatous human aortas are enriched in soluble lipid mediators that may induce neovascularization of the arterial wall through a PPAR-γ dependent mechanism (28). Clinically, such effects might predispose to plaque hemorrhage and rupture.
A potential synthesis of these seemingly contradictory data is that PPAR-γ may oppose atherosclerosis in its early stages but exert different and deleterious effects in advanced atherosclerotic lesions. Studies examining whether vascular PPAR-γ expression is altered in human atherosclerosis also provide conflicting evidence. In one study, PPAR-γ mRNA expression was lower in atheromatous carotid endarterectomy and abdominal aortic aneurysm specimens than in normal inferior mesenteric arteries excised during colectomy (126). In contrast, another study of human aortas sampled at autopsy found increased expression of PPAR-γ mRNA and protein in areas of atherosclerosis (127). These studies leave open the question of whether vascular PPAR-γ favorably modulates the progression of atherosclerosis, unfavorably modulates this process, or plays no direct pathophysiologic role. The first possibility is suggested by a study that demonstrated the potential of PPAR-γ agonists to promote differentiation of human monocytes into anti-inflammatory M2 macrophages and that showed a positive correlation between expression of PPAR-γ mRNA and M2 markers in human carotid atherosclerotic lesions (128).
Clinical studies have utilized vascular imaging techniques to evaluate the effects of TZD agents on atherosclerosis progression, as marked by cIMT, coronary atheroma volume by intravascular ultrasound (IVUS), coronary artery calcification by computed tomography, or arterial pulse wave velocity. Major trials are summarized in Table 2. In aggregate, these studies suggest that TZDs retard atherosclerosis progression, with more convincing evidence for pioglitazone than rosiglitazone.
TABLE 2.
Major randomized trials of TZD drugs with clinical or imaging outcomes
| Trial, Year (Ref.) | Patient Population | Drug | Comparator | Design | Sample Size; Follow-up | Primary Outcome Measure | Key Results |
| Clinical outcomes | |||||||
| PROACTIVE, 2005 (92) | Type 2 diabetes with evidence of macrovascular disease | Pioglitazone | Placebo | Double-blind | N = 5,248; 34.5 mo (mean) | Death, nonfatal acute MI, CVA, ACS, coronary or peripheral artery revascularization, or lower extremity amputation | Primary outcome: Pioglitazone 19.7%; placebo 21.7%; HR 0.90, P = 0.095. Principal secondary outcome (death, acute MI, or CVA): Pioglitazone 11.6%; placebo 13.6%; HR 0.84, P = 0.027. |
| DREAM, 2006 (191) | Impaired fasting glucose or impaired glucose tolerance without diabetes or prior CV disease | Rosiglitazone | Placebo | Double-blind; factorial design with ramipril/placebo | N = 5,269; 3 yr (median) | Incident diabetes or death | Primary outcome: Rosiglitazone 11.6%; placebo 26% ; HR 0.40, P < 0.0001. Death: Rosiglitazone 1.1%; placebo 1.3%; NS. CV death, acute MI, or CVA: Rosiglitazone 1.2%; placebo 0.9%; HR 1.39, NS. |
| ADOPT, 2006 (193) | Recently diagnosed type 2 diabetes | Rosiglitazone | Metformin or glyburide | Double-blind | N = 4,360; 4 yr (median) | Failure of monotherapy to control diabetes | Primary outcome: Rosiglitazone 15%, metformin 21%, glyburide 34%. Serious CV events (fatal or nonfatal acute MI, CVA, or CHF): Rosiglitazone 3.4%, metformin 3.2%, glyburide 1.8% (P < 0.05 vs. rosiglitazone). |
| RECORD, 2009 (94) | Type 2 diabetes | Rosiglitazone added to metformin or SU | Combination of metformin and SU | Open-label, noninferiority | N = 4,447; 5.5 yr (mean) | CV hospitalization or CV death | Primary outcome: 14.5% both groups, HR 0.99.CV death, acute MI, or CVA: Rosiglitazone + metformin or SU 6.9%; metformin + SU 7.4%; HR 0.93, NS. |
| Imaging outcomes | |||||||
| CHICAGO, 2006 (130) | Recently diagnosed type 2 diabetes without symptomatic atherosclerosis | Pioglitazone | Glimepiride | Double-blind | N = 361; up to 72 wk | cIMT: Change from baseline, posterior wall of common carotid arteries | Primary outcome: Pioglitazone mean −0.001 mm; glimepiride mean +0.012 mm, P = 0.02. |
| STARR, 2009 (129) | Substudy of DREAM | Rosiglitazone | Placebo | See DREAM | N = 1,256; up to 3 yr | cIMT: Aggregate change from baseline in 12 1-cm long carotid arterial segments | Primary outcome: Rosiglitazone mean 0.0064 mm; placebo mean 0.0088 mm; P = 0.10. Secondary outcome: Change of cIMT in posterior wall of common carotid arteries. Rosiglitazone mean 0.0017 mm; placebo mean 0.0054 mm, P = 0.03. |
| PERISCOPE, 2008 (133) | Type 2 diabetes with CHD | Pioglitazone | Glimepiride | Double-blind | N = 360; up to 18 mo | Coronary IVUS: Change from baseline in percentage atheroma volume | Primary outcome: Pioglitazone mean −0.16%; glimepiride +0.73%, P = 0.002. |
| APPROACH, 2010 (139) | Type 2 diabetes with CHD | Rosiglitazone | Glipizide | Double-blind | N = 462; up to 18 mo | Coronary IVUS: Change from baseline in percentage atheroma volume | Primary outcome: Rosiglitazone mean −0.21%; glipizide +0.43%, P = 0.12. |
CVA, cerebrovascular accident; HR, hazard ratio; NS, not significant; SU, sulfonylurea.
The Study of Atherosclerosis with Ramipril and Rosiglitazone (STARR) (129) compared effects of rosiglitazone with placebo on cIMT progression in 1,256 subjects with impaired glucose tolerance or impaired fasting glucose, but without diabetes or overt cardiovascular disease. The primary outcome was an aggregate measure of cIMT in 12 carotid arterial segments. After 3 years of follow-up, there was a trend (P = 0.10) to less cIMT progression in the rosiglitazone group. A secondary outcome, cIMT progression in the posterior wall of the common carotid arteries, showed significantly less progression in the rosiglitazone group compared with placebo (P = 0.03). The Carotid Intima-Media Thickness in Atherosclerosis Using Pioglitazone (CHICAGO) trial (130) compared effects of pioglitazone with glimepiride on posterior wall common carotid cIMT in 361 patients with type 2 diabetes. After observation up to 18 months, cIMT did not progress under pioglitazone treatment, but it increased with glimepiride (P = 0.02). However, in a subset of the CHICAGO cohort, pioglitazone had no effect on progression of coronary artery calcification (131). A meta-analysis of nine placebo or active comparator-controlled trials of pioglitazone or rosiglitazone (not including STARR and CHICAGO) in 1,400 patients with type 2 diabetes showed a significant favorable effect of TZD treatment on cIMT progression, without significant difference in the effects of the two drugs (132). A reduction of arterial pulse wave velocity was also demonstrated with TZD treatment, suggesting a favorable effect on arterial stiffness.
The Pioglitazone Effect on Regression of Intravascular Sonographic Coronary Obstruction Prospective Evaluation (PERISCOPE) trial compared 18 months of treatment with pioglitazone or glimepiride on coronary atheroma volume in 360 diabetic patients. Percentage atheroma volume decreased slightly with pioglitazone but progressed with glimepiride (difference P = 0.002) (133). Several small trials, each with 54 or fewer participants, have examined the effects of 6–8 months of treatment with pioglitazone or placebo on coronary atheroma progression and ultrasonic tissue characteristics in patients with type 2 diabetes. These studies have shown that pioglitazone reduces coronary atheroma progression and/or volume of necrotic core (134–137). In contrast, a trial comparing 12 months of treatment with rosiglitazone versus placebo in 193 patients (138) showed no significant effect of rosiglitazone on saphenous vein bypass graft atherosclerosis assessed by IVUS, and the Assessment on the Prevention of Progression by Rosiglitazone on Atherosclerosis in Diabetes Patients with Cardiovascular History (APPROACH) trial found no difference in progression of coronary atheroma volume after up to 18 months of treatment with rosiglitazone or glipizide in 462 patients (139).
ISCHEMIA/REPERFUSION INJURY
Some experimental evidence suggests that PPAR-γ activation mitigates ischemia/reperfusion injury. In normal or insulin-resistant rodents, treatment with PPAR-γ activators improved contractile recovery and/or reduced infarct size after ischemia and reperfusion (140–149). Intravenous troglitazone reduced myocardial infarct size in dogs (150). Chronic, oral troglitazone suppressed inflammatory responses and improved postischemic contractile function in pigs (151), but the protective effects of troglitazone may have been due to its α-tocopherol moiety rather than PPAR-γ activation, as treatment with equimolar α-tocopherol recapitulated the beneficial effects of troglitazone, whereas treatment with rosiglitazone (which does not have a tocopherol moiety) did not. Other studies have investigated whether TZDs affect postinfarction left ventricular remodeling. The evidence is mixed, with some studies demonstrating reduced postinfarction fibrosis and improved systolic function (152, 153), while others show neutral or adverse effects on left ventricular remodeling and survival (154, 155).
Rodent studies suggest that cerebral ischemia/reperfusion injury may also be favorably modified by PPAR-γ activation, manifest by improved recovery of neurologic function and/or decreased infarct size (156–161). In these studies, postulated mechanisms of cerebral protection by PPAR-γ include attenuation of inflammatory responses in injured tissue, promotion of signaling through protective stress kinases, diminished apoptosis, and enhanced tissue availability of nitric oxide. Cerebral-protective effects of PPAR-γ activators have been demonstrated with either systemic or local treatment (161) and with either TZD or non-TZD agents (156).
ADVERSE AND/OR OFF-TARGET CARDIOVASCULAR ACTIONS OF PPAR-γ ACTIVATORS
As described in preceding sections, the portfolio of potentially beneficial cardiovascular effects of PPAR-γ activation might lead to the expectation that TZDs would provide clinical benefit to patients with or at risk for cardiovascular disease. However, as discussed in succeeding sections, clinical trials have not clearly demonstrated such benefit. The apparent divergence between metabolic and vascular actions of PPAR-γ activation and clinical outcomes with TZD treatment raises a pivotal question: Do adverse cardiovascular effects of PPAR-γ activation and/or off-target effects of TZDs attenuate or nullify the potential clinical benefit of this therapeutic approach?
Plasma volume expansion and heart failure
Effects of PPAR-γ activation on sodium retention and plasma volume have the potential to cause edema and significant heart failure (22). Treatment of patients without preexisting heart failure with TZD agents leads to an increase in circulating natriuretic peptides (162–164), plasma volume (164–166), and extracellular fluid volume (167, 168). PPAR-γ is expressed in the nephron, particularly in the collecting duct (169). Mice with collecting duct-specific deletion of PPAR-γ are resistant to rosiglitazone-induced plasma volume expansion (166). Mechanisms of sodium and volume retention via PPAR-γ may be enhanced transcription of the kinase SGK1 (170), which in turn activates the renal epithelial sodium channel, as well as nontranscriptional effects of PPAR-γ in the proximal tubule (171), with both actions promoting sodium retention (169). Patients with underlying systolic and/or diastolic cardiac dysfunction or a history of congestive heart failure may be particularly prone to develop heart failure in response to treatment with a PPAR-γ activator, due to increased plasma volume. However, neither animal (151, 172) nor clinical studies (163, 173, 174) indicate that heart failure resulting from TZD treatment is due to direct, adverse effects of TZDs on left ventricular systolic function. Moreover, some studies indicate salutory effects of TZDs on diastolic function (175–177). Preclinical studies with novel, non-TZD PPAR-γ activators suggest that these agents may enhance insulin sensitivity to a similar degree as TZD agents, but with less expansion of plasma or extracellular volume (178, 179). Whether these novel PPAR-γ activators will pose a lower risk for congestive heart failure remains to be established.
Ion channels and electrophysiology
Increasing evidence suggests that TZD compounds may affect cardiac ion channels and arrhythmias through off-target mechanisms. In vitro studies indicate that TZDs block voltage-gated potassium channels and L-type calcium channels in isolated ventricular cardiomyocytes (25, 180) and block ATP-sensitive potassium current in channels from cardiac and noncardiac cells (181–185). Because these effects are immediate, they suggest nontranscriptional effects of the drugs rather than classical effects via nuclear PPAR-γ. In vivo studies indicate a potential for TZD drugs to influence cardiac arrhythmias. In dogs, troglitazone promotes dephosphorylation of connexin 43, a gap junction protein, in response to ischemia and reperfusion (150), an effect with possible pro-arrhythmic consequences. In pigs, acute treatment with troglitazone, rosiglitazone, or pioglitazone, resulting in clinically relevant plasma concentrations, blocks cardiac ATP-sensitive potassium (KATP) channels, promotes ischemic ventricular fibrillation, and impairs the success of defibrillation (24, 26). These pro-arrhythmic effects are recapitulated by the prototypical KATP blocker glyburide. The mechanism by which TZDs close the KATP channel may be via phosphorylation of its inward rectifying subunit (186). To date, no human cardiac electrophysiology studies have been performed before and after TZD treatment, and therefore, it remains unknown whether TZD treatment increases the propensity for cardiac arrhythmias in patients.
KEY CLINICAL TRIALS AND OBSERVATIONAL ANALYSES OF PIOGLITAZONE OR ROSIGLITAZONE IN PATIENTS WITH, OR AT RISK FOR CARDIOVASCULAR DISEASE
Although the balance of data from experimental studies and clinical studies with surrogate endpoints supports the plausibility of cardiovascular benefit from PPAR-γ activation, proof of efficacy depends on the results of large, randomized clinical trials that assess clinically relevant cardiovascular outcomes. To date, that proof remains elusive. This section draws inferences from key trials performed with pioglitazone and rosiglitazone (Table 2) and discusses limitations of the data.
PROACTIVE
PROACTIVE is the only completed trial to test a hypothesis of superiority of a TZD over placebo with regard to cardiovascular outcomes (92, 187). The trial compared pioglitazone 45 mg daily (or maximum tolerated dose) with placebo in 5,238 patients with type 2 diabetes, HbA1c greater than 6.5% despite treatment with other hypoglycemic agents, and evidence of macrovascular coronary, peripheral arterial, or cerebrovascular atherosclerotic disease. The primary outcome measure was the composite of all-cause mortality; nonfatal MI or acute coronary syndrome (ACS); stroke; coronary- or lower extremity arterial revascularization procedure; or amputation of a lower extremity above the ankle. At baseline, the median duration of diabetes was 8 years. Notably, only 43% of patients were treated with a statin at baseline and only 55% after randomization. In addition, imbalances in the use of insulin and metformin developed after randomization, with these agents used more often in the placebo group (46% and 64%) than in the pioglitazone group (36% and 58%). Insulin doses were also higher in the placebo group (188). After an average observation time of 34.5 months, the primary efficacy measure was not significantly affected by treatment with pioglitazone, with a hazard ratio of 0.90 (95% confidence interval 0.80–1.02, P = 0.095). Although no individual component of the primary composite endpoint was significantly affected by treatment assignment, there were trends toward reductions in nonfatal coronary events and stroke with pioglitazone. A prespecified secondary efficacy measure, time to first occurrence of death, MI (excluding silent), or stroke, was significantly reduced by pioglitazone with hazard ratio 0.84 (P = 0.03). Moreover, in a prespecified subgroup analysis, pioglitazone reduced the risk of recurrent MI by 28% among 2,445 patients with prior MI (189). However, investigator-reported heart failure and hospitalization for heart failure were significantly more frequent in the pioglitazone group.
Although PROACTIVE results support cardiovascular efficacy of pioglitazone, the evidence provided is inconclusive. First, although pioglitazone had a favorable effect on coronary events, an effect on the primary composite endpoint was diluted by null or negative-trending effects on lower extremity amputation or revascularization. Second, the Kaplan-Meier curves for the primary and key secondary cardiovascular efficacy measures showed no divergence for the first year but continuing divergence thereafter. This suggests that a longer observation period might have allowed a conclusive demonstration of efficacy of pioglitazone. Third, imbalances between the two treatment arms in the frequency and intensity of use of other diabetic therapies may have confounded the results if these agents also affected cardiovascular outcomes. Fourth, contemporary guidelines endorse statin use in the vast majority of patients who were included in PROACTIVE; however, only about half the patients in the trial were actually treated with a statin. In a posthoc analysis, the risk of death, MI, or stroke was reduced by 25% with pioglitazone among patients not treated with statin, but it was reduced by only 5% with pioglitazone among patients who were treated with a statin (43). Further studies will be required to address the question of whether pioglitazone or other PPAR-γ activators provide clinical benefit incremental to statins.
RECORD, DREAM, ADOPT, and ACT NOW
The RECORD trial (94) tested a noninferiority hypothesis for rosiglitazone as second-line therapy in type 2 diabetes. In 4,447 subjects with type 2 diabetes not optimally controlled on monotherapy with metformin or sulfonylurea, the trial compared the addition of rosiglitazone to either metformin or sulfonylurea with the combination of metformin and sulfonylurea. The primary endpoint was time to cardiovascular hospitalization (including hospitalization for heart failure) or cardiovascular death. During open-label, randomized treatment there were significant imbalances in the use of diuretics and statins (both used more frequently in the rosiglitazone group). The rosiglitazone group exhibited lower levels of HbA1c and higher levels of LDL-C, HDL-C, and weight. After mean follow up of 5.5 years, primary endpoint events occurred in 321 patients in the rosiglitazone group and 323 patients in the metformin/sulfonylurea group, thus meeting the criterion for noninferiority of rosiglitazone. Fatal or nonfatal heart failure occurred more frequently in the rosiglitazone group than in the active control group (61 versus 29 patients). Limitations of RECORD include an event rate that was substantially lower than that projected in trial design with consequent reduction of statistical power, potential confounding by differential use of statins and diuretics, and open-label design.
The Diabetes Reduction Assessment with Ramipril and Rosiglitazone Medication (DREAM) trial (190, 191) was 2-factor, placebo-controlled study designed to determine whether rampril and/or rosiglitazone treatment prevents onset of diabetes among 5,269 nondiabetic patients with impaired fasting glucose or abnormal glucose tolerance. Rosiglitazone significantly reduced the progression to new onset diabetes during active treatment with the drug. Despite that benefit, a prespecified cardiorenal outcome measure, including cardiovascular events (MI, stroke, cardiovascular death, revascularization, heart failure, angina) and renal events (progression to microalbuminuria or macroalbuminuria), showed no difference between rosiglitazone and placebo over a mean 3 years of observation (rosiglitazone 15.7%, control 16%). Rosiglitazone was associated with more frequent development of heart failure. There was no interaction between rosiglitazone and ramipril on cardiovascular outcomes. The Actos Now for the Prevention of Diabetes (ACT NOW) trial (192) addressed a similar question to DREAM in 602 patients with impaired glucose tolerance who were randomized to receive either pioglitazone 45 mg or placebo. After a mean follow-up of 2.2 years, progression to diabetes occurred in 5% of the pioglitazone group compared with 16.7% of the placebo group, but too few cardiovascular events occurred (pioglitazone 26, placebo 23) to draw any inferences regarding effect of treatment on cardiovascular outcomes.
A Diabetes Outcome Progression Trial (ADOPT) (193) compared glycemic durability of rosiglitazone, metformin, and glyburide as first-line treatment in 4,360 patients with newly diagnosed type 2 diabetes. The primary outcome was monotherapy treatment failure, defined by a fasting blood glucose greater than 10 mmol/l after 6 weeks at maximum dose of assigned agent. At 5 years of follow-up, significantly fewer patients had failed rosiglitazone monotherapy (15%) compared with either metformin (21%) or glyburide (34%). HbA1c levels remained lower and insulin sensitivity higher in the rosiglitazone group, but with higher levels of LDL-C. In this relatively low-risk population, few cardiovascular events occurred. Notwithstanding this limitation, event frequencies, including heart failure, were similar in the rosiglitazone and metformin groups but lower in the glyburide group.
In composite, RECORD, DREAM, and ADOPT suggest that improved glycemic control with rosiglitazone is not accompanied by a corresponding reduction of cardiovascular risk. The findings again call into question whether insulin resistance and/or hyperglycemia are primary determinants of cardiovascular risk and/or whether glycemic benefits of rosiglitazone were negated by other actions of the drug, including a greater propensity for heart failure and increased LDL-C.
Meta-analyses
Meta-analyses of clinical trials with pioglitazone or rosiglitazone provide further insight into their cardiovascular effects. An analysis of 19 randomized controlled trials with pioglitazone, including PROACTIVE and comprising 16,390 patients, showed that the drug was associated with a significantly lower risk of death, MI, or stroke compared with corresponding control groups (4.4% versus 5.7%, hazard ratio 0.82, P = 0.005) (194). Similarly, a meta-analysis of 94 trials with pioglitazone, excluding PROACTIVE and comprising over 20,000 patients, showed that pioglitazone was associated with significantly lower all-cause mortality (195), with the caveat that the trials comprised only 57 deaths. In contrast, a meta-analysis of 56 randomized controlled trials with rosiglitazone comprising 35,531 patients showed that the drug was associated with an excess risk of MI (odds ratio 1.28, P = 0.04), albeit without excess cardiovascular mortality (196). These findings contributed to decisions by regulatory agencies to withdraw rosiglitazone in Europe and limit its use in the United States (197).
Observational analyses
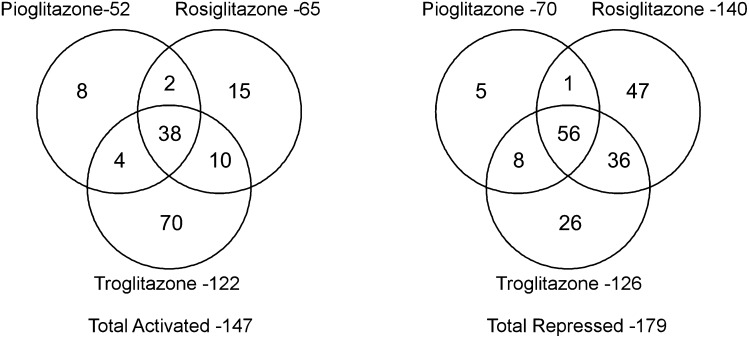
Juxtaposition of generally favorable cardiovascular outcomes associated with pioglitazone with neutral to unfavorable outcomes associated with rosiglitazone suggests divergent effects on cardiovascular risk. Unfortunately, no randomized comparison of the two drugs has been performed to test this hypothesis. Instead, inferences must be drawn from analyses of observational databases. Six large, retrospective cohort studies compared treatment with rosiglitazone to treatment pioglitazone (as monotherapy or in combination with other hypoglycemic agents). After correction for potential confounders, each of these analyses found a greater incidence of major adverse cardiovascular events among patients treated with rosiglitazone than pioglitazone (198–203). A meta-analysis of 16 observational studies comparing rosiglitazone with pioglitazone comprising 810,000 patients found significant adverse odds ratios associated with rosiglitazone (1.14 for death, 1.16 for MI, and 1.22 for congestive heart failure) (204). The implications of these findings are 2-fold: first, there is no reasonable basis for the ongoing use of rosiglitazone when pioglitazone provides a safer alternative, and second, the data reinforce the concept that safety and efficacy of new PPAR modulators must be evaluated on a compound-by-compound basis, rather than as a drug class. Compounds that nominally may be considered to be in the same class of PPAR activators may in fact exert very different effects on gene transcription, in part related to PPAR ligand-specific effects on coactivator or corepressor recruitment. Notwithstanding the limitations of an in vitro cell culture model, a recent study demonstrated that rosiglitazone, pioglitazone, and troglitazone regulated more genes discordantly than concordantly in adipocytes (Fig. 2) (205).
Fig. 2.
Venn diagram of genes regulated by pioglitazone, rosiglitazone, and troglitazone. In cultured 3T3-L1 adipocytes, expression of mRNA was assessed using microarrays (GeneChip, Affymetrix) after 24 h of exposure to vehicle (DMSO), pioglitazone (20 μM), rosiglitazone (1 μM), or troglitazone (20 μM), concentrations known to elicit maximal biological effects. Despite significant overlap in genes activated or repressed by the three TZD agents, a substantial number of genes were regulated discordantly. The findings emphasize that each PPAR ligand induces a unique transcriptional effect, and they may provide an explanation for differences in clinical outcomes with pioglitazone and rosiglitazone. Reproduced from Ref. 205 with permission.
NEW PPAR-γ ACTIVATORS IN DEVELOPMENT
Pioglitazone and rosiglitazone act as full PPAR-γ agonists. Selective partial PPAR-γ agonists are being developed in the hope that they will produce similar insulin-sensitizing effects as TZDs but with fewer of the unwanted effects of a full agonist, including adipogenic weight gain, fluid retention, heart failure, and possibly pathologic bone remodeling and fractures. One such compound is INT-131, which has been evaluated in an 8-week, phase II, placebo-controlled monotherapy study in 70 patients with type 2 diabetes but without evident cardiovascular or renal disease (206). Doses of 1 mg or 10 mg daily were evaluated. At 1 mg, average fasting blood glucose was reduced from 163 to 142 mg/dl without an increase in body weight but also without significant changes in fasting insulin, adiponectin, or NEFA. At 10 mg, average fasting blood glucose was reduced from 183 to 137 mg/dl, an effect similar to monotherapy with maximum doses of pioglitazone or rosiglitazone, accompanied by significant reductions in insulin, adiponectin, and NEFA, but with a mean 1 kg increase in body weight. At present, it remains uncertain whether there will be any meaningful advantage of partial PPAR-γ activators at doses that provide glycemic control equivalent to approved doses of pioglitazone or rosiglitazone. In addition, at least two new TZD PPAR-γ agonists, rivoglitazone, a potent agonist (207), and balaglitazone, a partial agonist (208), are being evaluated in type 2 diabetes, but any advantages of these compounds over pioglitazone also remain uncertain.
A future therapeutic approach to modulate PPAR-γ action may be to interfere with its phosphorylation by cyclin-dependent kinase (CDK)5. Obesity and high-fat feeding increase CDK5 activity and PPAR-γ phosphorylation, which attenuates its insulin-sensitizing effects (209). One of the actions of TZDs is to prevent phosphorylation of PPAR-γ by CDK5. SR1664, a compound in preclinical development, appears to block phosphorylation of PPAR-γ by CDK5 without the full PPAR-γ agonist effects of TZDs. Consequently, animal and in vitro studies suggest that SR1664 exerts antidiabetic effects without increasing adioposity, causing fluid retention, or affecting bone metabolism (210).
Considerable effort has been devoted to development of dual PPAR-α/γ activators that combine effects of PPAR-α to lower circulating triglycerides and raise HDL-C with effects of PPAR-γ to enhance insulin sensitivity. Glitazars are a class of drugs that activate both PPAR-α and PPAR-γ. Several glitazars progressed through phase II development but were then abandoned for various reasons, including development of bladder cancer in rodents (ragaglitazar), liver function abnormalities (imiglitazar), reduction in glomerular filtration rate (tesaglitazar), or an excess of adverse cardiovascular events in meta-analysis of small trials (muraglitazar) (211–213). Nonetheless, other dual PPAR-α/γ activators are in development. Among them, aleglitazar (214) has proceeded to phase III development.
PIVOTAL ONGOING CLINICAL TRIALS OF PPAR-γ ACTIVATORS IN PATIENTS WITH CARDIOVASCULAR DISEASE
Two large, ongoing multicenter international trials may provide important new information about the efficacy and safety of PPAR-γ activation in patients with cardiovascular disease. The Insulin Resistance Intervention after Stroke (IRIS) trial (www.clinicaltrials.gov NCT00091949) is a randomized comparison of pioglitazone (up to 45 mg daily) with placebo in nondiabetic, insulin-resistant patients with a recent ischemic stroke or transient ischemic attack. The criterion for insulin resistance is a HOMA-IR index greater than 3, measured 2 weeks to 6 months after the index event. Approximately 3,800 patients will be followed for a minimum of 3 years. The primary efficacy measure is time to first fatal or nonfatal stroke or MI. Recruitment will be completed in 2012, with results expected in 2015. IRIS may provide important information in several respects. First, because approximately half of nondiabetic patients with acute coronary or cerebrovascular syndromes are insulin resistant (215, 216), potential efficacy of pioglitazone in such populations would have broad applicability. Second, because patients in IRIS are not diabetic at enrollment, the trial is less likely to be confounded by differential use of other diabetic medications in the two treatment arms than prior trials of TZDs in diabetic subjects. Third, IRIS should determine whether pioglitazone adds clinical benefit in patients treated with statins, a question left open by PROACTIVE (43).
Aleglitazar is the first dual PPAR-α/γ activator to proceed to phase III development. Because aleglitazar is believed to be a balanced PPAR- α/γ activator (i.e., relatively less PPAR-α activation than evaluated doses of tesaglitazar and relatively less PPAR-γ activation than evaluated doses of muraglitazar), it is hoped that renal and cardiovascular safety concerns with those previous agents will be avoided (217). The Alecardio trial (www.clinicaltrials.gov NCT01042769) compares aleglitazar with placebo in approximately 7,000 patients with type 2 diabetes and recent ACS. The primary efficacy measure is time to first occurrence of death or nonfatal MI or stroke. The trial will continue until 950 primary endpoint events have accrued.
SUMMARY AND FUTURE DIRECTIONS
Each PPAR ligand interacts differently with PPAR-retinoic acid dimers and attracts coactivators and corepressors in a ligand-specific manner. Therefore, transcriptional and clinical effects of each PPAR activator may differ from others. Inability to assume class effects adds complexity to preclinical and clinical development of new PPAR activator drugs. Although there is strong biological rationale for PPAR-γ activation to reduce cardiovascular risk, current clinical evidence leaves this hypothesis unproven. At present, supporting evidence is greatest for a clinical benefit of pioglitazone. However, to establish a secure role in cardiovascular therapeutics for pioglitazone, other selective PPAR-γ agonists, or dual PPAR agonists, ongoing and future clinical trials must go beyond the limitations of prior studies. Specifically, trials must evaluate agents on the basis of important clinical outcomes, including cardiovascular mortality, nonfatal MI, stroke, and heart failure; allow long enough exposure to observe modification of atherosclerotic risk; assess benefit in addition to statins; and avoid confounding by differential use of other diabetic medications.
Footnotes
Abbreviations:
- ACS
- acute coronary syndrome
- CDK
- cyclin-dependent kinase
- CHD
- coronary heart disease
- cIMT
- carotid intima-media thickness
- CV
- cardiovascular
- EPC
- endothelial progenitor cell
- HDL-C
- high-density lipoprotein cholesterol
- LDL-C
- low density lipoprotein cholesterol
- MI
- acute myocardial infarction
- PPAR-γ
- peroxisome proliferator-activated receptor γ
- TZD
- thiazolidinedione
This work was supported by the Medical Research Service, US Department of Veterans Affairs and by National Institutes of Health Grant 5-R01-HL-049944 (to G.G.S.). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health. G. G. Schwartz received research grant support from Roche Laboratories.
REFERENCES
- 1.Adams M., Montague C. T., Prins J. B., Holder J. C., Smith S. A., Sanders L., Digby J. E., Sewter C. P., Lazar M. A., Chatterjee V. K., et al. 1997. Activators of peroxisome proliferator-activated receptor gamma have depot-specific effects on human preadipocyte differentiation. J. Clin. Invest. 100: 3149–3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Festuccia W. T., Blanchard P. G., Turcotte V., Laplante M., Sariahmetoglu M., Brindley D. N., Deshaies Y. 2009. Depot-specific effects of the PPARgamma agonist rosiglitazone on adipose tissue glucose uptake and metabolism. J. Lipid Res. 50: 1185–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gray S. L., Vidal-Puig A. J. 2007. Adipose tissue expandability in the maintenance of metabolic homeostasis. Nutr. Rev. 65: S7–S12. [DOI] [PubMed] [Google Scholar]
- 4.Sharma A. M., Staels B. 2007. Review: peroxisome proliferator-activated receptor gamma and adipose tissue–understanding obesity-related changes in regulation of lipid and glucose metabolism. J. Clin. Endocrinol. Metab. 92: 386–395. [DOI] [PubMed] [Google Scholar]
- 5.Weisberg S. P., McCann D., Desai M., Rosenbaum M., Leibel R. L., Ferrante A. W., Jr 2003. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Invest. 112: 1796–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hajer G. R., van Haeften T. W., Visseren F. L. 2008. Adipose tissue dysfunction in obesity, diabetes, and vascular diseases. Eur. Heart J. 29: 2959–2971. [DOI] [PubMed] [Google Scholar]
- 7.Doshi K. B., Kashyap S. R., Brennan D. M., Hoar B. M., Cho L., Hoogwerf B. J. 2009. All-cause mortality risk predictors in a preventive cardiology clinic cohort-examining diabetes and individual metabolic syndrome criteria: a PRECIS database study. Diabetes Obes. Metab. 11: 102–108. [DOI] [PubMed] [Google Scholar]
- 8.Malik S., Wong N. D., Franklin S. S., Kamath T. V., L'Italien G. J., Pio J. R., Williams G. R. 2004. Impact of the metabolic syndrome on mortality from coronary heart disease, cardiovascular disease, and all causes in United States adults. Circulation. 110: 1245–1250. [DOI] [PubMed] [Google Scholar]
- 9.Ninomiya J. K., L'Italien G., Criqui M. H., Whyte J. L., Gamst A., Chen R. S. 2004. Association of the metabolic syndrome with history of myocardial infarction and stroke in the Third National Health and Nutrition Examination Survey. Circulation. 109: 42–46. [DOI] [PubMed] [Google Scholar]
- 10.Bajaj M., Baig R., Suraamornkul S., Hardies L. J., Coletta D. K., Cline G. W., Monroy A., Koul S., Sriwijitkamol A., Musi N., et al. 2010. Effects of pioglitazone on intramyocellular fat metabolism in patients with type 2 diabetes mellitus. J. Clin. Endocrinol. Metab. 95: 1916–1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mayerson A. B., Hundal R. S., Dufour S., Lebon V., Befroy D., Cline G. W., Enocksson S., Inzucchi S. E., Shulman G. I., Petersen K. F. 2002. The effects of rosiglitazone on insulin sensitivity, lipolysis, and hepatic and skeletal muscle triglyceride content in patients with type 2 diabetes. Diabetes. 51: 797–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McLaughlin T. M., Liu T., Yee G., Abbasi F., Lamendola C., Reaven G. M., Tsao P., Cushman S. W., Sherman A. 2010. Pioglitazone increases the proportion of small cells in human abdominal subcutaneous adipose tissue. Obesity (Silver Spring). 18: 926–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miyazaki Y., Mahankali A., Matsuda M., Mahankali S., Hardies J., Cusi K., Mandarino L. J., DeFronzo R. A. 2002. Effect of pioglitazone on abdominal fat distribution and insulin sensitivity in type 2 diabetic patients. J. Clin. Endocrinol. Metab. 87: 2784–2791. [DOI] [PubMed] [Google Scholar]
- 14.Nam J. S., Nam J. Y., Yoo J. S., Cho M., Park J. S., Ahn C. W., Cha B. S., Lee E. J., Lim S. K., Kim K. R., et al. 2010. The effect of rosiglitazone on insulin sensitivity and mid-thigh low-density muscle in patients with Type 2 diabetes. Diabet. Med. 27: 30–36. [DOI] [PubMed] [Google Scholar]
- 15.Derosa G., Salvadeo S. A. 2008. Pioglitazone and rosiglitazone: effects of treatment with a thiazolidinedione on lipids and non conventional cardiovascular risk factors. Curr. Clin. Pharmacol. 3: 77–84. [DOI] [PubMed] [Google Scholar]
- 16.Martens F. M., Visseren F. L., Lemay J., de Koning E. J., Rabelink T. J. 2002. Metabolic and additional vascular effects of thiazolidinediones. Drugs. 62: 1463–1480. [DOI] [PubMed] [Google Scholar]
- 17.Riera-Guardia N., Rothenbacher D. 2008. The effect of thiazolidinediones on adiponectin serum level: a meta-analysis. Diabetes Obes. Metab. 10: 367–375. [DOI] [PubMed] [Google Scholar]
- 18.Wang N., Yin R., Liu Y., Mao G., Xi F. 2011. Role of peroxisome proliferator-activated receptor-gamma in atherosclerosis: an update. Circ. J. 75: 528–535. [DOI] [PubMed] [Google Scholar]
- 19.Sigmund C. D. 2010. Endothelial and vascular muscle PPARgamma in arterial pressure regulation: lessons from genetic interference and deficiency. Hypertension. 55: 437–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duan S. Z., Usher M. G., Mortensen R. M. 2008. Peroxisome proliferator-activated receptor-gamma-mediated effects in the vasculature. Circ. Res. 102: 283–294. [DOI] [PubMed] [Google Scholar]
- 21.Giaginis C., Tsourouflis G., Theocharis S. 2008. Peroxisome proliferator-activated receptor-gamma (PPAR-gamma) ligands: novel pharmacological agents in the treatment of ischemia reperfusion injury. Curr. Mol. Med. 8: 562–579. [DOI] [PubMed] [Google Scholar]
- 22.Rubenstrunk A., Hanf R., Hum D. W., Fruchart J. C., Staels B. 2007. Safety issues and prospects for future generations of PPAR modulators. Biochim. Biophys. Acta. 1771: 1065–1081. [DOI] [PubMed] [Google Scholar]
- 23.Goldberg R. B., Kendall D. M., Deeg M. A., Buse J. B., Zagar A. J., Pinaire J. A., Tan M. H., Khan M. A., Perez A. T., Jacober S. J. 2005. A comparison of lipid and glycemic effects of pioglitazone and rosiglitazone in patients with type 2 diabetes and dyslipidemia. Diabetes Care. 28: 1547–1554. [DOI] [PubMed] [Google Scholar]
- 24.Lu L., Reiter M. J., Xu Y., Chicco A., Greyson C. R., Schwartz G. G. 2008. Thiazolidinedione drugs block cardiac KATP channels and may increase propensity for ischaemic ventricular fibrillation in pigs. Diabetologia. 51: 675–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hancox J. C. 2011. Cardiac ion channel modulation by the hypoglycaemic agent rosiglitazone. Br. J. Pharmacol. 163: 496–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sarraf M., Lu L., Ye S., Reiter M. J., Greyson C. R., Schwartz G. G. 2012. Thiazolidinedione drugs promote onset, alter characteristics, and increase mortality of ischemic ventricular fibrillation in pigs. Cardiovasc. Drugs Ther.. 26: 195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thorp E., Kuriakose G., Shah Y. M., Gonzalez F. J., Tabas I. 2007. Pioglitazone increases macrophage apoptosis and plaque necrosis in advanced atherosclerotic lesions of nondiabetic low-density lipoprotein receptor-null mice. Circulation. 116: 2182–2190. [DOI] [PubMed] [Google Scholar]
- 28.Ho-Tin-Noé B., Le D. J., Gomez D., Louedec L., Vranckx R., El-Bouchtaoui M., Legres L., Meilhac O., Michel J. B. 2011. Early atheroma-derived agonists of peroxisome proliferator-activated receptor-gamma trigger intramedial angiogenesis in a smooth muscle cell-dependent manner. Circ. Res. 109: 1003–1014. [DOI] [PubMed] [Google Scholar]
- 29.Amin R. H., Mathews S. T., Camp H. S., Ding L., Leff T. 2010. Selective activation of PPARgamma in skeletal muscle induces endogenous production of adiponectin and protects mice from diet-induced insulin resistance. Am. J. Physiol. Endocrinol. Metab. 298: E28–E37. [DOI] [PubMed] [Google Scholar]
- 30.He W., Barak Y., Hevener A., Olson P., Liao D., Le J., Nelson M., Ong E., Olefsky J. M., Evans R. M. 2003. Adipose-specific peroxisome proliferator-activated receptor gamma knockout causes insulin resistance in fat and liver but not in muscle. Proc. Natl. Acad. Sci. USA. 100: 15712–15717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hevener A. L., He W., Barak Y., Le J., Bandyopadhyay G., Olson P., Wilkes J., Evans R. M., Olefsky J. 2003. Muscle-specific Pparg deletion causes insulin resistance. Nat. Med. 9: 1491–1497. [DOI] [PubMed] [Google Scholar]
- 32.Savage D. B., Tan G. D., Acerini C. L., Jebb S. A., Agostini M., Gurnell M., Williams R. L., Umpleby A. M., Thomas E. L., Bell J. D., et al. 2003. Human metabolic syndrome resulting from dominant-negative mutations in the nuclear receptor peroxisome proliferator-activated receptor-gamma. Diabetes. 52: 910–917. [DOI] [PubMed] [Google Scholar]
- 33.Perriello G., Pampanelli S., Di P. C., Brunetti P. 2006. Comparison of glycaemic control over 1 year with pioglitazone or gliclazide in patients with Type 2 diabetes. Diabet. Med. 23: 246–252. [DOI] [PubMed] [Google Scholar]
- 34.Pavo I., Jermendy G., Varkonyi T. T., Kerenyi Z., Gyimesi A., Shoustov S., Shestakova M., Herz M., Johns D., Schluchter B. J., et al. 2003. Effect of pioglitazone compared with metformin on glycemic control and indicators of insulin sensitivity in recently diagnosed patients with type 2 diabetes. J. Clin. Endocrinol. Metab. 88: 1637–1645. [DOI] [PubMed] [Google Scholar]
- 35.Herz M., Johns D., Reviriego J., Grossman L. D., Godin C., Duran S., Hawkins F., Lochnan H., Escobar-Jimenez F., Hardin P. A., et al. 2003. A randomized, double-blind, placebo-controlled, clinical trial of the effects of pioglitazone on glycemic control and dyslipidemia in oral antihyperglycemic medication-naive patients with type 2 diabetes mellitus. Clin. Ther. 25: 1074–1095. [DOI] [PubMed] [Google Scholar]
- 36.Carey D. G., Cowin G. J., Galloway G. J., Jones N. P., Richards J. C., Biswas N., Doddrell D. M. 2002. Effect of rosiglitazone on insulin sensitivity and body composition in type 2 diabetic patients. [corrected] Obes. Res. 10: 1008–1015. [DOI] [PubMed] [Google Scholar]
- 37.D'Souza A., Hussain M., Howarth F. C., Woods N. M., Bidasee K., Singh J. 2009. Pathogenesis and pathophysiology of accelerated atherosclerosis in the diabetic heart. Mol. Cell. Biochem. 331: 89–116. [DOI] [PubMed] [Google Scholar]
- 38.McGuire D. K., Newby L. K., Bhapkar M. V., Moliterno D. J., Hochman J. S., Klein W. W., Weaver W. D., Pfisterer M., Corbalan R., Dellborg M., et al. 2004. Association of diabetes mellitus and glycemic control strategies with clinical outcomes after acute coronary syndromes. Am. Heart J. 147: 246–252. [DOI] [PubMed] [Google Scholar]
- 39.Ahmed S., Cannon C. P., Murphy S. A., Braunwald E. 2006. Acute coronary syndromes and diabetes: Is intensive lipid lowering beneficial? Results of the PROVE IT-TIMI 22 trial. Eur. Heart J. 27: 2323–2329. [DOI] [PubMed] [Google Scholar]
- 40.Schwartz G. G., Olsson A. G., Szarek M., Sasiela W. J. 2005. Relation of characteristics of metabolic syndrome to short-term prognosis and effects of intensive statin therapy after acute coronary syndrome: an analysis of the Myocardial Ischemia Reduction with Aggressive Cholesterol Lowering (MIRACL) trial. Diabetes Care. 28: 2508–2513. [DOI] [PubMed] [Google Scholar]
- 41.Bartnik M., Malmberg K., Norhammar A., Tenerz A., Ohrvik J., Ryden L. 2004. Newly detected abnormal glucose tolerance: an important predictor of long-term outcome after myocardial infarction. Eur. Heart J. 25: 1990–1997. [DOI] [PubMed] [Google Scholar]
- 42.Stratton I. M., Adler A. I., Neil H. A., Matthews D. R., Manley S. E., Cull C. A., Hadden D., Turner R. C., Holman R. R. 2000. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 321: 405–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ferrannini E., Betteridge D. J., Dormandy J. A., Charbonnel B., Wilcox R. G., Spanheimer R., Erdmann E., DeFronzo R. A., Laakso M. 2011. High-density lipoprotein-cholesterol and not HbA1c was directly related to cardiovascular outcome in PROactive. Diabetes Obes. Metab. 13: 759–764. [DOI] [PubMed] [Google Scholar]
- 44.Patel A., MacMahon S., Chalmers J., Neal B., Billot L., Woodward M., Marre M., Cooper M., Glasziou P., Grobbee D., et al. 2008. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 358: 2560–2572. [DOI] [PubMed] [Google Scholar]
- 45.Gerstein H. C., Miller M. E., Byington R. P., Goff D. C., Jr, Bigger J. T., Buse J. B., Cushman W. C., Genuth S., Ismail-Beigi F., Grimm R. H., Jr, et al. 2008. Effects of intensive glucose lowering in type 2 diabetes. N. Engl. J. Med. 358: 2545–2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Duckworth W., Abraira C., Moritz T., Reda D., Emanuele N., Reaven P. D., Zieve F. J., Marks J., Davis S. N., Hayward R., et al. 2009. Glucose control and vascular complications in veterans with type 2 diabetes. N. Engl. J. Med. 360: 129–139. [DOI] [PubMed] [Google Scholar]
- 47.Frye R. L., August P., Brooks M. M., Hardison R. M., Kelsey S. F., MacGregor J. M., Orchard T. J., Chaitman B. R., Genuth S. M., Goldberg S. H., et al. 2009. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N. Engl. J. Med. 360: 2503–2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sarafidis P. A., Bakris G. L. 2007. Non-esterified fatty acids and blood pressure elevation: a mechanism for hypertension in subjects with obesity/insulin resistance? J. Hum. Hypertens. 21: 12–19. [DOI] [PubMed] [Google Scholar]
- 49.Staiger H., Staiger K., Stefan N., Wahl H. G., Machicao F., Kellerer M., Haring H. U. 2004. Palmitate-induced interleukin-6 expression in human coronary artery endothelial cells. Diabetes. 53: 3209–3216. [DOI] [PubMed] [Google Scholar]
- 50.Steinberg H. O., Tarshoby M., Monestel R., Hook G., Cronin J., Johnson A., Bayazeed B., Baron A. D. 1997. Elevated circulating free fatty acid levels impair endothelium-dependent vasodilation. J. Clin. Invest. 100: 1230–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Steinberg H. O., Paradisi G., Hook G., Crowder K., Cronin J., Baron A. D. 2000. Free fatty acid elevation impairs insulin-mediated vasodilation and nitric oxide production. Diabetes. 49: 1231–1238. [DOI] [PubMed] [Google Scholar]
- 52.Giacca A., Xiao C., Oprescu A. I., Carpentier A. C., Lewis G. F. 2011. Lipid-induced pancreatic beta-cell dysfunction: focus on in vivo studies. Am. J. Physiol. Endocrinol. Metab. 300: E255–E262. [DOI] [PubMed] [Google Scholar]
- 53.Samuel V. T., Petersen K. F., Shulman G. I. 2010. Lipid-induced insulin resistance: unravelling the mechanism. Lancet. 375: 2267–2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Savage D. B., Semple R. K. 2010. Recent insights into fatty liver, metabolic dyslipidaemia and their links to insulin resistance. Curr. Opin. Lipidol. 21: 329–336. [DOI] [PubMed] [Google Scholar]
- 55.Charnock J. S. 1994. Lipids and cardiac arrhythmia. Prog. Lipid Res. 33: 355–385. [DOI] [PubMed] [Google Scholar]
- 56.Pilz S., Scharnagl H., Tiran B., Wellnitz B., Seelhorst U., Boehm B. O., Marz W. 2007. Elevated plasma free fatty acids predict sudden cardiac death: a 6.85-year follow-up of 3315 patients after coronary angiography. Eur. Heart J. 28: 2763–2769. [DOI] [PubMed] [Google Scholar]
- 57.Golay A., Swislocki A. L., Chen Y. D., Reaven G. M. 1987. Relationships between plasma-free fatty acid concentration, endogenous glucose production, and fasting hyperglycemia in normal and non-insulin-dependent diabetic individuals. Metabolism. 36: 692–696. [DOI] [PubMed] [Google Scholar]
- 58.Kashyap S., Belfort R., Gastaldelli A., Pratipanawatr T., Berria R., Pratipanawatr W., Bajaj M., Mandarino L., DeFronzo R., Cusi K. 2003. A sustained increase in plasma free fatty acids impairs insulin secretion in nondiabetic subjects genetically predisposed to develop type 2 diabetes. Diabetes. 52: 2461–2474. [DOI] [PubMed] [Google Scholar]
- 59.Mook S., Halkes C. C., Bilecen S., Cabezas M. C. 2004. In vivo regulation of plasma free fatty acids in insulin resistance. Metabolism. 53: 1197–1201. [DOI] [PubMed] [Google Scholar]
- 60.Chaudhuri A., Rosenstock J., Digenio A., Meneghini L., Hollander P., McGill J. B., Dandona P., Ilgenfritz J., Riddle M. 2012. Comparing the effects of insulin glargine and thiazolidinediones on plasma lipids in type 2 diabetes: a patient-level pooled analysis. Diabetes Metab. Res. Rev. 28: 258–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Martens F. M., Visseren F. L., de Koning E. J., Rabelink T. J. 2005. Short-term pioglitazone treatment improves vascular function irrespective of metabolic changes in patients with type 2 diabetes. J. Cardiovasc. Pharmacol. 46: 773–778. [DOI] [PubMed] [Google Scholar]
- 62.Miyazaki Y., Glass L., Triplitt C., Matsuda M., Cusi K., Mahankali A., Mahankali S., Mandarino L. J., DeFronzo R. A. 2001. Effect of rosiglitazone on glucose and non-esterified fatty acid metabolism in Type II diabetic patients. Diabetologia. 44: 2210–2219. [DOI] [PubMed] [Google Scholar]
- 63.Abbasi F., Lima N. K., Reaven G. M. 2009. Relationship between changes in insulin sensitivity and associated cardiovascular disease risk factors in thiazolidinedione-treated, insulin-resistant, nondiabetic individuals: pioglitazone versus rosiglitazone. Metabolism. 58: 373–378. [DOI] [PubMed] [Google Scholar]
- 64.Brackenridge A. L., Jackson N., Jefferson W., Stolinski M., Shojaee-Moradie F., Hovorka R., Umpleby A. M., Russell-Jones D. 2009. Effects of rosiglitazone and pioglitazone on lipoprotein metabolism in patients with Type 2 diabetes and normal lipids. Diabet. Med. 26: 532–539. [DOI] [PubMed] [Google Scholar]
- 65.Miyazaki Y., Mahankali A., Wajcberg E., Bajaj M., Mandarino L. J., DeFronzo R. A. 2004. Effect of pioglitazone on circulating adipocytokine levels and insulin sensitivity in type 2 diabetic patients. J. Clin. Endocrinol. Metab. 89: 4312–4319. [DOI] [PubMed] [Google Scholar]
- 66.Yamanouchi T., Sakai T., Igarashi K., Ichiyanagi K., Watanabe H., Kawasaki T. 2005. Comparison of metabolic effects of pioglitazone, metformin, and glimepiride over 1 year in Japanese patients with newly diagnosed Type 2 diabetes. Diabet. Med. 22: 980–985. [DOI] [PubMed] [Google Scholar]
- 67.Rajagopalan R., Xu Y., Abbadessa M. 2006. The effect of pioglitazone on glycemic and lipid parameters and adverse events in elderly patients with type 2 diabetes mellitus: a post hoc analysis of four randomized trials. Am. J. Geriatr. Pharmacother. 4: 123–133. [DOI] [PubMed] [Google Scholar]
- 68.Abbasi F., Chen Y. D., Farin H. M., Lamendola C., Reaven G. M. 2008. Comparison of three treatment approaches to decreasing cardiovascular disease risk in nondiabetic insulin-resistant dyslipidemic subjects. Am. J. Cardiol. 102: 64–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Diabetes Atorvastin Lipid Intervention (DALI) Study Group. 2001. The effect of aggressive versus standard lipid lowering by atorvastatin on diabetic dyslipidemia: the DALI study: a double-blind, randomized, placebo-controlled trial in patients with type 2 diabetes and diabetic dyslipidemia. Diabetes Care. 24: 1335–1341. [DOI] [PubMed] [Google Scholar]
- 70.Brochu-Gaudreau K., Rehfeldt C., Blouin R., Bordignon V., Murphy B. D., Palin M. F. 2010. Adiponectin action from head to toe. Endocrine. 37: 11–32. [DOI] [PubMed] [Google Scholar]
- 71.Berg A. H., Combs T. P., Du X., Brownlee M., Scherer P. E. 2001. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat. Med. 7: 947–953. [DOI] [PubMed] [Google Scholar]
- 72.Xu A., Yin S., Wong L., Chan K. W., Lam K. S. 2004. Adiponectin ameliorates dyslipidemia induced by the human immunodeficiency virus protease inhibitor ritonavir in mice. Endocrinology. 145: 487–494. [DOI] [PubMed] [Google Scholar]
- 73.Miyazaki Y., DeFronzo R. A. 2008. Rosiglitazone and pioglitazone similarly improve insulin sensitivity and secretion, glucose tolerance and adipocytokines in type 2 diabetic patients. Diabetes Obes. Metab. 10: 1204–1211. [DOI] [PubMed] [Google Scholar]
- 74.Manning P. J., Sutherland W. H., Walker R. J., Williams S. M., de Jong S. A., Berry E. A. 2008. The effect of rosiglitazone on oxidative stress and insulin resistance in overweight individuals. Diabetes Res. Clin. Pract. 81: 209–215. [DOI] [PubMed] [Google Scholar]
- 75.Barac A., Campia U., Matuskey L. A., Lu L., Panza J. A. 2008. Effects of peroxisome proliferator-activated receptor-gamma activation with pioglitazone on plasma adipokines in nondiabetic patients with either hypercholesterolemia or hypertension. Am. J. Cardiol. 101: 980–985. [DOI] [PubMed] [Google Scholar]
- 76.Pfützner A., Marx N., Lubben G., Langenfeld M., Walcher D., Konrad T., Forst T. 2005. Improvement of cardiovascular risk markers by pioglitazone is independent from glycemic control: results from the pioneer study. J. Am. Coll. Cardiol. 45: 1925–1931. [DOI] [PubMed] [Google Scholar]
- 77.Forst T., Pfützner A., Lubben G., Weber M., Marx N., Karagiannis E., Koehler C., Baurecht W., Hohberg C., Hanefeld M. 2007. Effect of simvastatin and/or pioglitazone on insulin resistance, insulin secretion, adiponectin, and proinsulin levels in nondiabetic patients at cardiovascular risk–the PIOSTAT Study. Metabolism. 56: 491–496. [DOI] [PubMed] [Google Scholar]
- 78.Eguchi K., Tomizawa H., Ishikawa J., Hoshide S., Numao T., Fukuda T., Shimada K., Kario K. 2007. Comparison of the effects of pioglitazone and metformin on insulin resistance and hormonal markers in patients with impaired glucose tolerance and early diabetes. Hypertens. Res. 30: 23–30. [DOI] [PubMed] [Google Scholar]
- 79.Dorkhan M., Frid A., Groop L. 2008. Differences in effects of insulin glargine or pioglitazone added to oral anti-diabetic therapy in patients with type 2 diabetes: what to add–insulin glargine or pioglitazone? Diabetes Res. Clin. Pract. 82: 340–345. [DOI] [PubMed] [Google Scholar]
- 80.Foryst-Ludwig A., Hartge M., Clemenz M., Sprang C., Hess K., Marx N., Unger T., Kintscher U. 2010. PPARgamma activation attenuates T-lymphocyte-dependent inflammation of adipose tissue and development of insulin resistance in obese mice. Cardiovasc. Diabetol. 9: 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ohman M. K., Shen Y., Obimba C. I., Wright A. P., Warnock M., Lawrence D. A., Eitzman D. T. 2008. Visceral adipose tissue inflammation accelerates atherosclerosis in apolipoprotein E-deficient mice. Circulation. 117: 798–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Permana P. A., Zhang W., Wabitsch M., Fischer-Posovszky P., Duckworth W. C., Reaven P. D. 2009. Pioglitazone reduces inflammatory responses of human adipocytes to factors secreted by monocytes/macrophages. Am. J. Physiol. Endocrinol. Metab. 296: E1076–E1084. [DOI] [PubMed] [Google Scholar]
- 83.Ricote M., Li A. C., Willson T. M., Kelly C. J., Glass C. K. 1998. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature. 391: 79–82. [DOI] [PubMed] [Google Scholar]
- 84.Derosa G., D'Angelo A., Ragonesi P. D., Ciccarelli L., Piccinni M. N., Pricolo F., Salvadeo S. A., Montagna L., Gravina A., Ferrari I., et al. 2007. Metabolic effects of pioglitazone and rosiglitazone in patients with diabetes and metabolic syndrome treated with metformin. Intern. Med. J. 37: 79–86. [DOI] [PubMed] [Google Scholar]
- 85.Orasanu G., Ziouzenkova O., Devchand P. R., Nehra V., Hamdy O., Horton E. S., Plutzky J. 2008. The peroxisome proliferator-activated receptor-gamma agonist pioglitazone represses inflammation in a peroxisome proliferator-activated receptor-alpha-dependent manner in vitro and in vivo in mice. J. Am. Coll. Cardiol. 52: 869–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Qin S., Liu T., Kamanna V. S., Kashyap M. L. 2007. Pioglitazone stimulates apolipoprotein A-I production without affecting HDL removal in HepG2 cells: involvement of PPAR-alpha. Arterioscler. Thromb. Vasc. Biol. 27: 2428–2434. [DOI] [PubMed] [Google Scholar]
- 87.Ogata M., Tsujita M., Hossain M. A., Akita N., Gonzalez F. J., Staels B., Suzuki S., Fukutomi T., Kimura G., Yokoyama S. 2009. On the mechanism for PPAR agonists to enhance ABCA1 gene expression. Atherosclerosis. 205: 413–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pedersen T. R., Olsson A. G., Faergeman O., Kjekshus J., Wedel H., Berg K., Wilhelmsen L., Haghfelt T., Thorgeirsson G., Pyorala K., et al. 1998. Lipoprotein changes and reduction in the incidence of major coronary heart disease events in the Scandinavian Simvastatin Survival Study (4S). Circulation. 97: 1453–1460. [DOI] [PubMed] [Google Scholar]
- 89.Davidson M., Meyer P. M., Haffner S., Feinstein S., D'Agostino R., Sr, Kondos G. T., Perez A., Chen Z., Mazzone T. 2008. Increased high-density lipoprotein cholesterol predicts the pioglitazone-mediated reduction of carotid intima-media thickness progression in patients with type 2 diabetes mellitus. Circulation. 117: 2123–2130. [DOI] [PubMed] [Google Scholar]
- 90.Nicholls S. J., Tuzcu E. M., Wolski K., Bayturan O., Lavoie A., Uno K., Kupfer S., Perez A., Nesto R., Nissen S. E. 2011. Lowering the triglyceride/high-density lipoprotein cholesterol ratio is associated with the beneficial impact of pioglitazone on progression of coronary atherosclerosis in diabetic patients: insights from the PERISCOPE (Pioglitazone Effect on Regression of Intravascular Sonographic Coronary Obstruction Prospective Evaluation) study. J. Am. Coll. Cardiol. 57: 153–159. [DOI] [PubMed] [Google Scholar]
- 91.Yki-Järvinen H. 2004. Thiazolidinediones. N. Engl. J. Med. 351: 1106–1118. [DOI] [PubMed] [Google Scholar]
- 92.Dormandy J. A., Charbonnel B., Eckland D. J., Erdmann E., Massi-Benedetti M., Moules I. K., Skene A. M., Tan M. H., Lefebvre P. J., Murray G. D., et al. 2005. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet. 366: 1279–1289. [DOI] [PubMed] [Google Scholar]
- 93.Lachin J. M., Viberti G., Zinman B., Haffner S. M., Aftring R. P., Paul G., Kravitz B. G., Herman W. H., Holman R. R., Kahn S. E. 2011. Renal function in type 2 diabetes with rosiglitazone, metformin, and glyburide monotherapy. Clin. J. Am. Soc. Nephrol. 6: 1032–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Home P. D., Pocock S. J., Beck-Nielsen H., Curtis P. S., Gomis R., Hanefeld M., Jones N. P., Komajda M., McMurray J. J. 2009. Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): a multicentre, randomised, open-label trial. Lancet. 373: 2125–2135. [DOI] [PubMed] [Google Scholar]
- 95.Komajda M., Curtis P., Hanefeld M., Beck-Nielsen H., Pocock S. J., Zambanini A., Jones N. P., Gomis R., Home P. D. 2008. Effect of the addition of rosiglitazone to metformin or sulfonylureas versus metformin/sulfonylurea combination therapy on ambulatory blood pressure in people with type 2 diabetes: a randomized controlled trial (the RECORD study). Cardiovasc. Diabetol. 7: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Haider A. W., Larson M. G., Franklin S. S., Levy D. 2003. Systolic blood pressure, diastolic blood pressure, and pulse pressure as predictors of risk for congestive heart failure in the Framingham Heart Study. Ann. Intern. Med. 138: 10–16. [DOI] [PubMed] [Google Scholar]
- 97.Marx N., Bourcier T., Sukhova G. K., Libby P., Plutzky J. 1999. PPARgamma activation in human endothelial cells increases plasminogen activator inhibitor type-1 expression: PPARgamma as a potential mediator in vascular disease. Arterioscler. Thromb. Vasc. Biol. 19: 546–551. [DOI] [PubMed] [Google Scholar]
- 98.Desouza C. V., Hamel F. G., Bidasee K., O'Connell K. 2011. Role of inflammation and insulin resistance in endothelial progenitor cell dysfunction. Diabetes. 60: 1286–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fadini G. P., Sartore S., Agostini C., Avogaro A. 2007. Significance of endothelial progenitor cells in subjects with diabetes. Diabetes Care. 30: 1305–1313. [DOI] [PubMed] [Google Scholar]
- 100.Jialal I., Devaraj S., Singh U., Huet B. A. 2010. Decreased number and impaired functionality of endothelial progenitor cells in subjects with metabolic syndrome: implications for increased cardiovascular risk. Atherosclerosis. 211: 297–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liao Y. F., Chen L. L., Zeng T. S., Li Y. M., Fan Y., Hu L. J., Ling Y. 2010. Number of circulating endothelial progenitor cells as a marker of vascular endothelial function for type 2 diabetes. Vasc. Med. 15: 279–285. [DOI] [PubMed] [Google Scholar]
- 102.Hannan K. M., Dilley R. J., de Dios S. T., Little P. J. 2003. Troglitazone stimulates repair of the endothelium and inhibits neointimal formation in denuded rat aorta. Arterioscler. Thromb. Vasc. Biol. 23: 762–768. [DOI] [PubMed] [Google Scholar]
- 103.Makino H., Okada S., Nagumo A., Sugisawa T., Miyamoto Y., Kishimoto I., Akie T. K., Soma T., Taguchi A., Yoshimasa Y. 2008. Pioglitazone treatment stimulates circulating CD34-positive cells in type 2 diabetes patients. Diabetes Res. Clin. Pract. 81: 327–330. [DOI] [PubMed] [Google Scholar]
- 104.Pistrosch F., Herbrig K., Oelschlaegel U., Richter S., Passauer J., Fischer S., Gross P. 2005. PPARgamma-agonist rosiglitazone increases number and migratory activity of cultured endothelial progenitor cells. Atherosclerosis. 183: 163–167. [DOI] [PubMed] [Google Scholar]
- 105.Sorrentino S. A., Bahlmann F. H., Besler C., Muller M., Schulz S., Kirchhoff N., Doerries C., Horvath T., Limbourg A., Limbourg F., et al. 2007. Oxidant stress impairs in vivo reendothelialization capacity of endothelial progenitor cells from patients with type 2 diabetes mellitus: restoration by the peroxisome proliferator-activated receptor-gamma agonist rosiglitazone. Circulation. 116: 163–173. [DOI] [PubMed] [Google Scholar]
- 106.Werner C., Kamani C. H., Gensch C., Bohm M., Laufs U. 2007. The peroxisome proliferator-activated receptor-gamma agonist pioglitazone increases number and function of endothelial progenitor cells in patients with coronary artery disease and normal glucose tolerance. Diabetes. 56: 2609–2615. [DOI] [PubMed] [Google Scholar]
- 107.Boyle J. G., Logan P. J., Ewart M. A., Reihill J. A., Ritchie S. A., Connell J. M., Cleland S. J., Salt I. P. 2008. Rosiglitazone stimulates nitric oxide synthesis in human aortic endothelial cells via AMP-activated protein kinase. J. Biol. Chem. 283: 11210–11217. [DOI] [PubMed] [Google Scholar]
- 108.Mittermayer F., Schaller G., Pleiner J., Krzyzanowska K., Kapiotis S., Roden M., Wolzt M. 2007. Rosiglitazone prevents free fatty acid-induced vascular endothelial dysfunction. J. Clin. Endocrinol. Metab. 92: 2574–2580. [DOI] [PubMed] [Google Scholar]
- 109.Wong W. T., Tian X. Y., Xu A., Yu J., Lau C. W., Hoo R. L., Wang Y., Lee V. W., Lam K. S., Vanhoutte P. M., et al. 2011. Adiponectin is required for PPARgamma-mediated improvement of endothelial function in diabetic mice. Cell Metab. 14: 104–115. [DOI] [PubMed] [Google Scholar]
- 110.Fernandez M., Triplitt C., Wajcberg E., Sriwijilkamol A. A., Musi N., Cusi K., DeFronzo R., Cersosimo E. 2008. Addition of pioglitazone and ramipril to intensive insulin therapy in type 2 diabetic patients improves vascular dysfunction by different mechanisms. Diabetes Care. 31: 121–127. [DOI] [PubMed] [Google Scholar]
- 111.Patel S. R., Mailloux L. M., Coppola J. T., Mindrescu C., Staniloae C. S. 2008. Pioglitazone increases adiponectin levels in nondiabetic patients with coronary artery disease. Coron. Artery Dis. 19: 349–353. [DOI] [PubMed] [Google Scholar]
- 112.Tsuchiya K., Akaza I., Yoshimoto T., Hirata Y. 2009. Pioglitazone improves endothelial function with increased adiponectin and high-density lipoprotein cholesterol levels in type 2 diabetes. Endocr. J. 56: 691–698. [DOI] [PubMed] [Google Scholar]
- 113.Wöhrle J., Marx N., Koenig W., Hombach V., Kestler H. A., Hoher M., Nusser T. 2008. Impact of pioglitazone on coronary endothelial function in non-diabetic patients with coronary artery disease. Clin. Res. Cardiol. 97: 726–733. [DOI] [PubMed] [Google Scholar]
- 114.Kitahara H., Kobayashi Y., Iwata Y., Fujimoto Y., Komuro I. 2011. Effect of pioglitazone on endothelial dysfunction after sirolimus-eluting stent implantation. Am. J. Cardiol. 108: 214–219. [DOI] [PubMed] [Google Scholar]
- 115.Little P. J., Osman N., de Dios S. T., Cemerlang N., Ballinger M., Nigro J. 2007. Anti-proliferative activity of oral anti-hyperglycemic agents on human vascular smooth muscle cells: thiazolidinediones (glitazones) have enhanced activity under high glucose conditions. Cardiovasc. Diabetol. 6: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Law R. E., Goetze S., Xi X. P., Jackson S., Kawano Y., Demer L., Fishbein M. C., Meehan W. P., Hsueh W. A. 2000. Expression and function of PPARgamma in rat and human vascular smooth muscle cells. Circulation. 101: 1311–1318. [DOI] [PubMed] [Google Scholar]
- 117.Hong S. J., Kim S. T., Kim T. J., Kim E. O., Ahn C. M., Park J. H., Kim J. S., Lee K. M., Lim D. S. 2010. Cellular and molecular changes associated with inhibitory effect of pioglitazone on neointimal growth in patients with type 2 diabetes after zotarolimus-eluting stent implantation. Arterioscler. Thromb. Vasc. Biol. 30: 2655–2665. [DOI] [PubMed] [Google Scholar]
- 118.de Dios S. T., Bruemmer D., Dilley R. J., Ivey M. E., Jennings G. L., Law R. E., Little P. J. 2003. Inhibitory activity of clinical thiazolidinedione peroxisome proliferator activating receptor-gamma ligands toward internal mammary artery, radial artery, and saphenous vein smooth muscle cell proliferation. Circulation. 107: 2548–2550. [DOI] [PubMed] [Google Scholar]
- 119.Geng D. F., Jin D. M., Wu W., Wang Z., Wang J. F. 2009. Effect of thiazolidinediones on in-stent restenosis in patients after coronary stenting: a meta-analysis of randomized controlled trials. Atherosclerosis. 202: 521–528. [DOI] [PubMed] [Google Scholar]
- 120.Patel D., Walitt B., Lindsay J., Wilensky R. L. 2011. Role of pioglitazone in the prevention of restenosis and need for revascularization after bare-metal stent implantation: a meta-analysis. JACC Cardiovasc. Interv. 4: 353–360. [DOI] [PubMed] [Google Scholar]
- 121.Riche D. M., Valderrama R., Henyan N. N. 2007. Thiazolidinediones and risk of repeat target vessel revascularization following percutaneous coronary intervention: a meta-analysis. Diabetes Care. 30: 384–388. [DOI] [PubMed] [Google Scholar]
- 122.Cao Z., Zhou Y. 2008. Thiazolidinediones may be effective in the prevention of stent thrombosis with DES. Med. Hypotheses. 70: 329–332. [DOI] [PubMed] [Google Scholar]
- 123.Babaev V. R., Yancey P. G., Ryzhov S. V., Kon V., Breyer M. D., Magnuson M. A., Fazio S., Linton M. F. 2005. Conditional knockout of macrophage PPARgamma increases atherosclerosis in C57BL/6 and low-density lipoprotein receptor-deficient mice. Arterioscler. Thromb. Vasc. Biol. 25: 1647–1653. [DOI] [PubMed] [Google Scholar]
- 124.Chawla A., Boisvert W. A., Lee C. H., Laffitte B. A., Barak Y., Joseph S. B., Liao D., Nagy L., Edwards P. A., Curtiss L. K., et al. 2001. A PPAR gamma-LXR-ABCA1 pathway in macrophages is involved in cholesterol efflux and atherogenesis. Mol. Cell. 7: 161–171. [DOI] [PubMed] [Google Scholar]
- 125.Vucic E., Dickson S. D., Calcagno C., Rudd J. H., Moshier E., Hayashi K., Mounessa J. S., Roytman M., Moon M. J., Lin J., et al. 2011. Pioglitazone modulates vascular inflammation in atherosclerotic rabbits noninvasive assessment with FDG-PET-CT and dynamic contrast-enhanced MR imaging. JACC Cardiovasc. Imaging. 4: 1100–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Soumian S., Gibbs R., Davies A., Albrecht C. 2005. mRNA expression of genes involved in lipid efflux and matrix degradation in occlusive and ectatic atherosclerotic disease. J. Clin. Pathol. 58: 1255–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sueyoshi S., Mitsumata M., Kusumi Y., Niihashi M., Esumi M., Yamada T., Sakurai I. 2010. Increased expression of peroxisome proliferator-activated receptor (PPAR)-alpha and PPAR-gamma in human atherosclerosis. Pathol. Res. Pract. 206: 429–438. [DOI] [PubMed] [Google Scholar]
- 128.Bouhlel M. A., Derudas B., Rigamonti E., Dievart R., Brozek J., Haulon S., Zawadzki C., Jude B., Torpier G., Marx N., et al. 2007. PPARgamma activation primes human monocytes into alternative M2 macrophages with anti-inflammatory properties. Cell Metab. 6: 137–143. [DOI] [PubMed] [Google Scholar]
- 129.Lonn E. M., Gerstein H. C., Sheridan P., Smith S., Diaz R., Mohan V., Bosch J., Yusuf S., Dagenais G. R. 2009. Effect of ramipril and of rosiglitazone on carotid intima-media thickness in people with impaired glucose tolerance or impaired fasting glucose: STARR (STudy of Atherosclerosis with Ramipril and Rosiglitazone). J. Am. Coll. Cardiol. 53: 2028–2035. [DOI] [PubMed] [Google Scholar]
- 130.Mazzone T., Meyer P. M., Feinstein S. B., Davidson M. H., Kondos G. T., D'Agostino R. B., Sr, Perez A., Provost J. C., Haffner S. M. 2006. Effect of pioglitazone compared with glimepiride on carotid intima-media thickness in type 2 diabetes: a randomized trial. JAMA. 296: 2572–2581. [DOI] [PubMed] [Google Scholar]
- 131.Davidson M. H., Beam C. A., Haffner S., Perez A., D'Agostino R., Sr, Mazzone T. 2010. Pioglitazone versus glimepiride on coronary artery calcium progression in patients with type 2 diabetes mellitus: a secondary end point of the CHICAGO study. Arterioscler. Thromb. Vasc. Biol. 30: 1873–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Webb D. R., Davies M. J., Gray L. J., Abrams K. R., Srinivasan B., Das S., Taub N., Lawrence I., Sutton S., Khunti K. 2010. Searching for the right outcome? A systematic review and meta-analysis of controlled trials using carotid intima-media thickness or pulse wave velocity to infer antiatherogenic properties of thiazolidinediones. Diabetes Obes. Metab. 12: 124–132. [DOI] [PubMed] [Google Scholar]
- 133.Nissen S. E., Nicholls S. J., Wolski K., Nesto R., Kupfer S., Perez A., Jure H., De Larochelliere R., Staniloae C. S., Mavromatis K., et al. 2008. Comparison of pioglitazone vs glimepiride on progression of coronary atherosclerosis in patients with type 2 diabetes: the PERISCOPE randomized controlled trial. JAMA. 299: 1561–1573. [DOI] [PubMed] [Google Scholar]
- 134.You S. H., Kim B. S., Hong S. J., Ahn C. M., Lim D. S. 2010. The effects of pioglitazone in reducing atherosclerosis progression and neointima volume in type 2 diabetic patients: prospective randomized study with volumetric intravascular ultrasonography analysis. Korean Circ. J. 40: 625–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yang H. B., Zhao X. Y., Zhang J. Y., Du Y. Y., Wang X. F. 2012. Pioglitazone induces regression and stabilization of coronary atherosclerotic plaques in patients with impaired glucose tolerance. Diabet. Med. 29: 359–365. [DOI] [PubMed] [Google Scholar]
- 136.Ogasawara D., Shite J., Shinke T., Watanabe S., Otake H., Tanino Y., Sawada T., Kawamori H., Kato H., Miyoshi N., et al. 2009. Pioglitazone reduces the necrotic-core component in coronary plaque in association with enhanced plasma adiponectin in patients with type 2 diabetes mellitus. Circ. J. 73: 343–351. [DOI] [PubMed] [Google Scholar]
- 137.Nakayama T., Komiyama N., Yokoyama M., Namikawa S., Kuroda N., Kobayashi Y., Komuro I. 2010. Pioglitazone induces regression of coronary atherosclerotic plaques in patients with type 2 diabetes mellitus or impaired glucose tolerance: a randomized prospective study using intravascular ultrasound. Int. J. Cardiol. 138: 157–165. [DOI] [PubMed] [Google Scholar]
- 138.Bertrand O. F., Poirier P., Rodes-Cabau J., Rinfret S., Title L. M., Dzavik V., Natarajan M., Angel J., Batalla N., Almeras N., et al. 2010. Cardiometabolic effects of rosiglitazone in patients with type 2 diabetes and coronary artery bypass grafts: a randomized placebo-controlled clinical trial. Atherosclerosis. 211: 565–573. [DOI] [PubMed] [Google Scholar]
- 139.Gerstein H. C., Ratner R. E., Cannon C. P., Serruys P. W., Garcia-Garcia H. M., van Es G. A., Kolatkar N. S., Kravitz B. G., Miller D. M., Huang C., et al. 2010. Effect of rosiglitazone on progression of coronary atherosclerosis in patients with type 2 diabetes mellitus and coronary artery disease: the assessment on the prevention of progression by rosiglitazone on atherosclerosis in diabetes patients with cardiovascular history trial. Circulation. 121: 1176–1187. [DOI] [PubMed] [Google Scholar]
- 140.Yue T. L., Bao W., Gu J. L., Cui J., Tao L., Ma X. L., Ohlstein E. H., Jucker B. M. 2005. Rosiglitazone treatment in Zucker diabetic Fatty rats is associated with ameliorated cardiac insulin resistance and protection from ischemia/reperfusion-induced myocardial injury. Diabetes. 54: 554–562. [DOI] [PubMed] [Google Scholar]
- 141.Yue Tl T. L., Chen J., Bao W., Narayanan P. K., Bril A., Jiang W., Lysko P. G., Gu J. L., Boyce R., Zimmerman D. M., et al. 2001. In vivo myocardial protection from ischemia/reperfusion injury by the peroxisome proliferator-activated receptor-gamma agonist rosiglitazone. Circulation. 104: 2588–2594. [DOI] [PubMed] [Google Scholar]
- 142.Yasuda S., Kobayashi H., Iwasa M., Kawamura I., Sumi S., Narentuoya B., Yamaki T., Ushikoshi H., Nishigaki K., Nagashima K., et al. 2009. Antidiabetic drug pioglitazone protects the heart via activation of PPAR-gamma receptors, PI3-kinase, Akt, and eNOS pathway in a rabbit model of myocardial infarction. Am. J. Physiol. Heart Circ. Physiol. 296: H1558–H1565. [DOI] [PubMed] [Google Scholar]
- 143.Wynne A. M., Mocanu M. M., Yellon D. M. 2005. Pioglitazone mimics preconditioning in the isolated perfused rat heart: a role for the prosurvival kinases PI3K and P42/44MAPK. J. Cardiovasc. Pharmacol. 46: 817–822. [DOI] [PubMed] [Google Scholar]
- 144.Sidell R. J., Cole M. A., Draper N. J., Desrois M., Buckingham R. E., Clarke K. 2002. Thiazolidinedione treatment normalizes insulin resistance and ischemic injury in the zucker Fatty rat heart. Diabetes. 51: 1110–1117. [DOI] [PubMed] [Google Scholar]
- 145.Molavi B., Chen J., Mehta J. L. 2006. Cardioprotective effects of rosiglitazone are associated with selective overexpression of type 2 angiotensin receptors and inhibition of p42/44 MAPK. Am. J. Physiol. Heart Circ. Physiol. 291: H687–H693. [DOI] [PubMed] [Google Scholar]
- 146.Lotz C., Lange M., Redel A., Stumpner J., Schmidt J., Tischer-Zeitz T., Roewer N., Kehl F. 2011. Peroxisome-proliferator-activated receptor gamma mediates the second window of anaesthetic-induced preconditioning. Exp. Physiol. 96: 317–324. [DOI] [PubMed] [Google Scholar]
- 147.Liu H. R., Tao L., Gao E., Qu Y., Lau W. B., Lopez B. L., Christopher T. A., Koch W., Yue T. L., Ma X. L. 2009. Rosiglitazone inhibits hypercholesterolaemia-induced myeloperoxidase upregulation–a novel mechanism for the cardioprotective effects of PPAR agonists. Cardiovasc. Res. 81: 344–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Khandoudi N., Delerive P., Berrebi-Bertrand I., Buckingham R. E., Staels B., Bril A. 2002. Rosiglitazone, a peroxisome proliferator-activated receptor-gamma, inhibits the Jun NH(2)-terminal kinase/activating protein 1 pathway and protects the heart from ischemia/reperfusion injury. Diabetes. 51: 1507–1514. [DOI] [PubMed] [Google Scholar]
- 149.Cao Z., Ye P., Long C., Chen K., Li X., Wang H. 2007. Effect of pioglitazone, a peroxisome proliferator-activated receptor gamma agonist, on ischemia-reperfusion injury in rats. Pharmacology. 79: 184–192. [DOI] [PubMed] [Google Scholar]
- 150.Lee T. M., Chou T. F. 2003. Troglitazone administration limits infarct size by reduced phosphorylation of canine myocardial connexin43 proteins. Am. J. Physiol. Heart Circ. Physiol. 285: H1650–H1659. [DOI] [PubMed] [Google Scholar]
- 151.Zhu P., Lu L., Xu Y., Schwartz G. G. 2000. Troglitazone improves recovery of left ventricular function after regional ischemia in pigs. Circulation. 101: 1165–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Geng D. F., Wu W., Jin D. M., Wang J. F., Wu Y. M. 2006. Effect of peroxisome proliferator-activated receptor gamma ligand. Rosiglitazone on left ventricular remodeling in rats with myocardial infarction. Int. J. Cardiol. 113: 86–91. [DOI] [PubMed] [Google Scholar]
- 153.Shiomi T., Tsutsui H., Hayashidani S., Suematsu N., Ikeuchi M., Wen J., Ishibashi M., Kubota T., Egashira K., Takeshita A. 2002. Pioglitazone, a peroxisome proliferator-activated receptor-gamma agonist, attenuates left ventricular remodeling and failure after experimental myocardial infarction. Circulation. 106: 3126–3132. [DOI] [PubMed] [Google Scholar]
- 154.Frantz S., Hu K., Widder J., Bayer B., Witzel C. C., Schmidt I., Galuppo P., Strotmann J., Ertl G., Bauersachs J. 2004. Peroxisome proliferator activated-receptor agonism and left ventricular remodeling in mice with chronic myocardial infarction. Br. J. Pharmacol. 141: 9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lygate C. A., Hulbert K., Monfared M., Cole M. A., Clarke K., Neubauer S. 2003. The PPARgamma-activator rosiglitazone does not alter remodeling but increases mortality in rats post-myocardial infarction. Cardiovasc. Res. 58: 632–637. [DOI] [PubMed] [Google Scholar]
- 156.Pereira M. P., Hurtado O., Cardenas A., D. Alonso-Escolano, L. Bosca, J. Vivancos, F. Nombela, J. C. Leza, P. Lorenzo, I. Lizasoain, et al. 2005. The nonthiazolidinedione PPARgamma agonist L-796,449 is neuroprotective in experimental stroke. J. Neuropathol. Exp. Neurol. 64: 797–805. [DOI] [PubMed] [Google Scholar]
- 157.Sobrado M., Pereira M. P., Ballesteros I., Hurtado O., Fernandez-Lopez D., Pradillo J. M., Caso J. R., Vivancos J., Nombela F., Serena J., et al. 2009. Synthesis of lipoxin A4 by 5-lipoxygenase mediates PPARgamma-dependent, neuroprotective effects of rosiglitazone in experimental stroke. J. Neurosci. 29: 3875–3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Tureyen K., Kapadia R., Bowen K. K., Satriotomo I., Liang J., Feinstein D. L., Vemuganti R. 2007. Peroxisome proliferator-activated receptor-gamma agonists induce neuroprotection following transient focal ischemia in normotensive, normoglycemic as well as hypertensive and type-2 diabetic rodents. J. Neurochem. 101: 41–56. [DOI] [PubMed] [Google Scholar]
- 159.Wu J. S., Cheung W. M., Tsai Y. S., Chen Y. T., Fong W. H., Tsai H. D., Chen Y. C., Liou J. Y., Shyue S. K., Chen J. J., et al. 2009. Ligand-activated peroxisome proliferator-activated receptor-gamma protects against ischemic cerebral infarction and neuronal apoptosis by 14-3-3 epsilon upregulation. Circulation. 119: 1124–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zhang H. L., Xu M., Wei C., Qin A. P., Liu C. F., Hong L. Z., Zhao X. Y., Liu J., Qin Z. H. 2011. Neuroprotective effects of pioglitazone in a rat model of permanent focal cerebral ischemia are associated with peroxisome proliferator-activated receptor gamma-mediated suppression of nuclear factor-kappaB signaling pathway. Neuroscience. 176: 381–395. [DOI] [PubMed] [Google Scholar]
- 161.Zhao Y., Patzer A., Gohlke P., Herdegen T., Culman J. 2005. The intracerebral application of the PPARgamma-ligand pioglitazone confers neuroprotection against focal ischaemia in the rat brain. Eur. J. Neurosci. 22: 278–282. [DOI] [PubMed] [Google Scholar]
- 162.de Boer R. A., Martens F. M., Kuipers I., Boomsma F., Visseren F. L. 2010. The effects of the PPAR-gamma agonist pioglitazone on plasma concentrations of circulating vasoactive factors in type II diabetes mellitus. J. Hum. Hypertens. 24: 74–76. [DOI] [PubMed] [Google Scholar]
- 163.Narang N., Armstead S. I., Stream A., Abdullah S. M., See R., Snell P. G., McGavock J., Ayers C. R., Gore M. O., Khera A., et al. 2011. Assessment of cardiac structure and function in patients without and with peripheral oedema during rosiglitazone treatment. Diab. Vasc. Dis. Res. 8: 101–108. [DOI] [PubMed] [Google Scholar]
- 164.Ogawa S., Takeuchi K., Ito S. 2003. Plasma BNP levels in the treatment of type 2 diabetes with pioglitazone. J. Clin. Endocrinol. Metab. 88: 3993–3996. [DOI] [PubMed] [Google Scholar]
- 165.Kim H. J., Kang E. S., Kim D. J., Kim S. H., Ahn C. W., Cha B. S., Nam M., Chung C. H., Lee K. W., Nam C. M., et al. 2007. Effects of rosiglitazone and metformin on inflammatory markers and adipokines: decrease in interleukin-18 is an independent factor for the improvement of homeostasis model assessment-beta in type 2 diabetes mellitus. Clin. Endocrinol. (Oxf.). 66: 282–289. [DOI] [PubMed] [Google Scholar]
- 166.Zhang H., Zhang A., Kohan D. E., Nelson R. D., Gonzalez F. J., Yang T. 2005. Collecting duct-specific deletion of peroxisome proliferator-activated receptor gamma blocks thiazolidinedione-induced fluid retention. Proc. Natl. Acad. Sci. USA. 102: 9406–9411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Mudaliar S., Chang A. R., Aroda V. R., Chao E., Burke P., Baxi S., Griver K. A., O'Connor D. T., Henry R. R. 2010. Effects of intensive insulin therapy alone and with added pioglitazone on renal salt/water balance and fluid compartment shifts in type 2 diabetes. Diabetes Obes. Metab. 12: 133–138. [DOI] [PubMed] [Google Scholar]
- 168.Karalliedde J., Buckingham R., Starkie M., Lorand D., Stewart M., Viberti G. 2006. Effect of various diuretic treatments on rosiglitazone-induced fluid retention. J. Am. Soc. Nephrol. 17: 3482–3490. [DOI] [PubMed] [Google Scholar]
- 169.Guan Y., Hao C., Cha D. R., Rao R., Lu W., Kohan D. E., Magnuson M. A., Redha R., Zhang Y., Breyer M. D. 2005. Thiazolidinediones expand body fluid volume through PPARgamma stimulation of ENaC-mediated renal salt absorption. Nat. Med. 11: 861–866. [DOI] [PubMed] [Google Scholar]
- 170.Artunc F., Sandulache D., Nasir O., Boini K. M., Friedrich B., Beier N., Dicks E., Potzsch S., Klingel K., Amann K., et al. 2008. Lack of the serum and glucocorticoid-inducible kinase SGK1 attenuates the volume retention after treatment with the PPARgamma agonist pioglitazone. Pflugers Arch. 456: 425–436. [DOI] [PubMed] [Google Scholar]
- 171.Endo Y., Suzuki M., Yamada H., Horita S., Kunimi M., Yamazaki O., Shirai A., Nakamura M., Iso O., Li Y., et al. 2011. Thiazolidinediones enhance sodium-coupled bicarbonate absorption from renal proximal tubules via PPARgamma-dependent nongenomic signaling. Cell Metab. 13: 550–561. [DOI] [PubMed] [Google Scholar]
- 172.Xu Y., Gen M., Lu L., Fox J., Weiss S. O., Brown R. D., Perlov D., Ahmad H., Zhu P., Greyson C., et al. 2005. PPAR-gamma activation fails to provide myocardial protection in ischemia and reperfusion in pigs. Am. J. Physiol. Heart Circ. Physiol. 288: H1314–H1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Dargie H. J., Hildebrandt P. R., Riegger G. A., McMurray J. J., McMorn S. O., Roberts J. N., Zambanini A., Wilding J. P. 2007. A randomized, placebo-controlled trial assessing the effects of rosiglitazone on echocardiographic function and cardiac status in type 2 diabetic patients with New York Heart Association Functional Class I or II Heart Failure. J. Am. Coll. Cardiol. 49: 1696–1704. [DOI] [PubMed] [Google Scholar]
- 174.Naka K. K., Pappas K., Papathanassiou K., Papamichael N. D., Kazakos N., Kanioglou C., Makriyiannis D., Katsouras C. S., Liveris K., Tsatsoulis A., et al. 2010. Lack of effects of pioglitazone on cardiac function in patients with type 2 diabetes and evidence of left ventricular diastolic dysfunction: a tissue doppler imaging study. Cardiovasc. Diabetol. 9: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Von Bibra, H., M. Diamant, P. G. Scheffer, T. Siegmund, and P. M. Schumm-Draeger. 2008. Rosiglitazone, but not glimepiride, improves myocardial diastolic function in association with reduction in oxidative stress in type 2 diabetic patients without overt heart disease. Diab. Vasc. Dis. Res. 5: 310–318. [DOI] [PubMed] [Google Scholar]
- 176.Ordu S., Ozhan H., Alemdar R., Aydin M., Basar C., Caglar O., Yazici M., Yalcin S. 2010. Pioglitazone improves ventricular diastolic function in patients with diabetes mellitus: a tissue Doppler study. Acta Cardiol. 65: 401–406. [DOI] [PubMed] [Google Scholar]
- 177.Horio T., Suzuki M., Suzuki K., Takamisawa I., Hiuge A., Kamide K., Takiuchi S., Iwashima Y., Kihara S., Funahashi T., et al. 2005. Pioglitazone improves left ventricular diastolic function in patients with essential hypertension. Am. J. Hypertens. 18: 949–957. [DOI] [PubMed] [Google Scholar]
- 178.Acton J. J., III, Akiyama T. E., Chang C. H., Colwell L., Debenham S., Doebber T., Einstein M., Liu K., McCann M. E., Moller D. E., et al. 2009. Discovery of (2R)-2-(3-{3-[(4-Methoxyphenyl)carbonyl]-2-methyl-6-(trifluoromethoxy)-1H- indol-1-yl}phenoxy)butanoic acid (MK-0533): a novel selective peroxisome proliferator-activated receptor gamma modulator for the treatment of type 2 diabetes mellitus with a reduced potential to increase plasma and extracellular fluid volume. J. Med. Chem. 52: 3846–3854. [DOI] [PubMed] [Google Scholar]
- 179.Kim M. K., Chae Y. N., Choi S. H., Moon H. S., Son M. H., Bae M. H., Choi H. H., Hur Y., Kim E., Park Y. H., et al. 2011. PAM-1616, a selective peroxisome proliferator-activated receptor gamma modulator with preserved anti-diabetic efficacy and reduced adverse effects. Eur. J. Pharmacol. 650: 673–681. [DOI] [PubMed] [Google Scholar]
- 180.Szentandrássy N., Harmati G., Barandi L., Simko J., Horvath B., Magyar J., Banyasz T., Lorincz I., Szebeni A., Kecskemeti V., et al. 2011. Effects of rosiglitazone on the configuration of action potentials and ion currents in canine ventricular cells. Br. J. Pharmacol. 163: 499–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Sunaga Y., Inagaki N., Gonoi T., Yamada Y., Ishida H., Seino Y., Seino S. 1999. Troglitazone but not pioglitazone affects ATP-sensitive K(+) channel activity. Eur. J. Pharmacol. 381: 71–76. [DOI] [PubMed] [Google Scholar]
- 182.Rowe I. C., Lee K., Khan R. N., Ashford M. L. 1997. Effect of englitazone on KATP and calcium-activated non-selective cation channels in CRI-G1 insulin-secreting cells. Br. J. Pharmacol. 121: 531–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.McKay N. G., Kinsella J. M., Campbell C. M., Ashford M. L. 2000. Sensitivity of Kir6.2-SUR1 currents, in the absence and presence of sodium azide, to the K(ATP) channel inhibitors, ciclazindol and englitazone. Br. J. Pharmacol. 130: 857–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Lee K., Ibbotson T., Richardson P. J., Boden P. R. 1996. Inhibition of KATP channel activity by troglitazone in CRI-G1 insulin-secreting cells. Eur. J. Pharmacol. 313: 163–167. [DOI] [PubMed] [Google Scholar]
- 185.Lee K., Boden P. 1997. Troglitazone inhibits type 2KATP channel activity and depolarises tolbutamide-sensitive neurones in the rat ventromedial hypothalamus. Brain Res. 751: 165–168. [DOI] [PubMed] [Google Scholar]
- 186.Chang T. J., Chen W. P., Yang C., Lu P. H., Liang Y. C., Su M. J., Lee S. C., Chuang L. M. 2009. Serine-385 phosphorylation of inwardly rectifying K+ channel subunit (Kir6.2) by AMP-dependent protein kinase plays a key role in rosiglitazone-induced closure of the K(ATP) channel and insulin secretion in rats. Diabetologia. 52: 1112–1121. [DOI] [PubMed] [Google Scholar]
- 187.Charbonnel B., Dormandy J., Erdmann E., Massi-Benedetti M., Skene A. 2004. The prospective pioglitazone clinical trial in macrovascular events (PROactive): can pioglitazone reduce cardiovascular events in diabetes? Study design and baseline characteristics of 5238 patients. Diabetes Care. 27: 1647–1653. [DOI] [PubMed] [Google Scholar]
- 188.Charbonnel B., DeFronzo R., Davidson J., Schmitz O., Birkeland K., Pirags V., Scheen A. 2010. Pioglitazone use in combination with insulin in the prospective pioglitazone clinical trial in macrovascular events study (PROactive19). J. Clin. Endocrinol. Metab. 95: 2163–2171. [DOI] [PubMed] [Google Scholar]
- 189.Erdmann E., Dormandy J. A., Charbonnel B., Massi-Benedetti M., Moules I. K., Skene A. M. 2007. The effect of pioglitazone on recurrent myocardial infarction in 2,445 patients with type 2 diabetes and previous myocardial infarction: results from the PROactive (PROactive 05) Study. J. Am. Coll. Cardiol. 49: 1772–1780. [DOI] [PubMed] [Google Scholar]
- 190.Dagenais G. R., Gerstein H. C., Holman R., Budaj A., Escalante A., Hedner T., Keltai M., Lonn E., McFarlane S., McQueen M., et al. 2008. Effects of ramipril and rosiglitazone on cardiovascular and renal outcomes in people with impaired glucose tolerance or impaired fasting glucose: results of the Diabetes REduction Assessment with ramipril and rosiglitazone Medication (DREAM) trial. Diabetes Care. 31: 1007–1014. [DOI] [PubMed] [Google Scholar]
- 191.Gerstein H. C., Yusuf S., Bosch J., Pogue J., Sheridan P., Dinccag N., Hanefeld M., Hoogwerf B., Laakso M., Mohan V., et al. 2006. Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: a randomised controlled trial. Lancet. 368: 1096–1105. [DOI] [PubMed] [Google Scholar]
- 192.DeFronzo R. A., Tripathy D., Schwenke D. C., Banerji M., Bray G. A., Buchanan T. A., Clement S. C., Henry R. R., Hodis H. N., Kitabchi A. E., et al. 2011. Pioglitazone for diabetes prevention in impaired glucose tolerance. N. Engl. J. Med. 364: 1104–1115. [DOI] [PubMed] [Google Scholar]
- 193.Kahn S. E., Haffner S. M., Heise M. A., Herman W. H., Holman R. R., Jones N. P., Kravitz B. G., Lachin J. M., O'Neill M. C., Zinman B., et al. 2006. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N. Engl. J. Med. 355: 2427–2443. [DOI] [PubMed] [Google Scholar]
- 194.Lincoff A. M., Wolski K., Nicholls S. J., Nissen S. E. 2007. Pioglitazone and risk of cardiovascular events in patients with type 2 diabetes mellitus: a meta-analysis of randomized trials. JAMA. 298: 1180–1188. [DOI] [PubMed] [Google Scholar]
- 195.Mannucci E., Monami M., Lamanna C., Gensini G. F., Marchionni N. 2008. Pioglitazone and cardiovascular risk. A comprehensive meta-analysis of randomized clinical trials. Diabetes Obes. Metab. 10: 1221–1238. [DOI] [PubMed] [Google Scholar]
- 196.Nissen S. E., Wolski K. 2010. Rosiglitazone revisited: an updated meta-analysis of risk for myocardial infarction and cardiovascular mortality. Arch. Intern. Med. 170: 1191–1201. [DOI] [PubMed] [Google Scholar]
- 197.Woodcock J., Sharfstein J. M., Hamburg M. 2010. Regulatory action on rosiglitazone by the US Food and Drug Administration. N. Engl. J. Med. 363: 1489–1491. [DOI] [PubMed] [Google Scholar]
- 198.Ziyadeh N., McAfee A. T., Koro C., Landon J., Arnold C. K. 2009. The thiazolidinediones rosiglitazone and pioglitazone and the risk of coronary heart disease: a retrospective cohort study using a US health insurance database. Clin. Ther. 31: 2665–2677. [DOI] [PubMed] [Google Scholar]
- 199.Winkelmayer W. C., Setoguchi S., Levin R., Solomon D. H. 2008. Comparison of cardiovascular outcomes in elderly patients with diabetes who initiated rosiglitazone vs pioglitazone therapy. Arch. Intern. Med. 168: 2368–2375. [DOI] [PubMed] [Google Scholar]
- 200.Tzoulaki I., Molokhia M., Curcin V., Little M. P., Millett C. J., Ng A., Hughes R. I., Khunti K., Wilkins M. R., Majeed A., et al. 2009. Risk of cardiovascular disease and all cause mortality among patients with type 2 diabetes prescribed oral antidiabetes drugs: retrospective cohort study using UK general practice research database. BMJ. 339: b4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Lipscombe L. L., Gomes T., Levesque L. E., Hux J. E., Juurlink D. N., Alter D. A. 2007. Thiazolidinediones and cardiovascular outcomes in older patients with diabetes. JAMA. 298: 2634–2643. [DOI] [PubMed] [Google Scholar]
- 202.Hsiao F. Y., Huang W. F., Wen Y. W., Chen P. F., Kuo K. N., Tsai Y. W. 2009. Thiazolidinediones and cardiovascular events in patients with type 2 diabetes mellitus: a retrospective cohort study of over 473,000 patients using the National Health Insurance database in Taiwan. Drug Saf. 32: 675–690. [DOI] [PubMed] [Google Scholar]
- 203.Habib Z. A., Tzogias L., Havstad S. L., Wells K., Divine G., Lanfear D. E., Tang J., Krajenta R., Pladevall M., Williams L. K. 2009. Relationship between thiazolidinedione use and cardiovascular outcomes and all-cause mortality among patients with diabetes: a time-updated propensity analysis. Pharmacoepidemiol. Drug Saf. 18: 437–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Loke Y. K., Kwok C. S., Singh S. 2011. Comparative cardiovascular effects of thiazolidinediones: systematic review and meta-analysis of observational studies. BMJ. 342: d1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Sears D. D., Hsiao A., Ofrecio J. M., Chapman J., He W., Olefsky J. M. 2007. Selective modulation of promoter recruitment and transcriptional activity of PPARgamma. Biochem. Biophys. Res. Commun. 364: 515–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Dunn F. L., Higgins L. S., Fredrickson J., DePaoli A. M. 2011. Selective modulation of PPARgamma activity can lower plasma glucose without typical thiazolidinedione side-effects in patients with Type 2 diabetes. J. Diabetes Complications. 25: 151–158. [DOI] [PubMed] [Google Scholar]
- 207.Kong A. P., Yamasaki A., Ozaki R., Saito H., Asami T., Ohwada S., Ko G. T., Wong C. K., Leung G. T., Lee K. F., et al. 2011. A randomized-controlled trial to investigate the effects of rivoglitazone, a novel PPAR gamma agonist on glucose-lipid control in type 2 diabetes. Diabetes Obes. Metab. 13: 806–813. [DOI] [PubMed] [Google Scholar]
- 208.Henriksen K., Byrjalsen I., Qvist P., Beck-Nielsen H., Hansen G., Riis B. J., Perrild H., Svendsen O. L., Gram J., Karsdal M. A., et al. 2011. Efficacy and safety of the PPARgamma partial agonist balaglitazone compared with pioglitazone and placebo: a phase III, randomized, parallel-group study in patients with type 2 diabetes on stable insulin therapy. Diabetes Metab. Res. Rev. 27: 392–401. [DOI] [PubMed] [Google Scholar]
- 209.Choi J. H., Banks A. S., Estall J. L., Kajimura S., Bostrom P., Laznik D., Ruas J. L., Chalmers M. J., Kamenecka T. M., Bluher M., et al. 2010. Anti-diabetic drugs inhibit obesity-linked phosphorylation of PPARgamma by CDK5. Nature. 466: 451–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Choi J. H., Banks A. S., Kamenecka T. M., Busby S. A., Chalmers M. J., Kumar N., Kuruvilla D. S., Shin Y., He Y., Bruning J. B., et al. 2011. Antidiabetic actions of a non-agonist PPARgamma ligand blocking CDK5-mediated phosphorylation. Nature. 477: 477–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Balakumar P., Rose M., Ganti S. S., Krishan P., Singh M. 2007. PPAR dual agonists: are they opening Pandora's Box? Pharmacol. Res. 56: 91–98. [DOI] [PubMed] [Google Scholar]
- 212.Nissen S. E., Wolski K., Topol E. J. 2005. Effect of muraglitazar on death and major adverse cardiovascular events in patients with type 2 diabetes mellitus. JAMA. 294: 2581–2586. [DOI] [PubMed] [Google Scholar]
- 213.Oleksiewicz M. B., Southgate J., Iversen L., Egerod F. L. 2008. Rat urinary bladder carcinogenesis by dual-acting PPARalpha + gamma agonists. PPAR Res. 2008: 103167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Younk L. M., Uhl L., Davis S. N. 2011. Pharmacokinetics, efficacy and safety of aleglitazar for the treatment of type 2 diabetes with high cardiovascular risk. Expert Opin. Drug Metab. Toxicol. 7: 753–763. [DOI] [PubMed] [Google Scholar]
- 215.Caccamo G., Bonura F., Bonura F., Vitale G., Novo G., Evola S., Evola G., Grisanti M. R., Novo S. 2010. Insulin resistance and acute coronary syndrome. Atherosclerosis. 211: 672–675. [DOI] [PubMed] [Google Scholar]
- 216.Kernan W. N., Inzucchi S. E., Viscoli C. M., Brass L. M., Bravata D. M., Shulman G. I., McVeety J. C., Horwitz R. I. 2003. Impaired insulin sensitivity among nondiabetic patients with a recent TIA or ischemic stroke. Neurology. 60: 1447–1451. [DOI] [PubMed] [Google Scholar]
- 217.Charbonnel B. 2009. PPAR-alpha and PPAR-gamma agonists for type 2 diabetes. Lancet. 374: 96–98. [DOI] [PubMed] [Google Scholar]