Abstract
The invention of Free Electron X-ray Lasers has opened a new era for membrane protein structure determination with the recent first proof-of-principle of the new concept of femtosecond nanocrystallography. Structure determination is based on thousands of diffraction snapshots that are collected on a fully hydrated stream of nanocrystals. This review provides a summary of the method and describes how femtosecond X-ray crystallography overcomes the radiation damage problem in X-ray crystallography, avoids the need for growth and freezing of large single crystals while offering a new method for direct digital phase determination by making use of the fully coherent nature of the X-ray beam. We briefly review the possibilities for time-resolved crystallography, and the potential for making “molecular movies” of membrane proteins at work.
Introduction
Many of the most important processes in living cells are catalyzed by membrane proteins. They play vital roles in respiration, photosynthesis, cell communication, cell import/export, cell-growth and recognition. A critical step for the elucidation of the complex processes that are catalyzed by membrane proteins is an understanding of their structure, dynamics, and function. Membrane proteins also have an overwhelming impact on human health. Over 30% of human genes code for membrane proteins and 60% of all drug molecules target these proteins. Our knowledge of the processes catalyzed by membrane proteins suffers from a lack of information concerning their structure. Whereas the structures of more than 60,000 soluble proteins have been determined, less than 300 different membrane protein structures are known at present. One rate-limiting step for structure determination of membrane proteins is the growth of large well-ordered single crystals suitable for X-ray analysis, a process which may take decades. Many membrane proteins never provide large well-diffracting crystals, despite years of effort and thousands of optimization screens.
Microcrystals of membrane proteins: Opportunities and Challenges
Structure determination methods based on microcrystals are desired not only because large single crystals are difficult to grow but also because smaller crystals may be better ordered, since they contain less long range disorder [1]. The crystallization of human membrane proteins has proven especially difficult, and just 4 years ago the first structures of a G-protein coupled receptor were determined [2,3], [4], based on micro-crystals grown in lipid cubic phases [5]. Evidence from line-width analysis in powder protein crystallography [6] suggests that nanocrystals may be more perfect than large crystals, containing fewer of the defects which contribute to mosaicity. Nevertheless, the possibility cannot be excluded that it is the imperfections which limit growth, while nanocrystals also contain a larger proportion of atypical surface molecules. Membrane protein crystals are crystallized in the form of protein-detergent micelles or embedded in lipid phases, which generally leads to a restriction in the number of unit cell contacts. In this respect it is interesting to ask: How small can a membrane protein crystal be? It was shown in a recent powder diffraction study that 100nm protein nano-crystals of the large membrane protein Photosystem I exist,, containing less than 100 unit cells [•• 7]. Submicron crystals cannot be detected by light microscopy, so that high-throughput methods for detecting nanocrystals are highly desired. The new SONICC method does allow fast detection of crystals of submicron size [•• 8] and it has been shown recently that it is superior to all other methods for crystal detection in non-transparent media such as lipid cubic phases [•• 9].
Automated data collection on frozen microcrystals at Synchrotron sources has progressed steadily, while automated screening of protein crystals at microfocus beamlines has also been improved recently [10],[11]. However, the quality of data collected from microcrystals is limited by the inherent problem of X-ray-induced radiation damage, which occurs even if crystals are frozen [• 12]. X-ray induced radiation damage has a strong dependence on the type of protein and the packing of the protein in the crystals. As membrane proteins are crystallized in the form of protein-detergent micelles, bicelles or in lipid cubic phases, they often have fewer crystal contact sites than soluble proteins and are therefore more prone to radiation damage. It is not possible to simply expose a microcrystal for a proportionally longer time since the required X-ray dose causes structural modification before enough scattering can be acquired. This problem, and that of initial crystal quality, set the limits to resolution in conventional crystallography of membrane proteins The methods developed for freezing the crystals have reduced, but not eliminated, the damage problem [13], [14]. Another hurdle for X-ray structure determination based on microcrystals is that the screening for the optimal cryo-protectant must to be done at synchrotron sources and is a time-consuming process. In summary, difficulties with crystallization, together with resolution limits due to crystal quality and radiation damage have become a serious bottleneck in the structural biology of membrane proteins.
Origins of femtosecond nanocrystallography
Femtosecond X-ray lasers open the way for a new approach to the collection of diffraction data from nanocrystals. The first femtosecond soft-X-ray FEL operating in the world was FLASH at DESY in Germany. The key experiment that showed the potential of the new technology for structure determination was the experiment by Chapman and his colleagues in 2006 [15]. They demonstrated the “diffract-before-destruction” principle [16] for a target etched into a silicon-nitride film. It had also been shown at FLASH that diffraction can be obtained from biological nanoparticles [17]; however, with an X-ray wavelength of > 80 Å, FLASH did not allow the determination of molecular structures.
The first hard X-ray free-electron laser in the world, the Linac Coherent Light Source, at SLAC in Stanford, was completed and started operation in 2009 [•• 18] The short wavelength at LCLS opened the way to structural studies of molecules at atomic resolution, as proposed in 2004 [19]. LCLS provides a peak X-ray flux that is 109 higher than a 3rd generation synchrotron pulses [•• 18], [20], [21]. The focussed beam destroys any solid material, producing temperatures that are higher than those inside the sun. It was understood that the data aquisition rates depend on source, scatterer and detector (any of which can provide a bottleneck), and that methods of hydrated sample delivery in vacuum would be challenging. The “serial crystallography” approach [22] was therefore adopted, with a fresh supply of samples fed continuously across the pulsed beam. Unlike single particles, these samples need not be identical, allowing data from nanocrystals of different sizes to be merged.
The first crystallography experiments at LCLS were performed in December 2009 using one of the largest membrane proteins that has been crystallized to date, Photosystem I, [23]. The results provided an impressive proof-of-principle for this radically new approach [•• 24]. Millions of single crystal diffraction snapshot were collected from a liquid water microjet of fully hydrate nanocrystals of Photosystem I in their crystallization mother liquor at room temperature. As shown in figure 1, the nanocrystals run in single file across the pulsed X-ray beam, producing one snap-shot diffraction pattern per particle, which is read out before the arrival of the next pulse. The repetition rate for the first experiments was 60 Hz, but has been increased in 2011 to 120Hz so that thousands of patterns can be collected in a few hours. The diffraction patterns were indexed, and accurate structure factors determined based on Monte Carlo integration of the partial reflections from more than 10 000 patterns [•• 25]. The resolution of the structure determined at 8.4 Å was solely limited by the wavelength of the X-ray beam and the detector [•• 24]. The large interdisciplinary collaboration which led to these results spans many fields of science, from the new data analysis methods [•26], the development of new fast-readout detectors [•• 27], X-ray laser development [•• 18], to the design of the CAMP vacuum chamber [•• 27] and the development of the crystal-stream injector,.
Figure 1.
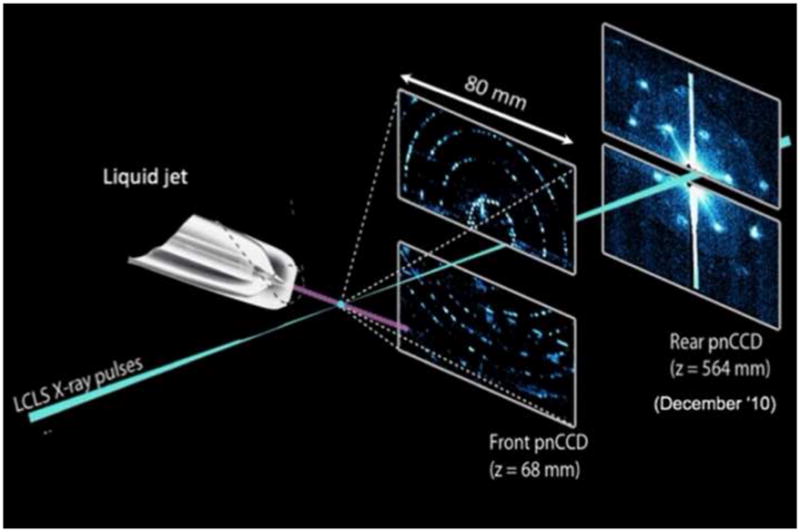
Principle of the experimental setup used for femtosecond crystallography.. The stream of crystals is provided by the injector with the gas focusing nozzle. The femtosecond X-ray beam intersect with the continous crystal stream; diffraction patterns are recorded with the frequency of the Free Electron Laser at LCLS (60 or 120 Hz) using either a one or two CCD detector setup. In the two detector setup, which was used in the CAMP camber at the AMO beamline at LCLS for the first fs crystallography experiments reported in [•• 24], the high order reflections are detected on the front detector. The back detector, which records the low order reflections, allows the resolution of the shape transforms of the reflections. The figure has been modified after [•• 24].
We now discuss in more detail the nanocrystal delivery system, the new methods of data analysis required as well as new phasing methods, and review the literature on the mechanisms of the destructive-readout process at the atomic level. We end with an outline of the future research opportunities which this exciting development offers for membrane protein structure analysis.
Delivery of the nanocrystals: the injector
Hydration is crucial for biological significance in structural biology, so that the essential problem to be solved for particle-delivery is that of synchronizing the arrival of wet particles into a micron-sized volume, in vacuum, with a repeating X-ray pulse focussed to micron dimensions. A highly collimated “high brightness” single-file particle beam is required. The particles will freeze at about 106 °/s in vacuum by evaporative cooling as they travel. The injector must run for days without clogging, and must provide sub-micron positional stability to match the X-ray beam and particle size. The highest possible “hit rate” (mean number of patterns per shot) is required, which depends on particle concentration. For micron-sized protein nanocrystals, neither a gas-phase, gas-focussed injector [17] (with perhaps 25 micron focus and 10% hit rate) or the electro-spray are suitable. The unsynchronized liquid jet used in late 2010 at LCLS [•• 24] provided a hit rate of about 40%, however all the protein flowing between X-ray pulses was not used for data collection. This injector, which is now fitted with a pump laser and an in-vacuum microscope, is described in detail elsewhere [28], [•• 29]. The microscope is essential for alignment purposes, since flashes of light could be seen in the control room on a large screen from the plasma formed as particles are hit and vaporized. A droplet stream of submicron dimensions [• 30] has been produced, well matched to future planned X-ray beam diameters. The design is based on a gas-dynamic virtual nozzle (GDVN), in which the nanocrystal/buffer solution flows along a hollow fiber-optic within a larger concentric capillary tube. The interstitial space is filled with high pressure helium, which emerges at the fiber-optic tip to focus the liquid from the 40 micron internal diameter of the fiber to micron dimensions or less, depending on the conditions of gas and liquid pressure. In this way a large nozzle, which does not clog, may be used to form a much finer droplet stream. (The gas speeds up the liquid, which -to conserve mass flux- shrinks in diameter). The fiber carrying the liquid can be fed from an HPLC system through a fluidic switching device providing alternate samples [•• 24]. New versions of this injector, including a synchronized “droplet-on-demand” type, and versions based on slower viscous host media, such as the lipid cubic phase or a sucrose solution, are under development. These will drastically reduce the amount of protein needed for the collection of a full data set.
Evaluation of fs crystallography data
The snapshot diffraction patterns contain “partial” Bragg reflections [31] unlike those at a synchrotron, where continuous rotation by a goniometer provides angular integration across the Bragg condition. The indexing and merging of millions of snapshot diffraction patterns therefore involves new challenges involving many terrabytes of data. Nanocrystals as small less than 10 unit cells on a side must be considered, for which the energy-and-momentum-conserving Ewald sphere will generate scattering in non-Bragg directions, complicating the use of auto-indexing software and the use of conventional mosaicity, energy-spread and beam-divergence corrections. A new “Monte-Carlo” approach has therefore been developed, [•26] in which it is first assumed that all nanocrystal orientations occur with equal frequency. Then the intensity within a spherical volume in reciprocal space around the same reflection from different nanocrystals is summed to give a quantity proportional to a squared structure factor, under the assumption that the nanocrystal is smaller than one mosaic block, and smaller than the X-ray beam. The results of this approach have been compared with data from large crystals of Photosystem I obtained at a synchrotron, and gave R-factors of about 20% for the data collected at 1.9 kV at the LCLS in 2009 [•• 24]. The LCLS data of Photosystem I crystals was phased by molecular replacement. This data was solely limited to about 8Å resolution by the X-ray wavelength. For a nanocrystal, the scattered intensity around a Bragg condition takes the form of a “shape transform”, whose width (inversely proportional to the crystal size) differs in each pattern (see Fig 2). It is important to show that this summation converges to structure factors for a range of crystal sizes. For details of the method as applied to the PSI data, see [•• 25] where the effects of particle size distribution and flow alignment are discussed. The energy of the LCLS has recently been increased to 9 kV, which has allowed data from nanocrystals to be recorded at higher resolution using this liquid stream flow-injector for sample delivery, indicating that the method of femtosecond nanocrystallography extends to near atomic resolution.
Figure 2.
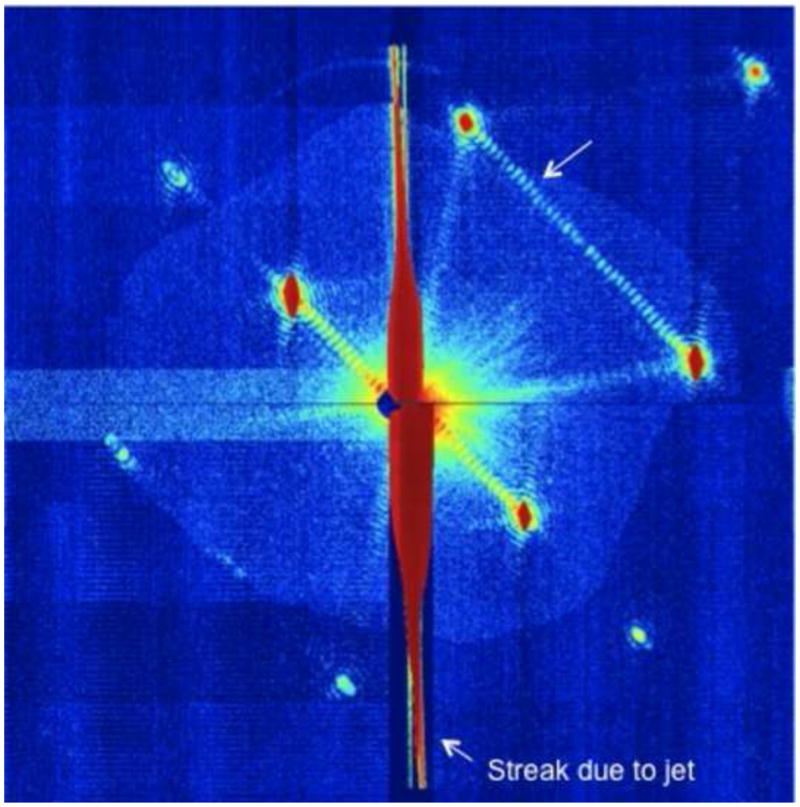
Example of a diffraction pattern from photosystem I which shows the shape transforms. Note the fringes between the Bragg peaks which indicate the number of unit cells in that direction. Thus the evaluation of the shape transforms can be used to determine the size of the crystals [•• 24], and for phase determination [•• 38]. This Figure has been modified after [•• 24].
New solutions to the phase problem
The use of “oversampling”, or scattering between Bragg peaks, to solve the phase problem has a long history going back to early work by Perutz [32] and Sayre [33]. A practical iterative solution to the non-crystallographic phase problem was first described by Feinup, whose methods were used to phase the first “lensless” X-ray image of a non-periodic object [34]. From that work has grown the field of X-ray diffractive imaging (CXDI), using “continuous” X-rays to provide three-dimensional, tomographic, lensless images at about 10nm resolution [35], [36], (see [37] for a review). All these studies provided the groundwork for solutions of the phase problem for XFEL scattering from non-periodic (or nanocrystalline) particles, where scattering is not confined to Bragg peaks. For the PSI nanocrystals, the particle sizes varied between about 0.1 microns and 2 microns, with a corresponding variation in the width of the shape transforms around Bragg peaks. Since the molecular transform modulating the Bragg peaks is the same for every nanocrystal (because each one has the same unit cell contents), whereas the shape transforms around the Braggs (which provide the size distribution) vary, these effects may be separated. The size distribution may thus be “divided out”, to isolate the molecular transform, and allow it to be phased by iterative methods [•• 38]. Phasing by more conventional methods (such as anomalous diffraction (MAD) and isomorphous replacement) may also be possible, and theoretical work on the computation of time-dependent absorption coefficients for MAD is under way.
Overcoming the radiation-damage problem in X-ray crystallography
The idea that radiation damage may be avoided by using very brief pulses of radiation is evident in the basic physics of photoelectron spectroscopy. The suggestion to use this effect in structural biology was made by Solem and Baldwin [39] for X-ray holography, and by Neutze et al [40]. This “diffract-and-destroy” principle was first demonstrated experimentally using soft X-rays for a macroscopic object in 2006 [15]. The underlying theory for this phenomena has been treated by several groups [41], [• 42], [• 43]. For sub-10 fs pulses (which contain about 1E13 photons at the LCLS), elastic scattering terminates before the damaging photoelectron cascade gets under way. A comparison of merged diffraction data for several pulse durations is shown in [•• 24] indicating that high resolution (sub nm) detail begins to be lost for pulse durations longer than about 40 fs, when significant elastic diffraction from the disturbed charge density can occur. The detailed form of this “spot fading” is being simulated using both molecular dynamics and plasma models in much current research. For nanocrystals smaller than the photoelectron mean free path, photoelectrons escape, and a Coulomb explosion results, while for large nanocrystals the photoelectrons thermalize, dumping so much heat energy into the crystal that it vaporizes at very high temperature.
In summary, the method of fs crystallography may overcome the problem of X-ray damage in crystallography by using a very brief pulse of X-rays with a duration of a few tens of femtoseconds (shorter than the period for atomic vibration). The detection of elastically scattered photons terminates before radiation damage, due to ejected photoelectrons, develops, so that damage is greatly reduced despite the use of a very high dose.
Conclusions
Femtosecond nanocrystallography provides the exciting potential for structure determination of difficult-to-crystallize proteins. The method uses nanocrystals, that are too small to be seen under an optical microscope, in their native crystallization-buffer solution, for structure analysis. This allows structure determination of proteins which may only produce showers of nanocrystals that resemble amorphous precipitate. The question now arises as to how many nanocrystals of membrane proteins may already be out there in labs all over the world? The method of SONICC [8,44] allows the detection of nanocrystals, and we predict that systematic screens with SONICC will reveal thousands of nanocrystal of different membrane proteins. Our group routinely uses reversible precipitation/crystallization as the last purification step for our membrane proteins [45]. A small test sample from our lab of “reversible precipitate” of membrane proteins of unknown structure characterized with SONICC (in collaboration with G. Simpson) indicated that up to 30% of the precipitates consisted of nanocrystals. We expect that developments of the injector will further drastically reduce the amount of sample needed, and we predict that in the next few years full fs X-ray data sets of nanocrystals consisting of several hundred thousand patterns will be collected from 10ul of a crystal suspension at a protein concentrations of about 1mg/ml. Developments are already underway towards high throughput data collection where the nanocrystal samples are injected into the jet using a modified HPLC autosampler injection system. Nanocrystal samples are currently being injected into the jet using a modified HPLC autosampler injection system. The number of patterns that must be collected for a full data set depends on the method of data evaluation. Currently, structure factors are determined using the Monte Carlo integration of all partial reflections with the same Miller indices over the full data set. Reconstruction of the peak profiles based on estimates of partiality of the reflections may further drastically reduce the amount of data needed for all but the smallest nanocrystals.
Nanocrystallography also suggests a new solution to the phase problem. Because the X-ray laser beam is fully spatially coherent, new diffraction effects are seen from nanocrystals, interference fringes running between Bragg beams (Fig 2), which provide a new solution to the phase problem. Many of these developments, including the higher repetition rates of new XFELs, will require further developments in detector technology, in readout speed, dynamic range and pixel density/detector area. The new CXI beamline at LCLS, which started operation in February 2011 uses a new type of detector developed by researchers at Cornell University, which is based on a modular design with high dynamic range [46].
A major limitation of conventional X-ray structure analysis is that it shows a static picture of the molecule. Pump-probe femtosecond nanocrystallography experiments open the possibility of collecting molecular “snapshots” of different conformational states of membrane proteins, with the final goal of obtaining molecular movies of molecules “at work”. A spectroscopic pump-probe experimental setup has been established at the AMO beamline of LCLS [• 47]. In June 2010 we conducted the first pump probe experiments on photoactive membrane protein crystals at LCLS, using a pump laser to excite nanocrystals in the liquid jet, before taking their X-ray snapshot, and the results indicate that large conformational changes occur in the crystals upon light excitation (publication in preparation). Time-resolved X-ray structure analysis using fs crystallography is especially interesting for the membrane protein complexes involved in photosynthesis, and opens new avenues to unravel the molecular mechanism of water splitting in Photosystem II. These experiments will be conducted at LCLS by two research teams in August and September 2011.
In addition to the use of optical triggers to initiate a reaction, this experimental arrangement also suggests the possibility of “snapshot chemistry”, in which a substrate fluid is used to drive, for example, an enzyme through its catalytic cycle. Using two fluids mixing at the tip of the liquid jet it will be possible to record snap-shot diffraction patterns at different points along the liquid stream as the reaction proceeds. We also envision that proteins locked in one conformation by a photo-labile substrate analog can be activated by light, upon which the conformational change is followed by fs nanocrystallography. These possibilities suggest an exciting future for time-resolved imaging using XFEL sources in future,
Figure 3.
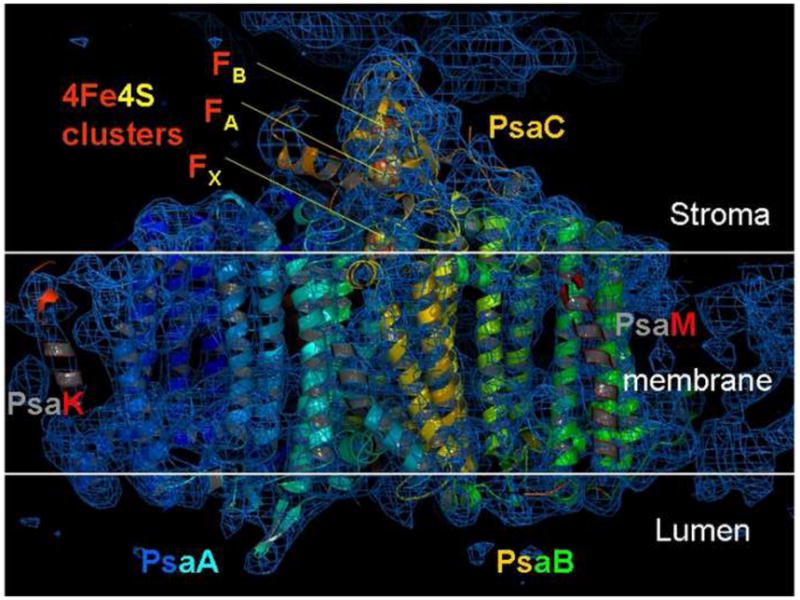
Electron density map of Photosystem I at 8 Åresolution, derived by fs crystallography. The phases were obtained using molecular replacement as described in [•• 24]. The section of the electron density in this picture shows transmembrane helices of the large PSI subunits PsaA, PsaB as well as helices of PsaK and PsaF. This Figure has been modified after [•• 25].
Research highlights.
Femtosecond nanocrystallography is based on Free Electron X-ray Lasers
The diffract-before-destroy- principle overcomes damage problem in crystallography
Nanocrystals are delivered at RT in stream by new type of gas-focusing injector
Coherently illuminated nanocrystals provide interference effects solving the phase problem
The method opens new avenues for time resolved studies showing proteins at work.
Acknowledgments
The work is supported by National Institute of Health (awards 1R01GM095583–01 and 1U54GM094625–01), the National Science Foundation (awards 0417142 and 1021557) and the Center for Bio-Inspired Solar Fuel Production, an Energy Frontier Research Center funded by the U. S. Department of Energy, Office of Science, Office of Basic Energy Sciences under award Number DE-SC0001016.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cusack S, Belrhali H, Bram A, Burghammer M, Perrakis A, Riekel C. Small is beautiful: protein micro-crystallography. Nat Struct Biol. 1998;5 (Suppl):634–637. doi: 10.1038/1325. [DOI] [PubMed] [Google Scholar]
- 2.Cherezov V, Caffrey M. Membrane protein crystallization in lipidic mesophases. A mechanism study using X-ray microdiffraction. Faraday Discuss. 2007;136:195–212. doi: 10.1039/b618173b. discussion 213–129. [DOI] [PubMed] [Google Scholar]
- 3.Cherezov V, Rosenbaum DM, Hanson MA, Rasmussen SG, Thian FS, Kobilka TS, Choi HJ, Kuhn P, Weis WI, Kobilka BK, et al. High-resolution crystal structure of an engineered human beta2-adrenergic G protein-coupled receptor. Science. 2007;318:1258–1265. doi: 10.1126/science.1150577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rasmussen SG, Choi HJ, Rosenbaum DM, Kobilka TS, Thian FS, Edwards PC, Burghammer M, Ratnala VR, Sanishvili R, Fischetti RF, et al. Crystal structure of the human beta2 adrenergic G-protein-coupled receptor. Nature. 2007;450:383–387. doi: 10.1038/nature06325. [DOI] [PubMed] [Google Scholar]
- 5.Rosenbaum DM, Cherezov V, Hanson MA, Rasmussen SG, Thian FS, Kobilka TS, Choi HJ, Yao XJ, Weis WI, Stevens RC, et al. GPCR engineering yields high-resolution structural insights into beta2-adrenergic receptor function. Science. 2007;318:1266–1273. doi: 10.1126/science.1150609. [DOI] [PubMed] [Google Scholar]
- 6.VonDreele R. J Appl Acryst. 2007;40:133. [Google Scholar]
- ••7.Hunter MS, DePonte DP, Shapiro DA, Kirian RA, Wang X, Starodub D, Marchesini S, Weierstall U, Doak RB, Spence JC, et al. X-ray diffraction from membrane protein nanocrystals. Biophys J. 2011;100:198–206. doi: 10.1016/j.bpj.2010.10.049. This study shows the existence of nanocrystals of 100nm size of Photosystem I by powder diffraction. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••8.Gualtieri EJ, Guo F, Kissick DJ, Jose J, Kuhn RJ, Jiang W, Simpson GJ. Detection of membrane protein two-dimensional crystals in living cells. Biophys J. 2011;100:207–214. doi: 10.1016/j.bpj.2010.10.051. This paper describes the detection of two-dimensional protein crystalline arrays in living cells by second-order nonlinear optical imaging of chiral crystals (SONNIC) [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••9.Kissick DJ, Gualtieri EJ, Simpson GJ, Cherezov V. Nonlinear optical imaging of integral membrane protein crystals in lipidic mesophases. Anal Chem. 2010;82:491–497. doi: 10.1021/ac902139w. In this study the authors show that SONICC is the superior method for sensitive detection of integral membrane protein crystals grown in opaque and turbid environments like lipid cubic phases. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aishima J, Owen RL, Axford D, Shepherd E, Winter G, Levik K, Gibbons P, Ashton A, Evans G. High-speed crystal detection and characterization using a fast-readout detector. Acta Crystallogr D Biol Crystallogr. 2010;66:1032–1035. doi: 10.1107/S0907444910028192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flot D, Mairs T, Giraud T, Guijarro M, Lesourd M, Rey V, van Brussel D, Morawe C, Borel C, Hignette O, et al. The ID23–2 structural biology microfocus beamline at the ESRF. J Synchrotron Radiat. 2010;17:107–118. doi: 10.1107/S0909049509041168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •12.Meents A, Gutmann S, Wagner A, Schulze-Briese C. Origin and temperature dependence of radiation damage in biological samples at cryogenic temperatures. Proc Natl Acad Sci U S A. 2010;107:1094–1099. doi: 10.1073/pnas.0905481107. This paper explores the mechanism of radiation damage of biological samples by ionizing radiation at cryogenic temperatures using a combination of single-crystal x-ray diffraction, small-angle scattering. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henderson R. The potential and limitations of neutrons, electrons and X-rays for atomic resolution microscopy of unstained biological molecules. Quart Rev Biophys. 1995;28:171–193. doi: 10.1017/s003358350000305x. [DOI] [PubMed] [Google Scholar]
- 14.Juers DH, Matthews BW. Cryo-cooling in macromolecular crystallography: advantages, disadvantages and optimization. Q Rev Biophys. 2004;37:105–119. doi: 10.1017/s0033583504004007. [DOI] [PubMed] [Google Scholar]
- 15.Chapman HN, Barty A, Bogan MJ, Boutet S, Frank M, Hau-Riege SP, Marchesini S, Woods BW, Bajt S, Benner H, et al. Femtosecond diffractive imaging with a soft-X-ray free-electron laser. Nature Physics. 2006;2:839–843. [Google Scholar]
- 16.Neutze R, Wouts R. Deconvoluting ultrafast structural dynamics: temporal resolution beyond the pulse length of synchrotron radiation. J Synchrotron Radiat. 2000;7:2226. doi: 10.1107/S0909049599012352. [DOI] [PubMed] [Google Scholar]
- 17.Bogan MJ, Benner WH, Boutet S, Rohner U, Frank M, Barty A, Seibert MM, Maia F, Marchesini S, Bajt S, et al. Single particle X-ray diffractive imaging. Nano Lett. 2008;8:310–316. doi: 10.1021/nl072728k. [DOI] [PubMed] [Google Scholar]
- ••18.Emma P, Akre R, Arthur J, Bionta R, Bostedt C, Bozek J, Brachmann A, Bucksbaum P, Coffee R, Decker F-J, et al. First lasing and operation of an Ångstrom-wavelength free-electron laser. Nature Photonics. 2010;4:641–647. This paper describes the development and operation of the first high energy Free Electron X-ray laser in the world, which has a peak photon flux that is 9 orders of magnitude stronger than 3rd generation synchrotron sources and provides femtosecond X-ray pulses that from the basis for the new structure determination method of femtosecond crystallography. [Google Scholar]
- 19.Cornacchia M, Arthur J, Bane K, Bolton P, Carr R, Decker FJ, Emma P, Galayda J, Hastings J, Hodgson K, et al. Future possibilities of the Linac Coherent Light Source. J Synchrotron Radiat. 2004;11:227–238. doi: 10.1107/S090904950400370X. [DOI] [PubMed] [Google Scholar]
- 20.Barty A, Soufli R, McCarville T, Baker SL, Pivovaroff MJ, Stefan P, Bionta R. Predicting the coherent X-ray wavefront focal properties at the Linac Coherent Light Source (LCLS) X-ray free electron laser. Opt Express. 2009;17:15508–15519. doi: 10.1364/OE.17.015508. [DOI] [PubMed] [Google Scholar]
- 21.Ding Y, Brachmann A, Decker FJ, Dowell D, Emma P, Frisch J, Gilevich S, Hays G, Hering P, Huang Z, et al. Measurements and simulations of ultralow emittance and ultrashort electron beams in the linac coherent light source. Phys Rev Lett. 2009;102:254801. doi: 10.1103/PhysRevLett.102.254801. [DOI] [PubMed] [Google Scholar]
- 22.Spence JC, Doak RB. Single molecule diffraction. Phys Rev Lett. 2004;92:198102–198104. doi: 10.1103/PhysRevLett.92.198102. [DOI] [PubMed] [Google Scholar]
- 23.Jordan P, Fromme P, Witt HT, Klukas O, Saenger W, Krauss N. Three-dimensional structure of cyanobacterial photosystem I at 2. 5 angstrom resolution. Nature. 2001;411:909–917. doi: 10.1038/35082000. [DOI] [PubMed] [Google Scholar]
- ••24.Chapman HN, Fromme P, Barty A, White TA, Kirian RA, Aquila A, Hunter MS, Schulz J, DePonte DP, Weierstall U, et al. Femtosecond X-ray protein nanocrystallography. Nature. 2011;470:73–77. doi: 10.1038/nature09750. This study provides the first proof of concept for a new avenue for structure determination of proteins, where damage free protein structure determination is based on ten-thousands of diffraction snapshots collected at LCLS, the worlds first high energy free electron femtosecond X-ray laser, on a stream of fully hydrated nanocrystals of proteins in their mother liquor at room temperature. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••25.Kirian RA, White TA, Holton JM, Chapman HN, Fromme P, Barty A, Lomb L, Aquila A, Maia FR, Martin AV, et al. Structure-factor analysis of femtosecond microdiffraction patterns from protein nanocrystals. Acta Crystallogr A. 2011;67:131–140. doi: 10.1107/S0108767310050981. This study describes how structure factors from an experimental data set consisting of millions of LCLS single crystal diffraction snapshots from nanocrystals of the large membrane protein complex Photosystem I are determined from partial reflections using a modified Monte Carlo Monte Carlo integration over crystallite size and orientation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •26.Kirian RA, Wang X, Weierstall U, Schmidt KE, Spence JC, Hunter M, Fromme P, White T, Chapman HN, Holton J. Femtosecond protein nanocrystallography-data analysis methods. Opt Express. 2010;18:5713–5723. doi: 10.1364/OE.18.005713. This paper describes the Monte Carlo method for recovering structure factors from tens of thousands of snapshot patterns from nanocrystals varying in size, shape and orientation, based on simulated FEL diffraction patterns. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••27.Struder L, Epp S, Rolles D, Hartmann R, Hollb P, Lutz G, Soltau H, Eckart R, Reich C, Heinzinger K, Thamm C, Rudenk A, Krasniqia F, Ku K, Bauer C, Schroter C, Moshammer R, Techert S, Miessner D, Porro M, Halker O, Meidinger N, Kimmel N, Andritschke R, Schopper F, Weidenspointner G, Ziegler A, Pietschner A, Herrmann S, Pietschh U, Walenta A, Leitenberger W, Bostedtf C, Moller T, Ruppf D, Adolphf M, Graafsma H, Hirsemanng H, Gartnerk K, Richter R, Foucar L, Shoeman R, Schlichting I, Ullrich J. Large-format, high-speed, X-ray pnCCDs combined with electron and ion imaging spectrometers in a multipurpose chamber for experiments at 4th generation light sources. Nucl Instr Meth A. 2010;614:483–496. This publication describes the design and development of the CAMP chamber which was essential to perform the first bio-imaging experiments at LCLS and describes high-speed X-ray pnCCD detectors that have a high sensitivity and can read out data in real time with frequencies corresponding to 60Hz frequency of the FEL. [Google Scholar]
- 28.DePonte DP, Weierstall U, Schmidt K, Warner J, Starodub D, Spence JCH, Doak RB. Gas dynamic virtual nozzle for generation of microscopic droplet streams. J Phys D: Appl Phys. 2008;41:195505. [Google Scholar]
- ••29.Doak RB, Weierstall U, De Ponte D, Spence JCH. A pump-probe XFEL particle injector for hydrated samples. Rev Sci Instr. 2011 Submitted. This study describes the liquid-stream and droplet-beam sample injectors with gas-focussing nozzles used for femtosecond nanocrystallography at the LCLS, including the pump-probe facilities for time-resolved nanocrystalloraphy. [Google Scholar]
- •30.DePonte DP, McKeown J, Weierstall U, Doak RB. Towards ETEM serial crystallography electron diffraction from liquid jets. Ultramic. 2011 doi: 10.1016/j.ultramic.2010.11.036. in press. This paper describes operation of the droplet beam injector in a TEM. [DOI] [PubMed] [Google Scholar]
- 31.Rossmann MG, Leslie AG, Sherin SA, Tsukihara T. Processing and postrefinement of oscillation camera data. J Appl Cryst. 1979:12. [Google Scholar]
- 32.Perutz M. The structure of haemoglobin. Proc Roy Sco Lond. 1954;A 225:264–286. [Google Scholar]
- 33.Sayre D. Some inplications of a theorem due to Shannon. Acta Cryst. 1952;5:843. [Google Scholar]
- 34.Miao H, Charalambous P, Kirz J, Sayre D. Extending the methodology of X-ray crystallography to allow imaging of micrometre-sized non-crystalline specimens. Nature. 1999;400:342–344. [Google Scholar]
- 35.Chapman HN, Barty A, Marchesini S, Noy A, Hau-Riege SP, Cui C, Howells MR, Rosen R, He H, Spence JC, et al. High-resolution ab initio three-dimensional x-ray diffraction microscopy. J Opt Soc Am A Opt Image Sci Vis. 2006;23:1179–1200. doi: 10.1364/josaa.23.001179. [DOI] [PubMed] [Google Scholar]
- 36.Shapiro D, Thibault P, Beetz T, Elser V, Howells M, Jacobsen C, Kirz J, Lima E, Miao H, Neiman AM, et al. Biological imaging by soft x-ray diffraction microscopy. Proc Natl Acad Sci U S A. 2005;102:15343–15346. doi: 10.1073/pnas.0503305102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spence JCH. Diffractive (lensless) imaging. In: Hawkes P, Spence JCH, editors. Science of Microscopy. Ch 19. Springer; New York: 2008. pp. 1196–1225. [Google Scholar]
- ••38.Spence JCH, Kirian RA, Wang X, Weierstall U, Schmidt KE, White T, Barty A, Chapman HN, Marchesini S, Holton J. Phasing of coherent femtosecond X-ray diffraction from size-varying nanocrystals. Optics Express. 2011;19:2866–2873. doi: 10.1364/OE.19.002866. This paper describes how to phase diffraction data from nanocrystals of many different sizes, using the interference fringes between Bragg spots to “divide out” the particle size distribution. [DOI] [PubMed] [Google Scholar]
- 39.Solem JC, Baldwin GC. Microholography of living organisms. Science. 1982;218:229–235. doi: 10.1126/science.218.4569.229. [DOI] [PubMed] [Google Scholar]
- 40.Neutze R, Wouts R, van der Spoel D, Weckert E, Hajdu J. Potential for biomolecular imaging with femtosecond X-ray pulses. Nature. 2000;406:752–757. doi: 10.1038/35021099. [DOI] [PubMed] [Google Scholar]
- 41.Hau-Riege SP, London RA, Chapman HN, Bergh M. Soft-x-ray free-electron-laser interaction with materials. Phys Rev E Stat Nonlin Soft Matter Phys. 2007;76:046403. doi: 10.1103/PhysRevE.76.046403. [DOI] [PubMed] [Google Scholar]
- •42.Caleman C, Ortiz C, Marklund E, Bultmark F, Gabrysch M, Parak F, Hajdu J, Klintenberg M, Timneanu N. Radiation damage in biological material: Electronic properties and electron impact ionization in urea. Europhysics Letters. 2009;85:18005. This paper describes simulations of the electronic and atomistic processes which occur in organic nanocrystals when irradiated by an intense femtosecond hard X-ray pulse. [Google Scholar]
- •43.Quiney H, Nugent K. Biomolecular imaging and electronic damage using X-ray free-electron lasers. Nature Physics. 2011;7:142–146. This paper provides an overview of the mechanism of electronic damage in Bio-imaging with X-ray Free Electron Lasers. [Google Scholar]
- 44.Wampler RD, Kissick DJ, Dehen CJ, Gualtieri EJ, Grey JL, Wang HF, Thompson DH, Cheng JX, Simpson GJ. Selective detection of protein crystals by second harmonic microscopy. J Am Chem Soc. 2008;130:14076–14077. doi: 10.1021/ja805983b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grotjohann I, Fromme P. Crystallization of Photosynthetic Membrane Proteins. In: DeLukas L, editor. Protein Crystallization. Academic Press, Elsevier; 2009. pp. 192–224. Benos D (Series Editor): Current Topics in Membranes. [Google Scholar]
- •46.Hugh T, Philipp LJ, Koerner MS, Hromalik Tate MW, Gruner SM. Femtosecond experiment radiation detector for XFEL coherent X-ray imaging. IEEE Transactions on Nuclear Science. 2010;57:3795. This paper describes the development of a new type of detector with a modular design especially designed for femtosecond coherent X-ray imaging with Free Electron Lasers. [Google Scholar]
- •47.Glownia JM, Cryan J, Andreasson J, Belkacem A, Berrah N, Blaga CI, Bostedt C, Bozek J, DiMauro LF, Fang L, et al. Time-resolved pump-probe experiments at the LCLS. Opt Express. 2010;18:17620–17630. doi: 10.1364/OE.18.017620. This paper describes the development of implementation of instrumentation that is designed to perform spectroscopic pump-probe experiments at LCLS. [DOI] [PubMed] [Google Scholar]


