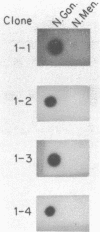
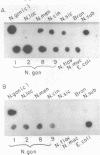
Abstract
We have developed a general procedure for the rapid and efficient enrichment of specific DNA, RNA or cDNA sequences. Biotinylated DNA or RNA is used as a hybridization probe in solution, avidin is then added to label both the probe and hybrid molecules, and the hybridization mixture chromatographed over cupric-iminodiacetic acid agarose beads. Avidin-probe and avidin-hybrid molecules are selectively retained on the column; non-hybridized sequences are contained in the flow-through fraction. Sequences retained on the column are recovered in high yield by the addition of ethylenediamine tetracetic acid in the buffer. The method can be used in both subtractive enrichment and positive selection protocols. Here we report its application to the isolation of Neisseria gonorrhoeae specific genomic DNA clones and the purification of a cDNA subpopulation representing mRNA sequences that are over-expressed in murine Friend cells after dimethylsulfoxide induction.
Full text
PDF






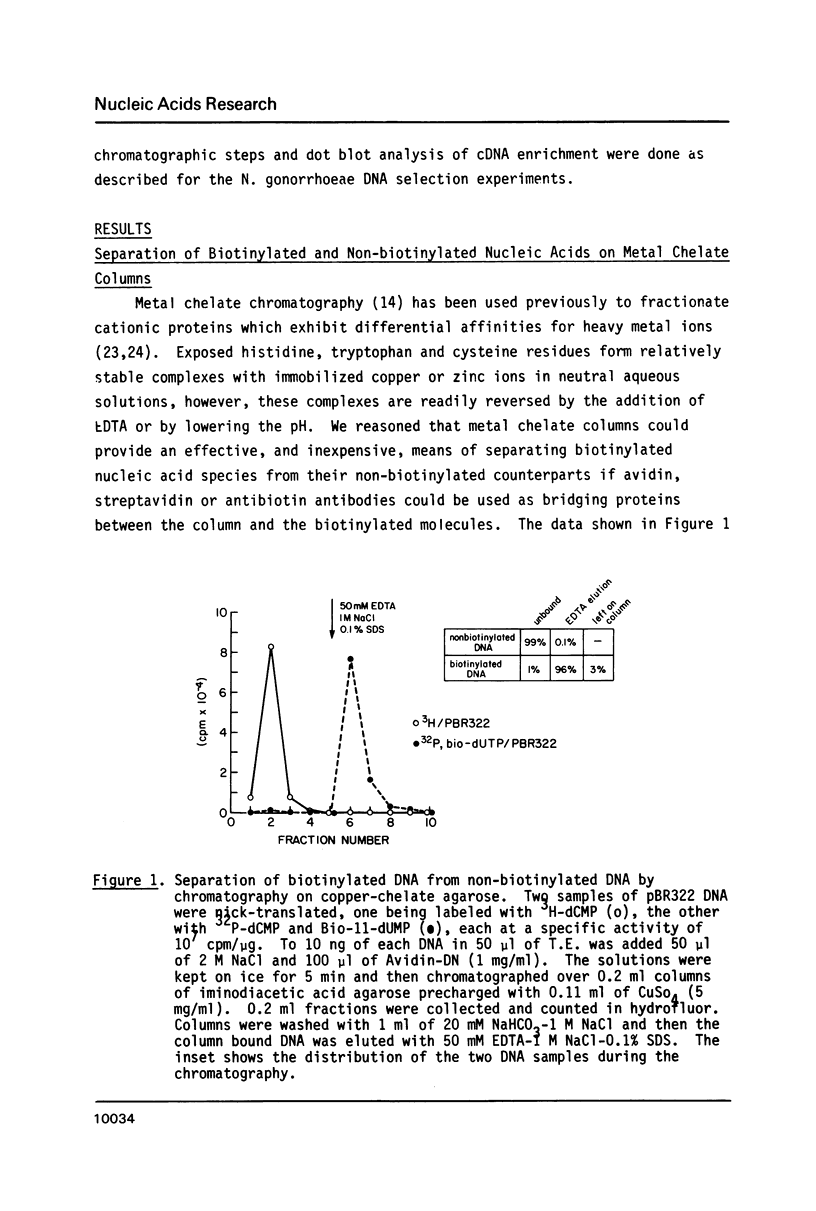


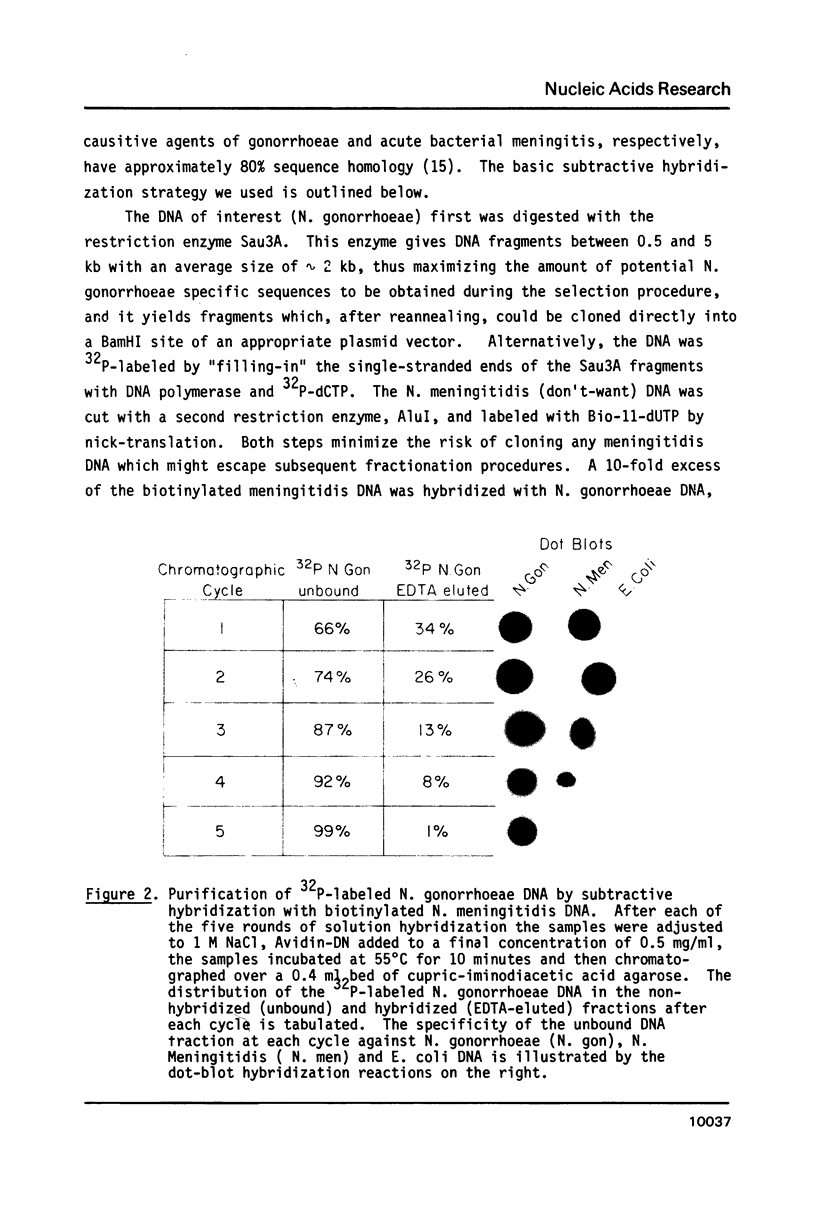






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Affara N., Daubas P. Regulation of a group of abundant mRNA sequences during Friend cell differentiation. Dev Biol. 1979 Sep;72(1):110–125. doi: 10.1016/0012-1606(79)90102-7. [DOI] [PubMed] [Google Scholar]
- Argaraña C. E., Kuntz I. D., Birken S., Axel R., Cantor C. R. Molecular cloning and nucleotide sequence of the streptavidin gene. Nucleic Acids Res. 1986 Feb 25;14(4):1871–1882. doi: 10.1093/nar/14.4.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigati D. J., Myerson D., Leary J. J., Spalholz B., Travis S. Z., Fong C. K., Hsiung G. D., Ward D. C. Detection of viral genomes in cultured cells and paraffin-embedded tissue sections using biotin-labeled hybridization probes. Virology. 1983 Apr 15;126(1):32–50. doi: 10.1016/0042-6822(83)90460-9. [DOI] [PubMed] [Google Scholar]
- Carlsson J., Porath J., Lönnerdal B. Isolation of lactoferrin from human milk by metal-chelate affinity chromatography. FEBS Lett. 1977 Mar 15;75(1):89–92. doi: 10.1016/0014-5793(77)80059-8. [DOI] [PubMed] [Google Scholar]
- Davis M. M., Cohen D. I., Nielsen E. A., Steinmetz M., Paul W. E., Hood L. Cell-type-specific cDNA probes and the murine I region: the localization and orientation of Ad alpha. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2194–2198. doi: 10.1073/pnas.81.7.2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster A. C., McInnes J. L., Skingle D. C., Symons R. H. Non-radioactive hybridization probes prepared by the chemical labelling of DNA and RNA with a novel reagent, photobiotin. Nucleic Acids Res. 1985 Feb 11;13(3):745–761. doi: 10.1093/nar/13.3.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green N. M. Avidin. Adv Protein Chem. 1975;29:85–133. doi: 10.1016/s0065-3233(08)60411-8. [DOI] [PubMed] [Google Scholar]
- Gusella J. F., Housman D. Induction of erythroid differentiation in vitro by purines and purine analogues. Cell. 1976 Jun;8(2):263–269. doi: 10.1016/0092-8674(76)90010-6. [DOI] [PubMed] [Google Scholar]
- Harrison P. R. Analysis of erythropoeisis at the molecular level. Nature. 1976 Jul 29;262(5567):353–356. doi: 10.1038/262353a0. [DOI] [PubMed] [Google Scholar]
- Kingsbury D. T. Deoxyribonucleic acid homologies among species of the genus Neisseria. J Bacteriol. 1967 Oct;94(4):870–874. doi: 10.1128/jb.94.4.870-874.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korman A. J., Knudsen P. J., Kaufman J. F., Strominger J. L. cDNA clones for the heavy chain of HLA-DR antigens obtained after immunopurification of polysomes by monoclonal antibody. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1844–1848. doi: 10.1073/pnas.79.6.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus J. P., Rosenberg L. E. Purification of low-abundance messenger RNAs from rat liver by polysome immunoadsorption. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4015–4019. doi: 10.1073/pnas.79.13.4015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel L. M., Monaco A. P., Middlesworth W., Ochs H. D., Latt S. A. Specific cloning of DNA fragments absent from the DNA of a male patient with an X chromosome deletion. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4778–4782. doi: 10.1073/pnas.82.14.4778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz D. T., Feigelson P. Multihormonal induction of hepatic alpha2u-globulin mRNA as measured by hybridization to complementary DNA. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4791–4795. doi: 10.1073/pnas.74.11.4791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnes J. R., Velan B., Felsenfeld A., Ramanathan L., Ferrini U., Appella E., Seidman J. G. Mouse beta 2-microglobulin cDNA clones: a screening procedure for cDNA clones corresponding to rare mRNAs. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2253–2257. doi: 10.1073/pnas.78.4.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porath J., Carlsson J., Olsson I., Belfrage G. Metal chelate affinity chromatography, a new approach to protein fractionation. Nature. 1975 Dec 18;258(5536):598–599. doi: 10.1038/258598a0. [DOI] [PubMed] [Google Scholar]
- Potuzak H., Dean D. G. Affinity chromatography on columns containing nucleic acids. FEBS Lett. 1978 Apr 15;88(2):161–166. doi: 10.1016/0014-5793(78)80165-3. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Shapiro S. Z., Young J. R. An immunochemical method for mRNA purification. Application to messenger RNA encoding trypanosome variable surface antigen. J Biol Chem. 1981 Feb 25;256(4):1495–1498. [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timberlake W. E. Developmental gene regulation in Aspergillus nidulans. Dev Biol. 1980 Aug;78(2):497–510. doi: 10.1016/0012-1606(80)90349-8. [DOI] [PubMed] [Google Scholar]
- Torres A. R., Peterson E. A., Evans W. H., Mage M. G., Wilson S. M. Fractionation of granule proteins of granulocytes by copper chelate chromatography. Biochim Biophys Acta. 1979 Feb 26;576(2):385–392. doi: 10.1016/0005-2795(79)90413-6. [DOI] [PubMed] [Google Scholar]
- Totten P. A., Holmes K. K., Handsfield H. H., Knapp J. S., Perine P. L., Falkow S. DNA hybridization technique for the detection of Neisseria gonorrhoeae in men with urethritis. J Infect Dis. 1983 Sep;148(3):462–471. doi: 10.1093/infdis/148.3.462. [DOI] [PubMed] [Google Scholar]
- Zimmermann C. R., Orr W. C., Leclerc R. F., Barnard E. C., Timberlake W. E. Molecular cloning and selection of genes regulated in Aspergillus development. Cell. 1980 Oct;21(3):709–715. doi: 10.1016/0092-8674(80)90434-1. [DOI] [PubMed] [Google Scholar]