Abstract
Many acute stressors reduce pain, a phenomenon called stress-induced antinociception (SIA). Stress also is associated with increased scratching in chronic itch conditions. We investigated effects of acute stressors on facial itch and pain using a recently-introduced rat model. Under baseline (no-swim) conditions, intradermal (id) cheek microinjection of the pruritogen serotonin (5-HT) selectively elicited hindlimb scratch bouts, while the algogen mustard oil (allyl isothiocyanate= AITC) selectively elicited ipsilateral forepaw swipes, directed to the cheek injection site. To test effects of swim stress, rats received id cheek microinjection of 5-HT (1%), AITC (10%), or vehicle, and were then subjected to one of the following swim conditions: (1) weak SIA (W-SIA), (2) naltrexone-sensitive SIA (intermediate or I-SIA), or (3) naltrexone-insensitive SIA (strong or S-SIA). After the swim, we recorded the number of hindlimb scratch bouts and forelimb swipes directed to the cheek injection site, as well as facial grooming by both forepaws. Under S-SIA, AITC-evoked swiping and 5-HT-evoked scratching were both reduced. I-SIA reduced AITC-evoked swiping with no effect on 5-HT-evoked scratching. Facial grooming immediately post-swim was suppressed by S-SIA, but not I- or W-SIA. W-SIA tended to equalize scratching and swiping elicited by 5-HT and AITC compared to no-swim controls, suggesting altered itch and pain processing. Exercise (wheel-running), novelty, cold exposure and fear (shaker table), key components of swim stress, differentially affected tailflick latencies and 5-HT-evoked swiping and scratching behavior. Thus, itch and pain can be simultaneously suppressed by a combination of acute stress-related factors via an opioid-independent mechanism.
Keywords: stress, opioid, itch, scratch, pain, antinociception
1. Introduction
Environmental stressors can reduce pain sensation and nocifensive responses of animals, a phenomenon called stress-induced antinociception (SIA) [5,10,23,26]. Two mechanisms of SIA have been proposed: lower levels of acute stress (such as intermittent cold-water swim) activate an endogenous μ-opioid-dependent analgesia system [7,8,13], whereas more intense stressors (such as continuous cold water swim) activate a μ-opioid-independent analgesia mechanism [6,10,16,23,34]. Swim stress is commonly used to investigate SIA since the swim parameters can be easily manipulated to induce the two distinct forms of SIA [32,33,43]. Cross-tolerance studies involving the μ-opioid antagonist naloxone identified the opioid dependency of the two forms of swim stress [6,13,34], but factors such as genotype and sex are also known to influence the quantity and quality of SIA [30,31,36].
While many previous studies have investigated SIA, the effect of stress on acute itch is poorly understood. Itch is defined as an unpleasant sensation which elicits the desire to scratch. Itch is associated with insect bites or exposure to certain plants, and frequently accompanies dermatitis, systemic diseases and a variety of other conditions [45]. Stress has been correlated with an increase in scratching associated with atopic dermatitis and psoriasis, thus contributing to the belief that stress worsens chronic itch conditions [2,3,44,46]. Despite this, there are few if any investigations of the effects of stress on itch. While pain inhibits itch, mounting evidence suggests that itch and pain are conveyed by partially-overlapping populations of dorsal horn neurons [19]. We reasoned that stress may either disinhibit itch, or suppress itch by inhibiting a common population of itch- and pain-signaling neurons.
We have presently investigated these possibilities using a recently-developed rodent model that distinguishes between itch- and pain-related behaviors. Intradermal (id) injection of pruritogens or algogens in the cheek of mice or rats selectively elicits hindlimb scratch bouts or ipsilateral forelimb swipes, respectively, that are directed to the injection site [1,19,39]. Pruritogen-evoked scratching was reduced by the μ-opioid antagonist naltrexone but not morphine, whereas algogen-evoked swiping was reduced by morphine but not naltrexone [1,40]. This model is advantageous over the traditional model of id injection of pruritogens in the nape of the neck, for which the only available response is hindlimb scratching [20]. In the present study, we first established parameters for naltrexone-sensitive and –insensitive types of swim SIA using the standard tail flick assay. We then tested the effects of these different stressors on pruritogen-evoked scratching and algogen-evoked swiping, as well as generalized facial grooming behavior, to determine if these behavioral responses are modulated in a similar or differential manner by acute stress. We additionally investigated various components of swim stress including temperature, exercise, novelty and fear. An abstract of this work has appeared [18].
2. Methods
2.1. Animals
Adult male Sprague Dawley rats from Simonsen and Charles River (weighing 365–445 g when testing began) were doubly housed, and had free access to food and water. All procedures were approved by the UC Davis Institutional Animal Care and Use Committee.
2.2. Swim Stress-Induced Antinociception
At the time of testing, all rats were subjected to one of three swim protocols to induce a strong level of stress antinociception (S-SIA), an intermediate level of stress antinociception (I-SIA), or a weak level of stress antinociception (W-SIA). Swim tanks consisted of a plastic cylindrical insulated water cooler filled with water to a depth such that rats were unable to touch the bottom of the tank with their hindpaws and/or tails while keeping their heads above water, thus forcing them to swim for the duration of the test. The only exception was the W-SIA “float” group in Fig. 1, in which the water depth was shallow enough that rats’ tails could touch the bottom, possibly explaining why some rats floated but did not swim.
Figure 1.
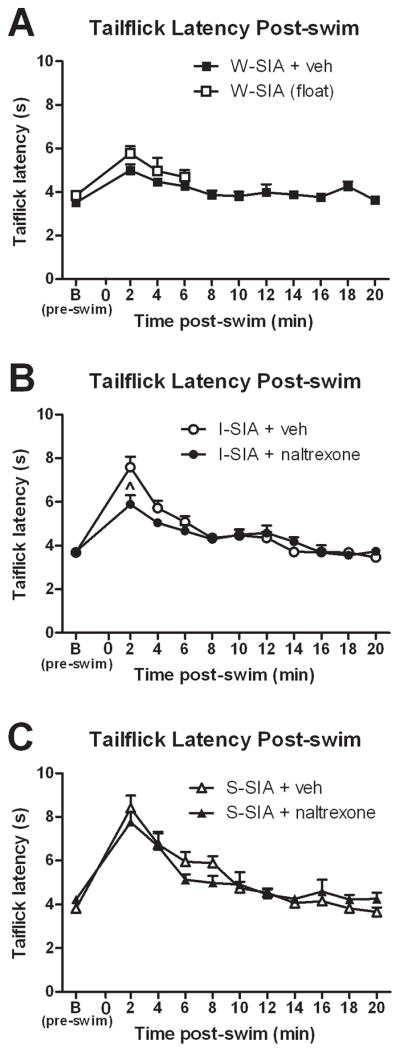
Opioid-dependent and –independent forms of stress-induced antinociception. (A) Rats displayed elevated tailflick latencies indicating a weak SIA (W-SIA), regardless of whether they actively swam (W-SIA + veh, N=8) or floated without swimming (W-SIA, float, N=9). (B) Systemic (ip) administration of the opioid antagonist naltrexone (14 mg/kg, N=7) partially prevented the elevation in tailflick latencies induced by I-SIA; (vehicle, N=8). (C) ip naltrexone (N=7) did not affect elevation in tailflick latency induced by S-SIA (vehicle, N=8). Mean ± SEM; repeated measures ANOVA, 1-way ANOVA, Tukey post-hocs; paired two-tailed t-tests. ^P≤0.05.
The water temperature and swim times were as follows for each condition: the W-SIA condition required a 2 min swim in 21°C water, the I-SIA condition required a 3 min swim in 12°C water, and the S-SIA condition required a 5 min swim in 10°C water. The temperature of the water was measured before and after each swim to ensure protocol uniformity. Temperature adjustments were made by adding ice, but rats were not placed in the tanks until the ice had melted and the temperature stabilized.
2.3. Tailflick Test
To determine the opioid dependency of the I-SIA and S-SIA forms of SIA, rats were first habituated for 15 minutes to Plexiglas restraint tubes in which their bodies were loosely restrained but their tails remained free. Baseline antinociception latencies were obtained for each rat using the Tailflick test [12], in which the time to withdraw his tail from a radiant heat source was quantified (IITC Inc., Woodland Hills, CA). In order to prevent tissue damage, the tailflick apparatus was programmed to cut off after a latency of 10 sec.
After baseline latencies were obtained, rats received an intraperitoneal (ip) injection of either vehicle (1:1:1:17, DMSO:ethanol:Tween-80:saline) or naltrexone 14 mg/kg (dissolved in vehicle; Dupont; Garden, NY). Next, the tailflick latencies of a sample of rats from each group were obtained 15 min after injection to verify that the injections had no effects on nociception prior to SIA.
Each rat was subjected to the I-SIA or S-SIA swim test 25 min after ip injection of either vehicle or naltrexone. After swimming, rats were quickly toweled off, replaced in the restraint tubes, and tested for antinociception. Tailflick latencies were recorded immediately following the swim and every two minutes up through 20 min post-stress.
Rats tested under other conditions (described below) were subjected to the same tailflick testing protocol, except that they were exposed to the condition for 5 min, 25 min post-ip injection of vehicle or naltrexone.
Since tail temperature can influence tailflick latency [11], we systematically measured tail temperature before and immediately after each swim condition or other stressor, prior to the first tailflick measurement. An infrared thermometer (CEN-TECH, Camarillo CA) was used to measure the tail surface temperature at approximately the same location on each rat’s tail for uniformity.
2.4 Behavioral Testing of Responses to Cheek Microinjections
After establishment of the opioid dependency of the various types of SIA assessed using the tailflick assay, rats were subjected to each of the same swim stress (or other) conditions and tested using the cheek behavioral model.
Prior to the day of testing, the fur from each rat’s cheek was shaved. At the time of testing, rats were habituated to an opaque Plexiglas chamber on a clear glass table through which the animals were videotaped from below for 15 minutes. Subsequently, rats were intradermally injected in the cheek with 10 μL 1% 5-HT or 10 μL 10% AITC via a Hamilton syringe connected to PE50 tubing and a 30 gauge hypodermic needle. Immediately following the injection, each rat was subjected to one of the following conditions: (a) swim stress (W-SIA, I-SIA, or S-SIA as described above). (b) Forced wheel running for 5 min. Note that these rats were not previously trained to run on wheels. An experimenter was therefore present to ensure the consistent rotation of the wheels at a constant rate (mean 29.4 +/− 1.9 [SEM] revolutions per 5 min; mean distance traveled 2580.1 +/− 165.5 cm) during the 5 min period. (c) Exposure to the immobile wheel for 5 min. (d) Cold exposure in a cold room (2.2 °C) for 5 min. (e) Loose restraint on a shaker platform that tilted 30° up and down at a rate of approximately 1 Hz (shaker) for 5 min. (f) Exposure to shaker platform while in the cold room (combination) for 5 min. The rationale for rats receiving id injections prior to each manipulation is that typically it takes 5–10 min for the pruritogen/algogen to initiate a behavioral response; if the id injection is given prior to the condition, then presumably the timing of the evoked behavioral responses will correspond with the peak of SIA if present. The only exception to this protocol was for the systematic investigation of the timing of the 5-HT cheek injection relative to the swim. For these experiments, rats were injected id (cheek) 10 min before, immediately before, or immediately after W-SIA. After exposure to each condition (and id injection), rats were immediately replaced in the Plexiglas testing chambers. Behaviors were recorded for 1 hr post-manipulation. Videotapes were scored by viewers blinded to the experimental condition. Viewers scored the number of bouts (events) of grooming, swiping, and scratching behaviors. Scratch bouts consisted of discrete episodes of back-and-forth hind claw movements across the cheek injection area, terminating when the rat brought the hind claws to the mouth and/or placed the hindpaw on the floor. Swiping behavior consisted of single, isolated unilateral swipes directed across the injected site. A bout of facial grooming consisted of a discrete episode of head- or face-washing by both forepaws. Swipes, scratches, and grooms of non-injected body regions were excluded from analyses.
2.5. Chemicals
The pruritogen serotonin (5-hydroxytryptamine, 5-HT-HCl, Sigma, St. Louis, MO) was tested at a concentration of 1% (47 mM) in saline, and the algogen allyl isothiocyanate (AITC, the pungent ingredient in mustard oil; Sigma) was tested at a concentration of 10% (1 M) in a 7% Tween-80 solution (in 0.9% saline). The opioid antagonist naltrexone (Sigma, St. Louis, MO) was tested at 14 mg/kg and dissolved in a vehicle containing DMSO, ethanol, Tween-80, and saline in a ratio of 1:1:1:17. Despite evidence that high doses of opioid antagonists may affect other neurotransmitter systems [38], we selected naltrexone (rather than naloxone) at this dose for reasons described by Maier et al. [25], and supported by subsequent studies [13,15,17]. Briefly, naltrexone is 3–8 times more potent than naloxone, has a longer duration of action, and the dose of 14 mg/kg prevents or reverses both short and long-term analgesic effects of SIA. These factors convinced us to use naltrexone at this dose in the present studies.
2.6. Statistics
For the tailflick test, three baseline values were averaged for each animal. Data are expressed as Mean ± SEM. Data were analyzed by repeated measures ANOVA and paired two-tailed t-tests. When Mauchly’s Test of Sphericity indicated significance, the Greenhouse-Geisser correction factor was applied to the interaction term of all repeated factors. When a significant interaction was present, 1-way ANOVAs were performed for each time point with Tukey post-hocs as needed if more than two groups were being compared. When repeated measures indicated a significant group effect, Tukey post-hocs were performed as indicated.
For the behavioral testing, data are expressed as Mean ± SEM. The data represented in bar graphs were analyzed via either 1-way ANOVAs and Tukey post-hocs as needed or unpaired two-tailed t-tests; these values represent the total amount of the specified behavior quantified over the entire 60-min post-stress period. The data represented in line graphs were analyzed via repeated measures ANOVA as described above; the values represent the total amount of specified behavior quantified per 5-min bin either during the baseline period (up through 0 min) or during the post-stress period (up through 60 min).
On the figures, each symbol designates a unique significance as described in the figure legends. Symbols set to the side of a line graph next to a bracket designate a significant group effect, while symbols above specific time points indicate the post-hoc results of a significant interaction.
Statistics were performed using the software SPSS 9.0, and for graphing purposes the software Graphpad Prism 5.0 was utilized.
3. Results
3.1. Different forms of swim SIA assessed by TF
Pre-swim baseline tailflick latencies were similar across groups (Figure 1A–C). Under the W-SIA condition, there was a significant elevation in tailflick latency immediately following the swim that declined to baseline within 8 min (Fig. 1A, ■; P=0.0004, 0.0012, 0.0064, 0.209 for times 2, 4, 6, and 8, respectively, compared to baseline). Some rats did not swim when placed into the 21°C water tank. These animals (N=9) nevertheless underwent tailflick testing upon removal from the tank. The mean tailflick latencies for this group (□, Figure 1A) were not significantly different from the W-SIA group immediately after the swim; thus, tailflick testing was not continued for this group past the 6 min timepoint.
The I-SIA group exhibited a significant elevation in tailflick latencies over the first 12 min post-swim (Fig. 1B, ●, P=0, 0.0003, 0.0024, 0.0329, 0.001, 0.015, 0.8158, through time 14, respectively). Overall, the I-SIA group was significantly different from W-SIA (repeated-measures ANOVA, F1,14=6.901, P=0.02); post-hoc tests due to a significant interaction (F10,140=9.773, P=0) revealed significant differences between the I-SIA and W-SIA groups at time points 2, 4, 6, and 18 min post-swim (P=0, 0.003, 0.011, 0.042, respectively). Pretreatment with naltrexone (14 mg/kg) significantly attenuated I-SIA (Fig. 1B, ●). There was a significant difference between I-SIA + vehicle and I-SIA + naltrexone groups (Fig. 1B; F10,130=4.071, P ≤ 0.001) with the difference occurring at time point 2 min (P=0.02).
The S-SIA group displayed an overall elevation in mean tailflick latency compared to baseline until time 14 min (Fig. 1C, △, P=0, 0.0006, 0.0018, 0.0003, 0.0346, 0.0069, and 0.2156, respectively) and was significantly different compared to the W-SIA group overall (F1,14=14.91, P=0.002) and over time (F5.139,71.946=14.841, P=0), including times 2–10 (P=0, 0, 0.002, 0, 0.022, respectively). The S-SIA group was not significantly different compared to the I-SIA group (F1,140=3.891, P=0.0686). Pretreatment with naltrexone (Fig. 1C, ▲) had no significant within-group (F4.10,53.23=1.87, P=0.13) or between-group effect (F1,13=0.007, P=0.93).
Vehicle-pretreated animals had a mean tail temperature of 22.63°C ± 0.06°C (Mean ± SEM) pre- and 22.93°C ± 0.22°C post-swim, at the time of the first tailflick measurement. Similarly, naltrexone-pretreated rats had tail temperatures of 22.60°C ± 0.29°C prior to and 22.90°C ± 0.23°C post-swim. Pre- vs. post-swim tail temperatures were not significantly different for either vehicle- or naltrexone-pretreatment groups (P>0.05 for both).
3.2. Different forms of swim SIA assessed by cheek injection of 5-HT or AITC
5-HT
Under baseline conditions (no swim), cheek microinjection of 5-HT elicited a significant increase in the number of hindlimb scratch bouts but not forelimb swipes, while vehicle injection elicited very low levels of scratching or swiping (Fig. 2A).
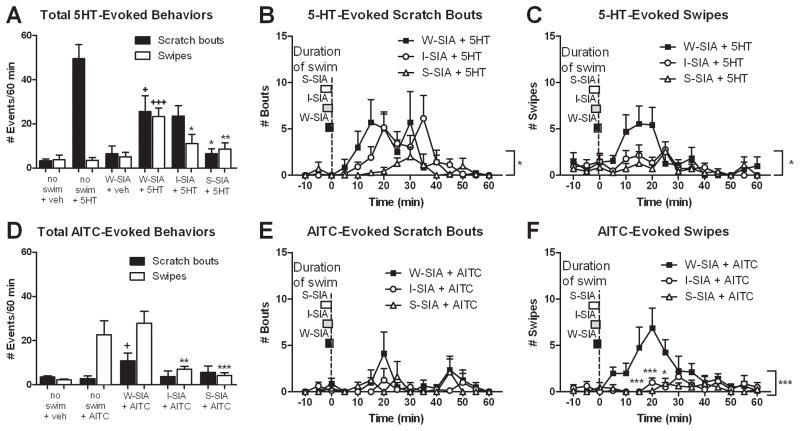
Figure 2.
Effects of swim stress on 5-HT- and AITC-evoked scratching and swiping behaviors. (A) Bar graph plots mean number of hindlimb scratch bouts (■) or ipsilateral forelimb swipes (□) directed to the cheek injection site under each condition (N=6–12). (B) Time course of 5-HT-evoked scratch bouts under each swim condition (N=7). Boxes to left indicate duration of swim under each condition, aligned with the end of the swim and beginning of tailflick testing at time 0 (dashed vertical line). 5-HT was injected in the cheek immediately prior to each swim condition. (C) As in B for 5-HT-evoked swipes (N=7). (D) As in A for AITC-evoked scratching and swiping (N=8). (E, F) As in B, C for AITC-evoked scratching and swiping (N=8). For all panels, error bars represent SEM. + P≤0.05, +++ P≤0.001 vs no swim + 5-HT; *P≤0.05, **P≤0.01, ***P≤0.001 vs. W-SIA + 5-HT; repeated measures ANOVA, 1-way ANOVA, Tukey post-hocs, or unpaired two-tailed t-tests as appropriate (see text).
Under the W-SIA condition, cheek injection of vehicle elicited very low numbers of scratch bouts or swipes that did not differ from the no-swim vehicle group (Fig. 2A). However, in animals receiving cheek injection of 5-HT immediately prior to exposure to W-SIA, there was a significant reduction in 5-HT-evoked scratch bouts compared to the no-swim + 5-HT group (t17=2.416, P=0.0272), and a significant increase in forelimb swipes (Fig. 2A; t17=5.96, P=0). Fig. 2B (■) shows the time course of 5-HT-evoked scratching under W-SIA, which peaked at 15–30 min post-swim and declined thereafter. Fig. 2C shows a similar time course for 5-HT-evoked swiping.
The finding that W-SIA tended to equalize 5-HT-evoked scratching and swiping was unexpected. We next tested if the expression of 5-HT-evoked behaviors depended on the timing of 5-HT cheek injection relative to the swim. When 5-HT was injected in the cheek 10 min prior to the W-SIA swim condition, there was reduced scratching and increased swiping relative to the no swim + 5-HT group (Fig. 3, left and middle bars). This was similar to the group receiving 5-HT cheek injection immediately prior to the W-SIA swim condition (Fig. 2A). When 5-HT was injected in the cheek immediately after the W-SIA swim condition (Fig. 3, right bars), scratching returned to baseline (no swim + 5-HT) and swiping increased significantly above levels observed in all other W-SIA conditions (t16=5.474, P=0).
Figure 3.
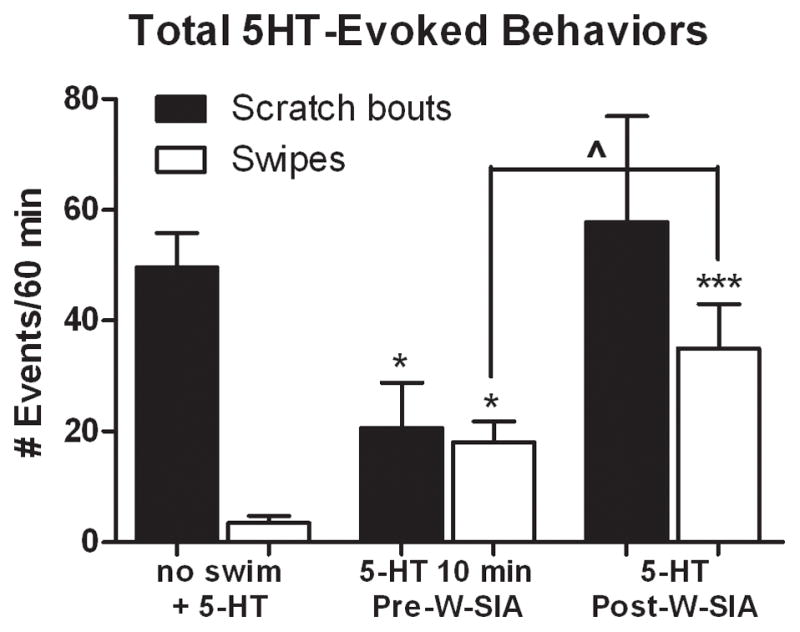
Scratching is reduced when 5-HT is injected before, but not after, W-SIA. Compared to no swim controls (left-hand bars; no swim + 5-HT data reproduced from Fig. 2A for convenience, N=12), the total number of 5-HT-evoked scratch bouts was reduced and swipes increased when 5-HT was injected in the cheek 10 min before rats were subjected to W-SIA (middle bars, N=8), as was the case when 5-HT was injected immediately prior to the swim (see Fig. 2A). When 5-HT was injected in the cheek after W-SIA (N=6), scratching was at control (no swim) levels but swiping was elevated. Mean ± SEM; unpaired two-tailed t-tests. ^P≤0.05 10 min Pre-W-SIA vs. Post-W-SIA; *P≤0.05, ***P≤0.001 vs. no swim + 5-HT.
Under the I-SIA condition there was a significant reduction in 5-HT-evoked scratch bouts (t17=2.864, P=0.0108) and a significant elevation in swipes (Fig. 2A, I-SIA + 5-HT; t17=2.185, P=0.0432), compared to the no-swim + 5-HT group. Compared with the W-SIA group, the I-SIA group exhibited significantly fewer 5-HT-evoked swipes (t12=2.164, P=0.05) consistent with the tailflick data, but no significant difference in the number of scratch bouts. The time courses of 5-HT-evoked scratch bouts and swipes under the I-SIA condition are shown in Fig. 2B and C (○), respectively.
Under the S-SIA condition, there was a significant reduction in the number of 5-HT-evoked scratch bouts (t17=5.058, P=0), compared to the no swim + 5-HT group (Fig. 2A). The time courses of 5-HT evoked scratch bouts and swipes are shown in Figs. 2B and C (△), respectively. Importantly, the number of 5-HT-evoked scratch bouts was significantly different compared to the W-SIA group (t12=2.53, P=0.0264), as was the number of 5-HT-evoked swipes (t12=3.125, P=0.0088).
AITC
Under baseline conditions (no swim), cheek microinjection of AITC elicited a significant increase in forelimb swipes, but not hindlimb scratch bouts, compared to vehicle controls (Fig. 2D). Under W-SIA, there was a significant increase in AITC-evoked scratch bouts, compared to the no swim + AITC condition (Fig. 2D; t18=2.394, P=0.0278). Under both I-SIA and H-SIA conditions, there was a significant reduction in AITC-evoked swipes consistent with antinociception, but no change in scratch bouts (compared to no swim + AITC). The time courses of AITC-evoked scratch bouts and swipes under each condition of SIA are shown in Figs. 2E and F, respectively. Importantly, there was a significant difference in the number of AITC-evoked swipes between the W-SIA group and both I-SIA and S-SIA groups (Fig. 2F, t14=3.759, P=0.0021 vs I-SIA, t14=4.291, P=0.0007 vs. S-SIA), consistent with antinociception.
Facial grooming
Under baseline (no swim) conditions, the mean number of grooming bouts following cheek injection of 5-HT or AITC (Fig. 4A, C, respectively, hatched bars) was not significantly different compared to that of naïve (untreated) rats (mean 21.3 bouts/60 min +/− 2.4 SEM; from 40).
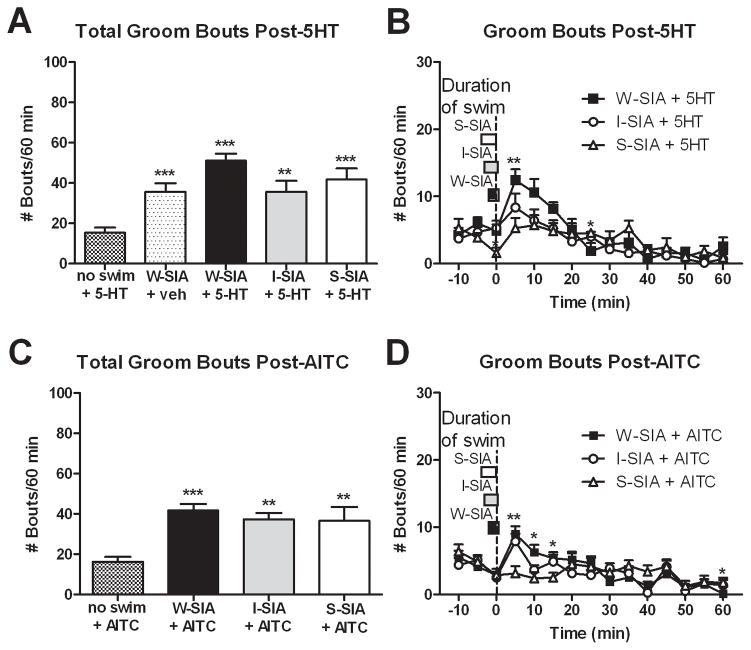
Figure 4.
Suppression of facial grooming behavior during the peak of SIA. (A, C) The total number of groom bouts after id 5-HT (N=7–12) or AITC (N=8,12) was similar between swim groups, but greater than the no swim group. (B, D) However, groom bouts were suppressed in the S-SIA groups compared to the W-SIA groups at the peak of SIA (N=7 for 5-HT groups, N=8 for AITC groups). Mean ± SEM; repeated measures ANOVA, 1-way ANOVA; Tukey post-hocs; unpaired two-tailed t-tests. *P≤0.05, **P≤0.01, ***P≤0.001 vs. no swim groups in bar graphs and vs. W-SIA groups in line graphs.
Each condition of SIA significantly increased the mean number of facial grooming bouts following cheek injection of 5-HT or AITC (Fig. 4A, C; P<0.05 for all groups compared to no swim + 5-HT and no swim + AITC, respectively). W-SIA also significantly increased facial grooming bouts following cheek vehicle injection (Fig. 4A). Increased grooming occurred during the first 10–15 min after the rat was removed from the water tank, and is likely attributed to removal of water from the wet fur. Importantly, there was no increase in grooming immediately post-swim in the S-SIA group (compared to W-SIA + 5-HT [F14,168=2.919, P=0.001] and W-SIA + AITC [F14,196=3.386, P=0], respectively).
3.3. Components contributing to swim stress assessed by tailflick
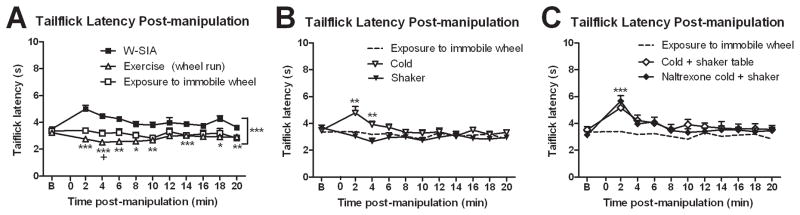
To attempt to tease apart some of the factors that may contribute to stress under the different swim conditions, we exposed rats to the following: exercise (forced wheel-running), exposure to the running wheel without running, cold (2.2°C cold room), shaker table, and a combination of shaker table plus cold room exposure. Following exposure to the immobile wheel, there was no significant change in tailflick latencies relative to baseline (Fig. 5A, □). When tested immediately following exercise (wheel-running), there was a significant decrease in tailflick latencies indicating hyperalgesia (Fig. 5A, ○). Both the exercise (wheel running) and exposure to immobile wheel groups were significantly different compared to the W-SIA group (reproduced in Fig. 5A [■] for convenience). Exposure to the cold room resulted in an increase in tailflick latency that was significant at 2 and 4 min post-stress (Fig. 5B, ○; P=0.0159, 0.0089, respectively), similar to the W-SIA group. Exposure to the shaker table did not significantly affect tailflick latencies. There was a significant interaction among the cold, shaker, and exposure to immobile wheel conditions (F9.011,108.126=2.705, P=0.007). We combined two conditions by placing rats on the shaker table in the cold room (cold + shaker). This group exhibited tailflick latencies that were elevated at 2 and 4 min post-stress compared to baseline (Fig. 5C, P=0.003, 0.0379, respectively). The antinociceptive effect of cold + shaker exposure was not affected by pretreatment with naltrexone (Fig. 5C, significant interaction between the three groups F10.868,114.115=3.637, P=0)
Figure 5.
Effects of other manipulations assessed by tailflick. (A) Exercise (5 min forced running, N=8) shortened tailflick latencies, while 5 min exposure to an immobile running wheel (N=8) had no effect. B refers to baseline (pre-manipulation). (B) Cold temperature (5 min, N=11) mildly elevated tailflick latencies, while 5 min loosely restrained on the shaker table (N=8) had no effect. Tailflick latencies for the exposure to the immobile wheel condition in A are reproduced in B and C as dashed lines for convenience. (C) Combining the conditions of shaker table and exposure to cold (N=8) also elevated tailflick latencies in a manner that was unaffected by naltrexone (14 mg/kg, N=8). Mean ± SEM; repeated measures ANOVA, 1-way ANOVA; Tukey post-hocs; paired two-tailed t-tests. *P≤0.05, **P≤0.01, ***P≤0.001 vs W-SIA group and +P≤0.05 vs novelty in A; * indicates significance vs novelty in B and C.
3.4. Effects of various stressors on 5-HT-evoked scratching, swiping and facial grooming
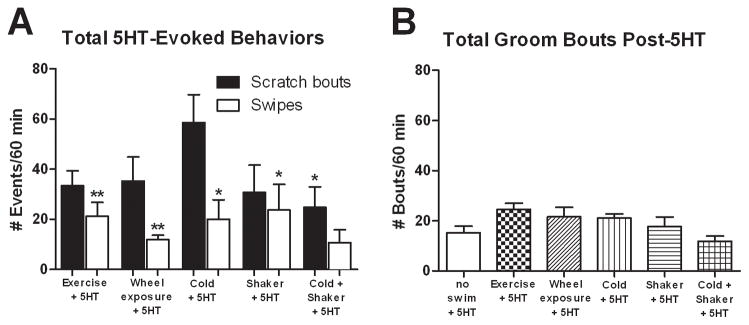
The S-SIA condition significantly attenuated 5-HT-evoked scratching behavior (Fig. 2A), so we were interested to determine which component(s) of this stress condition may have contributed to the antipruritic effect. Rats received cheek microinjection of 5-HT and were then immediately exposed to one of the conditions described in the preceding section. Fig. 6A shows that under each individual condition, there was no significant change in hindlimb scratch bouts but there was a significant increase in forelimb swipes (compared to the baseline no swim + 5-HT condition shown in Fig. 2A). Only in the combined cold + shaker condition was there a significant reduction in scratch bouts (t17=2.391, P=0.0286) that was not accompanied by a significant change in number of swipes (Fig. 6A, white bars).
Figure 6.
Effect of other manipulations assessed by cheek injection of 5-HT. (A) None of the individual manipulations affected the total number of scratch bouts compared to non-stressed 5-HT-injected rats, except for the combination of cold + shaker (which suppressed scratching) (N=7, 8). Similarly, only the cold + shaker group did not display elevated swiping behavior compared to non-stressed controls. (B) Total groom bouts were similar for all groups (N=7–12). Mean ± SEM; unpaired two-tailed t-tests. *P≤0.05, **P≤0.01 vs. no swim + 5-HT group.
There was no significant effect of any of the conditions on facial grooming behavior compared to baseline (no swim + 5-HT) (Fig. 6B). However, the number of grooming bouts in the cold + shaker + 5-HT group was significantly less compared to naïve rats (unpaired t-test, P<0.05; 11.9 +/− 2 SEM vs. 21.3 +/− 2.4 SEM, results from 40).
4. Discussion
A number of interesting findings emerged from this study. Most importantly, the naltrexone-insensitive form of stress-induced analgesia resulting from high stress conditions (S-SIA) significantly attenuated itch-related scratching as well as nociceptive behavior, implying the activation of an endogenous system that simultaneously inhibits both itch and pain transmission. This requires a combination of factors associated with cold-water swim, including cold temperature and fear. Curiously, opioid-dependent I-SIA did not enhance 5-HT-evoked scratching as would have been predicted by opioid enhancement of itch. It was noteworthy that W-SIA reduced 5-HT-evoked scratching and increased wiping, essentially equalizing these itch- and pain-related behaviors and suggesting a fundamental alteration in itch- and pain processing, compared to non-swimming rats. The data also suggest that W-SIA is distinct from I- and S-SIA, supporting the existence of multiple forms of opioid-dependent and -independent SIA.
The reduction in itch-related responses under S-SIA suggests that both itch- and pain-signaling pathways are under a common inhibitory effect during high levels of acute stress. This is surprising, since noxious stimuli usually suppress itch [47] and reduced pain might be expected to enhance itch [21,24]. However, there are reports that both itch and pain can be simultaneously reduced, for example by administration of α2-adrenoceptor agonists or gabapentin [14,41]. It is tempting to speculate that S-SIA activates an endogenous antinociceptive system that also suppresses itch signals that are transmitted by pruritogen-sensitive ascending sensory neurons.
The I-SIA condition differentially suppressed AITC-evoked wiping, but not 5-HT-evoked scratching. Conceivably, the I-SIA condition may have generated an intrinsically weaker antipuritic effect compared to S-SIA, although there was no significant difference between these conditions on tail flick latencies (Fig. 1). Moreover, it would be useful in future studies to test if naltrexone reduces the antinociceptive effect of I-SIA but not S-SIA on AITC-evoked wiping behavior, as predicted by the tail flick data.
Facial grooming was briefly elevated immediately after the end of the swim, presumably due to removal of excess water. Facial grooming, along with scratching behavior, is considered to be part of a larger repertoire of grooming behaviors. It is thus interesting that both behaviors were suppressed in the S-SIA condition, as well as by naltrexone [40], suggesting a common link. Consistent with this, the combination of cold exposure and shaker table also resulted in a decrease in facial grooming compared to naïve animals. This is not likely attributed to generalized motor suppression, since rats did not exhibit a significant change in locomotor activity during the peak of opioid-independent SIA elicited by footshock [42].
Stress is a well-known contributing factor to chronic itch conditions such as atopic dermatitis and psoriasis, and is typically associated with a worsening of scratching [2,3,44,46]. The present data show that S-SIA due to acute cold-water swim reduces itch-related scratching, seemingly in contradiction to stress effects on chronic itch. However, Corticotropin-Releasing Factor (CRF) may play a role in centrally suppressing stress-induced atopic dermatitis [2], and children with atopic dermatitis are thought to have a suppressed adrenocortical response, which may partly explain the higher correlation of stress and eruption of atopic dermatitis lesions [9]. Thus it is possible that stressors associated with SIA can suppress acute itch, but may enhance chronic itch in those individuals whose hypothalamic-pituitary axis has been compromised. Further work is needed to clarify the effects of acute and chronic stress on acute itch and pathological chronic itch.
Previous studies indicate that the facial model discriminates well between pruritogen- vs. algogen-evoked responses in rats and mice [1,19,39,40]. It was thus surprising that W-SIA reduced 5-HT-evoked scratching and increased AITC-evoked swiping. This implies the emergence of facial hyperalgesia during and after W-SIA, which is contrary to the observed antinociceptive effect of W-SIA as assessed by the tailflick assay. This difference might be attributable to the behaviors being assessed in the two assays. The tailflick reflex is a polysynaptic spinally-organized reflex, whereas hindlimb scratches and forelimb swipes directed to the cheek are highly integrated behaviors that require intact connections between the medullary trigeminal subnucleus caudalis where sensory input arrives, and cervical and lumbar spinal segments where the forelimb swipe and hindlimb scratch movements are respectively generated. It is conceivable that such complex behavioral responses are modulated differently compared to the overriding antinociceptive effect observed with the tailflick reflex.
W-SIA resulted in an equivalent elevation in tailflick latency whether the rats swam or not (Fig. 1A). Equivalent antinociception was also induced by exposure to the cold room (Fig. 5B). This suggests that cold temperature, even mildly hypothermic 21°C water, may be the most important factor inducing W-SIA. In the cheek injection assay, each individual stressor (exercise, exposure to immobile wheel, cold exposure and shaker table) significantly enhanced 5-HT-evoked forelimb swiping, suggesting hyperalgesia (Fig. 6A). In the tailflick assay, exercise also had a hyperalgesic effect (Fig. 4). Therefore, the increase in 5-HT-evoked swiping under W-SIA (Fig. 2A) may be attributed to the hyperalgesic effect of exercise during swimming. Only the combination of cold exposure and shaker reduced 5-HT-evoked scratching (Fig. 6A) relative to no-swim controls. Overall, these data suggest that swim stress induces fundamental alterations in itch and pain processing that involve multiple, interacting physiological and psychological factors. One speculation is that the pronociceptive effect of swim exercise inhibits itch, leading to reduced hindlimb scratch bouts and increased forelimb swipes. However, this interaction does not apply to all conditions, since cold exposure increased swiping without reducing scratch bouts (Fig. 6A).
Scratching was reduced when 5-HT was injected in the cheek before, but not after, the W-SIA swim condition (Fig. 3), suggesting that swimming attenuated the buildup of itch. Possible factors accounting for this include water temperature, exercise, fear, and novelty. However, none of the individually tested factors affected 5-HT-evoked scratching (Fig. 6A). Speculatively, swimming may have provided a distraction that reduced itch, consistent with observations that distraction suppressed spontaneous scratching in NC mice with dermatitis [49], and that virtual reality immersion and audiovisual distraction temporarily suppressed scratching in humans with atopic dermatitis or psoriasis vulgaris [22]. Cheek injection of 5-HT after the swim did not reduce scratching, indicating that the antipruritic effect did not outlast the swim period. However, swiping was significantly increased by 5-HT injected before or after the swim (Figs. 2a, 3), indicating that the hyperalgesic effect outlasted the period of swimming.
Opiates often induce pruritus which is preferentially localized to the face in humans [4]. Surprisingly, the opioid-dependent form of I-SIA did not increase itch. Perhaps opioid-induced pruritus is dependent on the method of administration and/or location of opioid release. The periaqueductal gray (PAG) region of the midbrain plays a pivotal role in descending modulation of pain and SIA, and opioid release from this region is important for it to occur [10,27,28,35]. In humans, the PAG is believed to play an important role in the central itch modulation system as evidenced by positron emission tomography testing of itchy and cold (noxious) stimuli [29].
Opioid-dependent and –independent forms of SIA were originally identified using electric shock as a stressor [48]. Intermittent footshock-evoked SIA as well as the present I-SIA were attenuated by μ-antagonists, whereas continuous footshock-evoked SIA and the present W- and S-SIA were not affected by μ-antagonists. However, it is possible that other neurotransmitters are indirectly modulated secondarily to μ-opioids via naltrexone administration [25]. Continuous footshock-evoked SIA was shown to be attenuated by endocannabinoid CB1 receptor antagonists [17]. There is also evidence for the role of NMDA in naloxone-insensitive SIA [30,37], although these studies were performed in mice and/or variable stress paradigms. It is otherwise not known what endogenous mediators may be involved in W- and S-SIA. The combination of cold exposure and shaker table induced SIA that was similar to W-SIA, and was unaffected by naltrexone (Fig. 5C). Further work is needed to confirm and elucidate the identity and roles of endogenous mediators that are released during the swim stress conditions that modulate pain- and itch-related behaviors. Moreover, there appear to be important sex differences in the expression of SIA [30, 31, 36], an issue that was not addressed in the present study. Again, future studies are needed to address possible gender differences in the effects of stress on itch as well as pain using the cheek model that can discriminate between these sensory qualities.
Summary.
Certain intense acute stressors, such as cold-water swim, suppress facial itch- and pain-related behavioral responses simultaneously in rats. This indicates that the endogenous antinociceptive system activated by acute stressors can also exert an antipruritic effect.
Acknowledgments
We thank Connie Duong, Dione Fernandez, Leland Gee, and Margaret Ivanov, for their assistance with operating the behavioral experiments and rodent handling, and Connie Duong, Dione Fernandez, Leland Gee, Margaret Ivanov, and Kathryn McLaughlin for their assistance with watching the videotapes for behavioral quantification. We also thank Drs. Yves Boucher and Tasuku Akiyama for reading the manuscript. This work was supported by NIH grant #AR-057194 and DE021183.
Footnotes
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Akiyama T, Carstens MI, Carstens E. Differential itch- and pain-related behavioral responses and μ-opoid modulation in mice. Acta Derm Venereol. 2010;90:575–581. doi: 10.2340/00015555-0962. [DOI] [PubMed] [Google Scholar]
- 2.Amano H, Negishi I, Akiyama H, Ishikawa O. Psychological stress can trigger atopic dermatitis in NC/Nga mice: an inhibitory effect of corticotrophin-releasing factor. Neuropsychopharmacology. 2008;33:566–73. doi: 10.1038/sj.npp.1301435. [DOI] [PubMed] [Google Scholar]
- 3.Arck P, Paus R. From the brain-skin connection: the neuroendocrine-immune misalliance of stress and itch. Neuroimmunomodulation. 2006;13:347–56. doi: 10.1159/000104863. [DOI] [PubMed] [Google Scholar]
- 4.Ballantyne JC, Loach AB, Carr DB. Itching after epidural and spinal opiates. Pain. 1988;33:149–60. doi: 10.1016/0304-3959(88)90085-1. [DOI] [PubMed] [Google Scholar]
- 5.Bodnar RJ, Kelly DD, Brutus M, Glusman M. Stress-induced analgesia: neural and hormonal determinants. Neurosci Biobehav Rev. 1980;4:87–100. doi: 10.1016/0149-7634(80)90028-7. [DOI] [PubMed] [Google Scholar]
- 6.Bodnar RJ, Kelly DD, Steiner SS, Glusman M. Stress-produced analgesia and morphine-produced analgesia: lack of cross-tolerance. Pharmacol Biochem Behav. 1978;8:661–6. doi: 10.1016/0091-3057(78)90263-0. [DOI] [PubMed] [Google Scholar]
- 7.Bodnar RJ, Kelly DD, Spiaggia A, Ehrenberg C, Glusman M. Dose-dependent reductions by naloxone of analgesia induced by cold-water stress. Pharmacol Biochem Behav. 1978;8:667–72. doi: 10.1016/0091-3057(78)90264-2. [DOI] [PubMed] [Google Scholar]
- 8.Bodnar RJ, Sikorszky V. Naloxone and cold-water swim analgesia: Parametric considerations and individual differences. Learn Motiv. 1983;14:223–237. [Google Scholar]
- 9.Buske-Kirschbaum A, Jobst S, Wustmans A, Kirschbaum C, Rauh W, Hellhammer D. Attenuated free cortisol response to psychosocial stress in children with atopic dermatitis. Psychosom Med. 1997;59:419–26. doi: 10.1097/00006842-199707000-00012. [DOI] [PubMed] [Google Scholar]
- 10.Butler RK, Finn DP. Stress-induced analgesia. Prog Neurobiol. 2009;88:184–202. doi: 10.1016/j.pneurobio.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 11.Carrive P, Churyukanov M, Le Bars D. A reassessment of stress-induced “analgesia” in the rat using an unbiased method. Pain. 2011;152:676–86. doi: 10.1016/j.pain.2010.12.019. [DOI] [PubMed] [Google Scholar]
- 12.D’Amour FE, Smith DL. A method for determining loss of pain sensation. J Pharmacol Exp Ther. 1941;72:74–9. [Google Scholar]
- 13.Giradot MN, Holloway FA. Intermittent cold water stress-analgesia in rats: cross-tolerance to morphine. Pharmacol Biochem Behav. 1984;4:631–3. doi: 10.1016/0091-3057(84)90315-0. [DOI] [PubMed] [Google Scholar]
- 14.Gotoh Y, Andoh T, Kuraishi Y. Clonidine inhibits itch-related response through stimulation of α (2)-adrenoceptors in the spinal cord in mice. Eur J Pharmacol. 2011;650:215–9. doi: 10.1016/j.ejphar.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 15.Grau JW, Hyson RL, Maier SF, Madden J, 4th, Barchas JD. Long-term stress-induced analgesia and activation of the opiate system. Science. 1981;213:1409–11. doi: 10.1126/science.7268445. [DOI] [PubMed] [Google Scholar]
- 16.Grisel JE, Fleshner M, Watkins LR, Maier SF. Opioid and nonopioid interactions in two forms of stress-induced analgesia. Pharmacol Biochem Behav. 1993;45:161–72. doi: 10.1016/0091-3057(93)90100-8. [DOI] [PubMed] [Google Scholar]
- 17.Hohmann AG, Suplita RL, Bolton NM, Neely MH, Fegley D, Mangieri R, Krey JF, Walker JM, Holmes RV, Crystal JD, Duranti A, Tontini A, Mor M, Tarzia G, Piomelli D. An endocannabinoid mechanism for stress-induced analgesia. Nature. 2005;435:1108–12. doi: 10.1038/nature03658. [DOI] [PubMed] [Google Scholar]
- 18.Iodi-Carstens M, Spradley JM, Carstens E. Program No. 180.15. 2011 Neuroscience Meeting Planner. Washington, DC: Society for Neuroscience; 2011. Swim stress-induced analgesia and its effects on scratching behavior in rats. Online. [Google Scholar]
- 19.Klein A, Carstens MI, Carstens E. Facial injections of pruritogens or algogens elicit distinct behavior responses in rats and excite overlapping populations of primary sensory and trigeminal subnucleus caudalis neurons. J Neurophysiol. 2011;106:1078–88. doi: 10.1152/jn.00302.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuraishi Y, Nagasawa T, Hayashi K, Satoh M. Scratching behavior induced by pruritogenic but not algesiogenic agents in mice. Eur J Pharmacol. 1995;275:229–33. doi: 10.1016/0014-2999(94)00780-b. [DOI] [PubMed] [Google Scholar]
- 21.Lagerström MC, Rogoz K, Abrahamsen B, Persson E, Reinius B, Nordenankar K, Olund C, Smith C, Mendez JA, Chen ZF, Wood JN, Wallén-Mackenzie A, Kullander K. VGLUT2-dependent sensory neurons in the TRPV1 population regulate pain and itch. Neuron. 2010;68:529–42. doi: 10.1016/j.neuron.2010.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leibovici V, Magora F, Cohen S, Ingber A. Effects of virtual reality immersion and audiovisual distraction techniques for patients with pruritus. Pain Res Manage. 2009;14:283–286. doi: 10.1155/2009/178751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lewis JW, Cannon JT, Liebeskind JC. Opioid and nonopioid mechanisms of stress analgesia. Science. 1980;208:623–5. doi: 10.1126/science.7367889. [DOI] [PubMed] [Google Scholar]
- 24.Liu Y, Abdel Samad O, Zhang L, Duan B, Tong Q, Lopes C, Ji RR, Lowell BB, Ma Q. VGLUT2-dependent glutamate release from nociceptors is required to sense pain and suppress itch. Neuron. 2010;68:543–56. doi: 10.1016/j.neuron.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maier SF, Davies S, Grau JW, Jackson RL, Morrison DH, Moye T, Madden J, 4th, Barchas JD. Opiate antagonists and long-term analgesic reaction induced by inescapable shock in rats. J Comp Physiol Psychol. 1980;94:1172–83. doi: 10.1037/h0077743. [DOI] [PubMed] [Google Scholar]
- 26.Maier SF. Stressor controllability and stress-induced analgesia. Ann N Y Acad Sci. 1986;467:55–72. doi: 10.1111/j.1749-6632.1986.tb14618.x. [DOI] [PubMed] [Google Scholar]
- 27.Mayer DJ, Wolfe TL, Akil H, Carder B, Liebeskind JC. Analgesia from electrical stimulation in the brainstem of the rat. Science. 1971;174:1351–4. doi: 10.1126/science.174.4016.1351. [DOI] [PubMed] [Google Scholar]
- 28.Miczek KA, Thompson ML, Shuster L. Naloxone injections into the periaqueductal grey area and arcuate nucleus block analgesia in defeated mice. Psychopharmacology (Berl) 1985;87:39–42. doi: 10.1007/BF00431775. [DOI] [PubMed] [Google Scholar]
- 29.Mochizuki H, Tashiro M, Kano M, Sakurada Y, Itoh M, Yanai K. Imaging of central itch modulation in the human brain using positron emission tomography. Pain. 2003;105:339–46. doi: 10.1016/s0304-3959(03)00249-5. [DOI] [PubMed] [Google Scholar]
- 30.Mogil JS, Belknap JK. Sex and genotype determine the selective activation of neurochemically-distinct mechanisms of swim stress-induced analgesia. Pharmacol Biochem Behav. 1997;56:61–6. doi: 10.1016/S0091-3057(96)00157-8. [DOI] [PubMed] [Google Scholar]
- 31.Mogil JS, Richards SP, O’Toole LA, Helms ML, Mitchell SR, Kest B, Belknap JK. Identification of a sex-specific quantitative trait locus mediating nonopioid stress-induced analgesia in female mice. J Neurosci. 1997;17:7995–8002. doi: 10.1523/JNEUROSCI.17-20-07995.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mogil JS, Sternberg WF, Balian H, Liebeskind JC, Sadowski B. Opioid and nonopioid swim stress-induced analgesia: a parametric analysis in mice. Physiol Behav. 1996;59:123–32. doi: 10.1016/0031-9384(95)02073-x. [DOI] [PubMed] [Google Scholar]
- 33.O’Connor P, Chipkin RE. Comparisons between warm and cold water swim stress in mice. Life Sci. 1984;35:631–9. doi: 10.1016/0024-3205(84)90258-3. [DOI] [PubMed] [Google Scholar]
- 34.Pavlovic Z, Bodnar RJ. Antinociceptive and hypothermic crosstolerance between continuous and intermittent cold-water swims in rats. Physiol Behav. 1993;54:1081–4. doi: 10.1016/0031-9384(93)90328-d. [DOI] [PubMed] [Google Scholar]
- 35.Reynolds DV. Surgery in the rat during electrical analgesia induced by focal brain stimulation. Science. 1969;164:444–5. doi: 10.1126/science.164.3878.444. [DOI] [PubMed] [Google Scholar]
- 36.Romero MT, Bodnar RJ. Gender differences in two forms of cold-water swim analgesia. Physiol Behav. 1986;37:893–7. [PubMed] [Google Scholar]
- 37.Sakurada S, Onodera K, Katsuyama S, Yonezawa A, Arai K, Hayashi T, Furuta S, Sato T, Kisara K. Effects of forced walking stress on formalin-induced paw licking in mice. Methods Find Exp Clin Pharmacol. 1999;21:467–70. [PubMed] [Google Scholar]
- 38.Sawynok J, Pinsky C, LaBella FS. On the specificity of naloxone as an opiate antagonist. Life Sci. 1979;25:1621–32. doi: 10.1016/0024-3205(79)90403-x. [DOI] [PubMed] [Google Scholar]
- 39.Shimada SG, LaMotte RH. Behavioral differentiation between itch and pain in mouse. Pain. 2008;139:681–7. doi: 10.1016/j.pain.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spradley JS, Davoodi A, Iodi Carstens M, Carstens E. Opioid modulation of facial itch- and pain-related responses and grooming behavior in rats. Acta Derm Venereol. 2012 doi: 10.2340/00015555-1364. in press. [DOI] [PubMed] [Google Scholar]
- 41.Ständer S, Weisshaar E, Luger TA. Neurophysiological and neurochemical basis of modern pruritus treatment. Exp Dermatol. 2008;17:161–9. doi: 10.1111/j.1600-0625.2007.00664.x. [DOI] [PubMed] [Google Scholar]
- 42.Suplita RL, 2nd, Eisenstein SA, Neely MH, Moise AM, Hohmann AG. Cross-sensitization and cross-tolerance between exogenous cannabinoid antinociception and endocannabinoid-mediated stress-induced analgesia. Neuropharmacology. 2008;54:161–71. doi: 10.1016/j.neuropharm.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Terman GW, Morgan MJ, Liebeskind JC. Opioid and non-opioid stress analgesia from cold water swim: importance of stress severity. Brain Res. 1986;372:167–71. doi: 10.1016/0006-8993(86)91472-1. [DOI] [PubMed] [Google Scholar]
- 44.Tran BW, Papoiu AD, Russoniello CV, Wang H, Patel TS, Chan YH, Yosipovitch G. Effect of itch, scratching and mental stress on autonomic nervous system function in atopic dermatitis. Acta Derm Venereol. 2010;90:354–61. doi: 10.2340/00015555-0890. [DOI] [PubMed] [Google Scholar]
- 45.Twycross R, Greaves MW, Handwerker H, Jones EA, Libretto SE, Szepietowski JC, et al. Itch: scratching more than the surface. Q J Med. 2003;96:7–26. doi: 10.1093/qjmed/hcg002. [DOI] [PubMed] [Google Scholar]
- 46.Verhoeven EW, Kraaimaat FW, de Jong EM, Schalkwijk J, van de Kerkhof PC, Evers AW. Individual differences in the effect of daily stressors on psoriasis: a prospective study. Br J Dermatol. 2009;161:295–9. doi: 10.1111/j.1365-2133.2009.09194.x. [DOI] [PubMed] [Google Scholar]
- 47.Ward L, Wright E, McMahon SB. A comparison of the effects of noxious and innocuous counterstimuli on experimentally induced itch and pain. Pain. 1996;64:129–38. doi: 10.1016/0304-3959(95)00080-1. [DOI] [PubMed] [Google Scholar]
- 48.Watkins LR, Mayer DJ. Organization of endogenous opiate and nonopiate pain control systems. Science. 1982;216:1185–92. doi: 10.1126/science.6281891. [DOI] [PubMed] [Google Scholar]
- 49.Yamaguchi T, Maekawa T, Nishikawa Y, Nojima H, Kaneko M, Kawakita T, Miyamoto T, Kuraishi Y. Characterization of itch-associated responses of NC mice with mite-induced chronic dermatitis. J Dermatol Sci. 2001;25:20–8. doi: 10.1016/s0923-1811(00)00099-2. [DOI] [PubMed] [Google Scholar]