Abstract
Study Objectives:
To examine the association of sleep complaints reported at baseline (insomnia complaints and excessive daytime sleepiness (EDS)) and medication, with cognitive decline in community-dwelling elderly.
Design:
An 8-yr longitudinal study.
Setting:
The French Three-City Study.
Participants:
There were 4,894 patients without dementia recruited from 3 French cities and having a Mini-Mental Status Examination (MMSE) score ≥ 24 points at baseline.
Measurements and Results:
Questionnaires were used to evaluate insomnia complaints (poor sleep quality (SQ), difficulty in initiating sleep (DIS), difficulty in maintaining sleep (DMS), early morning awakening (EMA)), EDS, and sleep medication at baseline. Cognitive decline was defined as a 4-point reduction in MMSE score during follow-up at 2, 4, and 8 yr. Logistic regression models were adjusted for sociodemographic, behavioral, physical, and mental health variables, and apolipoprotein E genotype. EDS independently increased the risk of cognitive decline (odds ratio (OR) = 1.26, 95% confidence interval (CI) = 1.02-1.56), especially for those patients who also developed dementia during the follow-up period (OR = 1.39, 95% CI = 1.00-1.97). The number of insomnia complaints and DMS were negatively associated with MMSE cognitive decline (OR = 0.77, 95% CI = 0.60-0.98 for 3-4 complaints, OR = 0.81, 95% CI = 0.68-0.96, respectively). The 3 other components of insomnia (SQ, DIS, EMA) were not significantly associated with MMSE cognitive decline.
Conclusions:
Our results suggest that EDS may be associated independently with the risk of cognitive decline in the elderly population. Such results could have important public health implications because EDS may be an early marker and potentially reversible risk factor of cognitive decline and onset of dementia.
Citation:
Jaussent I; Bouyer J; Ancelin ML; Berr C; Foubert-Samier A; Ritchie K; Ohayon MM; Besset A; Dauvilliers Y. Excessive sleepiness is predictive of cognitive decline in the elderly. SLEEP 2012;35(9):1201-1207.
Keywords: Sleepiness, cognitive decline, elderly, insomnia, dementia
INTRODUCTION
Major changes in cognitive functioning occur with age and vary considerably across individuals and cognitive domains.1 Sleep decreases physiologically in quantity and quality with age,2,3 and insomnia and excessive daytime sleepiness (EDS) are frequently reported in the elderly.4 Although sleep deprivation and fragmentation are known to decrease neuropsychologic performance,5 little is known about the predictive role of sleep complaints in relation to cognitive decline with some divergent results across studies. These inconsistencies could be attributed to differences in design (cross-sectional versus longitudinal studies), sample size, and heterogeneity in cognitive assessment and sleep evaluation. Several cross-sectional studies have reported an association between sleep disturbances and low cognitive performance.6–9 Of the few longitudinal studies performed in community-dwelling elderly, EDS was predictive of cognitive decline in a study of men10 and two other studies reported that insomnia was associated with increased cognitive decline.11,12 Conversely, the Nurses Health Study and the Honolulu-Asia Aging Study did not find significant associations between insomnia and cognitive decline.10,13 All of these studies evaluated global cognitive function over a short follow-up period (≤ 3 yr) and with rare evaluation of specific areas of cognition that may be earlier or more specific predictors of cognitive impairment. None examined all the insomnia subcomponents, i.e., sleep quality (SQ), difficulty in initiating sleep (DIS), difficulty in maintaining sleep (DMS), and early morning awakening (EMA), as well as the effect of sleep medication as independent risk factors for cognitive decline.
This large prospective study aims to examine the associations of sleep complaints (insomnia complaints and EDS) and medication with cognitive decline over an 8-yr follow-up period in community-dwelling elderly, taking into account multiple competing causes of cognitive decline.
METHODS
Study Population
Participants were recruited as part of the Three-City Study, an ongoing multisite longitudinal study involving three French cities (Bordeaux, Dijon, and Montpellier).14 Briefly, 9,294 participants age 65 yr or older were recruited from the electoral rolls between March 1999 and March 2001. The study protocol was approved by the ethical committee of the Kremlin-Bicêtre University Hospital (France), and written informed consent was obtained from each participant. The participants were interviewed and underwent clinical examinations at baseline and after 2, 4, and 8 yr.
Cognitive Decline and Dementia Diagnosis
Global cognitive function was assessed using the Mini-Mental Status Examination (MMSE),15 at baseline and at each follow-up (2, 4, and 8 yr). Those with persistent cognitive decline were defined as participants with a 4-point reduction in MMSE score during the follow-up and without any increase (i.e., improvement) of 3 points or more after the decline, which would suggest resolution of the cognitive decline.
The 4-point reduction is a criterion widely used in epidemiologic studies working on assessment of cognition,16,17 and corresponds in our sample to the lowest 5th percentile of the differences between all the scores (e.g., between any follow-up score and baseline score or between all follow-up scores). The second criterion (3 points increased) was chosen to exclude participants who may have fluctuating scores due to transient conditions (intermittent cognitive decline) and thus to select those with a persistent cognitive decline.
Diagnosis and classification of dementia at baseline and at each follow-up examination were made by Three-City study clinical investigators according to the criteria of the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, Text Revision, and was further validated by a national panel of neurologists independently of the Three-City investigators. Participants with initial cognitive impairment (MMSE score at baseline < 24) and those with dementia at baseline were excluded from the current analysis.
Two other cognitive tests were also administered at baseline and at each consecutive follow-up; the Benton Visual Retention Test (BVRT),18 which assesses visual memory, and the Isaacs Set Test (IST), which measures semantic access within a 30-sec time limit.19 Participants in the lowest 5th percentile of the differences between all the scores (i.e., 4 points and 14 points reduction in BVRT and IST score, respectively) and without increase of 3 or more points in BVRT score and 13 or more points in IST score after the decline were considered to have persistent cognitive decline.
Sleep Complaints
Sleep complaints were assessed at baseline by a face-to-face clinical interview followed by the completion of a sleep questionnaire.20 The participants self-rated as “never, rarely, frequently, or often” occurrence of (1) being excessively sleepy during the day (EDS); (2) having difficulties in initiating sleep (DIS); (3) having several awakenings during the night (DMS); and (4) having early morning awakening (EMA) without being able to go back to sleep. Participants also rated their sleep quality (SQ) as good, average, or poor.
In the analyses, the presence of EDS was defined as reporting frequently/often and the severity of insomnia was defined by the number of insomnia complaints (having poor SQ or having frequently/often DIS or DMS or EMA).
Medication
An inventory of all drugs (prescription and over-the-counter drugs including sleep medication as well as antipsychotic agents, cholinesterase inhibitors, and all antidepressant drugs) used during the preceding month was recorded. Medical prescriptions and the medications themselves were checked by the interviewer. Sleep medication was classified as prescribed medication; benzodiazepine and similar compounds (zolpidem, zopiclone), antihistaminic compounds (doxylamine, alimemazine, hydroxyzine), miscellaneous medications (including hypnotic agents from different pharmacologic families such as neuroleptic agents and antidepressants), and homeopathic and nonprescription treatments.
Others Variables Measured at Inclusion
A standardized interview included questions on sociodemographic characteristics and current health status, body mass index, and mobility. A lifestyle questionnaire was used to obtain information on current smoking status, alcohol intake, and consumption of coffee, tea, fruit, vegetables, and fish. Case-level depressive symptoms were defined as a score above the 16-point cutoff on the Center for Epidemiological Studies–Depression Scale (CES-D),21 or current antidepressant treatment. Information on the history of vascular disease, hypertension, hypercholesterolemia, diabetes, asthma, and thyroid disease was recorded. Participants were classified as having chronic disease if they suffered from one, two, or more of these illnesses. Apolipoprotein E (APOE) ϵ4, which is a genetic risk factor for late-onset Alzheimer disease and cognitive decline, was genotyped at baseline as described previously.22
Statistical Analyses
Associations between cognitive decline on MMSE test, BVRT, and IST over the 8-yr follow-up period and patient characteristics, sleep complaints (insomnia complaints, insomnia severity, and EDS) and sleep medication were quantified with odds ratios (OR) and their 95% confidence intervals (CI). Study center, sociodemographic, and clinical variables associated with MMSE cognitive decline (at P < 0.15) and the MMSE score at baseline were included in logistic regression models to estimate adjusted ORs for sleep complaints and sleep medication. Multinomial logistic regression was used to study the relationship between each of the sleep complaints and the type of MMSE cognitive decline: no cognitive decline at any follow-up, cognitive decline without dementia, and cognitive decline with incident dementia. When appropriate, the interaction terms were tested using Wald χ2 test given by the logistic regression model. Significance level was set at P < 0.05. Analyses were performed using SAS statistical software (version 9.2; SAS Inc, Cary, North Carolina).
RESULTS
The study sample included 4,894 participants free of dementia and not treated by cholinesterase inhibitors, with a baseline MMSE score ≥ 24 points, having fully completed the sleep questionnaire and without missing data in adjustment covariates at baseline, with at least one follow-up and without fluctuating MMSE scores during follow-up (shown in Figure 1). The 3,642 participants free of dementia with a MMSE score ≥ 24 points excluded from the study (Figure 1) had a lower education level, were older, more frequently female, lived alone, and had chronic diseases, depressive symptoms, and disability (P < 0.0001).
Figure 1.
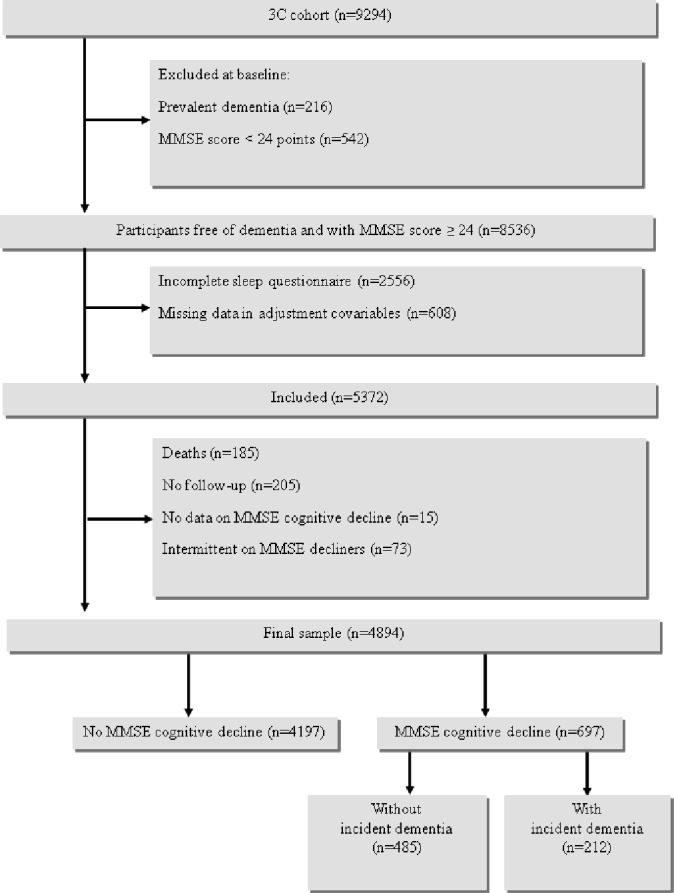
Three-City Study flow chart: Participants selection for longitudinal analyses.
Population Characteristics and Risk for MMSE Cognitive Decline Over 8-Yr Follow-up Period
At baseline, 12.4% complained of poor SQ, 33.8% had frequent/often DIS, 63.5% DMS, 35.7% EMA, and 22.3% reported 3 or 4 insomnia complaints. EDS was reported by 17.9% of the participants and 5.8% of the participants (n = 285) had both 3-4 insomnia complaints and EDS.
Only 16.4% of the participants used prescribed sleep medication (72.9% benzodiazepine, 24.1% benzodiazepine-like compounds, 8.6% antihistaminic compounds, and 21.1% miscellaneous medication), and 1.8% homeopathic and nonprescribed treatments for sleep (of whom more than half also took prescribed treatment). Among the participants often taking sleep medication, 43.3% had 3 or 4 insomnia complaints. Only 9.1% of those who were free of insomnia complaints were treated with sleep medication.
Over the 8-yr follow-up, 697 incident cases of cognitive decline (14.2%) were observed (among this group: 53.1% began their decline between baseline and 2-yr follow-up, 40.0% between 2- and 4-yr follow-up, and 14.9% after the 4-yr follow-up).
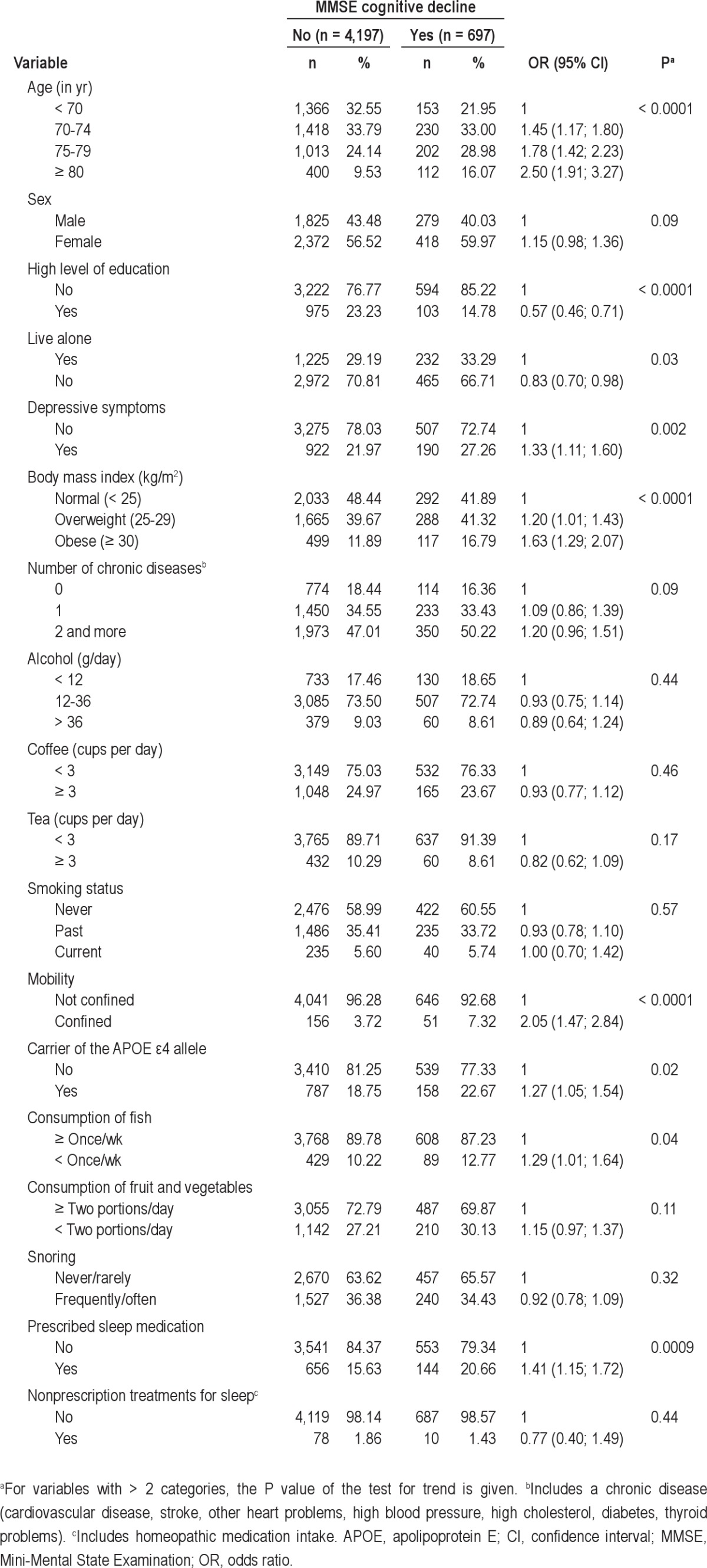
Baseline sociodemographic and clinical characteristics of the participants according to the cognitive decline in MMSE during the follow-up are described in Table 1. The risk of cognitive decline increased significantly with age from 70 yr and in participants with low level of education, who lived alone, and who had depressive symptoms, were overweight and obese and had more confined mobility, ate fish less than once a week, carried the APOE ϵ4 allele, and frequently used prescribed sleep medication. It also tended to be higher in women, in participants having two or more chronic diseases, and in those who ate fewer than two portions per day of fruit or vegetables. Subsequent analyses were thus adjusted for these factors. No significant relationship was found between MMSE cognitive decline and snoring, smoking status, or other lifestyle characteristics.
Table 1.
Baseline sociodemographic and clinical characteristics of participants with MMSE score ≥ 24 at baseline according to MMSE cognitive decline over 8-yr follow-up
Association Between Sleep Complaints and MMSE Cognitive Decline Over 8-Yr Follow-up Period
Table 2 shows the adjusted associations between insomnia complaints (SQ, DIS, DMS, EMA), number of insomnia components, EDS, and MMSE cognitive decline over the 8-yr follow-up. A significant positive association was observed between EDS and cognitive decline (OR = 1.33, 95% CI = 1.08-1.64) after adjustment for study center, sex, age, educational level, and MMSE score at baseline. This association persisted in the complete model adjusted for the others confounders including prescribed sleep medication (OR = 1.26, 95% CI = 1.02-1.56 (model 2)) as well as after subsequent adjustment for insomnia severity (OR = 1.30, 95% CI = 1.05-1.62, P = 0.02). No significant interactions were found between EDS and sex, age, depressive symptoms, or prescribed sleep medication for the risk of cognitive decline.
Table 2.
Adjusted associations between sleep complaints and MMSE cognitive decline over 8-yr follow-up
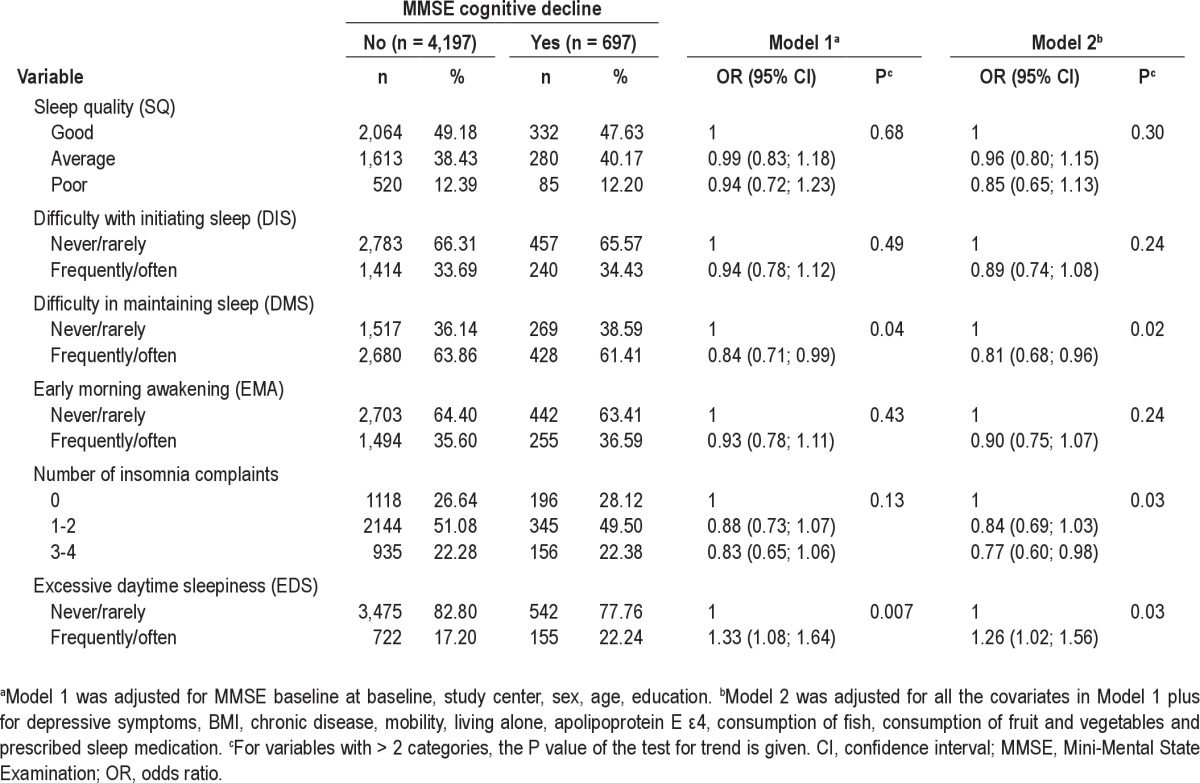
Whereas SQ, DIS, and EMA were not significantly associated with cognitive decline, a negative association was observed for DMS (OR = 0.81, 95% CI = 0.68-0.96 (model 2)). A negative dose-effect trend was observed between the number of insomnia complaints and cognitive decline (OR = 0.84, 95% CI = 0.69-1.03 for 1-2 complaints; OR = 0.77, 95% CI = 0.60-0.98 for 3-4 complaints; P-trend = 0.03 (model 2)). This association persisted after further adjustment for EDS (OR = 0.82, 95% CI = 0.67-1.01 for 1-2 complaints; OR = 0.74, 95% CI = 0.58-0.95 for 3-4 complaints; P-trend = 0.02). No significant interactions were found between DMS or insomnia severity and sex, age, depressive symptoms, prescribed sleep medication, and EDS for the MMSE cognitive decline. In addition, no significant association was observed between prescribed sleep medication and cognitive decline even after adjustment for confounders such as number of sleep complaints and EDS.
We also analyzed the relationships between sleep complaints and decline in specific cognitive domains, i.e. visual memory (BVRT) and verbal fluency (IST) over the 8-yr follow-up period. We did not find significant associations between sleep complaints (insomnia components and EDS) and BVRT or IST decline.
Association Between Sleep Complaints and Pattern of Cognitive Decline over 8-Yr Follow-up Period
We then evaluated the effect of EDS, number of insomnia components, and DMS according to the severity of the MMSE cognitive decline; participants were categorized into three groups: no decline at any follow-up (n = 4,197); decline without incident dementia (n = 485); and decline with incident dementia during the 8-yr follow-up period (n = 212). After adjustment for the same confounders of model 2 (Table 2), EDS was not significantly associated with MMSE decline without dementia (OR = 1.20, 95% CI = 0.93-1.54, P = 0.16) in contrast with decline with dementia (OR = 1.39, 95% CI = 1.00-1.97, P = 0.05). The number of insomnia components was not significantly associated with decline without dementia (OR = 0.91, 95% CI = 0.72-1.14 for 1-2 complaints and OR = 0.84, 95% CI = 0.63-1.13, for 3-4 complaints, P-trend = 0.27) but was negatively associated with decline with dementia (OR = 0.70, 95% CI = 0.49-1.00 for 1-2 complaints and OR = 0.60, 95% CI = 0.39-0.92 for 3-4 complaints, P-trend = 0.02). Likewise, DMS was not significantly associated with MMSE decline without dementia (OR = 0.88, 95% CI = 0.72-1.08, P = 0.20) but was found to have a negative association with decline with dementia (OR = 0.67, 95% CI = 0.49-0.90, P = 0.008).
As cholinesterase inhibitors may lead to inaccurate evaluation of cognitive decline status, additional analyses were performed after excluding participants initiating this treatment during the follow-up (n = 50). The same pattern of association was observed between EDS, the number of insomnia symptoms, and MMSE decline with dementia (OR = 1.57, 95% CI = 1.08-2.29, P = 0.02 for EDS; OR = 0.59, 95% CI = 0.36-0.96 for 3-4 complaints, P-trend = 0.03, respectively) whereas the association between DMS and decline with dementia failed to reach the significance level (OR = 0.74, 95% CI = 0.53-1.03, P = 0.06). Finally, no significant association between early MMSE cognitive decline (decline between baseline and the 1st 2-year follow-up) and late decline (decline only after the 2-year follow-up) was found for EDS, the number of insomnia symptoms, and DMS.
DISCUSSION
This study examined the relationships between EDS, insomnia complaints, medication, and incidence of cognitive changes at 8-yr follow-up in a large sample of participants age 65-85 yr. To the best of our knowledge, this is the first study that reports both EDS and the four different symptoms of insomnia (SQ, DIS, DMS, EMA) and their relationship with cognitive decline over a long follow-up period in an older cohort. EDS was significantly associated with a 30% increased risk in global cognitive decline at the MMSE score, independently of sociodemographic, behavioral, and clinical characteristics and prescribed sleep medication as well as APOE genotype. The number of insomnia complaints and DMS were found to be negatively associated with cognitive decline. In both conditions, the effects were specifically observed in those with cognitive decline who developed dementia during the follow-up period. Associations with EDS, number of insomnia symptoms, and DMS were independent of sleep medication use (prescribed and nonprescribed), but cholinesterase inhibitor intake during the follow-up period can at least partly confound the association between cognitive decline and DMS.
Few prospective epidemiologic studies have examined the association between EDS and cognitive decline in the elderly. One longitudinal study of 2,346 older Japanese-American men reported an association between EDS, cognitive decline (assessed by a global cognitive test–the Cognitive Abilities Screening Instrument), and dementia incidence over a 3-yr follow-up period.10 Our findings confirm and extend this finding in both sexes, within a larger sample of elderly persons and over a much longer follow-up period. Moreover, we observed that EDS was only associated with a decline in global cognitive function, with no significant association with decline on specific tasks of visual memory and verbal fluency.
The presence of EDS in elderly persons with some evidence of cognitive decline may be symptomatic of an early stage of brain lesions in areas that may initially cause sleep and/or circadian abnormalities. Thus, EDS may be considered as an early unspecific neurologic predictor of incident cognitive decline, particularly in cognitive decline devolving toward dementia. It may thus be part of the general adynamia syndrome observed in early dementia, characterized by a withdrawal from activity independently of functional ability, and a decrease in internalized cognitive activity.
We hypothesized a number of reasons for an apparent positive association between EDS and cognitive decline. In the Three-City population, EDS was previously reported to be associated with depression23 and cardiovascular and cerebrovascular disease24,25 as well as diabetes and metabolic syndrome (unpublished data), which are also risk factors for cognitive decline.26 We confirmed that an association between EDS and cognitive decline remains significant after adjustment for all these potential confounding factors. However, as EDS has been reported as a risk factor for fatal and nonfatal cardiovascular events24,25 as well as for vascular dementia,27 we may hypothesize that vascular events could be a mediator in the causal pathway between EDS and cognitive decline. Obstructive sleep apnea syndrome (OSAS), snoring, reduction of sleep duration, nighttime fragmentation, decreased circadian rhythm activity, psychotropic drug intake, bodily pain, and nocturia could also be possible determinants of EDS.28–32 OSAS has previously been found to be a risk factor for cognitive decline33 but confirmation of its presence required a polygraph, which was not performed in our study. Even if snoring was not associated with cognitive decline in our sample, we cannot exclude that OSAS could confound the association because habitual snoring was reported to be less predictive of OSA in the elderly population.34 We found no association between poor SQ, EMA, and DIS with cognitive decline. Surprisingly, we observed that the number of insomnia symptoms and DMS, the most frequent insomnia complaint in the elderly,20 were possible independent negative factors for decline on global cognitive function, but not visual memory or verbal fluency. A recent cross-sectional study also reported better executive function in elderly patients with DMS specifically but not with EMA.35 We can thus hypothesize that hyperarousal being closely related to insomnia per se may increase attentional abilities and enhance executive functioning,36,37 and consequently reduce cognitive decline or compensate for deficits in frontal attentional processing due to causes other than dementia. Although the analyses have been adjusted for depression using a clinical cutoff, the possibility remains that subclinical depression or dysthymia may be driving the association. However, sleep disturbances commonly associated with depressive disorders, notably EMA and DIS, are nonsignificant; depression is mainly reported as a risk factor of cognitive deficits in elderly.10,12 On the other hand, insomnia has been reported in some cognitively impaired patients taking cholinesterase inhibitors. Our sensitivity analysis shows that cholinesterase inhibitor intake may have at least partly confounded the association of cognitive decline with DMS, but not with the number of insomnia complaints and EDS.
The four longitudinal studies that have previously been conducted on insomnia and cognitive decline are inconsistent.10–13 Chronic insomnia was found to be associated with cognitive decline in the elderly (evaluated using the Short Portable Mental Status Questionnaire) over a 3-yr follow-up period in all men and in depressed women only.11 A population-based study of patients age 50 yr and older reported greater cognitive decline (measured by the MMSE) over 3 yr in patients reporting insomnia complaints, the association being confounded by depressive symptoms.12 Two prospective studies using the MMSE or one of its variants (Short Portable Mental Status Questionnaire), one in men and the other in women age 70 yr and older, did not report significant association between insomnia and cognitive decline over a period of 2 yr.10,13 Further studies focusing on the relationships between insomnia complaints, EDS, circadian rhythm activities, and the risk of cognitive deterioration are therefore required in the elderly.
Study Limitations
The current study has some limitations. Bias could have been introduced through the exclusion of participants with prevalent dementia or with severe cognitive impairment at baseline, those lost to follow-up also being more likely to have more chronic diseases with more depressive symptoms and to be female, older, and with lower education. Although a limitation to generalizability, the consequences on associations between sleep complaints and cognitive decline outcome were possibly underestimated. We were unable to measure anxiety and to distinguish depression being part of an anxiodepressive state. Sleep complaints were assessed only at baseline, excluding the possibility of monitoring their evolution in relation to cognitive decline. Validated measures of EDS such as the Epworth Sleepiness Scale were not available for this study. Potential confounding factors, such as bodily pain and nocturia, were not evaluated. Sleep apnea was not assessed and the confounding effect of an underlying OSAS cannot be excluded.
Study Strengths
The data used in the analysis come from a large, multicenter, population-based prospective study of people age 65 yr and older with study size and follow-up greater than any previous longitudinal studies. Medication was verified by examining the prescriptions and medications themselves, thus minimizing exposure misclassification. We have limited potential confounders by taking into account a wide range of risk factors for cognitive decline in the elderly, by controlling for sociodemographic, health (e.g., depression, vascular and chronic disorder), and lifestyle covariates and APOE genetic vulnerability. Another strength was the face-to-face clinical interview. We have used a global measure of cognitive functioning: the MMSE, which is a widely used screening instrument for assessing cognitive abilities in epidemiological studies6,8,10,13,15 as well as two other more specific cognitive tests of visual memory and semantic access. We have also considered participants with persistent and clinically relevant cognitive impairment during follow-up to avoid transient decline.
CONCLUSION
Sleep complaints are commonly underdiagnosed but constitute a significant source of concern in the geriatric population. Our findings suggest that EDS (but not insomnia) may be associated independently with the risk of developing cognitive decline in the elderly general population. Such results could potentially have important public health implications because EDS in older adults may be an early marker of cognitive decline and onset of dementia. It is thus important to recognize and treat daytime sleepiness to prevent its deleterious consequences.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Ritchie serves on scientific advisory boards for the Biomedical Research Centre, King's College London, and London and MRC Strategic Steering Committee (Longitudinal Health and Aging Research Unit); and serves on the editorial advisory boards of the International Journal of Geriatric Psychiatry, Dementia, International Psychogeriatrics, Journal of Clinical and Experimental Gerontology, Psychogeriatrics, Neuronale, Neurologie-Psychiatrie- Gériatrie, and Gerontology. Dr. Berr serves on a scientific advisory board and has received travel expenses from Janssen-Cilag, and research support from “Agence Nationale de la Recherche.” Dr. Foubert-Samier serves on a Novartis board and has received travel grants from Lundbeck and Janssen. Prof. Dauvilliers has received speaker's honoraria and supports for travel to meetings from UCB Pharma, Cephalon, Novartis, Jazz Pharmaceuticals, and Bioprojet. He has also participated in advisory boards of UCB and Bioprojet.
ACKNOWLEDGMENTS
The Three-City Study is conducted under a partnership agreement between Inserm, the Victor Segalen – Bordeaux II University, and Sanofi-Synthélabo. The Fondation pour la Recherche Médicale funded the preparation and first phase of the study. The Three-City Study is also supported by the Caisse Nationale Maladie des Travailleurs Salariés, Direction Générale de la Santé, MGEN, Institut de la Longévité, Agence Française de Sécurité Sanitaire des Produits de Santé, the Regional Governments of Aquitaine, Bourgogne and Languedoc-Roussillon and, the Fondation de France, the Ministry of Research-Inserm Programme “Cohorts and collection of biological material”. The Lille Génopôle received an unconditional grant from Eisai. Part of this project is financed by two grants from the Agence Nationale de la Recherche (ANR) (projects 07 LVIE 004 and 06-PNRA-005).
Footnotes
A commentary on this article appears in this issue on page 1189.
REFERENCES
- 1.Glisky EL. Changes in cognitive function in human aging. In: Riddle DR, editor. Brain aging: models, methods, and mechanisms. Boca Raton (FL): CRC Press,; 2007. [PubMed] [Google Scholar]
- 2.Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27:1255–73. doi: 10.1093/sleep/27.7.1255. [DOI] [PubMed] [Google Scholar]
- 3.Vitiello MV. Sleep disorders and aging: understanding the causes. J Gerontol A Biol Sci Med Sci. 1997;52:M189–91. doi: 10.1093/gerona/52a.4.m189. [DOI] [PubMed] [Google Scholar]
- 4.Foley DJ, Monjan AA, Brown SL, Simonsick EM, Wallace RB, Blazer DG. Sleep complaints among elderly persons: an epidemiologic study of three communities. Sleep. 1995;18:425–32. doi: 10.1093/sleep/18.6.425. [DOI] [PubMed] [Google Scholar]
- 5.Killgore WD. Effects of sleep deprivation on cognition. Prog Brain Res. 2010;185:105–29. doi: 10.1016/B978-0-444-53702-7.00007-5. [DOI] [PubMed] [Google Scholar]
- 6.Merlino G, Piani A, Gigli GL, et al. Daytime sleepiness is associated with dementia and cognitive decline in older Italian adults: a population-based study. Sleep Med. 2010;11:372–7. doi: 10.1016/j.sleep.2009.07.018. [DOI] [PubMed] [Google Scholar]
- 7.Nebes RD, Buysse DJ, Halligan EM, Houck PR, Monk TH. Self-reported sleep quality predicts poor cognitive performance in healthy older adults. J Gerontol B Psychol Sci Soc Sci. 2009;64:180–7. doi: 10.1093/geronb/gbn037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ohayon MM, Vecchierini MF. Daytime sleepiness and cognitive impairment in the elderly population. Arch Intern Med. 2002;162:201–8. doi: 10.1001/archinte.162.2.201. [DOI] [PubMed] [Google Scholar]
- 9.Schmutte T, Harris S, Levin R, Zweig R, Katz M, Lipton R. The relation between cognitive functioning and self-reported sleep complaints in nondemented older adults: results from the Bronx aging study. Behav Sleep Med. 2007;5:39–56. doi: 10.1207/s15402010bsm0501_3. [DOI] [PubMed] [Google Scholar]
- 10.Foley D, Monjan A, Masaki K, et al. Daytime sleepiness is associated with 3-year incident dementia and cognitive decline in older Japanese-American men. J Am Geriatr Soc. 2001;49:1628–32. doi: 10.1046/j.1532-5415.2001.t01-1-49271.x. [DOI] [PubMed] [Google Scholar]
- 11.Cricco M, Simonsick EM, Foley DJ. The impact of insomnia on cognitive functioning in older adults. J Am Geriatr Soc. 2001;49:1185–9. doi: 10.1046/j.1532-5415.2001.49235.x. [DOI] [PubMed] [Google Scholar]
- 12.Jelicic M, Bosma H, Ponds RW, Van Boxtel MP, Houx PJ, Jolles J. Subjective sleep problems in later life as predictors of cognitive decline. Report from the Maastricht Ageing Study (MAAS) Int J Geriatr Psychiatry. 2002;17:73–7. doi: 10.1002/gps.529. [DOI] [PubMed] [Google Scholar]
- 13.Tworoger SS, Lee S, Schernhammer ES, Grodstein F. The association of self-reported sleep duration, difficulty sleeping, and snoring with cognitive function in older women. Alzheimer Dis Assoc Disord. 2006;20:41–8. doi: 10.1097/01.wad.0000201850.52707.80. [DOI] [PubMed] [Google Scholar]
- 14.3C Study Group. Vascular factors and risk of dementia: design of the Three-City Study and baseline characteristics of the study population. Neuroepidemiology. 2003;22:316–25. doi: 10.1159/000072920. [DOI] [PubMed] [Google Scholar]
- 15.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 16.Soto ME, Andrieu S, Cantet C, et al. Predictive value of rapid decline in mini mental state examination in clinical practice for prognosis in Alzheimer's disease. Dement Geriatr Cogn Disord. 2008;26:109–16. doi: 10.1159/000144073. [DOI] [PubMed] [Google Scholar]
- 17.Tzourio C, Dufouil C, Ducimetiere P, Alperovitch A. Cognitive decline in individuals with high blood pressure: a longitudinal study in the elderly. EVA Study Group. Epidemiology of Vascular Aging. Neurology. 1999;53:1948–52. doi: 10.1212/wnl.53.9.1948. [DOI] [PubMed] [Google Scholar]
- 18.Benton A. Manuel pour l'Application du Test de Rétention Visuelle: Applications Cliniques et Expérimentales. Paris: Centre de Psychologie Appliquée,; 1965. [Google Scholar]
- 19.Isaacs B, Kennie AT. The Set test as an aid to the detection of dementia in old people. Br J Psychiatry. 1973;123:467–70. doi: 10.1192/bjp.123.4.467. [DOI] [PubMed] [Google Scholar]
- 20.Jaussent I, Dauvilliers Y, Ancelin ML, et al. Insomnia symptoms in older adults: associated factors and gender differences. Am J Geriatr Psychiatry. 2011;19:88–97. doi: 10.1097/JGP.0b013e3181e049b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Radloff LS. The CES-D Scale: a self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- 22.Dufouil C, Richard F, Fievet N, et al. APOE genotype, cholesterol level, lipid-lowering treatment, and dementia: the Three-City Study. Neurology. 2005;64:1531–8. doi: 10.1212/01.WNL.0000160114.42643.31. [DOI] [PubMed] [Google Scholar]
- 23.Jaussent I, Bouyer J, Ancelin ML, et al. Insomnia and daytime sleepiness are risk factors for depressive symptoms in the elderly. Sleep. 2011;34:1103–10. doi: 10.5665/SLEEP.1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blachier M, Dauvilliers Y, Jaussent I, et al. Excessive daytime sleepiness and vascular events: The Three City Study. Ann Neurol. 2012;71:661–7. doi: 10.1002/ana.22656. [DOI] [PubMed] [Google Scholar]
- 25.Empana JP, Dauvilliers Y, Dartigues JF, et al. Excessive daytime sleepiness is an independent risk indicator for cardiovascular mortality in community-dwelling elderly: the three city study. Stroke. 2009;40:1219–24. doi: 10.1161/STROKEAHA.108.530824. [DOI] [PubMed] [Google Scholar]
- 26.Raffaitin C, Feart C, Le Goff M, et al. Metabolic syndrome and cognitive decline in French elders: the Three-City Study. Neurology. 2011;76:518–25. doi: 10.1212/WNL.0b013e31820b7656. [DOI] [PubMed] [Google Scholar]
- 27.Elwood PC, Bayer AJ, Fish M, Pickering J, Mitchell C, Gallacher JE. Sleep disturbance and daytime sleepiness predict vascular dementia. J Epidemiol Community Health. 2011;65:820–4. doi: 10.1136/jech.2009.100503. [DOI] [PubMed] [Google Scholar]
- 28.Ferrie JE, Shipley MJ, Akbaraly TN, Marmot MG, Kivimaki M, Singh-Manoux A. Change in sleep duration and cognitive function: findings from the Whitehall II Study. Sleep. 2011;34:565–73. doi: 10.1093/sleep/34.5.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Foley DJ, Vitiello MV, Bliwise DL, Ancoli-Israel S, Monjan AA, Walsh JK. Frequent napping is associated with excessive daytime sleepiness, depression, pain, and nocturia in older adults: findings from the National Sleep Foundation `2003 Sleep in America' Poll. Am J Geriatr Psychiatry. 2007;15:344–50. doi: 10.1097/01.JGP.0000249385.50101.67. [DOI] [PubMed] [Google Scholar]
- 30.Tranah GJ, Blackwell T, Stone KL, et al. Circadian activity rhythms and risk of incident dementia and mild cognitive impairment in older women. Ann Neurol. 2011;70:722–32. doi: 10.1002/ana.22468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsuno N, Jaussent I, Dauvilliers Y, Touchon J, Ritchie K, Besset A. Determinants of excessive daytime sleepiness in a French community-dwelling elderly population. J Sleep Res. 2007;16:364–71. doi: 10.1111/j.1365-2869.2007.00606.x. [DOI] [PubMed] [Google Scholar]
- 32.Whitney CW, Enright PL, Newman AB, Bonekat W, Foley D, Quan SF. Correlates of daytime sleepiness in 4578 elderly persons: the Cardiovascular Health Study. Sleep. 1998;21:27–36. doi: 10.1093/sleep/21.1.27. [DOI] [PubMed] [Google Scholar]
- 33.Decary A, Rouleau I, Montplaisir J. Cognitive deficits associated with sleep apnea syndrome: a proposed neuropsychological test battery. Sleep. 2000;23:369–81. [PubMed] [Google Scholar]
- 34.Young T, Shahar E, Nieto FJ, et al. Predictors of sleep-disordered breathing in community-dwelling adults: the Sleep Heart Health Study. Arch Intern Med. 2002;162:893–900. doi: 10.1001/archinte.162.8.893. [DOI] [PubMed] [Google Scholar]
- 35.Altena E, Van Der Werf YD, Sanz-Arigita EJ, et al. Prefrontal hypoactivation and recovery in insomnia. Sleep. 2008;31:1271–6. [PMC free article] [PubMed] [Google Scholar]
- 36.Altena E, Ramautar JR, Van Der Werf YD, Van Someren EJ. Do sleep complaints contribute to age-related cognitive decline? Prog Brain Res. 2010;185:181–205. doi: 10.1016/B978-0-444-53702-7.00011-7. [DOI] [PubMed] [Google Scholar]
- 37.Vincent NK, Walker JR. Perfectionism and chronic insomnia. J Psychosom Res. 2000;49:349–54. doi: 10.1016/s0022-3999(00)00175-6. [DOI] [PubMed] [Google Scholar]


