Abstract
Study Objectives:
Quantify the short-term stability of multiple indices of sleep and nocturnal physiology in good sleeper controls and primary insomnia patients.
Design:
Intra-class correlation coefficients (ICC) were used to quantify the short-term stability of study outcomes.
Setting:
Sleep laboratory.
Participants:
Fifty-four adults with primary insomnia (PI) and 22 good sleeper controls (GSC).
Measurements:
Visually scored sleep outcomes included indices of sleep duration, continuity, and architecture. Quantitative EEG outcomes included power in the delta, theta, alpha, sigma, and beta bands during NREM sleep. Power spectral analysis was used to estimate high-frequency heart rate variability (HRV) and the ratio of low- to high-frequency HRV power during NREM and REM sleep.
Results:
With the exception of percent stage 3+4 sleep; visually scored sleep outcomes did not exhibit short-term stability across study nights. Most QEEG outcomes demonstrated short-term stability in both groups. Although power in the beta band was stable in the PI group (ICC = 0.75), it tended to be less stable in GSCs (ICC = 0.55). Both measures of cardiac autonomic tone exhibited short-term stability in GSCs and PIs during NREM and REM sleep.
Conclusions:
Most QEEG bandwidths and HRV during sleep show high short-term stability in good sleepers and patients with insomnia alike. One night of data is, thus, sufficient to derive reliable estimates of these outcomes in studies focused on group differences or correlates of QEEG and/or HRV. In contrast, one night of data is unlikely to generate reliable estimates of PSG-assessed sleep duration, continuity or architecture, with the exception of slow wave sleep.
Citation:
Israel B; Buysse DJ; Krafty RT; Begley A; Miewald J; Hall M. Short-term stability of sleep and heart rate variability in good sleepers and patients with insomnia: For some measures, one night is enough. SLEEP 2012;35(9):1285–1291.
Keywords: Sleep, QEEG, heart rate variability, polysomnography, insomnia, good sleepers
INTRODUCTION
Some dimensions of sleep and indices of nocturnal physiology vary considerably from night to night while others are relatively stable. The stability of one person's sleep and nocturnal physiology carries both theoretical and practical implications. Highly stable signals that distinguish individuals from one another suggest a genetic or permanently acquired component whereas measures that vary widely from night to night suggest susceptibility to a variety of subject and environmental factors. From a practical perspective, the high nightly variability of some components of sleep has made multi-night studies a common practice. Yet, emerging evidence suggests that some components of sleep and nocturnal physiology fluctuate less from night to night, suggesting that multiple recordings may not be necessary.
The concept of sleep stability has existed since at least the mid-1960s, when Agnew and colleagues noticed that sleep during the first night in a series of all-night polysomnography (PSG) was more disrupted than sleep on the following nights.1 Merica and Gaillard later built on Webb's and Agnew's exploration of differences among individual sleepers by conducting early analyses of the stability of each sleep stage.2,3 In their study of 147 non-related subjects, Merica and Gaillard reported that only stage 4 sleep produced reliable measurements across consecutive study nights. Subsequently, a modest body of research has focused on the stability of sleep, at times considering that some characteristics of sleep may even be “trait-like.”4,5 For instance, Achermann6,7 and De Gennaro8 pioneered quantitative EEG (QEEG) “fingerprinting” methods and showed that QEEG signals remain stable in healthy sleepers under experimental conditions such as sleep deprivation. Buckelmüller9 later produced visual proof of concept for distinguishing individual sleepers from each other but did not assess individual differences quantitatively, leaving the magnitude of the observed differences an open question. The fingerprinting studies were further limited in that none incorporated visual PSG sleep parameters and therefore could not offer a comprehensive appraisal of stability. Tucker and colleagues sought to address these concerns in a study of 21 healthy adults in which traditional PSG outcomes and QEEG were assessed over eight consecutive sleep periods.5 To vary sleep conditions, sleep periods were punctuated by bouts of physical activity or extended wakefulness. The authors used intraclass correlation coefficients (ICC) and ANOVA analyses to argue for a trait-like stability in some components of sleep, particularly in EEG delta power. While this study employed quantitative techniques, the authors were limited by a small sample size. Furthermore, they did not explore pathologic states or QEEG stability outside of the delta band.
Even less is known about the short term stability of other indices of nocturnal physiology important to health and functioning. Cardiac autonomic tone, as measured by heart rate variability (HRV), has been identified as a physiologic mechanism through which sleep disturbances and disorders may potentially influence morbidity and mortality. Heart rate variability varies as a function of sleep such that power in the high frequency band (HF-HRV), interpreted as a measure of parasympathetic tone, correlates with the depth of NREM sleep and is highest in stage N3. Conversely, REM sleep and lighter stages of NREM sleep are characterized by decreased power in the HF-HRV band.10 Heart rate variability may also vary as a function of sleep disorders, including insomnia and sleep disordered breathing (SDB), and experimental sleep restriction.11 Some, but not all, studies have reported decreased HF-HRV power in patients with insomnia compared to good sleeper controls.12,13 Patients with insomnia may also exhibit a higher ratio of low-to-high frequency power (LF:HF-HRV), interpreted as an index of sympathovagal tone. Sleep-related changes in HRV are associated with other physiological changes. For example, increased sympathovagal tone during NREM sleep following experimental sleep restriction in healthy young adults was associated with a lower glucose tolerance, lower thyrotropin concentrations, and elevated levels of cortisol.14 Cardiac autonomic dysfunction in obstructive sleep apnea syndrome has been suggested as a mechanism of increased cardiovascular mortality and malignant arrhythmias,15 while sustained ventricular tachyarrhythmias in the morning are associated with decreased parasympathetic power during sleep.16 Higher mortality among patients with SDB has been associated with decreased HRV.17 Finally, altered HRV during sleep is known to coincide with conditions such as PTSD, alcohol dependence and acute stress.18–20 While HRV during wakefulness is known to exhibit short-term stability,21–23 to our knowledge, no one has evaluated whether data from a single night offers reliable estimates of HRV during sleep.
The present study presents a multi-component quantitative analysis of night-to-night stability of sleep and nocturnal physiology including visually scored sleep, QEEG, and cardiac autonomic tone as measured by power spectral analysis of HRV during sleep. Our goal was to evaluate the night-to-night stability of these measures and to compare their stability in two distinct populations: patients with primary insomnia (PI) and good sleeper controls (GSC). Short-term stability of each of these parameters was assessed by intra-class correlation coefficients (ICC).
METHODS
Data were drawn from 2 studies of primary insomnia conducted at the University of Pittsburgh (D. Buysse, PI; MH024652). The University of Pittsburgh Institutional Review Board approved these studies, in which all participants provided written informed consent and completed eligibility screening.
Participants were recruited through media advertisements, fliers, and clinical referrals. Study clinicians conducted medical and psychiatric interviews to confirm eligibility. Diagnoses of insomnia were based on Diagnostic and Statistical Manual of Mental Disorders24 criteria, established by clinical consensus review of interview data regarding self-reported complaints of difficulty maintaining sleep (≥ 30 min wakefulness after sleep onset; WASO) or poor quality sleep for the majority of nights during the 30 days immediately preceding screening. Pursuant to diagnostic guidelines, PSG criteria were not used to diagnose insomnia. However, PSG values may be used to identify insomnia subtypes as follows: 11% presented with PSG-assessed sleep onset difficulties (sleep latency ≥ 30 min), 35% presented with PSG-assessed sleep maintenance difficulties (WASO ≥ 30 min), and 22% presented with both. The good sleeper control group consisted of individuals who did not meet diagnostic criteria for insomnia. General exclusion criteria for all participants included significant or unstable medical conditions (e.g., unstable hypertension, hyper- or hypothyroidism, seizure disorders, neurodegenerative diseases), current psychiatric disorders (e.g., major depression, drug dependence), other current sleep disorders, use of medications known to affect sleep or wake function, and excessive caffeine and alcohol use.
Sample characteristics including age, sex, race, and body mass index (BMI) were collected in conjunction with sleep studies. The Inventory of Depressive Symptomatology (IDS)25 was used to quantify self-reported symptoms of depression. IDS scores were calculated without the sleep items. Sleep studies were conducted across 3 consecutive nights in the Neuroscience Clinical and Translational Research Center (N-CTRC) at the University of Pittsburgh Western Psychiatric Institute and Clinic. Participants slept at their habitual sleep times as determined by sleep diary responses the week prior to sleep studies.26 Signals were acquired with Harmonie hardware and records were scored using locally developed analysis software.27 Signals were filtered during acquisition (0.3 Hz high pass, 100 Hz low pass, with 60 Hz notch), and additional filtering was performed prior to computing power spectral analysis of the EEG. A Hamming Window (v^2/Hz and zero overlap) was used to reduce spectral leakage.27 The PSG montage included bilateral central EEG leads with C3 and C4 referenced to linked mastoids (A1-A2), electro-oculogram (upper right outer canthus, lower left outer canthus), submentalis electromyogram and a 2-lead EKG channel (upper right clavicle, lower left rib cage). On the first night of sleep studies, participants were additionally monitored for sleep disordered breathing and periodic limb movements.
Visual sleep stage scoring was based on Rechtschaffen and Kales criteria and scored in 20-sec epochs,28 as data were collected prior to the updated American Academy of Sleep Medicine manual.29 Participants with an apnea-hypopnea index or leg movement arousal index ≥ 15 were excluded from further participation (n = 3). EEG signals were digitized at a rate of 256 Hz and decimated to 128 Hz.27 Relevant PSG outcomes included total sleep time (TST), sleep latency (SL), wakefulness after sleep onset (WASO), sleep efficiency (SE), and percent NREM (stages 1, 2, 3+4) and REM sleep. Four-second epochs were used to compute power spectral analysis of the EEG during NREM sleep. Epochs corresponding to “wake” or REM sleep were not included in the analyses. In addition, an automated artifact rejection program was run to remove epochs containing EMG artifacts from the EEG record.30 On average, artifact rejection resulted in a data loss of less than 2% in both groups of participants (range = 0.33%–4.46%). Measures included mean relative power in the delta (0.5–4.0 Hz), theta (4.0–8.0 Hz), alpha (8.0–12.0 Hz), sigma (12.0–16.0 Hz), and beta (16.0–32.0 Hz) bands during NREM sleep.27,30
Power spectral analysis was also used to estimate HF-HRV and LF:HF-HRV power during NREM and REM sleep as previously described.20 Briefly, an automated algorithm was used to calculate interbeat intervals (IBI) from the EKG signal (1,024 Hz).31 Artifacts and ectopic beats were edited manually by interpolating preceding-successive beats.32 Two-minute IBI epochs were transformed from the time domain (milliseconds) into the frequency domain (Hz) where autoregressive models were used to estimate HRV power in the LF (0.04–0.15 Hz) and HF (0.15–0.40 Hz) bands.19,33–35 Epochs scored as awake or movement time were excluded and power in the LF and HF bands was averaged across all epochs containing NREM or REM sleep exclusively. Heart rate variability outcomes included normalized HF-HRV power (0.15–0.40 Hz/0.04–0.40 Hz) as a measure of parasympathetic tone and the LF:HF ratio (0.04–0.15 Hz/0.15–0.40 Hz) as an index of sympathovagal tone.36
Analyses
Prior to analyses, distributions for all variables were examined for normality and to check for outliers. Transformations were used where necessary. For descriptive purposes, T-tests and Fisher exact tests were used to evaluate continuous and categorical sample characteristics, respectively. Intra-class correlation coefficients (ICC) were used to examine the within-participant short-term stability of PSG, QEEG and QEKG outcomes across the 3 sleep study nights. In these analyses, participant group was the blocking variable and night was treated as the within-subject variable and assumed to be a random subset of all possible nights (ICC(2,1)).37 The ICC was computed separately for each outcome and each group. A bootstrap method was used to examine the distribution of the ICC. One thousand independent samples were generated using SAS PROC SURVEYSELECT. Each independent sample had the same sample size and group proportions of the original sample and was generated using unrestricted random sampling. A participant had an equal probability of being selected within the group and could be selected more than once. The ICC was run on each of the 1000 bootstrap samples for each group, and the difference between the group ICCs was calculated. The distribution of the 1000 samples gave the mean and standard error (SE) of each group and the mean and 5-95th percentile of the difference between ICCs. If the 5–95th percentile did not include 0, then the group ICC was considered to differ between groups. Though investigators have previously attempted to stratify ICC results based on a standard system,38,39 setting universal “high” versus “low” ICC values is inherently problematic as an ICC is particular to both the test and the sample in question.40 For simplicity, and to minimize confounding by measurement error, we treat measures in this study with ICCs ≥ 0.60 as demonstrating reliable short-term stability.
RESULTS
Sample characteristics are shown in Tables 1 and 2. Groups differed by age, sex and symptoms of depression not related to sleep. Compared to GSCs, participants with insomnia were older, fewer were female, and depressive symptoms were more pronounced. The majority of participants in both groups were Caucasian, with a trend for increased BMI in the PI group. Despite diagnostic differences, diary-assessed habitual sleep times did not differ between groups. On average, controls went to bed at 23:58 (± 0.52 h) and awakened at 07:51 (± 1.01 h). Similarly, participants with insomnia went to bed at 23:39 (± 1.15 h) and awakened at 07:22 (± 1.18 h). As shown in Table 2, participants with insomnia had higher alpha and beta EEG power during NREM sleep compared to controls. None of the remaining sleep or HRV outcomes differed by group.
Table 1.
Sample characteristics: Sociodemographics
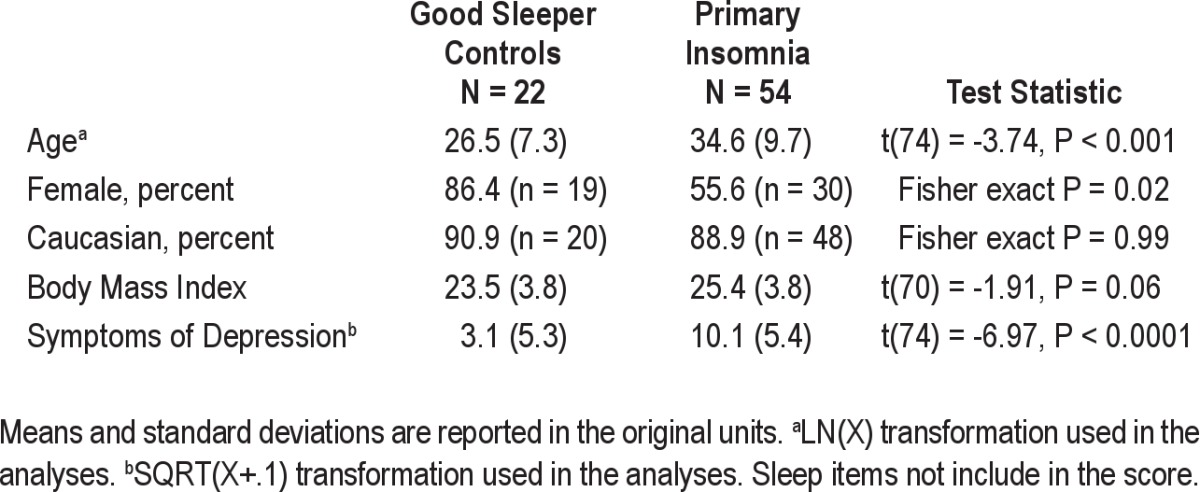
Table 2.
Sample characteristics: Sleep and Heart Rate Variability
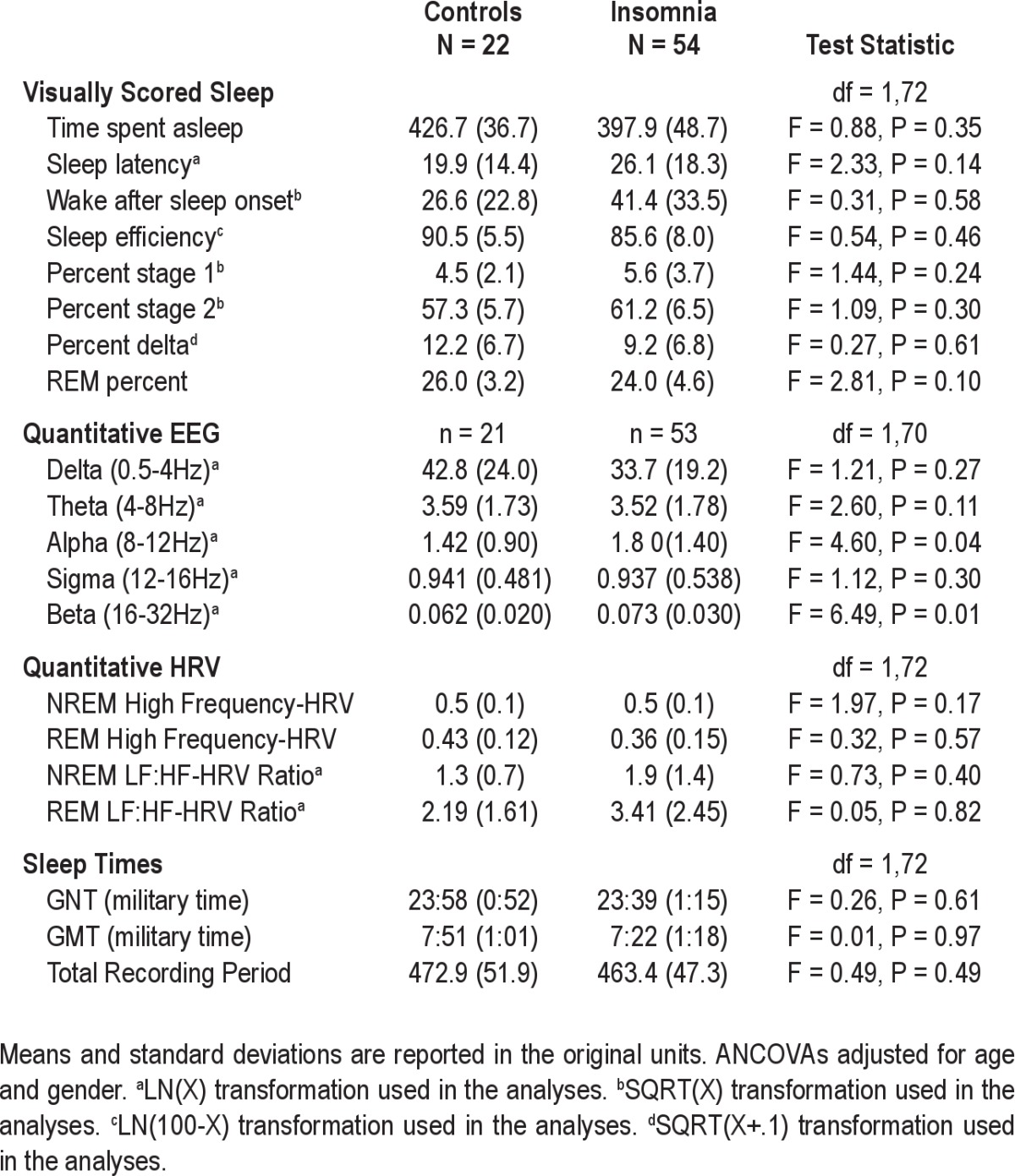
With the exception of percent Stage 3+4 NREM sleep, visually scored sleep outcomes exhibited low stability compared with QEEG outcomes, as indicated by ICC values < 0.60 (see Table 2). Reliability of visually scored sleep and QEEG outcomes was similar for both groups as revealed by the bootstrapping method for group mean difference. Most QEEG outcomes (power in the delta, theta, and alpha bands) demonstrated high short-term stability in both groups as indicated by ICC values of > 0.60. In contrast, the short-term stability of power in the beta band was 0.55 in GSCs and 0.75 in PIs, although this difference was not statistically significant. Both measures of cardiac autonomic tone exhibited high short-term stability, with ICC values > 0.88 for GSCs and > 0.89 for PIs during NREM and REM sleep. Moreover, results for HF-HRV and the LF:HF-HRV ratio were similar for both groups. Bootstrap means were similar to sample means for all visually scored sleep, QEEG and HRV outcomes.
DISCUSSION
We evaluated the short-term stability of visually scored sleep, QEEG, and QEKG outcomes across three consecutive nights in 22 good sleepers and 54 patients with primary insomnia. In both groups, most visually scored sleep outcomes demonstrated low short-term stability while QEEG and HRV outcomes showed high short-term stability across study nights. To our knowledge, these findings represent the first quantitative comparison of the short-term stability of multiple indices of sleep and nocturnal physiology in two distinct adult populations. The high short-term stability for a number of outcomes suggests that a single night of measurement is sufficient to yield reliable estimates of these outcomes. In other words, recordings from subsequent nights would not be required to generate reproducible results.
For the most part, visually scored measures traditionally used to characterize sleep showed poor short-term stability across nights. This was true for both groups of participants. Nightly fluctuation in measures of sleep duration, continuity and some measures of sleep architecture suggests that these indices of sleep may be more strongly influenced by a variety of subject and environmental factors than are other indices of sleep, such as percent stage 3+4 NREM sleep. The short-term stability of sleep duration, continuity, percent stages 1 and 2 of NREM sleep, and REM percent is likely to vary with changes in surroundings,41,42 and daily variability in health behaviors (e.g., exercise,43,44 caffeine consumption,45–47 mood,48 and psychological stress49–54).The short-term stability of percent stage 3+4 NREM sleep is consistent with previous reports that delta power displays high intra-individual stability across nights and circumstance.5 Our results indicate that this is true for good sleepers and patients with insomnia alike. Apart from stage 3+4 NREM sleep, our data support the current methodological practice of obtaining more than one night of sleep if one's outcomes of interest are based on visually scored sleep.
Quantitative EEG outcomes generally showed high short-term stability across nights. These data extend previous studies of EEG delta power to show that other EEG bandwidths may be reliably measured with one night of data. Intra-class correlation values in the present sample (Table 3) were similar to those reported in healthy children, adolescents, and adults studied between 2 and 11 consecutive nights.5,55,56 These studies reported ICC values between 0.72-0.96 for QEEG outcomes, further emphasizing the short-term stability of the EEG power spectrum during NREM sleep. The beta EEG band, which has been associated with physiological hyperarousal and active cognitive processes,57,58 demonstrated high short-term stability in the insomnia group only. When we compared both groups of participants, the short-term stability of beta power tended to be lower in the good sleeper group compared to the insomnia group. This trend may be measurement-related given lower overall power in the beta band as well as the smaller sample size in the GS group. The modest decrease in the stability of NREM beta power in the GS group may also underlie a true group difference. Cortical arousal during NREM sleep in good sleepers, as measured by fast frequency EEG activity, may largely be a function of circumstance such as negative affect or psychological stressors, whereas heightened cortical arousal in individuals with insomnia may persist independently of circumstance. This hypothesis could be tested in future studies by comparing the short-term stability of EEG beta power in patients with insomnia prior to and following effective treatment.
Table 3.
Intraclass Correlations (ICC) for Study Outcomes
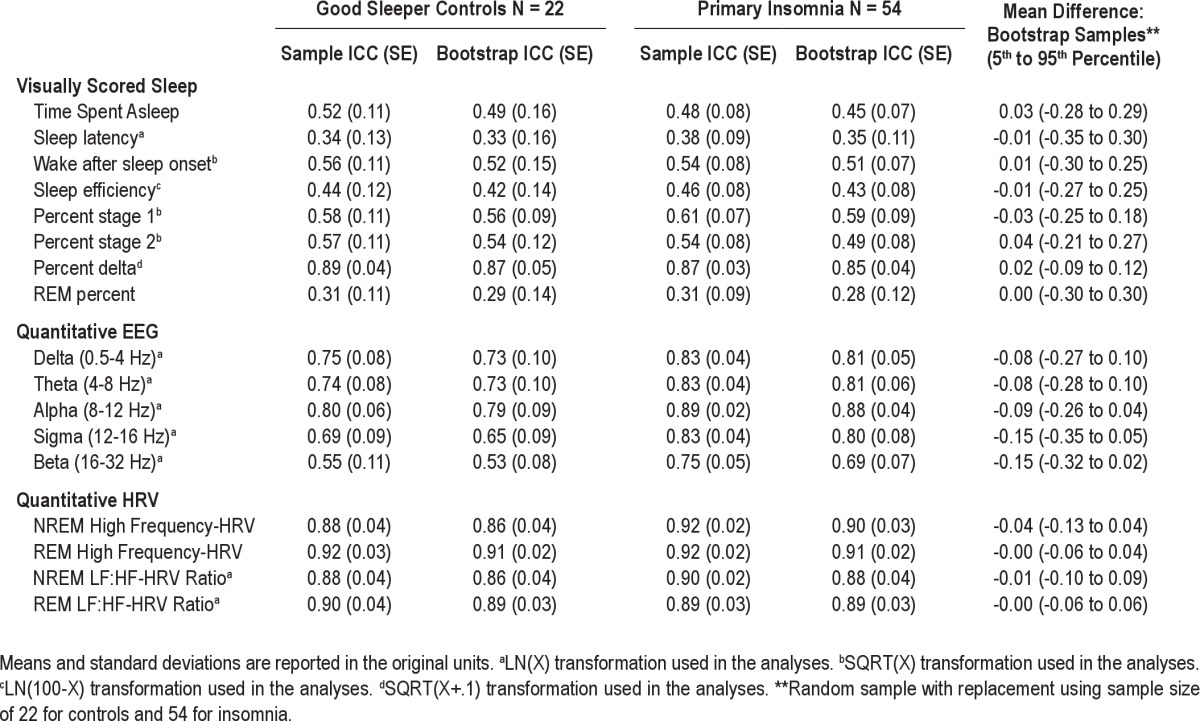
Both parameters of cardiac autonomic tone, HF-HRV and the LF:HF-HRV ratio showed short-term stability across nights in both groups of participants. These results extend the extant literature on HRV during wakefulness, which also shows intra-individual short-term stability as measured by cross-sample correlations above 0.87 and ICC's greater than 0.79.21,22 Taken as a whole, these data suggest that indices of cardiac autonomic tone, as measured by HRV, show short-term stability across wakefulness and sleep. The study of cardiac autonomic tone during sleep as measured by heart rate variability provides researchers the opportunity to quantify a physiological mechanism through which sleep disturbances and disorders may affect health and functioning. In contrast to blood pressure59,60 and blood-based assays,61 HRV offers the advantage of continuous and noninvasive monitoring throughout sleep. In the short term, autonomic dysregulation may be associated with weight gain and metabolic disease given that increased sympathovagal tone during experimental sleep restriction has been associated with lower glucose tolerance and increased craving for carbohydrate rich foods.14 If stable over the long term, autonomic dysregulation during sleep may contribute to cardiovascular disease which develops over years and decades.
Several limitations to the present study impact its generalizability. First, results apply only to the short-term stability of study outcomes. Other studies are needed to determine the extent to which the intra-individual stability of sleep and indices of nocturnal physiology change over longer time intervals in association with development, aging, and morbidity including other primary sleep disorders. This issue bears on the “trait-like” nature of sleep as well as the role of sleep in health and functioning, especially with respect to diseases that develop over time such as cardiovascular disease and cancer. Second, the sample included mostly non-Hispanic Caucasians. While it would be most parsimonious to hypothesize that the short-term stability of sleep and cardiac autonomic tone do not differ as a function of race or ethnicity, additional studies are needed in light of notable racial and ethnic differences in sleep62–67 and risk for morbidity and mortality.68–72 Limitations notwithstanding, these quantitative analyses show that most QEEG bandwidths and HRV during NREM sleep show high short-term stability in good sleepers and patients with insomnia alike. One night of data is, thus, sufficient to derive reliable estimates of these outcomes in studies focused on group differences or correlates of QEEG and/or HRV. In contrast, one night of data is unlikely to generate reliable estimates of PSG-assessed sleep duration, continuity or architecture, with the exception of slow wave sleep.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Buysse serves as a paid consultant for Eisai, Merck, Purdue Pharma, L.P., Pfizer, Philips Respironics and Sanofi-Aventis. He has also been paid for lectures at non-CME educational meetings supported by Servier. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGEMENTS
This research was supported in part by NIH grants HL104607, MH024652, HL082610, UL1RR024153 and RR000056
ABBREVIATIONS
- GSC
good sleeper control
- PI
patients with primary insomnia
- ICC
intra-class correlation coefficient
- PSG
polysomnography
- QEEG
quantitative electroencephalography
- EKG
electrocardiogram
- HRV
heart rate variability
- HF-HRV
high frequency heart rate variability
- LF:HF-HRV
ratio of low-to-high frequency heart rate variability
- AHI
apnea-hypopnea index
REFERENCES
- 1.Agnew HW, Webb WB, Williams RL. The first night effect: An EEG study of sleep. Psychophysiology. 1966;2:263–6. doi: 10.1111/j.1469-8986.1966.tb02650.x. [DOI] [PubMed] [Google Scholar]
- 2.Merica H, Gaillard JM. Statistical description and evaluation of the interrelationships of standard sleep variables for normal subjects. Sleep. 1985;8:261–73. doi: 10.1093/sleep/8.3.261. [DOI] [PubMed] [Google Scholar]
- 3.Webb WB, Agnew HW. Measurement and characteristics of nocturnal sleep. In: Abt LE, Riess BF, editors. Progress in clinical psychology. New York: Grune and Stratton; 1968. pp. 2–27. [Google Scholar]
- 4.Van Dongen HP, Vitellaro KM, Dinges DF. Individual differences in adult human sleep and wakefulness: Leitmotif for a research agenda. Sleep. 2005;28:479–96. doi: 10.1093/sleep/28.4.479. [DOI] [PubMed] [Google Scholar]
- 5.Tucker AM, Dinges DF, Van Dongen HP. Trait interindividual differences in the sleep physiology of healthy young adults. J Sleep Res. 2007;16:170–80. doi: 10.1111/j.1365-2869.2007.00594.x. [DOI] [PubMed] [Google Scholar]
- 6.Finelli LA, Achermann P, Borbely AA. Individual 'fingerprints' in human sleep EEG topography. Neuropsychopharmacology. 2001;25:S57–S62. doi: 10.1016/S0893-133X(01)00320-7. [DOI] [PubMed] [Google Scholar]
- 7.Tinguely G, Finelli LA, Landolt HP, Borbely AA, Achermann P. Functional EEG topography in sleep and waking: state-dependent and state-independent features. Neuroimage. 2006;32:283–92. doi: 10.1016/j.neuroimage.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 8.De Gennaro L, Ferrara M, Vecchio F, Curcio G, Bertini M. An electroencephalographic fingerprint of human sleep. Neuroimage. 2005;26:114–22. doi: 10.1016/j.neuroimage.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 9.Buckelmuller J, Landolt HP, Stassen HH, Achermann P. Trait-like individual differences in the human sleep EEG. Neuroscience. 2006;138:351–6. doi: 10.1016/j.neuroscience.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 10.Bonnet MH, Arand DL. Heart rate variability: sleep stage, time of night, and arousal influences. Electroencephalogr Clin Neurophysiol. 1997;102:390–6. doi: 10.1016/s0921-884x(96)96070-1. [DOI] [PubMed] [Google Scholar]
- 11.Stein PK, Pu Y. Heart rate variability, sleep and sleep disorders. Sleep Med Rev. doi: 10.1016/j.smrv.2011.02.005. (in press) [DOI] [PubMed] [Google Scholar]
- 12.Bonnet MH, Arand DL. Heart rate variability in insomniacs and matched normal sleepers. Psychosom Med. 1998;60:610–5. doi: 10.1097/00006842-199809000-00017. [DOI] [PubMed] [Google Scholar]
- 13.Hall M, Thayer JF, Germain A, et al. Psychological stress is associated with heightened physiological arousal during NREM sleep in primary insomnia. Behav Sleep Med. 2007;5:178–93. doi: 10.1080/15402000701263221. [DOI] [PubMed] [Google Scholar]
- 14.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435–9. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 15.Wiklund U, Olofsson BO, Franklin K, Blom H, Bjerle P, Niklasson U. Autonomic cardiovascular regulation in patients with obstructive sleep apnoea: a study based on spectral analysis of heart rate variability. Clin Physiol. 2000;20:234–41. doi: 10.1046/j.1365-2281.2000.00251.x. [DOI] [PubMed] [Google Scholar]
- 16.Fries R, Hein S, nig K. Reversed circadian rhythms of heart rate variability and morning peak occurrence of sustained ventricular tachyarrhythmias in patients with implanted cardioverter defibrillator. Med Sci Monit. 2002;8:CR751–6. [PubMed] [Google Scholar]
- 17.Bauer T, Ewig S, Schafer H, Jelen E, Omran H, Luderitz B. Heart rate variability in patients with sleep-related breathing disorders. Cardiology. 1996;87:492–6. doi: 10.1159/000177144. [DOI] [PubMed] [Google Scholar]
- 18.Mellman TA, Knorr BR, Pigeon WR, Leiter JC, Akay M. Heart rate variability during sleep and the early development of posttraumatic stress disorder. Biol Psychiatry. 2004;55:953–6. doi: 10.1016/j.biopsych.2003.12.018. [DOI] [PubMed] [Google Scholar]
- 19.Irwin MR, Valladares EM, Motivala S, Thayer JF, Ehlers CL. Association between nocturnal vagal tone and sleep depth, sleep quality, and fatigue in alcohol dependence. Psychosom Med. 2006;68:159–66. doi: 10.1097/01.psy.0000195743.60952.00. [DOI] [PubMed] [Google Scholar]
- 20.Hall M, Vasko R, Buysse DJ, et al. Acute stress affects heart rate variability during sleep. Psychosom Med. 2004;66:56–62. doi: 10.1097/01.psy.0000106884.58744.09. [DOI] [PubMed] [Google Scholar]
- 21.Pardo Y, Merz CN, Paul-Labrador M, et al. Heart rate variability reproducibility and stability using commercially available equipment in coronary artery disease with daily life myocardial ischemia. Am J Cardiol. 1996;78:866–70. doi: 10.1016/s0002-9149(96)00458-4. [DOI] [PubMed] [Google Scholar]
- 22.Kobayashi H. Does paced breathing improve the reproducibility of heart rate variability measurements? J Physiol Anthropol. 2009;28:225–30. doi: 10.2114/jpa2.28.225. [DOI] [PubMed] [Google Scholar]
- 23.Tarkiainen TH, Timonen KL, Tiittanen P, et al. Stability over time of short-term heart rate variability. Clin Auton Res. 2005;15:394–9. doi: 10.1007/s10286-005-0302-7. [DOI] [PubMed] [Google Scholar]
- 24.American Psychiatric Association. 4th ed. Washington, DC: American Psychiatric Association; 1994. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) [Google Scholar]
- 25.Rush AJ, Trivedi MH, Ibrahim HM, et al. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry. 2003;54:573–83. doi: 10.1016/s0006-3223(02)01866-8. [DOI] [PubMed] [Google Scholar]
- 26.Monk TH, Reynolds CF, Kupfer DJ, et al. The Pittsburgh Sleep Diary. J Sleep Res. 1994;3:111–20. [PubMed] [Google Scholar]
- 27.Vasko RC, Brunner DP, Monahan JP, et al. Power spectral analysis of EEG in a multiple-bedroom, multiple-polygraph sleep laboratory. Int J Med Inform. 1997;46:175–84. doi: 10.1016/s1386-5056(97)00064-6. [DOI] [PubMed] [Google Scholar]
- 28.Rechtschaffen A, Kales A. Washington, DC: U.S. Government Printing Office, Department of Health Education and Welfare; 1968. Manual of standardized terminology, techniques and scoring system for sleep stages of human subjects NIH Publication 204. [Google Scholar]
- 29.American Academy of Sleep Medicine. Westchester, IL: American Academy of Sleep Medicine; 2007. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. [Google Scholar]
- 30.Brunner DP, Vasko RC, Detka CS, Monahan JP, Reynolds CF, Kupfer DJ. Muscle artifacts in the sleep EEG: Automated detection and effect on all-night EEG power spectra. J Sleep Res. 1996;5:155–64. doi: 10.1046/j.1365-2869.1996.00009.x. [DOI] [PubMed] [Google Scholar]
- 31.Friesen GM, Jannett TC, Jadallah MA, Yates SL, Quint SR, Nagle HT. A comparison of the noise sensitivity of nine QRS detection algorithms. IEEE Trans Biomed Eng. 1990;37:85–98. doi: 10.1109/10.43620. [DOI] [PubMed] [Google Scholar]
- 32.Kamath MV, Fallen EL. Correction of the heart rate variablity signal for ectopics – missing beats. In: Malik M, Camm AJ, editors. Heart rate variability. Armonk, NY: Futura; 1995. pp. 75–85. [Google Scholar]
- 33.Pagani M, Lombardi F, Guzzetti S, et al. Power spectral analysis of heart rate and arterial pressure variabilities as a marker of sympatho-vagal interaction in man and conscious dog. Circ Res. 1986;59:178–93. doi: 10.1161/01.res.59.2.178. [DOI] [PubMed] [Google Scholar]
- 34.Thayer JF, Friedman BH, Borkovec TD. Autonomic characteristics of generalized anxiety disorder and worry. Biol Psychiatry. 1996;39:255–66. doi: 10.1016/0006-3223(95)00136-0. [DOI] [PubMed] [Google Scholar]
- 35.European Task Force Society of Cardiology – the North American Society of Pacing – Electrophysiology. Heart rate variability-standards of measurement, physiological interpretation, and clinical use. Circulation. 1996;93:1043–65. [PubMed] [Google Scholar]
- 36.Bonnet MH, Arand DL. Situational insomnia: consistency, predictors, and outcomes. Sleep. 2003;26:1029–36. doi: 10.1093/sleep/26.8.1029. [DOI] [PubMed] [Google Scholar]
- 37.Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979;86:420–8. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- 38.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–74. [PubMed] [Google Scholar]
- 39.Shrout PE. Measurement reliability and agreement in psychiatry. Stat Methods Med Res. 1998;7:301–17. doi: 10.1177/096228029800700306. [DOI] [PubMed] [Google Scholar]
- 40.Traub RE, Rowley GL. Understanding reliability. Educational Measurement: Issues and Practice. 1991;10:37–45. [Google Scholar]
- 41.Toussaint M, Luthringer R, Schaltenbrand N, et al. Changes in EEG power density during sleep laboratory adaptation. Sleep. 1997;20:1201–7. doi: 10.1093/sleep/20.12.1201. [DOI] [PubMed] [Google Scholar]
- 42.Sharpley AL, Solomon RA, Cowen PJ. Evaluation of first night effect using ambulatory monitoring and automatic sleep stage analysis. Sleep. 1988;11:273–6. doi: 10.1093/sleep/11.3.273. [DOI] [PubMed] [Google Scholar]
- 43.Browman CP. Sleep following sustained exercise. Psychophysiology. 1980;17:577–80. doi: 10.1111/j.1469-8986.1980.tb02300.x. [DOI] [PubMed] [Google Scholar]
- 44.Walker JM, Floyd TC, Fein G, Cavness C, Lualhati R, Feinburg I. Effects of exercise on sleep. J Appl Physiol. 1978;44:945–51. doi: 10.1152/jappl.1978.44.6.945. [DOI] [PubMed] [Google Scholar]
- 45.Karacan I, Thornby JI, Anch M, Booth GH, Williams RL, Salis PJ. Dose-related sleep disturbances induced by coffee and caffeine. Clin Pharmacol Ther. 1976;20:682–9. doi: 10.1002/cpt1976206682. [DOI] [PubMed] [Google Scholar]
- 46.Nicholson AN, Stone BM. Heterocyclic amphetamine derivatives and caffeine on sleep in man. Br J Clin Pharmacol. 1980;9:195–203. doi: 10.1111/j.1365-2125.1980.tb05833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Torsvall L, Akerstedt T, Lindbeck G. Effects on sleep stages and EEG power density of different degrees of exercise in fit subjects. Electroencephalogr Clin Neurophysiol. 1984;57:347–53. doi: 10.1016/0013-4694(84)90158-5. [DOI] [PubMed] [Google Scholar]
- 48.Benca RM, Obermeyer WH, Thisted RA, Gillin JC. Sleep and psychiatric disorders: A meta-analysis. Arch Gen Psychiatry. 1992;49:651–68. doi: 10.1001/archpsyc.1992.01820080059010. [DOI] [PubMed] [Google Scholar]
- 49.Hall M, Buysse DJ, Nofzinger EA, et al. Financial strain is a significant correlate of sleep continuity disturbances in late-life. Biol Psychol. 2008;77:217–22. doi: 10.1016/j.biopsycho.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hall M, Buysse DJ, Dew MA, Prigerson HG, Kupfer DJ, Reynolds CF. Intrusive thoughts and avoidance behaviors are associated with sleep disturbances in bereavement-related depression. Depress Anxiety. 1997;6:106–12. [PubMed] [Google Scholar]
- 51.Hall M, Buysse DJ, Nowell PD, et al. Symptoms of stress and depression as correlates of sleep in primary insomnia. Psychosom Med. 2000;62:227–30. doi: 10.1097/00006842-200003000-00014. [DOI] [PubMed] [Google Scholar]
- 52.Kecklund G, Akerstedt T. Apprehension of the subsequent working day is associated with a low amount of slow wave sleep. Biol Psychol. 2004;66:169–76. doi: 10.1016/j.biopsycho.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 53.Reynolds CF, Hoch CC, Buysse DJ, et al. Sleep after spousal bereavement: a study of recovery from stress. Biol Psychiatry. 1993;34:791–7. doi: 10.1016/0006-3223(93)90068-o. [DOI] [PubMed] [Google Scholar]
- 54.Soderstrom M, Ekstedt M, Akerstedt T, Nilsson J, Axelsson J. Sleep and sleepiness in young individuals with high burnout scores. Sleep. 2004;27:1369–77. doi: 10.1093/sleep/27.7.1369. [DOI] [PubMed] [Google Scholar]
- 55.Geiger A, Huber R, Kurth S, Ringli M, Jenni OG, Achermann P. The sleep EEG as a marker of intellectual ability in school age children. Sleep. 2011;34:181–9. doi: 10.1093/sleep/34.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tarokh L, Carskadon MA, Achermann P. Trait-like characteristics of the sleep EEG across adolescent development. J Neurosci. 2011;31:6371–8. doi: 10.1523/JNEUROSCI.5533-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lutzenberger W, Pulvermuller F, Birbaumer N. Words and pseudowords elicit distinct patterns of 30-Hz EEG responses in humans. Neurosci Lett. 1994;176:115–8. doi: 10.1016/0304-3940(94)90884-2. [DOI] [PubMed] [Google Scholar]
- 58.Makeig S, Jung TP. Tonic, phasic, and transient EEG correlates of auditory awareness in drowsiness. Brain Res Cogn Brain Res. 1996;4:15–25. doi: 10.1016/0926-6410(95)00042-9. [DOI] [PubMed] [Google Scholar]
- 59.Dimsdale JE, Coy TV, ncoli-Israel S, Clausen J, Berry CC. The effect of blood pressure cuff inflation on sleep. A polysomnographic examination. Am J Hypertens. 1993;6:888–91. doi: 10.1093/ajh/6.10.888. [DOI] [PubMed] [Google Scholar]
- 60.Imai Y, Abe K, Sasaki S, et al. Determination of clinical accuracy and nocturnal blood pressure pattern by new portable device for monitoring indirect ambulatory blood pressure. Am J Hypertens. 1990;3:293–301. doi: 10.1093/ajh/3.4.293. [DOI] [PubMed] [Google Scholar]
- 61.Vitiello MV, Larsen LH, Moe KE, Borson S, Schwartz RS, Prinz PN. Objective sleep quality of healthy older men and women is differentially disrupted by nighttime periodic blood sampling via indwelling catheter. Sleep. 1996;19:304–11. doi: 10.1093/sleep/19.4.304. [DOI] [PubMed] [Google Scholar]
- 62.Blazer DG, Hays JC, Foley DJ. Sleep complaints in older adults: a racial comparison. J Gerontol A Biol Sci Med Sci. 1995;50:M280–M284. doi: 10.1093/gerona/50a.5.m280. [DOI] [PubMed] [Google Scholar]
- 63.Jean-Louis G, Magai CM, Cohen CI, et al. Ethnic differences in self-reported sleep problems in older adults. Sleep. 2001;24:926–33. doi: 10.1093/sleep/24.8.926. [DOI] [PubMed] [Google Scholar]
- 64.Profant J, Ancoli-Israel S, Dimsdale JE. Are there ethnic differences in sleep architecture? Am J Hum Biol. 2002;14:321–6. doi: 10.1002/ajhb.10032. [DOI] [PubMed] [Google Scholar]
- 65.Rao U, Poland RE, Lutchmansingh P, Ott GE, McCracken JT, Lin KM. Relationship between ethnicity and sleep patterns in normal controls: implications for psychopathology and treatment. J Psychiatr Res. 1999;33:419–26. doi: 10.1016/s0022-3956(99)00019-9. [DOI] [PubMed] [Google Scholar]
- 66.Redline S, Kirchner HL, Quan SF, Gottlieb DJ, Kapur V, Newman A. The effects of age, sex, ethnicity, and sleep-disordered breathing on sleep architecture. Arch Intern Med. 2004;164:406–18. doi: 10.1001/archinte.164.4.406. [DOI] [PubMed] [Google Scholar]
- 67.Poland RE, Rao U, Lutchmansingh P, et al. REM sleep in depression is influenced by ethnicity. Psychiatry Res. 1999;88:95–105. doi: 10.1016/s0165-1781(99)00080-3. [DOI] [PubMed] [Google Scholar]
- 68.Hall M, Matthews KA, Kravitz HM, et al. Race and financial strain are independent correlates of sleep in mid-life women: the SWAN sleep study. Sleep. 2009;32:73–82. [PMC free article] [PubMed] [Google Scholar]
- 69.Crawford PB, Story M, Wang MC, Ritchie LD, Sabry ZI. Ethnic issues in the epidemiology of childhood obesity. Pediatr Clin North Am. 2001;48:855–78. doi: 10.1016/s0031-3955(05)70345-x. [DOI] [PubMed] [Google Scholar]
- 70.Dee DL, Bensyl DM, Gindler J, et al. Racial and ethnic disparities in hospitalizations and deaths associated with 2009 pandemic influenza A (H1N1) virus infections in the United States. Ann Epidemiol. 2011;21:623–30. doi: 10.1016/j.annepidem.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 71.Hausmann LR, Ibrahim SA, Mehrotra A, et al. Racial and ethnic disparities in pneumonia treatment and mortality. Med Care. 2009;47:1009–17. doi: 10.1097/MLR.0b013e3181a80fdc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Byrd WM, Clayton LA. An American health dilemma: race, medicine, and health care in the United States 1900-2000. 1st ed. New York: Routledge; 2001. [Google Scholar]


