Abstract
Background
Use of a reduced intensity conditioning regimen before an allogeneic hematopoietic cell transplantation is frequently associated with an early state of mixed hematopoietic chimerism. Such a co-existence of both host and donor hematopoietic cells may influence post-transplant alloreactivity and may affect the occurrence and severity of acute and chronic graft-versus-host disease (GVHD) as well as the intensity of the graft-versus-leukemia effect. Here we evaluated the relation between chimerism state after reduced intensity conditioning transplantation (RICT), auto-antibody production and chronic GVHD (cGVHD)-related pathology.
Methods
Chimerism state, circulating anti-cardiolipin and anti-double stranded DNA auto-antibody (Ab) titers as well as occurrence of cGVHD-like lesions were investigated in a murine RICT model.
Results
We observed a novel association between mixed chimerism state, high levels of pathogenic IgG auto-Abs and subsequent development of cGVHD-like lesions. Furthermore, we found that the persistence of host B cells, but not dendritic cell origin or subset, was a factor associated with the appearance of cGVHD-like lesions. The implication of host B cells was confirmed by a host origin of auto-Abs.
Conclusions
Recipient B cell persistence may therefore contribute to the frequency and/or severity of cGVHD after RICT.
Keywords: Animals; Autoantibodies; blood; Autoimmunity; Bone Marrow Transplantation; immunology; pathology; Hematopoietic Stem Cells; physiology; Immunoglobulin G; blood; Immunoglobulin M; blood; Interferon-gamma; blood; Interleukin-13; blood; Mice; Mice, Inbred BALB C; Transplantation Chimera; immunology
Keywords: chimerism, GVHD, autoimmunity, B cells
INTRODUCTION
The recent introduction of reduced intensity conditioning (RIC) regimens has modified allogeneic hematopoietic cell (HC) transplantation. While shifting away from toxic myeloablative conditioning regimens, such an approach can result in increased graft failure (1). In addition, reduced intensity conditioning transplantation (RICT) might be associated with less frequent incidence, less severe clinical form and/or delayed onset of acute graft-versus-host disease (GVHD) than conventional myeloablative allograft while a similar frequency of chronic graft-versus-host disease (cGVHD) has been reported (1–4). Allogeneic RICT are associated with delayed conversion to full-donor chimerism with longer host HC persistence. In experimental murine models, this state of mixed chimerism observed after allogeneic RICT offers several advantages over full-donor chimerism, including immunological competence (5), reciprocal graft-host tolerance (5, 6) and superior graft-versus-leukemia (GvL) effect (7). All these advantages have been related to the presence of both recipient and donor antigen-presenting cells (APC) (5–7). Dendritic cells (DC) can be considered as the major APC involved in the control of allo-immune responses after BMT (as discussed in Ref.8). Donor DC homing in the thymus may participate to negative selection of alloreactive T cells together with host DC. In peripheral organs, host APC and notably DC seems to be critical to initiate acute GVHD (9, 10) and to favor GvL effect (7). However, other –immunologically active –host HC subsets than DC persist and may influence the alloreactive interactions between donor and host immune cells. Here, we observed in a murine bone marrow transplantation (BMT) model that mixed chimeric mice presented higher levels of circulating pathogenic IgG auto-antibodies (Abs) than in non- or full-donor chimeric mice and preferentially developed cGVHD-like lesions. This increase of IgG auto-Abs in mixed chimeric mice was associated with an increase of total IgG levels. This suggests a polyclonal activation in these mixed chimeric recipients that can be related to the alloreactive conflict observed after transplantation. The persistence of host B cells together with donor T cells was identified as a factor associated with cGVHD occurrence. The involvement of host B cells in mixed chimeric mice was confirmed by the recipient origin of circulating auto-Abs. Such observation might be clinically relevant and contribute to cGVHD occurrence and/or severity after allogeneic RICT.
MATERIALS AND METHODS
Bone marrow transplantation
Pathogen-free male (5–6 weeks) BALB/c mice (Janvier, Le Genest-St-Isle, France) received a limited number of bone marrow (BM) cells (106) from FVB donors following a sub-lethal 6 Gy total body irradiation. Experiments were performed according to institutional guidelines. All mice described in this study corresponded initially to control recipients used to evaluate novel cell-based therapy approaches favoring HC engraftment. The initial observation –consisting of a retrospective analysis involving six independent experiments– was done by chance when some of these control receiving only a limited number of BM cells developed late GVHD-like lesions (> 45 days). These lesions were identical to those encountered in the chronic form of GVHD, as attested by pathological examination. Then, three prospective experiments were performed to further explore cellular factors involved in the occurrence of cGVHD in mixed chimeric mice.
Chimerism analysis
Engraftment was evaluated on either splenocytes or thymocytes at time of sacrifice or on peripheral blood mononuclear cells (kinetic studies) by flow cytometry using specific anti-H2 monoclonal Abs (mAbs), as previously reported (11). Recipients were considered full-donor chimeric when > 98% of cells exhibited donor phenotype, non-chimeric in the absence (< 2%) of donor cells, and mixed in all other cases. In some additional experiments (prospective studies), allophycocyanin-conjugated anti-CD3 (145-2C11), -CD8 (53-6.7), -CD45 (30-F11), -H2q (KH117), FITC-conjugated anti-CD44 (IM7), -H2q (KH117), -B220 (RA3-6B2), -IgM (R6-60.2), phycoerythrin (PE)-conjugated anti-CD3 (145-2C11), -CD5 (53-7.3), -CD11c (HL3), -CD19 (1D3), -H2d (SF1-1.1), -CD62L (MEL-14), peridinin chlorophyll-a protein (PerCP)-conjugated anti-CD4 (RM4-5), -B220 (RA3-6B2), PerCP-cyanin 5.5-conjugated CD11b (M1/70) (BD Biosciences PharMingen, Le Pont de Claix, France) and PE-cyanin 5-conjugated anti-F4/80 (A3-1, Serotec, Oxford, UK) mAbs were used to evaluate engraftment in T, B, and DC subsets. Absolute counts of circulating leukocyte subsets were determined using a single-platform approach based on cytometry and tubes containing a defined number of microbeads (TruCOUNT®, BD Biosciences) (12). In this “lyse no wash” method, side scatter (SSC) vs. CD45 fluorescence gating permits the discrimination of leukocytes from nucleated erythroid cells and debris (12). The kinetics of engraftment in this BMT model was determined by sequential sacrifices of grafted mice. Donor cells with a myeloid morphology (forward scatter [FSC]/SSC gating) first appeared in the BM 2 days after BMT. At day 8 post-BMT, donor cells disappeared in the BM of non-engrafted mice while persisted in the marrow of engrafted mice. Donor cells with both lymphoid and myeloid morphology were detected only after day 6–8 in other sites including the spleen and blood of engrafted mice. The only exception was donor T cells likely to persist and circulate in non-engrafted mice until sufficient autologous immuno-hematological reconstitution occurred.
Auto-antibody detection
Anti-double stranded (ds) DNA and anti-cardiolipin (AcL) auto-Abs were assessed by ELISA, as described (13). Briefly, Maxisorp™ immunoplates (Nunc, Naperville, IL) were coated overnight at 4°C with 10 μg/mL of calf thymus DNA (Sigma, Saint Quentin Fallavier, France) in borate buffered saline. The plates were washed with phosphate buffer saline (PBS) and then saturated with a blocking buffer containing 0.5% fetal calf serum (FCS, Boehringer Mannheim, Meylan, France) and 0.2% of sodium azide (Sigma) in PBS. Different dilutions of serum were added and incubated for 2 hours. Immune complexes (IC) were detected with biotinylated goat anti-mouse IgG or IgM (Jackson ImmunoResearch Laboratories, West Grove, PA) followed by Horseradish Peroxidase conjugated Streptavidin (BD Biosciences PharMingen). Then a solution of TMB substrate (3,3′,5,5′-tetramethylbenzidine, Moss Inc, Pasadena, MD) was added. The presence of anti-dsDNA Abs was determined by measuring optical density at 620 nm. For the detection of AcL auto-Abs, Polysorp™ immunoplates (Nunc) were coated overnight with 50 μg/mL of cardiolipin (Sigma) in ethanol. After saturation of the plates with a 10% FCS PBS solution, different dilutions of serum were added and incubated for 3 hours. Revelation of AcL auto-Abs (IgG and IgM) was performed as described for anti-dsDNA Abs. Normal mouse serum (Sigma) was used to obtain a standard curve for natural IgM Ab titer determination. Serum from autoimmune-prone MRL lpr/lpr mice (provided by Luc Reininger, Marseille, France) was used as positive control for IgG auto-Ab detection and to obtain a standard curve for IgG Ab titer determination. Experimental values from separate experiments were normalized to a single MRL-lpr/lpr-positive control serum used in every assay. The origin of auto-Abs can be determine since the mouse strains used differ at the Igh locus: BALB/c mice carry the “a” allotype (Igh-Ca gene haplotype), while C57BL/c mice carry the Igh-Cb haplotype (b allotype) and FVB mice carry neither the Igh-Ca nor Igh-Cb haplotype (non a non b allotype). Allotype-specific ELISA (14) were performed using rabbit anti-mouse 1a allotype or anti-1b allotype anti-serum (Nordic Immunology, Tilburg, The Netherlands) instead of biotinylated goat anti-mouse IgG. Serum from (NZB x NZW)F1 mice was used as positive control for Igh-1a allotype.
Total IgG and IgM antibody determination
Commercial ELISA kits (Bethyl Laboratories, Montgomery, TX) were used according to the manufacturer’ recommendations.
ELISA for IL-13 and IFN-γ detection
Commercial ELISA kits for mouse IL-13 and IFN-γ (R&D Systems, Minneapolis, MN) were used according to the manufacturer’ recommendations. Fifty-three serums were analyzed for IL-13 and IFN-γ and IL-13/IFN-γ ratio was calculated to appreciate type 1/type 2 polarization.
Pathological examination and immunohistochemical staining
All grafted mice involved in this study were prospectively followed for the development of clinical signs of GVHD according to the scoring system initially proposed by Ferrara’s group (15). All mice developing clinical signs of GVHD and in particular abnormality in fur texture (severe ruffling) or in skin integrity (areas of denuded skin) were sacrificed and pathological examination of the skin, kidney, liver and gastro-intestinal tract was performed. Healthy mice grafted the same day as mice developing GVHD-like lesions were randomly selected and analyzed for chimerism and by a pathologist blinded to the nature of the mice being examined. Lesions were assessed on paraffin-embedded tissues including skin, kidney, salivary gland, stomach and liver. Sections (5 μm) were stained with either hematoxylin eosin saffron (HES), periodic acid Schiff (PAS) or Masson’s trichrome (MT) reagents. Immune complex deposition was detected on OCT-embedded snap-frozen kidney section after staining with either FITC-conjugated goat anti-mouse IgG, IgA, Kappa or Lambda light chain Abs (Interchim, Montluçon, France) and UV fluorescence microscopy examination.
Statistical analysis
Statistical analysis was performed using SigmaStat 2.0 software (SPSS Inc., Jandel Scientific, Erkrath, Germany). Mann-Whitney Rank Sum, Student t or Fisher’s exact tests as well as Pearson product moment correlation test were used as indicated. P-Values <.05 were considered as significant.
RESULTS
Mixed chimeric recipients exhibit higher levels of circulating IgG auto-antibodies after allogeneic BMT than full-donor- or non-chimeric mice
In experimental conditions designed to limit donor engraftment, 284 BM recipients from 9 independent experiments were analyzed for splenic engraftment 7–9 week post-BMT. Among these mice, 58.4% (166/284) had rejected their graft, whereas 9.9% (28/284) presented full-donor engraftment and 31.7% (90/284) were mixed chimeric mice with 5% to 98% of donor-derived cells. In most cases, engraftment level was found similar in the lymphoid and myeloid populations identified on their FSC/SSC profile (data not shown). Circulating anti-dsDNA and AcL IgG auto-Ab titers were determined since they were frequently encountered in murine cGVHD (16). At the beginning of the study, both AcL and anti-dsDNA Ig auto-Ab titers were measured at the time of chimerism analysis. Since we observed a good correlation between circulating anti-dsDNA and AcL IgG auto-Ab titers (P=.0065, n=210; Pearson Product Moment Correlation, Figure 1A), either AcL or anti-dsDNA Ig auto-Ab titers were then determined in recipients. Higher levels of circulating IgG auto-Abs were found in mixed chimeric mice (anti-dsDNA auto-Abs: 192 U/mL [1-1716] (median [range]), n=52 AcL auto-Abs: 834 U/mL [17-7466], n=70) than in non-chimeric (anti-dsDNA Abs: 75 U/mL [2-825], n=108, P<.01; AcL Auto-Abs: 111 U/mL [10-4398], n=117, P<.01) or full-donor chimeric mice (anti-dsDNA Abs: 73 U/mL [2-266], n=19, P<.01; AcL auto-Abs: 164 U/mL [3-831], n=24, P<.01, Mann-Whitney Rank Sum Test). No statistical difference was observed between auto-Ab levels found in full-donor- or in non-chimeric mice (P=.91 for anti-dsDNA and P=.57 for AcL auto-Abs, Mann-Whitney Rank Sum Test). The high median auto-Ab levels observed in mixed chimeric mice resulted from the presence of high levels (> to the mean + 2 SD of Ab levels in naive mice) of auto-immune IgGs in nearly half of the mixed chimeric recipients (19/52 for anti-DNA and 31/70 for AcL auto-Abs) (Figure 1B). Such high levels of auto-Ab IgG were seldom found in full-donor- (anti-DNA Abs: 0/19; AcL Abs: 5/24) or non-chimeric mice (anti-DNA Abs: 8/108; AcL Abs: 9/117) (P<.01 between mixed chimeric mice and other groups, except for AcL auto-Abs between mixed- and full-donor chimeric mice where P=.07; Mann-Whitney Rank Sum Test, Figure 1B).
Figure 1. Auto-immunity is preferentially observed in mixed chimeric mice.
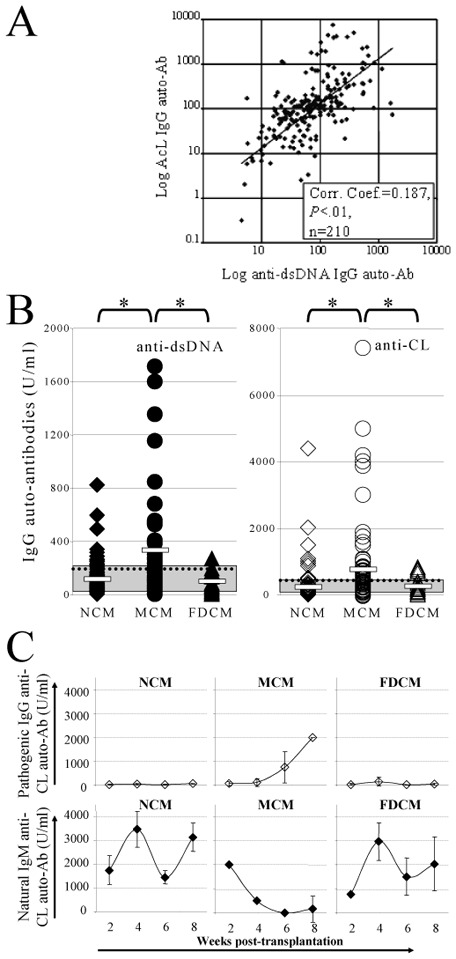
(A) A correlation between the levels of circulating anti-cardiolipin (AcL, x axis, Logarithmic representation) and anti-dsDNA (y axis, Logarithmic representation) IgG auto-Abs assessed by ELISA was found in recipient mice 9 weeks post-BMT. The solid line represents the calculated linear regression line of the observed data. Correlation coefficient =0.187, P<.01, n=210 recipient mice (Pearson Product Moment Correlation). (B) Circulating anti-dsDNA (black symbols - left hand side panel) and AcL (open symbols - right hand side panel) IgG auto-Abs were significantly higher 9 weeks post-BMT in mixed chimeric mice (MCM, circles) than in non- (NCM, diamonds) or full-donor chimeric mice (FDCM, triangles). White bars: mean for each group; doted line: mean of all groups; gray area: serum auto-Ab titers in 10 age-matched naive mice; *: P<.01 (Mann-Whitney Rank Sum Test). (C) Kinetics of both IgG (open diamonds) and IgM (black diamonds) AcL auto-Abs demonstrated a self-immunization in 4 out of 8 tested MCM, as attested by a simultaneous decrease of natural IgM auto-Abs and an increase of pathogenic IgG auto-Abs. In contrast, low levels of pathogenic IgG auto-Abs and normal levels of natural IgM auto-Abs (as compared to age-matched naive mice, range of AcL IgM auto-Ab titers: 2210.8–4358.3 U/ml) were observed in NCM (n=8) and FDCM (n=8). Error bars: SEM.
Self-immunization is preferentially observed in mixed chimeric recipients
An isotype switch from natural IgM auto-Abs –that react weakly with self-antigens (Ags)– to high affinity pathogenic IgG is observed during self-Ag immunization (17, 18). Kinetics of IgM and IgG auto-Ab production after BMT were analyzed in 8 randomly selected mice from each group (non-chimeric, full-donor and mixed chimeric mice). In a majority of mice (20/24), low levels of both anti-dsDNA and AcL IgGs (< 151 U/mL) were detected, as observed in age-matched naive mice (Figure 1C). In these mice, natural IgM auto-Ab serum concentration reached the levels found in age-matched naive mice 2–4 week post-BMT, therefore suggesting the absence of immunization (Figure 1C). In contrast, a decrease of both anti-dsDNA and AcL auto-Ab IgM levels simultaneously to an increase of auto-Ab IgG levels was observed in 4 mixed chimeric mice out of 8 analyzed (Figure 1C). This suggested a self-immunization in these 4 mixed chimeric mice.
Mixed chimeric recipients exhibit higher levels of total circulating IgGs than full-donor- or non-chimeric mice
Total IgG Ab titers were measured 7–9 weeks post-BMT in the 3 different groups of recipients. Significant higher levels of total IgGs were detected in mixed chimeric mice (0.59 μg/L [0.21–0.77] (median [range]), n=10) than in non-chimeric (0. 30 μg/L [0.09–0.72]), n=6, P<.05, Student t test) or full-donor chimeric mice (0.23 μg/L [0.07–1.05]), n=10, P<.05, Student t test) (Figure 2A). No difference was observed between non- and full-donor chimeric recipients (P=.4, Mann-Whitney Rank Sum Test). Only mixed chimeric mice exhibited significant higher levels of total IgGs than naive BALB/c mice (0.11 μg/L [0.07–0.12], n=6, P<.01, Student t test). Altogether, increase of circulating auto-IgG observed in mixed chimeric mice is associated with an increase of total IgGs, suggesting a polyclonal activation related to the alloreactive conflict. This hypothesis is supported by the correlation existing between the levels of total IgGs and anti-DNA IgG Abs (Correlation coefficient =0.566, P<.01, n=26; Pearson Product Moment Correlation, Figure 2B).
Figure 2. Significant higher levels of circulating total IgGs correlated with higher levels of IgG auto-Abs are preferentially found in mixed chimeric mice.
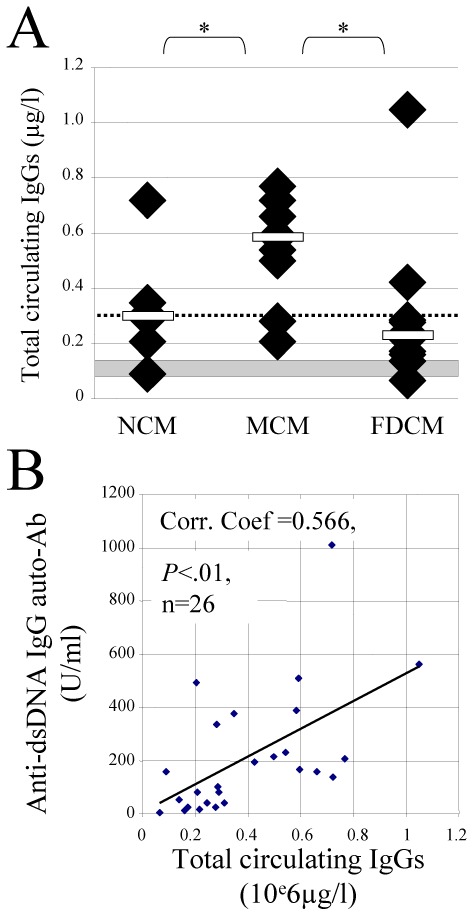
(A) Circulating total IgGs (μg/l) 9 weeks post-BMT were significantly higher in mixed chimeric mice (MCM; n=10) than in non- (NCM; n=6) or in full-donor chimeric mice (FDCM; n=10). White bars: median for each group; doted line: median of all groups; grey area: median + 2 SEM total IgGs found in 6 age-matched naive BALB/c mice; *: P<.01 (Mann-Whitney Rank Sum Test for NCM vs FDCM, otherwise Student t test). (B) Correlation between the levels of circulating total IgGs (x axis, 106 μg/l) and anti-dsDNA (y axis, U/ml) IgG auto-Ab assessed by ELISA in recipient mice 9 weeks post-BMT. The solid line represents the calculated linear regression line of the observed data. Correlation coefficient =0.566, P<.01, n=26 recipient mice (Pearson Product Moment Correlation).
Chronic-GVHD-like lesions are preferentially observed in mixed chimeric mice and correlate with high levels of circulating auto-Abs
Since auto-immunization may or may not result in autoimmune-like pathology (19), recipients of each group (non-chimeric, full-donor and mixed chimeric mice) were sacrificed 7–9 months post-BMT and examined for pathology. Chronic GVHD-like lesions (Figure 3A-B) were exclusively found in 13 out of 21 mixed chimeric recipients tested, whereas no lesions were observed in 13 full-donor- (P<.01; Mann-Whitney Rank Sum Test) or 37 non-chimeric mice (P<.01, Mann-Whitney Rank Sum Test). Furthermore higher levels of IgG auto-Abs (anti-dsDNA Abs: 25-1599 U/mL, median: 931; AcL Abs: 136-4078 U/mL, median: 779) were found in these 13 mixed chimeric recipients developing cGVHD-like lesions when compared to healthy mixed chimeric mice (P<.01 for anti-dsDNA). This strongly suggests a correlation between mixed chimeric state, high levels of IgG auto-Abs and cGVHD-like lesion occurrence.
Figure 3. Chronic GVHD-like lesions are detected in mixed chimeric recipient mice.
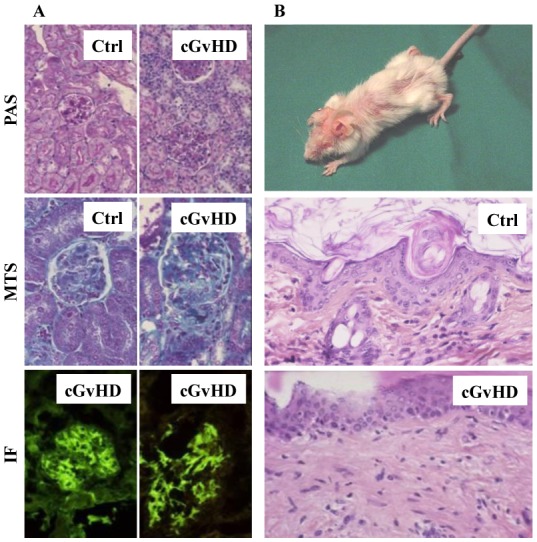
(A) Mixed chimeric mice (8/10) developed glomerulonephritis as attested by renal mononuclear cell infiltrate (noted cGVHD, periodic acid Schiff [PAS] staining, original magnification [OM] x80), a thickening of the glomerulus basal membrane due to IC deposition and a crescent formation due to epithelial cell proliferation (noted cGVHD, Masson’s trichrome [MT] staining, OM x128). Glomerulus from a BM grafted mouse non-developing cGVHD stained with PAS and MT (noted Ctrl) was shown as control. Immune complex deposition is identified by UV fluorescent microscopy after labeling with either FITC-conjugated anti-kappa (right hand side immunofluorescence panel, OM x128) or anti-lambda Abs (left hand side immunofluorescence panel, OM x128). (B) Sclerodermatous cGVHD-like lesions in a mixed chimeric mouse were attested by hair loss (upper panel) and skin structure alterations including fibrosis and mononuclear cell infiltrate (noted cGVHD, lower panel, HES staining, OM x80). Skin section from a healthy BM grafted mouse was shown as control (noted Ctrl, HES staining, OM x80).
Chronic GVHD-like lesions are preferentially found in mixed chimeric recipients demonstrating a divergent T and B chimerism with mostly donor T cells and host B cells
To further explore cellular factors explaining the occurrence of cGVHD in mixed chimeric mice, we also analyzed the presence of donor-derived cells in the thymus. Donor-derived cells in T lymphoid lineage were comparable in mixed chimeric mice developing or not developing cGVHD-like lesions (Table 1). Reciprocal graft-host tolerance is linked to co-existence of thymic host and donor CD11c+ DC participating to the negative selection (5, 20). However, engraftment in thymic DC was similar whatever the considered mixed chimeric mice (Table 2). Because patients with cGVHD exhibited significantly higher plasmacytoid DC (pDC) numbers (21), DC origin and subsets (myeloid vs. pDC) were also analyzed in the spleen of these mice. Based on B220 expression, myeloid DC (CD11c+B220−) can be distinguished from pDC (CD11c+B220+) (22, 23). The repartition of splenic DC subsets or origin was also not different in mice developing cGVHD-like lesions or not (Table 2).
TABLE 1.
T and B cell subset and chimerism analysis
| Chimeric state§ | CD19+ | CD3+ | Spleen TCD4+ | TCD8+ | CD4/CD8 ratio | Thymus thymocytes | |
|---|---|---|---|---|---|---|---|
| NCM | 0 [0–5] (n= 64) | 0 [0–7] (n= 67) | 1 [0 – 7] (n= 21) | 0 [0 – 3] (n= 21) | 2.3 [1.4 – 3.2] (n= 21) | 1 [0 – 5] (n= 25) | |
| MCM | healthy | 85 [32 – 100] (n= 20) | 76 [27 – 98] (n= 23) | 77 [15 – 80] (n= 8) | 74.5 [5 – 80] (n= 8) | 3.0 [1 .2 – 5.3] (n= 8) | 63.5 [10 – 90] (n= 12) |
| MCM | cGvHD | 17.5 [0 – 76]* (n= 16) | 80 [42 – 100] (n= 19) | 75 [24 – 80] (n= 8) | 78.5 [72 – 86] (n= 8) | 0.7 [0.3 – 1.4]* (n=8) | 61 [7 – 87] (n= 7) |
| FDCM | 100 [94 – 100] (n= 34) | 100 [91 – 100] (n= 37) | 98 [90 – 98] (n= 7) | 95 [90 – 98] (n= 7) | 3.0 [0.8 – 4.6] (n = 7) | 98 [88 – 100] (n= 10) | |
Results from 2–4 independent experiments are expressed as median [range] of donor derived cells, except for CD4/CD8 ratio that represent the percentageof CD4 T cells divided by the percentage of CD8 T cells whatever their origin. Representative cytometric profiles for B and T chimerism were shown Figure 4. Staining was performed on fresh cells, except in three mice per group for which CD3/CD4/CD8/H-2q staining was performed on frozen spleen cells. Chimericstate is defined by cytometry on lymphoid spleen cells; thymocytes represent double positive thymocytes as well as single positive thymocytes.
The difference in the values between healthy MCM and cGVHD MCM groups is statistically significant (P < 0.05, Student t test).
NCM, nonchimeric mice; MCM, mixed chimeric mice; FDCM, full donor chimeric mice.
TABLE 2.
Dendritic cell subset and chimerism analysis
| Chimeric state§ | mDC | Spleen pDC | mDC/pDC ratio | Thymus DC (CD11c+) | |
|---|---|---|---|---|---|
| NCM | n= 22 | 1.5 [0 – 4] | 1 [0 – 5] | 3.3 [0.8 – 18] | 1 [0 – 5] |
| MCM: | |||||
| healthy | n= 10 | 74.5 [5 – 99] | 84 [3 – 100] | 2.2 [1.2–21.3] | 50 [10 – 90] |
| cGvHD | n= 9 | 78 [27 – 96] | 86 [15 – 97] | 3.3 [0.4 – 30] | 47 [20 – 84] |
| FDCM | n= 9 | 93 [92 – 97] | 98 [92 – 100] | 1.4 [0.7 – 6.5] | 100 [95 – 100] |
Results from 3 independent experiments are expressed as median [range] of donor derived cells, except for mDC/pDC ratio, which represents the percentageof mDC divided by the percentage of pDC whatever their origin. Dendritic cell gating was performed by cytometry as previously reported (12). Chimeric stateis defined by cytometry on lymphoid spleen cells. No statistical difference was observed between any groups. NCM: nonchimeric mice; MCM: mixed chimeric mice; FDCM: full donor chimeric mice; myeloid dendritic cells (mDC) were defined as CD11c+ B220− cells; plasmacytoid dendritic cells (pDC) were defined as CD11c+ B220+.
Host B-cell stimulation induced by alloreactive donor T cells is believed to induce and maintain cGVHD in a murine model involving parental T cell transfer (without BM) into unirradiated F1 mice (16). We therefore determined B-cell chimerism in the peripheral blood and compared it to T-cell chimerism, 9 weeks post-BMT. Among 134 recipients from 4 independent experiments, 36 were mixed chimeric mice (Table 1). Whereas similar B- and T-cell engraftment (in a given mouse) was found in 20 healthy mixed chimeric mice, the 16 recipients developing cGVHD exhibited a divergent B- and T-cell chimerism with mostly host B cells (17.5% [0–76] of donor CD19+ B cells) and donor T cells (79% [42–100] of donor CD3+ T cells, Table 1). This dissociated state of lymphoid chimerism was never observed in full-donor chimeric mice since all the cells were from donor origin (Figure 4 and Table 1). Chimerism analysis in mixed chimeric mice was extended to T cell subsets. Comparable levels of donor chimerism were found in splenic CD4+ T cells and in CD8+ T cells from healthy mixed chimeric mice and mixed chimeric mice developing cGvHD-like lesions (Table 1). However, CD4/CD8 imbalance was detected in mice developing such lesions while healthy recipients exhibited a CD4/CD8 ratio higher than 1 (P<.05, Table 1).
Figure 4. Chronic GVHD is associated with the presence of host B cells and donor T cells.
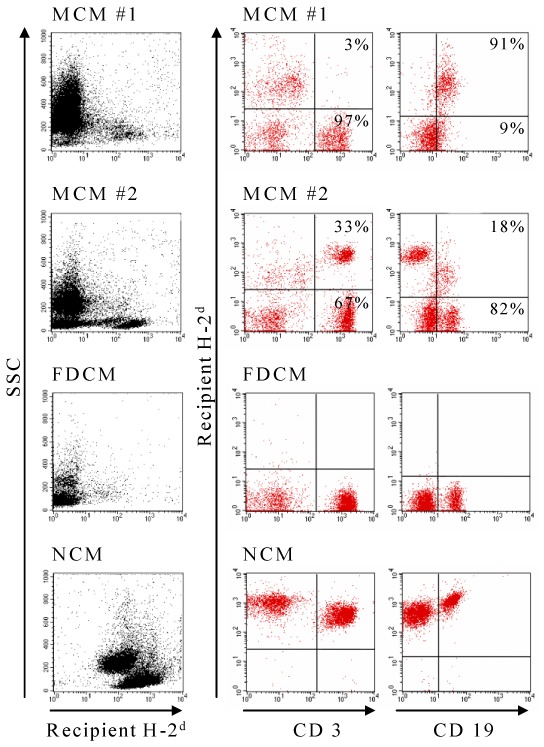
Recipient H-2d expression on CD3+ T cells (middle column panel) and CD19+ B cells (last column panel) was analyzed by flow cytometry on peripheral blood cells at time of sacrifice. Left hand side panels represented lymphoid chimerism obtained using an anti-recipient H-2 mAb versus SSC gating. A representative flow cytometry profile from a mixed chimeric mouse among 16 mice developing cGVHD (MCM#1) shows B cells from recipient origin and T cells from donor origin. A representative flow cytometry profile from a healthy mixed chimeric mouse out of 20 mice (MCM#2) shows similar proportions of T and B cells from both origins. Representative flow cytometry profiles for full-donor- (FDCM, n=34) and non-chimeric (NCM, n=64) mice are also shown.
Type 1 (IFN-γ) and 2 (IL-13) cytokine profiles were also determined in 53 grafted mice (11 healthy mixed chimeric mice, 10 mixed chimeric mice developing cGVHD-like lesions, 23 non chimeric and 9 full donor chimeric mice). No significant differences were observed between the IL-13/IFN-γ ratio of healthy mixed chimeric mice (median 16.4; range: 1.7–368.5, n=11) and that of mixed chimeric mice developing cGVHD-like lesions (median 12.7; range: 1.2–60, n=10). Among these 53 mice tested, only one (a full donor chimeric mouse) exhibited an IL-13/IFN-γ ratio lower than 1. Other grafted mice exhibited an elevated IL-13/IFN-gamma ratio. To confirm the origin of pathogenic auto-Abs presented in mixed chimeric recipients, allotype-specific ELISA were performed where auto-IgG were revealed by allotype-specific anti-serum instead of non-specific goat anti-mouse IgGs. More than 85% of anti-dsDNA auto-Abs were from host BALB/c origin (median: 89.1% [range: 48–98%] of anti-dsDNA auto-IgG detected with non-allotype specific anti-IgG Abs were detected with anti-1a allotype anti-serum; n=20). This demonstrates that persistence of host B cells is one potential factor involved in auto-Ab production.
DISCUSSION
Several advantages have been reported to be associated with mixed chimerism state following RICT (5–7). Among them, reciprocal graft-host tolerance appears prominent (5, 6, 24, 25). Here, we show that about half of the mixed chimeric mice presented preferentially higher levels of host-derived pathogenic IgG auto-Abs than full-donor or non-chimeric mice and that such mice developed subsequently cGVHD-like lesions. Healthy mixed chimeric mice could be distinguished from those developing cGVHD-like lesions on the basis of B- and T-cell chimerism. Indeed, mixed chimeric mice presenting cGVHD-like lesions exhibited a divergent B- and T-chimerism with mostly host B cells and donor T cells while healthy chimeric mice showed similar engraftment in B and T cell populations. In addition, mixed chimeric mice developing cGVHD-like lesions presented a CD4/CD8 imbalance. In this experimental model, we did not correlate the occurrence of such cGVHD-like lesions with a particular distribution of DC subset or origin. Altogether, these results show that mixed chimeric state is not always advantageous and suggest that such a state is not constantly associated with tolerance.
Chronic GVHD is an alloimmune disease associated with autoimmune features (26) and is closely related to systemic autoimmune disease in mice (16). As such, cGVHD may result from the occurrence of multiple factors (26, 28). Whereas some differences exists (for an extensive review, see Ref.28), both human and mouse cGVHD share several features, including B-cell dysregulation (16, 26, 28), with a high prevalence of anti-nuclear auto-Abs (16, 29) as well as clinical manifestations of autoimmune diseases (16, 30), such as scleroderma. In addition, certain ongoing clinical forms of human cGVHD can be treated by B-cell depletion (31–33) as also described in murine model (16) and in the treatment of some human autoimmune diseases (34). A well-described murine model of cGVHD consisting in administration of parental T cells to F1 mice showed that cGVHD occurrence resulting from auto-Ab mediated injury required host B cell activation and Th2 cytokine secretion (16, 35). In contrast, activation of donor CD8+ CTL in the context of Th1 cytokine production eradicated self-reactive B cells rather leading to acute GVHD (16, 35, 36). In this model, in contrast to the clinical situation, acute or chronic GVHD occurs in the same delay after transplantation (depending on T cell polarization) and no BM was infused (16, 35, 36). Here, we used a different model in which fully major histocompatibility complex-mismatched BM containing approximately 2% T cells was administrated and cGVHD-like lesions appeared several months after allograft. We found a correlation between host B cell persistence, elevated levels of auto-Abs (mostly from host origin) and of total circulating IgG as well as cGVHD occurrence, since only mixed chimeric mice with dissociated donor T-cell and recipient B-cell chimerism develop cGVHD-like lesions. These observations fit well with the parental T cell transfer into F1 mice model (16, 35, 36). Increasing percentage of splenic activated CD4+ T cells and increased levels of total IgGs, in addition to IgG auto-Abs, strongly suggest a polyclonal activation of host B cells due to alloreactive conflict.
We could not correlate a cGVHD episode with a particular distribution of DC subset or origin. All mixed chimeric mice whatever their status (healthy or none) exhibited host as well as donor splenic myeloid and pDC (Table 2). This suggests that such cells do not influence cGVHD occurrence in our model. Nevertheless, autoreactive B cell activation may occur independently of DC in response to alloreactive donor T cell stimulation (16). Activated host B cells may be potent APC for indirect allorecognition (37). This may contribute to alloreactive donor T cell activation creating therefore an amplification loop.
There are some discrepancies with the studies reporting reciprocal host-graft tolerance in a murine model (24, 25). There main differences between this (24, 25) and our model include: recipient strain (BALB/c mice –in our study –which are prone to autoimmune disease and present an intrinsic type 2 cytokine bias, see Ref.38, 39), conditioning regimens (9.5 Gy lethal irradiation that ablate completely the host immune system (24, 25) vs. 6 Gy sublethal irradiation that transiently deplete circulating immune cells in our model) and BM graft (co-administration of a mixture of 2x107 donor and recipient BM cells (24, 25) vs. a restricted number of donor BM cells, 106). In these different 2 models, one can imagine that host B cell populations were totally different after BMT. Host immature B cells derived from host BM (i.e., B-2 and B-1b cells, Ref.40) can be preferentially found in conditions associating lethal conditioning regimens and rich donor plus host BM graft. In contrast, host mature B –B-1a cells– or long-lived plasma cells that resist to γ-irradiation (i.e., RIC regimens) (41, 42) are observed after partial donor engraftment. The persistence of non dividing long-lived plasma despite cyclophosphamide treatment is responsible from anti-dsDNA auto-Ab production in NZB/NZW lupus prone mice (43), demonstrating the role of these long-lived plasma cells in auto-Ab production. Preliminary results suggested an increased number of B-1a cells in mice developing cGVHD-like lesion occurrence: 10-fold more splenic B-1a cells were found in mixed chimeric mice developing cGVHD than healthy mixed chimeric, non-chimeric or full-donor chimeric mice. Together with the absence of donor B cells in such recipients developing cGVHD-like lesions, excess of B-1a cells suggests that insufficient donor B cell engraftment can be implicated in the development of such lesions. One may hypothesize that continuous activation of residual host mature B cells during the alloreactive conflict results in increased auto-Ab production leading to autoimmune disease.
The hypothesis of altered immune reconstitution in mixed chimeric mice presenting cGVHD-like lesions is also sustained by absolute count determination of circulating lymphocyte subsets. Mixed chimeric mice developing cGVHD exhibited severe lymphopenia 8 weeks post-BMT when compared with healthy mixed chimeric mice (52 [18–71] (median [range] vs. 447 [47–2686] CD19+ cells/μl; 65 [51–562] vs. 837 [284–2638] CD3+ cells/μl, n=6 mice/group). In addition, these mice developing cGVHD showed evidence of a CD4/CD8 imbalance. However, it was difficult to ascertain whether such altered immune reconstitution was due to GVHD or was responsible for GVHD, as GVHD may lead to lymphopenia (16).
The relation between mixed chimerism, persistence of host B cells and cGVHD after RICT in humans is presently difficult to assess. Detailed clinical and chimerism data is scarce. In addition, several confounding factors such as the type of RIC regimen (i.e., with or without anti-lymphocyte globulin, affecting B cells or not), the presence or absence of acute GVHD or the use of donor lymphocyte infusions in the presence of mixed chimerism render data interpretation difficult. However, on the basis of a study involving 12 patients, full donor B-cell chimerism was delayed when compared to full donor T-cell chimerism (44). Moreover, although B cell chimerism was not directly addressed, cGVHD occurrence in 2 patients with persisting mixed lymphoid chimerism was reported (45). Although a study involving a myeloablative conditioning regimen failed to find an association between circulating auto-Abs and cGVHD (46), auto-Abs, including lupus anticoagulant and AcL Abs, have been described in patients developing cGVHD (47, 48). Finally, a recent interesting clinical report shows that host-derived Abs persist much longer in the setting of RICT than after myeloablative allografts (49). These authors reported recently that this was due to the persistence of host plasma cells that resist to RIC regimen associating cyclophosphamide and fludarabine (50). In the 12 patients analyzed, host plasma cells detected in the bone marrow persisted whereas all T cells were from donor origin. Disappearance of recipient plasma cells was correlated with decrease of anti-donor Ab titers (50). Overall, our results suggest that mixed chimerism after RICT could be associated with recipient B cell persistence, increased auto-immunity and cGVHD. Such a novel association could be clinically highly relevant and could suggest that mixed chimerism is not always associated with bi-directional tolerance. In addition, B-cell chimerism monitoring may contribute to cGVHD prevention by anticipating appropriate immunosuppression.
Acknowledgments
We also thank Amandine Radlovic, Magali Leuvrey and Christelle Personeni for their excellent technical assistance, Patricia Leiby and Thierry Martin (Strasbourg, France) for helpful advice concerning anti-cardiolipin antibody detection, Luc Reininger (Marseille, France) for reagent and helpful discussion, Marcelo de Carvalho Bittencourt (Fort de France, France) for critical reading of the manuscript, Patrick Hervé, Estelle Seilles, Charles Pellegrinelli (Besançon, France) for their support and Jackie Kerveillant for her help in preparing this manuscript.
Abbreviations
- Ab
antibody
- AcL
anti-cardiolipin
- Ag
antigen
- APC
Antigen-presenting cell
- BM
bone marrow
- BMT
bone marrow transplantation
- cGVHD
chronic graft-versus-host disease
- DC
dendritic cell
- ds
double stranded
- FCS
fetal calf serum
- FSC
forward scatter
- GVHD
graft-versus-host disease
- GvL
graft-versus-leukemia
- HC
hematopoietic cell
- HES
hematoxylin eosin saffron
- IC
immune complexes
- mAb
monoclonal antibody
- MT
Masson’s trichrome
- OM
original magnification
- PAS
periodic acid Schiff
- PBS
phosphate buffer saline
- pDC
plasmacytoid dendritic cell
- PerCP
peridinin chlorophyll-a protein
- PE
phycoerythrin
- RIC
reduced intensity conditioning
- RICT
reduced intensity conditioning transplantation
- SSC
side scatter
- TMB
3,3′,5,5′-tetramethylbenzidine
Footnotes
This work was supported by grants from the Association pour la Recherche sur le Cancer (#4508), the Comité Départemental de la Ligue contre le Cancer du Jura - Comité de Besançon, the Conseil Régional de Franche-Comté and the Etablissement Francais du Sang (Appel d’offres 2004). SP and FK received financial support from the Association pour la Recherche sur le Cancer and from Inserm (poste CCA), respectively.
References
- 1.McSweeney PA, Niederwieser D, Shizuru JA, et al. Hematopoietic cell transplantation in older patients with hematologic malignancies: replacing high-dose cytotoxic therapy with graft-versus-tumor effects. Blood. 2001;97:3390. doi: 10.1182/blood.v97.11.3390. [DOI] [PubMed] [Google Scholar]
- 2.Khouri IF, Saliba RM, Giralt SA, et al. Nonablative allogeneic hematopoietic transplantation as adoptive immunotherapy for indolent lymphoma: low incidence of toxicity, acute graft-versus-host disease, and treatment-related mortality. Blood. 2001;98:3595. doi: 10.1182/blood.v98.13.3595. [DOI] [PubMed] [Google Scholar]
- 3.Martino R, Caballero MD, Simon JA, et al. Evidence for a graft-versus-leukemia effect after allogeneic peripheral blood stem cell transplantation with reduced-intensity conditioning in acute myelogenous leukemia and myelodysplastic syndromes. Blood. 2002;100:2243. doi: 10.1182/blood-2002-02-0400. [DOI] [PubMed] [Google Scholar]
- 4.Mielcarek M, Martin PJ, Leisenring W, et al. Graft-versus-host disease after nonmyeloablative versus conventional hematopoietic stem cell transplantation. Blood. 2003;102:756. doi: 10.1182/blood-2002-08-2628. [DOI] [PubMed] [Google Scholar]
- 5.Sykes M. Mixed chimerism and transplant tolerance. Immunity. 2001;14:417. doi: 10.1016/s1074-7613(01)00122-4. [DOI] [PubMed] [Google Scholar]
- 6.Ildstad ST, Sachs DH. Reconstitution with syngeneic plus allogeneic or xenogeneic bone marrow leads to specific acceptance of allografts or xenografts. Nature. 1984;307:168. doi: 10.1038/307168a0. [DOI] [PubMed] [Google Scholar]
- 7.Mapara MY, Kim YM, Wang SP, Bronson R, Sachs DH, Sykes M. Donor lymphocyte infusions mediate superior graft-versus-leukemia effects in mixed compared to fully allogeneic chimeras: a critical role for host antigen-presenting cells. Blood. 2002;100:1903. doi: 10.1182/blood-2002-01-0023. [DOI] [PubMed] [Google Scholar]
- 8.Fresnay S, Garnache-Ottou F, Plumas J, Seilles E, Tiberghien P, Saas P. Can tolerogenic dendritic cells help to modulate allo-immune responses in the setting of hematopoietic cell transplantation? Transpl Immunol. 2003;11:259. doi: 10.1016/S0966-3274(03)00053-4. [DOI] [PubMed] [Google Scholar]
- 9.Shlomchik WD, Couzens MS, Tang CB, et al. Prevention of graft versus host disease by inactivation of host antigen-presenting cells. Science. 1999;285:412. doi: 10.1126/science.285.5426.412. [DOI] [PubMed] [Google Scholar]
- 10.Duffner UA, Maeda Y, Cooke KR, et al. Host dendritic cells alone are sufficient to initiate acute graft-versus-host disease. J Immunol. 2004;172:7393. doi: 10.4049/jimmunol.172.12.7393. [DOI] [PubMed] [Google Scholar]
- 11.Bittencourt MC, Perruche S, Contassot E, et al. Intravenous injection of apoptotic leukocytes enhances bone marrow engraftment across major histocompatibility barriers. Blood. 2001;98:224. doi: 10.1182/blood.v98.1.224. [DOI] [PubMed] [Google Scholar]
- 12.Perruche S, Kleinclauss F, Lienard A, Robinet E, Tiberghien P, Saas P. A single-platform approach using flow cytometry and microbeads to evaluate immune reconstitution in mice after bone marrow transplantation. J Immunol Methods. 2004;294:53. doi: 10.1016/j.jim.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 13.Perruche S, Kleinclauss F, Angonin R, et al. A single intravenous infusion of apoptotic cells, an alternative cell-based therapy approach facilitating hematopoietic cell engraftment, did not induce autoimmunity. J Hematother Stem Cell Res. 2003;12:451. doi: 10.1089/152581603322286088. [DOI] [PubMed] [Google Scholar]
- 14.Cohen PL, Maldonado MA. Animals models for SLE. In: Coligan JEKA, Margulies DH, Shevach EM, Strober W, Coico R, editors. Current Protocols in Immunology. New York, NY: John Wiley & Sons Inc; 1998. p. 15.20.7. [Google Scholar]
- 15.Cooke KR, Kobzik L, Martin TR, et al. An experimental model of idiopathic pneumonia syndrome after bone marrow transplantation: I. The roles of minor H antigens and endotoxin. Blood. 1996;88:3230. [PubMed] [Google Scholar]
- 16.Hakim F, Fowler DH, Shearer GM, Gress RE. Animal models of acute and chronic graft-versus-host disease. In: Coligan JEKA, Margulies DH, Shevach EM, Strober W, Coico R, editors. Current Protocols in Immunology. New York, NY: John Wiley & Sons Inc; 1998. p. 4.3.1. [Google Scholar]
- 17.Hahn BH. Antibodies to DNA. N Engl J Med. 1998;338:1359. doi: 10.1056/NEJM199805073381906. [DOI] [PubMed] [Google Scholar]
- 18.Levine JS, Subang R, Koh JS, Rauch J. Induction of anti-phospholipid autoantibodies by beta2-glycoprotein I bound to apoptotic thymocytes. J Autoimmun. 1998;11:413. doi: 10.1006/jaut.1998.0235. [DOI] [PubMed] [Google Scholar]
- 19.Parry SL, Hall FC, Olson J, Kamradt T, Sonderstrup G. Autoreactivity versus autoaggression: a different perspective on human autoantigens. Curr Opin Immunol. 1998;10:663. doi: 10.1016/s0952-7915(98)80086-1. [DOI] [PubMed] [Google Scholar]
- 20.Brocker T, Riedinger M, Karjalainen K. Targeted expression of major histocompatibility complex (MHC) class II molecules demonstrates that dendritic cells can induce negative but not positive selection of thymocytes in vivo. J Exp Med. 1997;185:541. doi: 10.1084/jem.185.3.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clark FJ, Freeman L, Dzionek A, et al. Origin and subset distribution of peripheral blood dendritic cells in patients with chronic graft-versus-host disease. Transplantation. 2003;75:221. doi: 10.1097/01.TP.0000041783.34083.11. [DOI] [PubMed] [Google Scholar]
- 22.Ferrero I, Held W, Wilson A, Tacchini-Cottier F, Radtke F, MacDonald HR. Mouse CD11c(+) B220(+) Gr1(+) plasmacytoid dendritic cells develop independently of the T-cell lineage. Blood. 2002;100:2852. doi: 10.1182/blood-2002-01-0214. [DOI] [PubMed] [Google Scholar]
- 23.Martin P, Del Hoyo GM, Anjuere F, et al. Characterization of a new subpopulation of mouse CD8alpha+ B220+ dendritic cells endowed with type 1 interferon production capacity and tolerogenic potential. Blood. 2002;100:383. doi: 10.1182/blood.v100.2.383. [DOI] [PubMed] [Google Scholar]
- 24.Ildstad ST, Wren SM, Bluestone JA, Barbieri SA, Stephany D, Sachs DH. Effect of selective T cell depletion of host and/or donor bone marrow on lymphopoietic repopulation, tolerance, and graft-vs-host disease in mixed allogeneic chimeras (B10 + B10. D2----B10) J Immunol. 1986;136:28. [PubMed] [Google Scholar]
- 25.Sykes M, Eisenthal A, Sachs DH. Mechanism of protection from graft-vs-host disease in murine mixed allogeneic chimeras. I. Development of a null cell population suppressive of cell-mediated lympholysis responses and derived from the syngeneic bone marrow component. J Immunol. 1988;140:2903. [PubMed] [Google Scholar]
- 26.Ferrara JL, Deeg HJ. Graft-versus-host disease. N Engl J Med. 1991;324:667. doi: 10.1056/NEJM199103073241005. [DOI] [PubMed] [Google Scholar]
- 27.Kamradt T, Mitchison NA. Tolerance and autoimmunity. N Engl J Med. 2001;344:655. doi: 10.1056/NEJM200103013440907. [DOI] [PubMed] [Google Scholar]
- 28.Lee SJ, Vogelsang G, Flowers ME. Chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2003;9:215. doi: 10.1053/bbmt.2003.50026. [DOI] [PubMed] [Google Scholar]
- 29.Kier P, Penner E, Bakos S, et al. Autoantibodies in chronic GVHD: high prevalence of antinucleolar antibodies. Bone Marrow Transplant. 1990;6:93. [PubMed] [Google Scholar]
- 30.Sullivan KM, Agura E, Anasetti C, et al. Chronic graft-versus-host disease and other late complications of bone marrow transplantation. Semin Hematol. 1991;28:250. [PubMed] [Google Scholar]
- 31.Ratanatharathorn V, Ayash L, Reynolds C, et al. Treatment of chronic graft-versus-host disease with anti-CD20 chimeric monoclonal antibody. Biol Blood Marrow Transplant. 2003;9:505. doi: 10.1016/s1083-8791(03)00216-7. [DOI] [PubMed] [Google Scholar]
- 32.Shimoni A, Hardan I, Avigdor A, et al. Rituximab reduces relapse risk after allogeneic and autologous stem cell transplantation in patients with high-risk aggressive non-Hodgkin’s lymphoma. Br J Haematol. 2003;122:457. doi: 10.1046/j.1365-2141.2003.04446.x. [DOI] [PubMed] [Google Scholar]
- 33.Canninga-van Dijk MR, van der Straaten HM, Fijnheer R, Sanders CJ, van den Tweel JG, Verdonck LF. Anti-CD20 monoclonal antibody treatment in 6 patients with therapy-refractory chronic graft-versus-host disease. Blood. 2004;104:2603. doi: 10.1182/blood-2004-05-1855. [DOI] [PubMed] [Google Scholar]
- 34.Martin F, Chan AC. Pathogenic roles of B cells in human autoimmunity; insights from the clinic. Immunity. 2004;20:517. doi: 10.1016/s1074-7613(04)00112-8. [DOI] [PubMed] [Google Scholar]
- 35.Rus V, Svetic A, Nguyen P, Gause WC, Via CS. Kinetics of Th1 and Th2 cytokine production during the early course of acute and chronic murine graft-versus-host disease. Regulatory role of donor CD8+ T cells. J Immunol. 1995;155:2396. [PubMed] [Google Scholar]
- 36.Shustov A, Luzina I, Nguyen P, et al. Role of perforin in controlling B-cell hyperactivity and humoral autoimmunity. J Clin Invest. 2000;106:R39. doi: 10.1172/JCI8876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schultz KR, Paquet J, Bader S, HayGlass KT. Requirement for B cells in T cell priming to minor histocompatibility antigens and development of graft-versus-host disease. Bone Marrow Transplant. 1995;16:289. [PubMed] [Google Scholar]
- 38.Hequet O, Vocanson M, Saint-Mezard P, Kaiserlian D, Nicolas JF, Berard F. CD4+ T cells prevent skin autoimmunity during chronic autologous graft-versus-host-disease. Am J Transplant. 2004;4:872. doi: 10.1111/j.1600-6143.2004.00439.x. [DOI] [PubMed] [Google Scholar]
- 39.Bix M, Wang ZE, Thiel B, Schork NJ, Locksley RM. Genetic regulation of commitment to interleukin 4 production by a CD4(+) T cell-intrinsic mechanism. J Exp Med. 1998;188:2289. doi: 10.1084/jem.188.12.2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kantor AB, Stall AM, Adams S, Herzenberg LA, Herzenberg LA. Differential development of progenitor activity for three B-cell lineages. Proc Natl Acad Sci U S A. 1992;89:3320. doi: 10.1073/pnas.89.8.3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hayakawa K, Asano M, Shinton SA, et al. Positive selection of natural autoreactive B cells. Science. 1999;285:113. doi: 10.1126/science.285.5424.113. [DOI] [PubMed] [Google Scholar]
- 42.Manz RA, Arce S, Cassese G, Hauser AE, Hiepe F, Radbruch A. Humoral immunity and long-lived plasma cells. Curr Opin Immunol. 2002;14:517. doi: 10.1016/s0952-7915(02)00356-4. [DOI] [PubMed] [Google Scholar]
- 43.Hoyer BF, Moser K, Hauser AE, et al. Short-lived plasmablasts and long-lived plasma cells contribute to chronic humoral autoimmunity in NZB/W mice. J Exp Med. 2004;199:1577. doi: 10.1084/jem.20040168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Childs R, Clave E, Contentin N, et al. Engraftment kinetics after nonmyeloablative allogeneic peripheral blood stem cell transplantation: full donor T-cell chimerism precedes alloimmune responses. Blood. 1999;94:3234. [PubMed] [Google Scholar]
- 45.Peggs KS, Thomson K, Hart DP, et al. Dose-escalated donor lymphocyte infusions following reduced intensity transplantation: toxicity, chimerism, and disease responses. Blood. 2004;103:1548. doi: 10.1182/blood-2003-05-1513. [DOI] [PubMed] [Google Scholar]
- 46.Rouquette-Gally AM, Boyeldieu D, Prost AC, Gluckman E. Autoimmunity after allogeneic bone marrow transplantation. A study of 53 long-term-surviving patients. Transplantation. 1988;46:238. doi: 10.1097/00007890-198808000-00010. [DOI] [PubMed] [Google Scholar]
- 47.Sanmarco M, Vialettes B, Maraninchi D, Bernard D. Autoantibody formation after bone marrow transplantation: a comparison between autologous and allogeneic grafts. Autoimmunity. 1991;11:712. doi: 10.3109/08916939108994702. [DOI] [PubMed] [Google Scholar]
- 48.Quaranta S, Shulman H, Ahmed A, et al. Autoantibodies in human chronic graft-versus-host disease after hematopoietic cell transplantation. Clin Immunol. 1999;91:106. doi: 10.1006/clim.1998.4666. [DOI] [PubMed] [Google Scholar]
- 49.Bolan CD, Leitman SF, Griffith LM, et al. Delayed donor red cell chimerism and pure red cell aplasia following major ABO-incompatible nonmyeloablative hematopoietic stem cell transplantation. Blood. 2001;98:1687. doi: 10.1182/blood.v98.6.1687. [DOI] [PubMed] [Google Scholar]
- 50.Griffith LM, McCoy JP, Jr, Bolan CD, et al. Persistence of recipient plasma cells and anti-donor isohaemagglutinins in patients with delayed donor erythropoiesis after major ABO incompatible non-myeloablative haematopoietic cell transplantation. Br J Haematol. 2005;128:668. doi: 10.1111/j.1365-2141.2005.05364.x. [DOI] [PubMed] [Google Scholar]


