Abstract
The study aim was to compare sleep, sleepiness, fatigue, and neurobehavioral performance among first-time mothers and fathers during their early postpartum period. Participants were 21 first-time postpartum mother-father dyads (N=42) and seven childless control dyads (N=14). Within their natural environment, participants completed one week of wrist actigraphy monitoring, along with multi-day self-administered sleepiness, fatigue, and neurobehavioral performance measures. The assessment week was followed by an objective laboratory based test of sleepiness. Mothers obtained more sleep compared to fathers, but mothers’ sleep was more disturbed by awakenings. Fathers had greater objectively measured sleepiness than mothers. Mothers and fathers did not differ on subjectively measured sleep quality, sleepiness, or fatigue; however, mothers had worse neurobehavioral performance than fathers. Compared to control dyads, postpartum parents experienced greater sleep disturbance, sleepiness, and sleepiness associated impairments. Study results inform social policy, postpartum sleep interventions, and research on postpartum family systems and mechanisms that propagate sleepiness.
Keywords: mother, father, couples, newborn, family, postpartum, sleep, disturbance, impairment, fatigue, neurobehavioral performance
The Centers for Disease Control and Prevention Statistics reported that across the last decade, there have been over four million childbirths each year in the United States (Martin et al., 2011). With the birth of each child, new parents are required to adjust to the demands of parenthood. Accordingly, sleep disturbance is commonly experienced among new parents during the early postpartum period, and sleep disturbance is known to have a multitude of adverse effects on health and functioning (see reviews: Alvarez & Ayas, 2004; Copinschi, 2005; Harrison & Horne, 2000).
Postpartum sleep disturbance is caused by factors that include infant signaling during nocturnal periods (Nishihara, Horiuchi, Eto, & Uchida, 2000; Nishihara, Horiuchi, Eto, & Uchida, 2001), maternal postpartum endocrine and physiological changes that affect sleep (Manber & Armitage, 1999; Santiago, Nolledo, Kinzler, & Santiago, 2001), and the sleep disturbances experienced by the other bed partner within the postpartum parent dyad (Meijer & vanden Wittenboer, 2007). Sleep within the family system can be interrupted by multiple factors, all of which result in sleep disturbance experienced by both mothers and fathers.
Maternal postpartum sleep profiles have been well described (see reviews: Ross, Murray, & Steiner, 2005; Hunter, Rychnovsky, & Yount, 2009). Maternal sleep worsens progressively throughout pregnancy and is most affected, primarily by sleep fragmentation immediately following delivery after which it improves steadily throughout the postpartum period (Shinkoda, Matsumoto, & Park, 1999; Kang, Matsumoto, Shinkoda, Mishima, & Seo, 2002; Rychnovsky & Hunter, 2009; Montgomery-Downs, Insana, Clegg-Kraynok, & Mancini, 2010). Paternal sleep profiles during the postpartum period have been less extensively profiled. Among the existing literature, there are reported changes in postpartum fathers’ sleep from the prenatal to the postpartum period (Condone, Boyce, & Corkindale, 2004). Gay and colleagues reported that both mothers and fathers had less sleep, more self-reported sleep disturbance, and higher ratings of fatigue during the first month postpartum when compared to their levels during the pregnancy period (Gay, Lee, & Lee, 2004).
Although postpartum maternal, and to a lesser extent, paternal sleep disturbances are recognized, to date no investigations have objectively quantified the extent of sleepiness, and functional impact of sleep disturbance experienced among postpartum mothers or fathers. The study objective was to fill this void through examination of sleep, sleepiness, fatigue, and neurobehavioral performance among healthy first-time mothers and fathers during their early postpartum period. All values were compared within postpartum couples, and between postpartum and childless control couples.
METHOD
Participants
The study was approved by the West Virginia University Office of Research Compliance (Institutional Review Board). Participants provided informed consent and Health Insurance Portability and Accountability Act (1996) authorization prior to participation. Participants included cohabitating primiparous (n = 21 [postpartum]) and nulliparous (n = 7 [childless]) control couples. Postpartum couples were recruited from a larger laboratory study of normative maternal postpartum sleep (Montgomery-Downs et al., 2010). For the current study, nulliparous couples were recruited via community advertisements. Potential postpartum and control participant couples were excluded from participation on the basis of a history of major depressive or anxiety disorder, a score ≥ 16 on the Center for Epidemiological Studies of Depression (Radloff, 1977), or prior diagnosis of a sleep disorder on the part of either partner in the couple. Postpartum couples were excluded if the mother was pregnant with multiples, had a premature delivery, or if the infant was admitted to the neonatal intensive care unit.
Postpartum and control sample characteristics are shown on Table 1. Postpartum mothers and control women did not differ in age or education (F [1, 25] = .24, p = .63; F [1, 25] = 1.21, p = .28, respectively). Postpartum fathers and control men did not differ in age (F [1, 26] = .46, p = .51), but control men obtained more education than postpartum fathers (F [1, 26] = 3.42, p = .08). Control women and men did not differ from each other on any study variable (range: p = .102–.95).
Table 1.
Postpartum and control sample characteristics during study participation
| Mothers (n = 21) | Fathers (n = 21) | Women (n = 7) | Men (n = 7) | |||||
| Characteristic | M | SD | M | SD | M | SD | M | SD |
| Age (years) | 26.8 | 4.8 | 29.1 | 5.1 | 25.8 | 3.5 | 27.7 | 3.2 |
| Education (years) | 15.3 | 3.5 | 15.7 | 3.8 | 17 | 3.0 | 18.7 | 3.6 |
| Annual Household Income (thousand) | $56 | $34 | (same) | (same) | $52 | $39 | (same) | (same) |
| Characteristic | n | % | n | % | n | % | n | % |
| White | 19 | 90.5 | 20 | 95.2 | 7 | 100 | 7 | 100 |
| Black or Multiracial | 2 | 9.5 | 1 | 4.8 | - | - | - | - |
| *Family leave | 12 | 57.1 | 1 | 4.8 | - | - | - | - |
| *Fulltime | 1 | 4.8 | 15 | 71.4 | 6 | 85.7 | 7 | 100 |
| *Part-time | 2 | 9.5 | 4 | 19.1 | 1 | 14.3 | 0 | 0 |
| *Unemployed | 6 | 28.6 | 1 | 4.8 | - | - | - | - |
| Infant Characteristics | n | % | M | SD | - | - | - | - |
| Age at birth (weeks) | - | - | 39.6 | 1.1 | - | - | - | - |
| Age during study (weeks) | - | - | 6.9 | 1.3 | - | - | - | - |
| †Vaginal | 14 | 66.6 | - | - | - | - | - | - |
| †C-section | 7 | 33.3 | - | - | - | - | - | - |
| ‡Breast | 13 | 61.9 | - | - | - | - | - | - |
| ‡Formula | 3 | 14.3 | - | - | - | - | - | - |
| ‡Breast & Formula | 5 | 23.8 | - | - | - | - | - | - |
Note. One woman did not provide her age or education. Postpartum mothers were significantly younger than fathers (F [1, 20] = 5.01, p = .04, d = .46); they did not differ on years of education (p = .38). Control women were significantly younger than control men (F [1, 5] = 4.82, p = .08, d = .56); they did not differ on years of education (p = .13).
= work status;
= delivery method;
= feeding method
Measures
Actigraphy
Sleep was objectively estimated using continuous, nonintrusive activity monitoring recorded with Mini Mitter’s Actiwatch-64 (AW-64) actigraphs (Respironics; Bend, OR). The highest sampling resolution of 15-second epochs was used.
Sleep periods on the actigram output were identified (Acebo & LeBourgeois, 2006) using an electronic sleep diary (Palm Zire 72 personal digital assistants [PDA]) with customized software (Bruner Consulting Co.; Longmont, Colorado). The beginning of a sleep period was identified as the first of two consecutive minutes of actigraphically identified inactivity that followed the sleep diary-reported ‘bedtime’. The end of a sleep period was identified as the last of two consecutive minutes of actigraphically identified inactivity that preceded the diary-reported ‘wake time’. Participants asked to report ‘bedtime’ but not their precise ‘lights-off’ moment. Therefore, absolute time-in-bed and corresponding measures (e.g., sleep onset latency) could not be reported.
Sleep periods were analyzed with Actiware Software Version 5.5 (Respironics; Bend, Oregon) using the default ‘wake threshold value’ parameter setting = 40 (medium). Variables that were calculated within the sleep period included: (1) twenty-four hour sleep time (number of minutes identified as sleep during a 24-hour period [including daytime naps]), (2) total sleep time (number minutes identified as sleep during a nocturnal sleep period), (3) sleep fragmentation (index of movement during sleep during a nocturnal sleep period), (4) wake after sleep onset (number of minutes identified as wake during nocturnal sleep period), and (5) sleep efficiency (or percentage of time spent asleep, was the minutes of sleep during a nocturnal sleep period divided by the minutes in the sleep period, multiplied by 100). Wake after sleep onset was the absolute time awake during the sleep period, whereas sleep efficiency was the standardized percentage of wakefulness during the sleep period. Participants provided 7.5 (SD = 0.9) days of actigraphy data.
Pittsburgh Sleep Quality Index
Subjective sleep quality from the previous month was examined with the Pittsburgh Sleep Quality Index (PSQI) (Buysse, Reynolds, Monk, Berman, & Kupfer, 1989). The PSQI is comprised of 19 items that yield seven component scores; component scores are summed to create a total score. The total score can range from 0 (best) to 21 (worst); scores ≤ 5 are associated with good sleep quality whereas scores > 5 are associated with poor sleep quality. The PSQI sensitivity and specificity are 89.6% and 86.5%, respectively (Buysse et al., 1989). Although not designed specifically for use among postpartum parents, the PSQI has been used among women to study population-based postpartum samples (a. Dørheim, Bondevik, Eberhard-Gran, & Bjorvatn, 2009), culturally diverse postpartum samples (Li et al., 2011), and postpartum depression (b. Dørheim, Bondevik, Eberhard-Gran, & Bjorvatn, 2009; Okun, Hanusa, Hall, & Wisner, 2009; Okun, Luther, Prather, Perel, Wisniewski, & Wisner, 2011); it has also been used among caregiving fathers (Shaki, Goldbart, Daniel, Fraser, & Shorer, 2011).
Multiple Sleep Latency Test
Sleepiness was objectively measured using the four-nap Multiple Sleep Latency Test (MSLT) (Carskadon & Dement, 1977) that was conducted in the sleep research laboratory at West Virginia University. The polysomnography (PSG) montage included four channels of electroencephalography (C3/M2, C4/M1, O1/M2, and O2/M1), bilateral electrooculography, submental electromyography, and electrocardiography (American Academy of Sleep Medicine, 2007). PSG recordings were made with the Embla N7000 system (Medcare; Broomfield, Colorado); data were managed, analyzed, and archived with Rembrandt software (Medcare; Broomfield, Colorado).
MSLT data were visually scored according to the AASM scoring criteria (American Academy of Sleep Medicine, 2007). Sleep onset was identified as the first of three consecutive 30-second epochs that were unequivocally scored as sleep. Sleep onset latency (SOL) was scored as the time from the initiation of the MSLT trial (“lights out”) to sleep onset. The four SOL nap scores were averaged to yield the MSLT score with a possible range from 0 (greatest sleepiness) – 20 (least sleepiness) minutes. The MSLT demonstrates a test-retest reliability of .97 within a normative sample (Littner et al., 2005). MSLT scores ≤ 5 minutes indicate pathological daytime sleepiness, > 5 to 10 minutes indicate moderate sleepiness, > 10 to 15 minutes indicate mild sleepiness, and >15 minutes indicate normative levels of sleepiness (Richardson et al., 1978; Thorpy et al., 1992).
Neurobehavioral Performance
Neurobehavioral performance was objectively measured using the psychomotor vigilance test (PVT) that was self-administered each morning using customized software on the PDA (Bruner Consulting Co.; Longmont, Colorado). This PVT is similar to a previously validated version of a Palm-based PVT (Thorne, Johnson, Redmond, Sing, & Belenky, 2005) and its use is supported by a study that validated PVT administrations that were <10 minutes in duration (Loh, Lamond, Dorrian, Roach, & Dawson, 2004). This PVT has been reported previously (Neylan et al., 2010) as a simple reaction time task developed to measure sustained attention using a bull’s-eye stimulus. Each 5-minute trial consists of the presentation of approximately 39–56 stimuli at random interstimulus intervals and has a 10 ms sensitivity resolution. Lapse frequency (reaction times ≥ 500 ms) is an outcome variable for the PVT (Basner & Dinges, 2011). Greater lapse frequency values are associated with greater performance impairment. Postpartum participants and control couples provided 7.2 (SD = 1.4), and 7.1 (SD = 0.5) days of PVT data, respectively.
Subjective sleepiness
Subjective sleepiness was measured on the PDA with the Stanford Sleepiness Scale (SSS). The SSS ranges from 1–7 and higher scores represent greater sleepiness (Hoddes, Zarcone, Smythe, Phillips, & Dement, 1973). A SSS score ≥ 3 obtained during times other than sleep onset indicates significant sleep debt (Hoddes et al., 1973). The SSS has been used previously among samples of healthy postpartum women (Groër et al., 2004; Insana & Montgomery-Downs, 2010). Postpartum participants self-administered the computerized SSS an average of 3.2 (SD = 0.7) times each day to provide 7.3 (SD = 1.4) days of PDA based data. Control participants self-administered the computerized SSS an average of 2.8 (SD = 0.5) times each day to provide 6.9 (SD = 0.8) days of PDA based data.
Fatigue
Subjective fatigue was measured on the PDA with the 100-point Visual Analogue of Fatigue Scale (VAFS) (Monk, 1989) that was used to rate, “How tired/fatigued do you feel RIGHT NOW?” (0 = not at all tired/fatigued, 100 = very tired/fatigued). The VAFS had a stylus-movable anchor point that was initially presented in the middle of the scale; the anchor could be moved according to 1-point increments on the display. An extended form of the VAFS has previously been used among samples of healthy postpartum women (Lee & Zaffke, 1999). Participants’ VAFS self-administration completion rate was the same as that for the SSS indicated above.
Procedure
Postpartum couples participated for one continuous week, between their third to eighth postpartum weeks (range: 3.6–8.6 weeks). Postpartum and control couples completed the same one-week protocol. During the protocol, both participants in each couple wore an actigraph continuously on their non-dominant wrist, and concurrently completed their PDA-based sleep diary in real-time. Participants were instructed to self-administer sleepiness and fatigue measures approximately four times throughout the day—mothers, every time they fed their baby. Participants were asked to complete the PVT each morning within two hours of awakening and before consuming caffeine; postpartum and control participants self-administered the PVT 76.7 (SD = 49.2), and 128.7 (SD = 210.4) minutes from wake, respectively.
Immediately following the one-week of actigraphy and daily assessments at home, each couple came to the sleep research laboratory approximately one hour after awakening for their MSLT. Postpartum parent nap opportunities were staggered so that one parent was able to care for their infant in a room adjacent to the sleep laboratory. During the MSLT day participants completed the PSQI. During the MSLT procedure all participants were provided lunch from a local restaurant. Following the study procedures, postpartum and control couples were provided a $100 and a $70 honorarium, respectively.
Statistical Analyses
SPSS 18.0 (SPSS Inc, Chicago, IL) was used for statistical analyses. A p < .05 was considered statistically significant for the within postpartum couple comparisons (i.e. postpartum mother and postpartum father differences). Due to the small control group sample size, a p < .10 was considered statistically significant for the between-group comparisons (e.g. postpartum mothers and control women differences) and control group within-group comparisons (i.e. control women and control men). Cohen’s d effect sizes (Cohen, 1988) were calculated for equal sample sizes (e.g., postpartum mothers-postpartum fathers), and Hedges g (Hedges, 1981) effect sizes were calculated for unequal sample sizes (e.g., postpartum mothers-control women). Effect sizes are interpreted according to standard convention (trivial = < .20; small = .20–.30; medium = >.30–.80; large = >.80) (Cohen, 1988). Medium to large effect sizes are emphasized when interpreting the results (Cohen, 1994). In accordance with the stated study aims, we only made direct comparisons and did not examine interactions that would yield irrelevant comparisons (e.g. postpartum mothers-control men). ANOVAs were calculated for the between-group comparisons because the groups were considered independent (e.g., postpartum mothers-control mothers). Repeated measures analyses of variance (ANOVA) were calculated for the within-couple comparisons because the groups were considered dependent (e.g., postpartum mothers-postpartum fathers).
Daily actigraphically measured sleep and PVT, as well as multi-daily SSS and VAF measures were each averaged within the study week to provide stable measures. Descriptive statistics were calculated for demographic and all study outcome measures. Missing data were handled via pairwise deletion to retain all available data for analysis.
Preliminary Analyses
For the MSLT analyses two mothers’ MSLT-SOL values, for one nap each, and one father’s full MSLT were excluded due to non-adherence to the research protocol and equipment malfunction, respectively. Thus sample sizes for MSLT analyses were: n = 21 postpartum mothers, n = 20 postpartum fathers (20 complete postpartum couples), and n = 7 complete control couples. For the actigraphy analyses one mothers’ and one fathers’ actigraphy data were excluded due to equipment malfunction and non-adherence to the research protocol, respectively. Thus, the sample sizes for actigraphy analyses were n = 20 mothers, n = 20 fathers (19 complete postpartum couples), and n = 7 complete control couples. For PVT comparisons one control couples’ data were excluded due to non-adherence to the research protocol. Thus, the full postpartum sample (n = 42 [21 complete couples]), n = 6 control women, and n = 6 control men (6 complete couples) were examined.
PSG sleep onset scoring was conducted with high internal reliability across all nap opportunities (Cronbach's α = .96, p < .001). Across postpartum and nulliparous samples, MSLT first nap opportunities occurred approximately three hours from sleep offset (Women, M = 207, SD = 45 minutes; Men, M = 196, SD = 47 minutes). A MSLT start time within three hours from awakening is in concordance with standard practice parameters (Littner et al., 2005).
Postpartum mothers’ 24-hour and nocturnal sleep time during the night prior to the MSLT was shorter than their previous one-week averages (p = .003, p < .001, respectively). However, the residuals between the night-prior and the week-prior sleep values were not significantly correlated with their MSLT scores (r = .18, r = .17, respectively). Control women’s WASO during the night prior to the MSLT was shorter than their previous one-week averages (p = .04), but the residuals between the night-prior and the week-prior were not associated with their MSLT scores (r = .02). No differences were found between the night- and week-prior to the MSLT within postpartum fathers, or control men. This assessment indicates that sleep from the night prior to the MSLT did not artificially drive the MSLT results, which is important to note considering the field-based nature of this study.
RESULTS
Sleep
Within couple comparisons on all postpartum actigraphically measured sleep variables are on Table 2. Mothers obtained more sleep (i.e. 24-hour sleep [d = 1.30, g = .87] and nocturnal sleep time [d = 1.43, g = .71]), but their sleep was also more disturbed (i.e. higher sleep fragmentation [d = 1.12, g = 1.93] and WASO [d = 1.19, g = 1.41]) compared to both fathers and control women, respectively. Fathers’ sleep efficiency was worse than control mens’ (d = 1.49).
Table 2.
Descriptive data and difference statistics within postpartum couples
| Mothers | Fathers | Difference Statistic | ||||||
| Variable | M | SD | M | SD | F | df | p | d |
| *24 hour sleep time | 424.8 | 42.0 | 375.1 | 34.1 | 17.31 | 1, 18 | < .001 | 1.30 |
| *Nocturnal sleep time | 412.8 | 39.3 | 366.7 | 37.3 | 1.06 | 1, 18 | < .001 | 1.43 |
| †Sleep Fragmentation | 18.9 | 3.3 | 14.3 | 4.6 | 13.17 | 1, 18 | .002 | 1.12 |
| *WASO | 88.3 | 29.6 | 55.6 | 25.0 | 21.41 | 1, 18 | < .001 | 1.19 |
| ‡Sleep efficiency | 77.8 | 6.1 | 79.6 | 6.9 | 1.11 | 1, 18 | .31 | 0.26 |
| PSQI | 7.0 | 3.4 | 8.8 | 3.7 | 3.60 | 1, 20 | .07 | 0.50 |
| MSLT-SOL | 11.8 | 4.6 | 8.0 | 3.9 | 11.85 | 1, 19 | .003 | 0.88 |
| PVT-Lapses | 11.6 | 8.5 | 6.7 | 5.3 | 11.25 | 1, 20 | .003 | 0.70 |
| SSS | 3.2 | 0.8 | 3.0 | 1.0 | 1.01 | 1, 20 | .33 | 0.23 |
| VAFS | 50.4 | 12.5 | 48.8 | 15.1 | 0.15 | 1, 20 | .71 | 0.11 |
Note. One father had missing MSLT data, and one mother and one father had missing actigraphy data. Notable differences are bolded.
= Minutes;
= index;
= percent;
- = value already reported in table.
WASO = Wake After Sleep Onset; PSQI = Pittsburgh Sleep Quality Index; MSLT = Multiple Sleep Latency Test; SOL = Sleep Onset Latency; PVT = Psychomotor Vigilance Test; RT = Reaction Time; SSS = Stanford Sleepiness Scale; ESS = Epworth Sleepiness Scale; VAFS = Visual Analogue of Fatigue Scale.
Within couple comparisons on subjective sleep quality are indicated on Table 2. Mothers and fathers did not differ on subjective sleep quality; neither did mothers and control women. Fathers reported worse sleep quality than control men (g = .91).
Objective Sleepiness
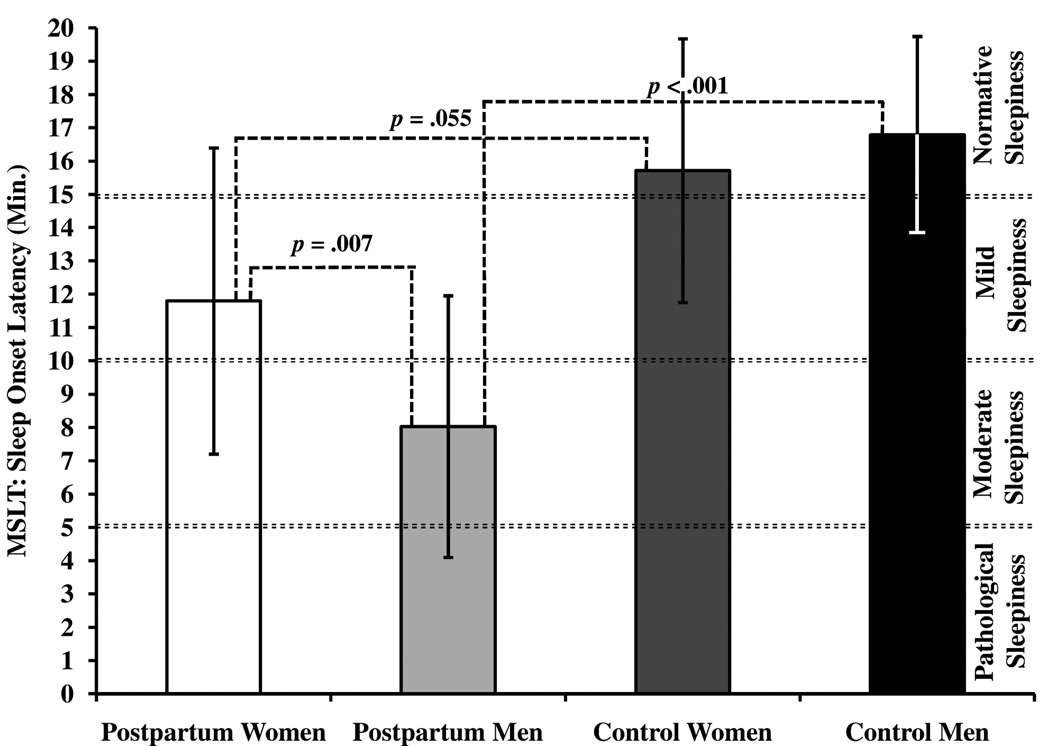
Among mothers, 10% were in the pathological sleepiness range, 29% were in the moderate sleepiness range, 42% were in the mild sleepiness range, and 19% were in the normative sleepiness range (SOL range: 3.1 – 18.6 minutes). Among fathers 25% were in the pathological sleepiness range, 50% were in the moderate sleepiness range, 20% were in the mild sleepiness range, and 5% were in the normative sleepiness range (SOL range: 3.6 – 20.0 minutes). Among control women, 43% were in the mild sleepiness range, and 57% were in the normative sleepiness range (SOL range: 10.3 – 20.0 minutes). Among control men, 29% were in the mild sleepiness range, and 71% were in the normative sleepiness range (SOL range: 11.8 – 20.0 minutes). Comparisons within postpartum couples’ MSLT scores are indicated on Table 2 and Figure 1. Postpartum within-dyad MSLT scores are shown on Supplement 1. Comparisons between postpartum mothers and control women, as well as between postpartum fathers and control men are indicated on Table 3. Postpartum mothers had significantly shorter sleep onset latency compared to control women (g = .85). Postpartum fathers had significantly shorter sleep onset latency than both postpartum mothers and control men (d = .88 and g = 2.29, respectively).
Figure 1.
MSLT sleepiness values compared within postpartum couples and between postpartum and control couples
Table 3.
Descriptive data and difference statistics between postpartum parents and control couples
| Mothers | Control Women | Difference Statistic | ||||||
| Variable | M | SD | M | SD | F | df | p | g |
| *24 hour sleep time | 426.1 | 41.3 | 388.0 | 45.8 | 4.18 | 1, 25 | .052 | 0.87 |
| *Nocturnal sleep time | 414.7 | 39.2 | 384.6 | 46.1 | 2.80 | 1, 25 | .107 | 0.71 |
| †Sleep Fragmentation | 18.9 | 3.3 | 12.1 | 3.7 | 20.61 | 1, 25 | < .001 | 1.93 |
| *WASO | 89.8 | 29.5 | 48.4 | 25.2 | 10.90 | 1, 25 | .003 | 1.41 |
| ‡Sleep efficiency | 77.8 | 6.0 | 89.1 | 4.4 | 21.02 | 1, 25 | < .001 | 1.95 |
| PSQI | 7.0 | 3.4 | 5.5 | 2.4 | 1.16 | 1, 26 | .29 | 0.46 |
| MSLT-SOL | 11.8 | 4.6 | 15.7 | 4.0 | 4.05 | 1, 26 | .055 | 0.85 |
| PVT-Lapses | 11.6 | 8.5 | 4.7 | 2.2 | 3.76 | 1, 25 | .06 | 0.87 |
| SSS | 3.2 | 0.8 | 2.6 | 0.5 | 3.55 | 1, 26 | .07 | 0.80 |
| VAFS | 50.4 | 12.5 | 37.4 | 18.7 | 4.35 | 1, 26 | .047 | 0.88 |
| Fathers | Control Men | Difference Statistic | ||||||
| Variable | M | SD | M | SD | F | df | p | g |
| *24 hour sleep time | 373.1 | 34.4 | 366.1 | 48.6 | .17 | 1, 25 | .68 | 0.18 |
| *Nocturnal sleep time | 365.1 | 37.0 | 366.1 | 48.6 | .003 | 1, 25 | .96 | 0.02 |
| †Sleep Fragmentation | 14.3 | 4.5 | 12.7 | 2.0 | .84 | 1, 25 | .37 | 0.39 |
| *WASO | 54.4 | 25.0 | 43.3 | 10.7 | 1.27 | 1, 25 | .27 | 0.48 |
| ‡Sleep efficiency | 80.0 | 7.0 | 89.5 | 2.7 | 12.19 | 1, 25 | .002 | 1.49 |
| PSQI | 8.8 | 3.7 | 5.6 | 2.2 | 4.56 | 1, 26 | .04 | 0.91 |
| MSLT-SOL | 8.0 | 3.9 | 16.8 | 3.0 | 28.81 | 1, 25 | < .001 | 2.29 |
| PVT-Lapses | 6.7 | 5.3 | 2.1 | 2.7 | 4.16 | 1, 25 | .052 | 0.92 |
| SSS | 3.0 | 1.0 | 2.8 | 0.6 | .44 | 1, 26 | .51 | 0.28 |
| VAFS | 48.8 | 15.1 | 48.2 | 14.3 | .009 | 1, 26 | .93 | 0.04 |
Note. One father had missing MSLT data, one mother and one father had missing actigraphy data, and one control woman and one control man had missing PVT data. Notable differences are bolded.
= Minutes;
= index;
= percent;
- = value already reported in table.
WASO = Wake After Sleep Onset; PSQI = Pittsburgh Sleep Quality Index; MSLT = Multiple Sleep Latency Test; SOL = Sleep Onset Latency; PVT = Psychomotor Vigilance Test; RT = Reaction Time; SSS = Stanford Sleepiness Scale; ESS = Epworth Sleepiness Scale; VAFS = Visual Analogue of Fatigue Scale.
Neurobehavioral Performance, and Subjective Sleepiness and Fatigue
Within couple comparisons on postpartum objective performance variables as well as subjective sleepiness and fatigue are indicated on Table 2. Postpartum mothers’ neurobehavioral performance was significantly more impaired than fathers (d = .70). Between couple comparisons indicated that both postpartum mothers and fathers had significantly worse neurobehavioral performance impairment compared to control women (g = .87) and men (g = .92), respectively. Postpartum mothers and fathers did not differ from each other on subjective sleepiness or fatigue reports. Postpartum mothers reported significantly worse subjective sleepiness (g = .80) and fatigue (g = .88) than control women; postpartum fathers did not differ from control men on any subjective sleepiness or fatigue reports.
Post-hoc Examination of Postpartum Experience
Among mothers, neither work status nor delivery method had a significant effect on any study variable. However, there was a main effect for infant feeding method on PVT lapse frequency (F [2, 18] = 4.83, p = .02). Tukey’s post-hoc analyses indicated that mothers who bottle fed (M = 22.7, SD = 7.2) had significantly more lapses than mothers who breast fed (M = 8.5, SD = 7.5, g = 1.80). Among fathers, neither feeding method nor work status had a significant effect on sleep or daytime functioning variables. Infant age during the study was not associated with any study variable among mothers or fathers.
DISCUSSION
The current study provides a description of the sleep, sleepiness, and daytime impairment experienced by new mothers and fathers during the early postpartum period, a time when many new parents return to work. Mothers obtained more sleep compared to fathers; however, mothers’ sleep was also more disturbed by awakenings. Both mothers and fathers experienced high levels of objectively measured sleepiness, while fathers experienced higher levels than mothers. Postpartum mothers and fathers did not differ on subjective sleep quality, sleepiness, or fatigue ratings, but mothers demonstrated worse neurobehavioral performance. Overall, both postpartum mothers and fathers experienced higher levels of sleep disturbance, sleepiness, and sleepiness associated impairments relative to control women and men, respectively.
The primary findings from this study describe the magnitude of sleepiness experienced by both postpartum mothers and fathers, and that fathers had a greater propensity to fall asleep during the MSLT than mothers. According to conventional MSLT interpretations, postpartum parents spanned all levels of sleepiness, but mothers primarily clustered in the ‘mild’ to ‘moderate’ range while fathers primarily clustered in the ‘moderate’ to ‘pathological’ range.
There are three primary explanations for why fathers had a higher propensity to fall asleep during the MSLT than mothers. First, longer total sleep time that mothers obtained may have partially countered the effects of their high sleep fragmentation, over and beyond the capacity for fathers’ consolidated sleep to counter the effects of their short sleep time. Although both parents experienced sleep disturbance (i.e. fragmentation and deprivation), mothers’ sleep profile may have permitted better recovery from their sleep disturbance than fathers’ sleep profile. Second, mothers’ sleep latency may have been influenced by worry about their infant’s safety or needs during her nap opportunities—despite the infant being under fathers’ care. Conversely, fathers may have interpreted MSLT nap opportunities as true opportunities to sleep because they perceived themselves as “off-duty.” A third possible explanation is that following birth postpartum mothers naturally develop a biological resistance to sleepiness, or heightened arousal. During the early postpartum period new mothers experience a multitude of physical and hormonal changes (Manber & Armitage, 1999; Gjerdingen, Froberg, Chaloner, & McGovern, 1993; Stremler & Wolfson, 2011), some of which may promote a lessened propensity to fall, or stay asleep. This postulation is supported by a report that when mothers slept away from their infants, maternal sleep disruption continued (Karacan, Williams, Hursch, McCaulley, & Heine, 1969).
Decreased propensity to fall asleep during the MSLT does not however translate into better physical or cognitive functioning. For instance, postpartum mothers demonstrated worse neurobehavioral performance than fathers, but fathers had a higher propensity to fall asleep during the MSLT than mothers. These findings suggest that mothers may be differentially affected by sleep disturbance than fathers (Baker, O’Brien, & Armitage, 2011). Alternatively, the difference in daytime consequences may reflect a specific mode and intensity of sleep disturbance that accumulated over time (e.g. deprivation or fragmentation) (Franzen, Siegle, & Buysse, 2008; Bei, Milgrom, Ericksen, & Trinder, 2010; Cohen et al., 2010; Insana, Williams, & Montgomery-Downs, under review).
Postpartum mothers and fathers both had impaired sleep quality, high sleepiness, and poor neurobehavioral performance. As expected, postpartum mothers reported worse sleepiness and fatigue, had worse neurobehavioral performance, and had worse MSLT scores than control women; however, they did not differ on self-reported sleep quality. Similarly, as expected, postpartum fathers reported worse subjective sleep quality, had worse neurobehavioral performance, and had worse MSLT scores than control men; however, they did not differ on self-reports of sleepiness or fatigue. Nulliparous women and men seemed to adequately serve as a control group. Although, control women and men had PSQI scores (M = 5.5, and M = 5.6, respectively) that were above the recommended threshold of 5 for ‘good sleep quality’ (within a 0–21 point scale [Buysse et al., 1989]), PSQI scores commonly exceed the 5-point threshold among normative community based samples. For example, Buysse and colleagues reported that among their community based sample of 187 healthy adults, the mean PSQI score was 6.3. Additionally, Knutson and colleagues reported on PSQI stability and demonstrated that among a sample of 600 participants, the mean PSQI score in year 1 was 5.7, and year 2 was 5.9 (Knutson, Rathouz, Yan, Liu, & Lauderdale, 2006). The comparison of postpartum to control participants provided an appropriate relative reference to indicate the extent of postpartum sleep disturbance and sleep related impairments.
Implications
The postpartum sleepiness and performance impairments reveal a societal problem at large. Following the birth of a child, postpartum parents are almost immediately required to go back to work to provide for their family; yet, parents have critically high levels of sleepiness. Within the United States, the Family Medical Leave Act (FMLA) permits new parents 12 weeks of unpaid family leave for medical conditions that include care for a newborn, if they are employed by a company with more than 50 employees (United States Department of Labor, Family and Medical Leave Act). However, even if eligible for medical leave, many new families cannot afford to go without pay for an extended period. Furthermore, the United States is the only industrialized country that does not have a policy for paid, job-protected maternity and paternity leave (Kamerman, 2000). For a review of the benefits that a longer family medical leave has on various infant outcomes see (Cabrera, Tamis-LeMonda, Bradley, Hofferth, & Lamb, 2000; Schor, 2003; Nepomnyaschy & Waldfogel, 2007; Staehelin, Bertea, & Stutz, 2007).
Postpartum mothers have been shown to acquire an increased workload across the first postpartum year, which is associated with less sleep, worse mental health, and decreased family caregiving activities (McGovern et al., 2011). Postpartum mothers describe the necessity to persevere through their sleep disturbance in order to work and meet essential economical demands (Doering & Dufor, 2011). Similarly, new fathers have been shown to return to work despite their high level of fatigue, which is consequently inversely associated with work safety behaviors (Mellor & St John, 2012). There is clear acknowledgement that sleepiness has adverse effects on safety and performance in the workforce (see reviews: Walsh, Dement, & Dinges, 2005; McDonald, Patel, & Belenky, 2011). Consequently, the current 1993 FMLA policy is not practical for most families without putting at least one postpartum parent back into the workforce, albeit with likely sleepiness associated impairments. Experts describe an urgent need for changes in policy that will increase practices that facilitate alertness, which will in turn stimulate safety, health, and productivity within society (Rosekind, 2005); however, postpartum parents were not discussed among the vulnerable populations that are susceptible to sleepiness. Therefore, in light of the current findings, future work should be designed to identify whether the current 1993 FMLA policy is efficacious for new families, and adequately safe for society.
Acknowledgement of postpartum sleep disturbances has resulted in the development of maternal postpartum sleep interventions that implement education strategies (Stremler et al., 2006), as well as behavioral (Hiscock, Bayer, Hampton, Ukoumunne, & Wake, 2008) and environmental modifications (Lee & Gay, 2011). The availability of sleep interventions and strategies targeted to help new fathers obtain more restful sleep are scant, if not currently nonexistent. The lack of focus on improving fathers’ sleep is likely due to limited empirical knowledge about their sleep experience and functioning during the early postpartum period. The current work articulates the necessity for, and creation of, sleep interventions for new fathers.
As discussed above, postpartum mothers may develop a biological resistance to falling, and staying asleep. Although currently constrained to speculation, converging evidence supports the possibility that distinctive biological alterations may play a role in maternal postpartum sleep regulation. The discovery of novel biological mechanisms for sleep regulation, and possibly sleep resistance, would have far reaching implications for application.
Limitations
The current study had considerable strengths. All study findings were in their expected directions, and all outcome measures aggregated together to bolster study interpretations. Sleep and sleepiness were measured from a multimethod approach with field- and laboratory-based procedures through the application of subjective and objective measurement instruments. Nevertheless, methodological limitations must be considered when interpreting the study results. The small control group sample size was a limitation. However, differences between the control and postpartum groups were detected at α = .10; the range of differences above the conventional α = .05, and up-to .10, were augmented by large effect sizes (range: .80 – .92). Another limitation posed by the small sample size was the interpretation of correlations. Specifically, a larger sample size would have likely detected significant correlations among infant age and study variables. The cross-sectional study design did not provide insight into changes in sleep and sleepiness across the transition to parenthood, or throughout the early postpartum period. In light of the current findings, a particularly important future direction would be to describe when postpartum parents’ sleep and sleepiness values return to normal. The absence of infant data is an unfortunate limitation. The prevalence of and potential impact that infant bed sharing had on parental sleep and sleepiness is unknown. Due to a low ethnic diversity among the current sample, it is inconclusive whether these results would generalize to postpartum parents from ethnicities other than White-Non-Hispanic. Future investigations could build upon the current study through examinations of: sleep and sleepiness among multiparous postpartum couples, additional ethnicities, and different cultures; a multitude of infant outcomes that may result from parental sleep and sleepiness; different biological mechanisms that may influence postpartum sleep and sleepiness; the effects that sleeping arrangements, postpartum mood disturbances, and psychological factors may have on postpartum sleep and sleepiness, and vise-versa; and intervention methods to effectively optimize postpartum sleep to decrease their daytime sleepiness.
Finally, it is important to note that the postpartum period is subject to the same dynamic systems that influence all times of change throughout normal lifespan development. The factors that are important to consider in future studies – which our study was not designed or statistically powered to address - include parental work status, infant age, infant feeding method, and infant delivery method.
Of particular interest, mothers in our study whose infants were exclusively bottle-fed had worse neurobehavioral performance than mothers whose infants were breastfed. This difference was unexpected because previous work has shown no differences in sleep measures, sleepiness, or fatigue based on infant feeding methods (Montgomery-Downs, Clawges, & Santy, 2010). However, as discussed above, different daytime behaviors—such as neurobehavioral performance—may be sensitive to specific sleep profiles. Currently, literature on the impact of infant feeding method on paternal sleep and daytime functioning is underdeveloped.
Among mothers in this sample, infant delivery method did not appear to have an effect on any study variable. However, according to previous work, mothers who had cesarean deliveries also experienced more sleep disturbances and fatigue during the early postpartum period (i.e., < 5 days and < 8 weeks) compared to mothers who had vaginal deliveries (Lee & Lee, 2007; Thompson, Roberts, Currie, & Ellwood, 2002, respectively). Differences on the current study variables, as a function of delivery method, would have likely been detected with a larger sample size studied earlier in the postpartum period. To further characterize the postpartum experience future studies should be designed to identify what, and how, certain characteristics drive postpartum parents’ sleep disturbance, sleepiness, fatigue, and performance impairment.
Conclusion
The current study reports on the sleep and functioning within the postpartum family system. The early postpartum period is an important context for these findings because it is a time when one—or both—parents go back to work to function as productive members of society, and they have a new infant to care for. Yet, new parents appear to experience a multitude of severe sleep and sleepiness-associated impairments which may interfere with their responsibilities. The results from this study should be used to: improve the wellbeing among new families by promoting consideration of current social policy (i.e., FMLA); inform the search for mechanisms that propagate postpartum sleep and sleepiness (e.g., biological differences, work status, infant age); inform the development of appropriate postpartum sleep interventions (e.g., for fathers); and inform future investigations of sleep, sleepiness, and safety within the family context.
Supplementary Material
Supplement 1. Postpartum within-dyad MSLT sleepiness values
Acknowledgments
Support: NIH grant R21HD053836 (HMD); T32GM081741 Behavioral and Biomedical Sciences Training Scholarship (SI); APAGS Basic Psychological Science Research Grant (SI); WVU Doctoral Student Research Support & Alumni Fund (SI).
Footnotes
Competing Interests: The authors declare that they have no competing interests.
REFERENCES
- Acebo C, LeBourgeois MK. Actigraphy. Respiratory Care Clinics of North America. 2006;12:23–30. doi: 10.1016/j.rcc.2005.11.010. [DOI] [PubMed] [Google Scholar]
- Alvarez GG, Ayas NT. The impact of daily sleep duration on health: A review of the literature. Progress in Cardiovascular Nursing. 2004;19:56–59. doi: 10.1111/j.0889-7204.2004.02422.x. [DOI] [PubMed] [Google Scholar]
- American Academy of Sleep Medicine. Westchester, IL: Author; 2007. The AASM Manual for the Scoring of Sleep and Associated Events. [Google Scholar]
- Baker FC, O’Brien LM, Armitage R. Sex differences and menstrual-related changes in sleep and circadian rhythms. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. 5th ed. St. Louis, Mo: Elsevier Saunders; 2011. pp. 1562–1571. [Google Scholar]
- Basner M, Dinges DF. Maximizing sensitivity of the psychomotor vigilance test (PVT) to sleep loss. Sleep. 2011;34:581–591. doi: 10.1093/sleep/34.5.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bei B, Milgrom J, Ericksen J, Trinder J. Subjective perception of sleep, but not its objective quality is associated with immediate postpartum mood disturbances in healthy women. Sleep. 2010;33:531–538. doi: 10.1093/sleep/33.4.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Research. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Hall ML, Strollo PJ, Kamarck TW, Owens J, Lee L, et al. Relationships between the Pittsburgh Sleep Quality Index (PSQI), Epworth Sleepiness Scale (ESS), and clinical/polysomnographic measures in a community sample. Journal of Clinical Sleep Medicine. 2008;4:563–571. [PMC free article] [PubMed] [Google Scholar]
- Cabrera NJ, Tamis-LeMonda CS, Bradley RH, Hofferth S, Lamb ME. Fatherhood in the twenty-first century. Child Development. 2000;71:127–136. doi: 10.1111/1467-8624.00126. [DOI] [PubMed] [Google Scholar]
- Carskadon MA, Dement WC. Sleep tendency: an objective measure of sleep loss. Sleep Research. 1977;6:200. [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- Cohen J. The earth is round (p < .05) American Psychologist. 1994;49:997–1003. [Google Scholar]
- Cohen DA, Wang W, Wyatt JK, Kronauer RE, Dijk D, Czeisler CA, Klerman EB. Uncovering residual effects of chronic sleep loss on human performance. Science Translational Medicine. 2010;2 doi: 10.1126/scitranslmed.3000458. 14ra3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condone JT, Boyce P, Corkindale CJ. The first-time fathers study: a prospective study of the mental health and wellbeing of men during the transition to parenthood. The Australian and New Zealand Journal of Psychiatry. 2004;38:56–64. doi: 10.1177/000486740403800102. [DOI] [PubMed] [Google Scholar]
- Copinschi G. Metabolic and endocrine effects of sleep deprivation. Essential Psychopharmacology. 2005;6:341–347. [PubMed] [Google Scholar]
- Doering J, Dufor SL. The process of “persevering toward normalcy” after childbirth. MCN The American Journal of Maternal Child Nursing. 2011;36:258–265. doi: 10.1097/NMC.0b013e31821826e7. [DOI] [PubMed] [Google Scholar]
- a.Dørheim SK, Bondevik GT, Eberhard-Gran M, Bjorvatn B. Sleep and depression in postpartum women: a population-based study. Sleep. 2009;32:847–855. doi: 10.1093/sleep/32.7.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- b.Dørheim SK, Bondevik GT, Eberhard-Gran M, Bjorvatn B. Subjective and objective sleep among depressed and non-depressed postnatal women. Acta Psychiatrica Scandinavica. 2009;119:128–136. doi: 10.1111/j.1600-0447.2008.01272.x. [DOI] [PubMed] [Google Scholar]
- Franzen PL, Siegle GJ, Buysse DJ. Relationships between affect, vigilance, and sleepiness following sleep deprivation. Journal of Sleep Research. 2008;17:34–41. doi: 10.1111/j.1365-2869.2008.00635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay CL, Lee KA, Lee SY. Sleep patterns and fatigue in new mothers and fathers. Biological Research for Nursing. 2004;5:311–318. doi: 10.1177/1099800403262142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gjerdingen DK, Froberg DG, Chaloner KM, McGovern PM. Changes in women’s physical health during the first postpartum year. Archives of Family Medicine. 1993;2:277–283. doi: 10.1001/archfami.2.3.277. [DOI] [PubMed] [Google Scholar]
- Groër M, Davis M, Casey K, Short B, Smith K, Groër S. Neuroendocrine and immune relationships in postpartum fatigue. The American Journal of Maternal Child Nursing. 2005;30:133–138. doi: 10.1097/00005721-200503000-00012. [DOI] [PubMed] [Google Scholar]
- Harrison Y, Horne JA. The impact of sleep deprivation on decision making: A review. Journal of Experimental Psychology: Applied. 2000;6:236–249. doi: 10.1037//1076-898x.6.3.236. [DOI] [PubMed] [Google Scholar]
- Hedges LV. Distribution theory for Glass’s estimator of effect size and related estimators. Journal of Educational Statistics. 1981;6:107–128. [Google Scholar]
- Hiscock H, Bayer JK, Hampton A, Ukoumunne OC, Wake M. Long-term mother and child mental health effects of a population-based infant sleep intervention: cluster-randomized, controlled trial. Pediatrics. 2008;122:e621–e627. doi: 10.1542/peds.2007-3783. [DOI] [PubMed] [Google Scholar]
- Hoddes E, Zarcone V, Smythe H, Phillips R, Dement WC. Quantification of sleepiness: A new approach. Psychophysiology. 1973;10:431–436. doi: 10.1111/j.1469-8986.1973.tb00801.x. [DOI] [PubMed] [Google Scholar]
- Hunter LP, Rychnovsky JD, Yount SM. A selective review of maternal sleep characteristics in the postpartum period. Journal of Obstetric, Gynecological, and Neonatal Nursing. 2009;38:60–68. doi: 10.1111/j.1552-6909.2008.00309.x. [DOI] [PubMed] [Google Scholar]
- Insana S, Montgomery-Downs H. Maternal postpartum sleepiness and fatigue: Associations with objectively measured sleep variables. Journal of Psychosomatic Research. 2010;69:467–473. doi: 10.1016/j.jpsychores.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insana SP, Williams KB, Montgomery-Downs HE. Maternal psychomotor vigilance performance worsens across the first three postpartum months despite improved sleep. (under review) [Google Scholar]
- Kamerman SB. From maternity to parental leave policies: women’s health, employment, and child and family well-being. Journal of the American Medical Women’s Association. 2000;55:96–99. [PubMed] [Google Scholar]
- Kang MJ, Matsumoto K, Shinkoda H, Mishima M, Seo YJ. Longitudinal Study for Sleep-Wake Behaviours of Mothers from Pre-Partum to Post-Partum Using Actigraph and Sleep Logs. Psychiatry and Clinical Neurosciences. 2002;56:251–252. doi: 10.1046/j.1440-1819.2002.00992.x. [DOI] [PubMed] [Google Scholar]
- Karacan I, Williams RL, Hursch CJ, McCaulley M, Heine MW. Some implications of the sleep patterns of pregnancy for postpartum emotional disturbances. The British Journal of Psychiatry. 1969;115:929–935. doi: 10.1192/bjp.115.525.929. [DOI] [PubMed] [Google Scholar]
- Knutson KL, Rathouz PJ, Yan LL, Liu K, Lauderdale DS. Stability of the Pittsburgh Sleep Quality Index and the Epworth Sleepiness Questionnaires over 1 year in early middle-aged adults: The CARDIA study. 2006 doi: 10.1093/sleep/29.11.1503. [DOI] [PubMed] [Google Scholar]
- Lee KA, Gay CL. Can modifications to the bedroom environment improve the sleep of new parents? Two randomized controlled trials. Research in Nursing and Health. 2011;34:17–19. doi: 10.1002/nur.20413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SY, Lee KA. Early postpartum sleep and fatigue for mothers after cesarean delivery compared with vaginal delivery: an exploratory study. The Journal of Perinatal & Neonatal Nursing. 2007;21:109–113. doi: 10.1097/01.JPN.0000270627.73993.b0. [DOI] [PubMed] [Google Scholar]
- Lee KA, Zaffke ME. Longitudinal changes in fatigue and energy during pregnancy and the postpartum period. Journal of Obstetric, Gynecologic, and Neonatal Nursing. 1999;28:183–191. doi: 10.1111/j.1552-6909.1999.tb01983.x. [DOI] [PubMed] [Google Scholar]
- Littner MR, Kushida C, Wise M, Davila DG, Morgenthaler T, Lee-Chiong T, et al. Practice parameters for clinical use of the Multiple Seep Latency Test and the Maintenance of Wakefulness Test. Sleep. 2005;28:113–121. doi: 10.1093/sleep/28.1.113. [DOI] [PubMed] [Google Scholar]
- Loh S, Lamond N, Dorrian J, Roach G, Dawson D. The validity of psychomotor vigilance tasks of less than 10-minute duration. Behavior Research Methods, Instruments, and Computers. 2004;36:339–346. doi: 10.3758/bf03195580. [DOI] [PubMed] [Google Scholar]
- Manber R, Armitage R. Sex, Steroids, and Sleep: A Review. Sleep. 1999;22:540–555. [PubMed] [Google Scholar]
- Martin JA, Hamilton BE, Ventura SJ, Osterman MJK, Kirmeyer S, Matthews TJ, et al. National Vital Statistics Reports. no 1. vol 60. Hyattsville, MD: National Center for Health Statistics; 2011. Births: Final data for 2009. [PubMed] [Google Scholar]
- McDonald J, Patel D, Belenky G. Sleep and performance monitoring in the workplace: The basis for fatigue risk management. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. 5th ed. St. Louis, Mo: Elsevier Saunders; 2011. pp. 775–783. [Google Scholar]
- McGovern P, Dagher RK, Roeber-Rice H, Gjerdingen D, Dowd B, Ukestad LK, et al. A longitudinal analysis of total workload and women’s health after childbirth. Journal of Occupational and Environmental Medicine. 2011;53:497–505. doi: 10.1097/JOM.0b013e318217197b. [DOI] [PubMed] [Google Scholar]
- Meijer AM, vandenWittenboer GL. Contribution of infants’ sleep and crying to marital relationship of first-time parent couples in the 1st year after childbirth. Journal of Family Psychology. 2007;21:49–57. doi: 10.1037/0893-3200.21.1.49. [DOI] [PubMed] [Google Scholar]
- Monk TH. A visual analogue scale technique to measure global vigor and affect. Psychiatry Research. 1989;27:89–99. doi: 10.1016/0165-1781(89)90013-9. [DOI] [PubMed] [Google Scholar]
- Montgomery-Downs HE, Clawges HM, Santy EE. Infant feeding methods and maternal sleep and daytime functioning. Pediatrics. 2010;126:e1562–e1568. doi: 10.1542/peds.2010-1269. [DOI] [PubMed] [Google Scholar]
- Montgomery-Downs HE, Insana SP, Clegg-Kraynok MM, Mancini LM. Normative longitudinal maternal sleep: The first 4 postpartum months. American Journal of Obstetrics and Gynecology. 2010;203:465e1–465e7. doi: 10.1016/j.ajog.2010.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nepomnyaschy L, Waldfogel J. Paternity leave and fathers’ involvement with their young children: Evidence from the American ECLS-B. Community, Work & Family. 2007;10:427–453. [Google Scholar]
- Neylan TC, Metzler TJ, Henn-Haase C, Blank Y, Tarasovsky G, McCaslin SE, et al. Prior night sleep duration is associated with psychomotor vigilance in a healthy sample of police academy recruits. Chronobiology International. 2010;27:1493–1508. doi: 10.3109/07420528.2010.504992. [DOI] [PubMed] [Google Scholar]
- Nishihara K, Horiuchi S, Eto H, Uchida S. Mothers’ wakefulness at night in the post-partum period is related to their infants’ circadian sleep-wake rhythm. Psychiatry and Clinical Neurosciences. 2000;54:305–306. doi: 10.1046/j.1440-1819.2000.00689.x. [DOI] [PubMed] [Google Scholar]
- Nishihara K, Horiuchi S, Eto H, Uchida S. Comparisons of sleep patterns between mothers in post-partum from 9 to 12 weeks and non-pregnant women. Psychiatry and Clinical Neurosciences. 2001;55:227–228. doi: 10.1046/j.1440-1819.2001.00835.x. [DOI] [PubMed] [Google Scholar]
- Okun ML, Luther J, Prather AA, Perel JM, Wisniewski S, Wisner KL. Changes in sleep quality but not hormones predict time to postpartum depression recurrence. Journal of Affective Disorders. 2011;130:378–384. doi: 10.1016/j.jad.2010.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okun ML, Hanusa BH, Hall M, Wisner KL. Sleep complaints in late pregnancy and the recurrence of postpartum depression. Behavioral Sleep Medicine. 2009;7:106–117. doi: 10.1080/15402000902762394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LA. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Richardson GS, Carskadon MA, Flagg W, Van Den Hoed J, Dement WC, Mitler MM. Excessive daytime sleepiness in man: Multiple Sleep Latency Measurement in narcoleptic and control subjects. Electroencephalography and Clinical Neurophysiology. 1978;45:621–627. doi: 10.1016/0013-4694(78)90162-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosekind MR. Underestimating the societal costs of impaired alertness: safety, health and productivity risks. Sleep Medicine. 2005;6:S21–S25. doi: 10.1016/s1389-9457(05)80005-x. [DOI] [PubMed] [Google Scholar]
- Ross LE, Murray BJ, Steiner M. Sleep and perinatal mood disorders: a critical review. Journal of Psychiatry and Neurosciences. 2005;30:347–256. [PMC free article] [PubMed] [Google Scholar]
- Rychnovsky J, Hunter LP. The relationship between sleep characteristics and fatigue in healthy postpartum women. Women’s Health Issues. 2009;19:38–44. doi: 10.1016/j.whi.2008.07.015. [DOI] [PubMed] [Google Scholar]
- Santiago JR, Nolledo MS, Kinzler W, Santiago TV. Sleep and sleep disorders in pregnancy. Annals of Internal Medicine. 2001;134:396–408. doi: 10.7326/0003-4819-134-5-200103060-00012. [DOI] [PubMed] [Google Scholar]
- Schor EL. Family pediatrics: report of the Task Force on the Family. Pediatrics. 2003;111:1541–1571. [PubMed] [Google Scholar]
- Shaki D, Goldbart A, Daniel S, Fraser D, Shorer Z. Pediatric epilepsy and parental sleep quality. Journal of Clinical Sleep Medicine. 2011;156:502–506. doi: 10.5664/JCSM.1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinkoda H, Matsumoto K, Park YM. Changes in sleep-wake cycle during the period from late pregnancy to puerperium identified through the wrist actigraph and sleep logs. Psychiatry and Clinical Neurosciences. 1999;53:133–135. doi: 10.1046/j.1440-1819.1999.00518.x. [DOI] [PubMed] [Google Scholar]
- Staehelin K, Bertea PC, Stutz EZ. Length of maternity leave and health of mother and child–a review. International Journal of Public Health. 2007;52:202–209. doi: 10.1007/s00038-007-5122-1. [DOI] [PubMed] [Google Scholar]
- Stremler R, Hodnett E, Lee K, MacMillan S, Mill C, Ongcangco L, Willan A. A behavioral-educatoin intervention to promote maternal and infant sleep: a pilot randomized, controlled trial. Sleep. 2006;29:1609–1615. doi: 10.1093/sleep/29.12.1609. [DOI] [PubMed] [Google Scholar]
- Stremler R, Wolfson AR. The postpartum period. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. 5th ed. St. Louis, Mo: Elsevier Saunders; 2011. pp. 1587–1590. [Google Scholar]
- Thompson JF, Roberts CL, Currie M, Ellwood DA. Prevalence and persistence of health problems after childbirth: associations with parity and method of birth. Birth. 2002;29:83–94. doi: 10.1046/j.1523-536x.2002.00167.x. [DOI] [PubMed] [Google Scholar]
- Thorne DR, Johnson DE, Redmond DP, Sing HC, Belenky G. The Walter Reed palm-held psychomotor vigilance test. Behavior Research Methods. 2005;37:111–118. doi: 10.3758/bf03206404. [DOI] [PubMed] [Google Scholar]
- Thorpy MJ, Westbrook P, Ferber R, Fredrickson P, Mahowald M, Perez-Guerra F, Reite M, Smith P. The clinical use of the Multiple Sleep Latency Test. Sleep. 1992;15:268–276. doi: 10.1093/sleep/15.3.268. [DOI] [PubMed] [Google Scholar]
- United States Department of Labor, Family and Medical Leave Act. Available online at: http://www.dol.gov/esa/whd/fmla/.
- Walsh JK, Dement WC, Dinges DF. Sleep medicine, public policy, and public health. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. 4th ed. Philadelphia, PA: Elsevier Saunders; 2005. pp. 648–656. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement 1. Postpartum within-dyad MSLT sleepiness values