Abstract
Objectives:
Previous studies have shown that high serum ceramides are associated with memory impairment and hippocampal volume loss, but have not examined dementia as an outcome. The aim of this study was to examine whether serum ceramides and sphingomyelins (SM) were associated with an increased risk of all-cause dementia and Alzheimer disease (AD).
Methods:
Participants included 99 women without dementia aged 70–79, with baseline serum SM and ceramides, enrolled in a longitudinal population-based study and followed for up to 6 visits over 9 years. Baseline lipids, in tertiles, were examined in relation to all-cause dementia and AD using discrete time Cox proportional survival analysis. Lipids were analyzed using electrospray ionization tandem mass spectrometry.
Results:
Twenty-seven (27.3%) of the 99 women developed incident dementia. Of these, 18 (66.7%) were diagnosed with probable AD. Higher baseline serum ceramides, but not SM, were associated with an increased risk of AD; these relationships were stronger than with all-cause dementia. Compared to the lowest tertile, the middle and highest tertiles of ceramide d18:1–C16:0 were associated with a 10-fold (95% confidence interval [CI] 1.2–85.1) and 7.6-fold increased risk of AD (95% CI 0.9–62.1), respectively. The highest tertiles of ceramide d18:1–C24:0 (hazard ratio [HR] = 5.1, 95% CI 1.1–23.6) and lactosylceramide (HR = 9.8, 95% CI 1.2–80.1) were also associated with risk of AD. Total and high-density lipoprotein cholesterol and triglycerides were not associated with dementia or AD.
Conclusions:
Results from this preliminary study suggest that particular species of serum ceramides are associated with incident AD and warrant continued examination in larger studies.
Lipidomic, metabolomic, and targeted approaches have identified pathways and products of sphingolipid metabolism that are altered early in the course of Alzheimer disease (AD).1–5 Ceramides facilitate the regulation of β-site APP cleaving enzyme 1 (BACE-1) and γ-secretase activity and amyloid precursor protein (APP) processing and trafficking. Evidence also suggests that glycosphingolipids bind amyloid-β (Aβ) at the cell surface and form domains that facilitate the oligomerization and fibril formation of Aβ.6–10 In addition to these roles, ceramide is a potent regulator of cell survival. Upon activation, ceramide-associated protein kinases and phosphatases evoke proapoptotic signaling pathways,11–15 leading to neurodegeneration.
Despite the abundant laboratory and animal findings linking sphingolipids and AD pathology, little research has extended these findings to examine the role of sphingolipids in AD pathogenesis among humans. The few postmortem, and 1 CSF, studies suggest ceramide and SM levels, and gene expression patterns of enzymes participating in the sphingolipid pathway, vary by AD severity.1–3,16,17 We have shown that blood ceramide levels vary by cognitive status, and that elevations of ceramides in subjects with amnestic mild cognitive impairment (MCI) predict cognitive decline and hippocampal volume loss.18 In a separate study we also found that high blood ceramides in cognitively normal women predicted memory impairment.19 However, not all individuals with memory impairment progress to dementia or AD.20 Therefore, we were unable to determine whether increased blood ceramides predicted dementia or AD, or were a general indicator of cognitive impairment. The goal of the present pilot study was to determine whether serum ceramides predicted all-cause dementia or were specifically associated with AD.
METHODS
Study sample.
The Women's Health and Aging Study II (WHAS II) is a prospective study of physical functioning among the two-thirds least disabled 70- to 79-year-old community-dwelling women in Baltimore, MD. The sampling and recruitment of this cohort have been previously described.21,22 Using the Health Care Financing Administration's Medicare eligibility lists for 12 zip code areas in Eastern Baltimore City and County, age-stratified (70–74, 75–79) random samples were drawn by Westat, Inc. in 1994–1995. Trained interviewers screened 1,630 women and determined eligibility according to whether women 1) were aged 70 to 79 years; 2) had sufficient hearing and English proficiency to be interviewed; 3) could be contacted by telephone; 4) had a Mini-Mental State Examination score ≥24; and 5) reported no, or limited, difficulty in only 1 of the following 4 domains: mobility and exercise tolerance, upper extremity function, high-functioning tasks, and basic self-care. Of 880 women screened eligible, 436 (49.5%) agreed to participate in the baseline examination at the Johns Hopkins Hospital and to prospective follow-up. Those agreeing to participate were more educated and had more diseases than those who refused, but did not differ on other characteristics.21,22 For the present study, 223 women had adequate serum baseline samples remaining in storage. Due to the limited blood reserved, we randomly selected 100 participants' serum samples for the lipid assays. Follow-ups were conducted 1.5, 3, 6, 7.5, and 9 years after baseline. Each examination consisted of a comprehensive medical history, medication inventory, physical and neurologic examination, neuropsychological battery, and blood draw.
Standard protocol approvals, registrations, and patient consent.
The study was approved by The Johns Hopkins University institutional review board. Written informed consent was obtained for all participants who were examined as part of the study.
Dementia adjudication.
The pool of WHAS II participants for dementia adjudication was chosen from the larger WHAS II cohort (n = 436) based on 3 criteria: Mini-Mental State Examination (MMSE) score <24, history of stroke (either self-report or diagnosed), or a decrease in MMSE score of >2 points between 2 consecutive rounds (with the exception of the extended 3-year window between visits 3 and 4). A total of 239 WHAS II participants met these criteria. These participants were adjudicated through a consensus conference in collaboration with the Johns Hopkins AD Research Center and included neuropsychologists, geriatric psychiatrists, geriatricians, epidemiologists, and faculty and staff involved in WHAS II. During each adjudication session, all available information on each participant was reviewed, including up to 9 years of medical and neurologic history, cognitive test performance, and proxy reports of daily functioning and cognitive problems. Consensus conferences diagnosed mild cognitive impairment, dementia, and dementia type using the National Alzheimer's Coordinating Center approach, which is used in all AD Research Centers. Dementia was initially diagnosed according to DSM-IV criteria23 at each WHAS II visit. The age at onset was assigned as the age when each participant unambiguously met DSM-IV criteria for dementia. Possible and probable AD were diagnosed according to National Institute of Neurological and Communicative Disorders and Stroke and Alzheimer's Disease and Related Disorders Association criteria.24 In order to account for the differences in the adjudication process over time, 20% (approximately 50 participants) were adjudicated twice in a masked fashion. There was 85% agreement (κ = 0.68) between adjudication sessions.
Cholesterol and triglyceride assays.
Nonfasting blood was drawn at baseline and serum was frozen at −80°C until processing. Total and high-density lipoprotein (HDL) cholesterol and triglycerides were determined using standard enzymatic techniques at Quest Diagnostics.
Sphingolipid assays.
A crude lipid extract was performed using previously published methods.25 In brief, methanol containing 30 mM ammonium acetate (3 volumes/weight) was added to each serum sample containing internal standards ceramide d18:1–C12:0 and sphingomyelin d18:1–C12:0 (1.3 μg/mL of extraction solvent) and the mixture was vortexed. Chloroform (4 volumes/weight) was added and the mixture was vortexed and centrifuged at 1,000 g for 10 minutes. The chloroform layer was removed and dried in a vacuum oven. Dried samples were resuspended in 100% methanol (200 μL) just prior to analysis by high-pressure liquid chromatography coupled tandem mass spectrometry.
Samples were injected using a Harvard Apparatus pump at the rate of 15 μL/min into a Sciex API3000 electrospray ionization triple stage quadruple tandem mass spectrometer (ESI-MS/MS; Thornhill, Ontario, Canada) operated in the positive mode. The ESI-MS/MS scanned from 300 to 1,000 atomic mass units (amu) per second at a step of 0.1 amu. Each lipid species was initially identified by a Q1 mass scan, then by precursor ion scanning or neutral loss scanning of a purified standard. Samples were injected into the ESI-MS/MS for 3 minutes, where the mass counts accumulate and the sum of the total counts under each peak were used to quantitate each analyte. SM and ceramide reference standards were purchased from Avanti Polar Lipids (Alabaster, AL).
Covariates.
To examine whether sphingolipids levels varied by demographic and health-related characteristics, we first assessed the association between these variables and tertiles of SM and ceramide species using analysis of variance for continuous variables and Fisher exact test for dichotomous variables. Potential covariates included baseline age, race, education, smoking status, and minutes of exercise per week; medical conditions and symptoms such as systolic and diastolic blood pressure, diabetes, myocardial infarction, stroke, angina, peripheral artery disease, and depression; statins and other medications; and serum total and HDL cholesterol, triglycerides, blood glucose, creatinine, and albumin levels. Of these variables, blood glucose was consistently higher and body mass index (BMI) lower in the highest tertile of all sphingolipids. Multivariable analyses controlled for baseline blood glucose levels, BMI, and age.
Statistical analysis.
SM species were highly correlated (p < 0.0001) after Bonferroni correction. We therefore summed all SM species to create a single (total) SM variable. Individual ceramides species were less correlated so these species were examined separately. SM and ceramides were analyzed in tertiles because they were highly skewed to the right. t Tests and Fisher exact tests were used to compare the 100 randomly selected women with available baseline samples and the 123 women with available samples who were not selected. Differences between the 100 women with assayed lipids and the rest of the population (n = 336), regardless of sample availability, were also examined.
Among the 100 participants with assayed serum sphingolipids, 1 person with prevalent dementia at baseline was excluded from the analysis, leaving a total of 99 individuals. Additionally, 9 participants developed non-AD dementia, and were therefore excluded from the analysis examining SM and ceramides as a risk factor for AD. Due to the time interval feature of WHAS II data, the discrete time Cox proportional hazards model with a complementary log-log link was used to assess the effect of baseline lipid levels on the risk of developing all-cause dementia or AD.26 Exponentiated coefficients from the model can be interpreted as hazard ratios (HRs). Participants were included in longitudinal analyses if they received a baseline evaluation and at least 1 additional follow-up. For each outcome, participants contributed information up to the examination at which they first developed dementia, died, or were lost to follow-up and therefore censored. Multivariate models controlled for age, blood glucose, and BMI. The a priori p value was p < 0.05. Analyses were conducted using SAS version 9.2 (SAS Institute Inc., Cary, NC).
RESULTS
The 100 participants with SM and ceramide assays were slightly younger (74.0 vs 74.7; p = 0.036) and had lower baseline systolic blood pressure (148.6 vs 153.5; p = 0.047) compared to the rest of the sample (n = 336). No other health or demographic characteristics differed between the 2 groups, including baseline cognitive test scores. Among the 223 women with available baseline serum samples, there were no differences between the 100 women randomly selected for the study and the 123 women with available stored bloods but who were not randomly selected. Of the 99 women in the all-cause dementia analyses, 27 (27.3%) developed incident dementia over the 9-year follow-up (this percentage is similar to the 24.8% that developed dementia in the full cohort); 18 of the 27 (66.7%) dementia cases were diagnosed with probable AD. There were no baseline demographic or health-related differences between those who did and those who did not develop all-cause dementia or AD (table 1). The average participant follow-up time in our study sample was 8.2 years (SD = 2.4) with a mean of 5.3 study visits (SD = 1.2) for individuals without dementia and 6 years (SD = 2.5) and 4.1 study visits (SD = 1.2) for those with incident dementia. Total risk time evaluated was 755.1 risk years for all-cause dementia vs no dementia analyses and 710.4 risk years for AD vs no dementia analyses.
Table 1.
Baseline characteristics of participants without dementia, with any dementia type, and with Alzheimer disease

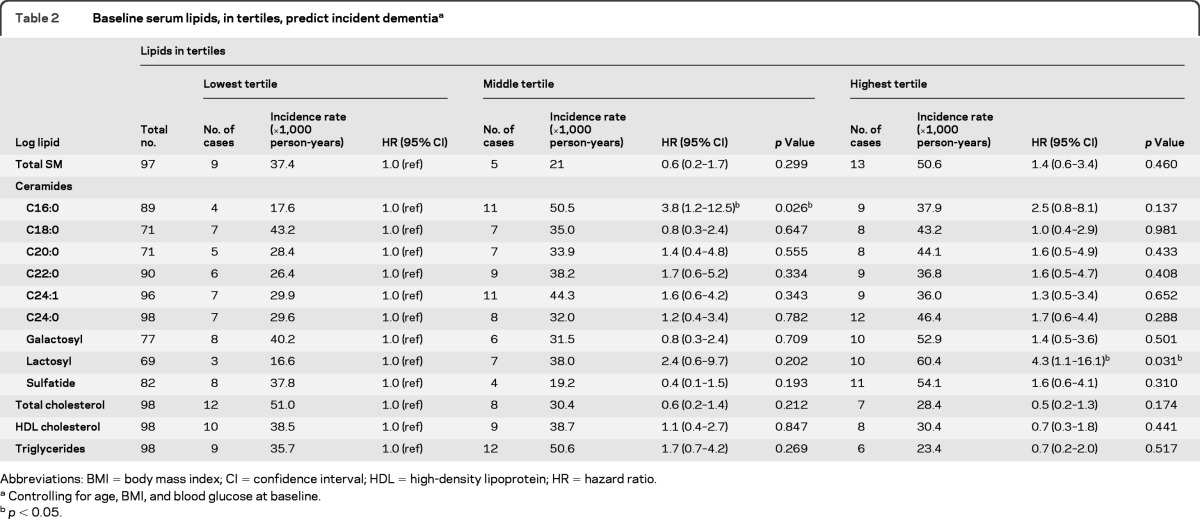
The number of events in each lipid tertile and corresponding incidence rates for all-cause dementia and AD are shown in tables 2 and 3, respectively. Using multivariable discrete time survival analysis to examine serum lipids as predictors of incident dementia (table 2), the highest tertile of lactosylceramide was associated with an increased risk of all-cause dementia (HR = 4.3; 95% confidence interval [CI] 1.1–16.1). Additionally, compared to the lowest tertile, the middle tertile of ceramide d18:1–C16:0 (HR = 3.8; 95% CI 1.2–12.5) was associated with an increased risk of all-cause dementia, while the effect of the highest tertile was attenuated and not significantly different from the lowest tertile (p = 0.137). Figure 1 displays the Kaplan-Meier plots for incident dementia by baseline tertiles of ceramide d18:1–C16:0 and lactosylceramide.
Table 2.
Baseline serum lipids, in tertiles, predict incident dementiaa
Abbreviations: BMI = body mass index; CI = confidence interval; HDL = high-density lipoprotein; HR = hazard ratio;
Controlling for age, BMI, and blood glucose at baseline.
p < 0.05.
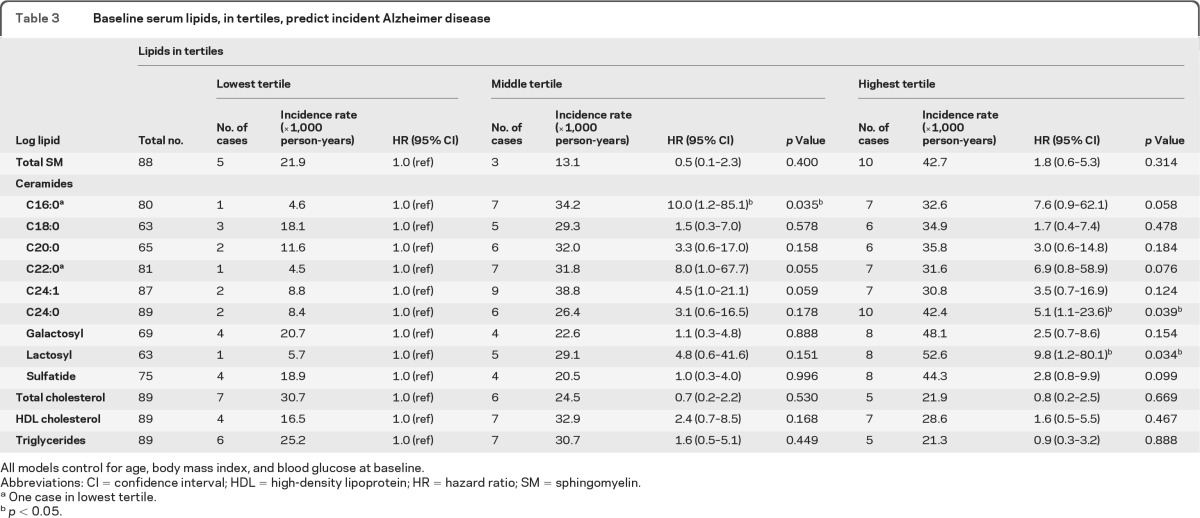
Table 3.
Baseline serum lipids, in tertiles, predict incident Alzheimer disease
All models control for age, body mass index, and blood glucose at baseline.
Abbreviations: CI = confidence interval; HDL = high-density lipoprotein; HR = hazard ratio; SM = sphingomyelin.
One case in lowest tertile.
p < 0.05.
Figure 1. Kaplan-Meier plot for incident dementia.
Kaplan-Meier plot for incident dementia by (A) ceramide C16:0 tertiles and (B) glycosyl C12:0 tertiles.
Higher baseline serum ceramides were also associated with an increased risk of AD, and the relationships were stronger than that seen when examining all-cause dementia as the outcome (table 3). Notably, there was only one case of AD in the lowest tertile of ceramides d18:1–C16:0, d18:1–C22:0, and lactosylceramide. There was also a threshold effect for most ceramides such that both the second and third tertiles exhibited an increased risk of AD, although the results were not always significant at the p < 0.05 level. For example, compared to the lowest tertile, the second tertile of ceramide d18:1–C16:0 was associated with a 10-fold increased risk of AD (95% CI 1.2–85.1) and the highest tertile was associated with a 7.6-fold increased risk (95% CI 0.9–62.1). The highest tertiles of ceramide d18:1–C24:0 (HR = 5.1; 95% CI 1.1–23.6) and lactosylceramide (HR = 9.8; 95% CI 1.2–80.1) were also associated with an increased risk of AD. Figure 2 displays the Kaplan-Meier plots for incident AD by baseline tertiles of lactosylceramides and ceramides d18:0–C16:0, d18:1–C22:0, and d18:1–C24:0.
Figure 2. Kaplan-Meier plot for incident Alzheimer disease (AD).
Kaplan-Meier plot for incident AD by (A) ceramide C16:0 tertiles, (B) ceramide C22:0 tertiles, (C) ceramide C24:0 tertiles, (D) lactosyl C12:0 tertiles.
To determine the specificity of the associations, we also examined other baseline serum lipid levels, including total and HDL cholesterol and triglycerides, but did not find associations between these lipids and incident all-cause dementia or AD (tables 2 and 3). In additional analyses, baseline SM and ceramide levels were not associated with loss to follow-up (data not shown).
DISCUSSION
In this population-based sample of older women, high serum ceramide levels (especially ceramides d18:1–C16:0, d18:1–C24:0, and lactosylceramide) were associated with an increased risk of all-cause dementia independent of age, blood glucose, and BMI. Importantly, the relationship between these lipids and AD was much stronger than for all-cause dementia, with an HR of about 10 and an apparent threshold effect. Only 1 person in the lowest tertile of serum ceramides d18:1–C16:0 and d18:1–C22:0 and lactosylceramide developed AD dementia. These findings suggest that high levels of serum ceramides increase the risk of developing AD.
Accumulating evidence suggests that ceramide metabolism may be perturbed early in the pathogenesis of AD.2,16,17,27 While the exact mechanisms by which this happens is an active area of research, a number of studies have identified pathogenic links between ceramides and amyloid-β (Aβ). First, exposure of cultured neurons to Aβ1–42 directly increases ceramide levels by activating neutral sphingomyelinase28–30; inhibiting this ceramide production protects neurons from Aβ1–42-induced cell death.16 Second, Aβ1–42 indirectly increases ceramide production through an oxidative stress-mediated mechanism.16,31 Ceramides then increase inflammatory and reactive oxygen species, further enhancing the pathology in a self-sustaining way. Finally, increased levels of ceramides accelerate the formation of pathogenic forms of amyloid by increasing β- and γ-cleavage of APP.32–35 These results suggest that a disruption of ceramide metabolism may be an early and critical event involved in Aβ production and the neuronal dysfunction associated with AD.4
We have previously reported that high blood ceramide levels varied by AD severity and were predictive of cognitive decline and hippocampal volume loss among clinically adjudicated patients with amnestic MCI.18 The present study supports these findings by showing that high ceramide levels, particularly ceramides d18:1–C16:0, d18:1–C24:0, and lactosylceramide, are most strongly associated with an increased risk of AD dementia. Notably in previous shotgun and targeted studies of blood and brain ceramides,1,16,18,19 the ceramide d18:1–C24:0 has consistently been altered.
There was a threshold effect for both incident dementia and AD such that the HR of the highest tertile was similar or lower than the middle tertile. Thus, it is possible that low ceramide levels reduce the risk of dementia/AD, rather than that high levels are detrimental. Future studies with larger sample sizes are needed to better determine this threshold effect. Additionally, normal ceramide and SM levels have not been adequately established. Thus, we used tertiles because our data were in cycles per second, and not in easily quantifiable clinical units. Ongoing research is examining normal levels in the population and will be essential to define abnormal values, whether high or low, for risk of dementia and AD.
The exact mechanism by which blood ceramides could contribute to AD is currently unknown, but both direct and indirect mechanisms have been suggested.4 Among HIV-infected participants we have found a significant correlation between plasma and CSF ceramides,36 suggesting that there is a relation between blood measures of ceramides and brain levels. As HIV disruptions of the blood–brain barrier could be driving this association, additional research examining the blood–CSF relationship of sphingolipids in AD is ongoing. Indirect mechanisms may also contribute. Both ceramides and SM increase the risk of cardiovascular disease and insulin resistance,37–39 both of which are associated with an increased risk of AD. In the present study, we examined several vascular factors as mediators of the ceramide–AD relationship and found no attenuation of the relationship between serum ceramides and AD, but additional examination is needed in a larger study.
Several limitations warrant consideration. First, this sample was composed of women and may not be generalizable to men. However, there are currently no results from other studies to suggest the relationship should be different among men. Second, serum lipids were only assayed at baseline, leaving open the possibility that change in these biomarkers may be a better indicator of progression. Third, lipids were nonfasting and the effect of fasting on SM and ceramides is not clear. Correcting for fasting status by controlling for blood glucose had little effect on point estimates. Finally, while there was a high retention rate in this cohort (55.7% of the 436 participants completed all 6 examinations), over 9 years 90 participants died21 and some individuals were lost to follow-up (n = 103) and may have developed dementia, leading to a potential underdetection of cases and a conservative estimate of the lipid–dementia associations. The findings from this small study of women warrant replication in a larger population-based sample to verify the results.
Despite these limitations, there are several strengths. WHAS II is a longitudinal, population-based study that allowed us to examine the specificity of associations between blood SM and ceramides and incident dementia. Women had up to 6 examinations, and 9 years of follow-up. Second, dementia diagnoses were conducted via consensus conference and in collaboration with the Johns Hopkins AD Research Center. Finally, despite the small sample size, effect sizes were quite large and specific to ceramides.
In this preliminary study, high serum ceramide levels were associated with an increased risk of AD and warrant replication in a larger study. Additional research is also needed in larger studies to determine whether there are mediating vascular factors or whether the timing of the measurement (midlife vs late life) is important. The present results, combined with the current literature, call for additional examination into ceramide metabolites as potential new targets for the prevention or treatment of AD.
GLOSSARY
- Aβ
amyloid-β
- AD
Alzheimer disease
- amu
atomic mass units
- APP
amyloid precursor protein
- BACE-1
β-site APP cleaving enzyme 1
- BMI
body mass index
- CI
confidence interval
- DSM-IV
Diagnostic and Statistical Manual of Mental Disorders, 4th edition
- ESI-MS/MS
electrospray ionization tandem mass spectrometry
- HDL
high-density lipoprotein
- HR
hazard ratio
- MCI
mild cognitive impairment
- MMSE
Mini-Mental State Examination
- SM
sphingomyelin
- WHAS II
Women's Health and Aging Study II.
AUTHOR CONTRIBUTIONS
Dr. Mielke: drafting/revising the manuscript for content, including medical writing for content, study concept or design, analysis and interpretation of data, obtaining funding. Dr. Bandaru: drafting/revising the manuscript for content, analysis or interpretation of data. Dr. Haughey: drafting/revising the manuscript for content, including medical writing for content, study concept or design, analysis or interpretation of data. Ms. Xia: drafting/revising the manuscript for content, statistical analysis, Interpretation of the data. Dr. Fried: drafting/revising the manuscript for content, including medical writing for content, study concept or design. Dr. Yasar: drafting/revising the manuscript for content, including medical writing for content, study concept or design, interpretation of data. Dr. Albert: drafting/revising the manuscript for content, including medical writing for content, interpretation of data. Mr. Varma: study concept or design, drafting/revising the manuscript for content, including medical writing for content. Mr. Harris: study concept or design, drafting/revising the manuscript for content, including medical writing for content. Dr. Schneider: study concept or design, drafting/revising the manuscript for content, including medical writing for content. Dr. Rabins: drafting/revising the manuscript for content, including medical writing for content, interpretation of data. Dr. Bandeen-Roche: analysis and interpretation of data, drafting/revising the manuscript for content, including medical writing for content. Dr. Lyketsos: drafting/revising the manuscript for content, including medical writing for content, interpretation of the data. Dr. Carlson: drafting/revising the manuscript for content, including medical writing for content, study concept or design, interpretation of the data, obtaining funding.
DISCLOSURE
M. Mielke, V.V.R. Bandaru, N. Haughey, J. Xia, L. Fried, S. Yasar, M. Albert, V. Varma, G. Harris, E. Schneider, P. Rabins, and K. Bandeen-Roche report no disclosures. C. Lyketsos has received grant support (research or CME) from the following organizations: NIMH, NIA, Associated Jewish Federation of Baltimore, Weinberg Foundation, Forest, GlaxoSmithKline, Eisai, Pfizer, Astra-Zeneca, Lilly, Ortho-McNeil, Bristol-Myers, and Novartis. Dr. Lyketsos has served as a consultant/advisor for Astra-Zeneca, GlaxoSmithKline, Eisai, Novartis, Forest, Supernus, Adlyfe, Takeda, Wyeth, Lundbeck, Merz, Lilly, and Genentech. Dr. Lyketsos has received honorarium or travel support from Pfizer, Forest, GlaxoSmithKline, and Health Monitor. M. Carlson reports no disclosures. Go to Neurology.org for full disclosures.
REFERENCES
- 1. Han X, Rozen S, Boyle SH, et al. Metabolomics in early Alzheimer's disease: identification of altered plasma sphingolipidome using shotgun lipidomics. PLoS One 2011; 6: e21643 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. He X, Huang Y, Li B, Gong CX, Schuchman EH. Deregulation of sphingolipid metabolism in Alzheimer's disease. Neurobiol Aging 2010; 31: 398– 408 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Katsel P, Li C, Haroutunian V. Gene expression alterations in the sphingolipid metabolism pathways during progression of dementia and Alzheimer's disease: a shift toward ceramide accumulation at the earliest recognizable stages of Alzheimer's disease? Neurochem Res 2007; 32: 845– 856 . [DOI] [PubMed] [Google Scholar]
- 4. Mielke MM, Lyketsos CG. Alterations of the sphingolipid pathway in Alzheimer's disease: new biomarkers and treatment targets? Neuromolecular Med 2010; 12: 331– 340 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Haughey NJ, Bandaru VV, Bae M, Mattson MP. Roles for dysfunctional sphingolipid metabolism in Alzheimer's disease neuropathogenesis. Biochim Biophys Acta 2010; 1801: 878– 886 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chi EY, Ege C, Winans A, et al. Lipid membrane templates the ordering and induces the fibrillogenesis of Alzheimer's disease amyloid-beta peptide. Proteins 2008; 72: 1– 24 . [DOI] [PubMed] [Google Scholar]
- 7. Chi EY, Frey SL, Lee KY. Ganglioside G(M1)-mediated amyloid-beta fibrillogenesis and membrane disruption. Biochemistry 2007; 46: 1913– 1924 . [DOI] [PubMed] [Google Scholar]
- 8. Kim SI, Yi JS, Ko YG. Amyloid beta oligomerization is induced by brain lipid rafts. J Cell Biochem 2006; 99: 878– 889 . [DOI] [PubMed] [Google Scholar]
- 9. Kakio A, Nishimoto S, Yanagisawa K, Kozutsumi Y, Matsuzaki K. Interactions of amyloid beta-protein with various gangliosides in raft-like membranes: importance of GM1 ganglioside-bound form as an endogenous seed for Alzheimer amyloid. Biochemistry 2002; 41: 7385– 7390 . [DOI] [PubMed] [Google Scholar]
- 10. Ikeda K, Yamaguchi T, Fukunaga S, Hoshino M, Matsuzaki K. Mechanism of amyloid beta-protein aggregation mediated by GM1 ganglioside clusters. Biochemistry 2011; 50: 6433– 6440 . [DOI] [PubMed] [Google Scholar]
- 11. Bourbon NA, Yun J, Kester M. Ceramide directly activates protein kinase C zeta to regulate a stress-activated protein kinase signaling complex. J Biol Chem 2000; 275: 35617– 35623 . [DOI] [PubMed] [Google Scholar]
- 12. Oh HL, Seok JY, Kwon CH, Kang SK, Kim YK. Role of MAPK in ceramide-induced cell death in primary cultured astrocytes from mouse embryonic brain. Neurotoxicology 2006; 27: 31– 38 . [DOI] [PubMed] [Google Scholar]
- 13. Detre C, Kiss E, Varga Z, et al. Death or survival: membrane ceramide controls the fate and activation of antigen-specific T-cells depending on signal strength and duration. Cell Signal 2006; 18: 294– 306 . [DOI] [PubMed] [Google Scholar]
- 14. Stoica BA, Movsesyan VA, Lea PM, 4th, Faden AI. Ceramide-induced neuronal apoptosis is associated with dephosphorylation of Akt, BAD, FKHR, GSK-3beta, and induction of the mitochondrial-dependent intrinsic caspase pathway. Mol Cell Neurosci 2003; 22: 365– 382 . [DOI] [PubMed] [Google Scholar]
- 15. Shen YH, Godlewski J, Zhu J, et al. Cross-talk between JNK/SAPK and ERK/MAPK pathways: sustained activation of JNK blocks ERK activation by mitogenic factors. J Biol Chem 2003; 278: 26715– 26721 . [DOI] [PubMed] [Google Scholar]
- 16. Cutler RG, Kelly J, Storie K, et al. Involvement of oxidative stress-induced abnormalities in ceramide and cholesterol metabolism in brain aging and Alzheimer's disease. Proc Natl Acad Sci USA 2004; 101: 2070– 2075 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Satoi H, Tomimoto H, Ohtani R, et al. Astroglial expression of ceramide in Alzheimer's disease brains: a role during neuronal apoptosis. Neuroscience 2005; 130: 657– 666 . [DOI] [PubMed] [Google Scholar]
- 18. Mielke MM, Haughey NJ, Ratnam Bandaru VV, et al. Plasma ceramides are altered in mild cognitive impairment and predict cognitive decline and hippocampal volume loss. Alzheimers Dement 2010; 6: 378– 385 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mielke MM, Bandaru VV, Haughey NJ, Rabins PV, Lyketsos CG, Carlson MC. Serum sphingomyelins and ceramides are early predictors of memory impairment. Neurobiol Aging 2010; 31: 17– 24 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Manly JJ, Tang MX, Schupf N, Stern Y, Vonsattel JP, Mayeux R. Frequency and course of mild cognitive impairment in a multiethnic community. Ann Neurol 2008; 63: 494– 506 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Carlson MC, Fried LP, Xue QL, Bandeen-Roche K, Zeger SL, Brandt J. Association between executive attention and physical functional performance in community-dwelling older women. J Gerontol B Psychol Sci Soc Sci 1999; 54: S262– 270 . [DOI] [PubMed] [Google Scholar]
- 22. Fried LP, Bandeen-Roche K, Chaves PH, Johnson BA. Preclinical mobility disability predicts incident mobility disability in older women. J Gerontol A Biol Sci Med Sci 2000; 55: M43– 52 . [DOI] [PubMed] [Google Scholar]
- 23. American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders, 4th ed Washington, DC: American Psychiatric Association; 2000. .. [Google Scholar]
- 24. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 1984; 34: 939– 944 . [DOI] [PubMed] [Google Scholar]
- 25. Bandaru VV, Troncoso J, Wheeler D, et al. ApoE4 disrupts sterol and sphingolipid metabolism in Alzheimer's but not normal brain. Neurobiol Aging 2009; 30: 591– 599 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cox DR. Regression models and life-tables (with discussion). J Royal Stat Soc B 1972; 34: 187– 202 . [Google Scholar]
- 27. Han X, Holtzman DM, McKeel DW, Jr, Kelley J, Morris JC. Substantial sulfatide deficiency and ceramide elevation in very early Alzheimer's disease: potential role in disease pathogenesis. J Neurochem 2002; 82: 809– 818 . [DOI] [PubMed] [Google Scholar]
- 28. Grimm MO, Grimm HS, Patzold AJ, et al. Regulation of cholesterol and sphingomyelin metabolism by amyloid-beta and presenilin. Nat Cell Biol 2005; 7: 1118– 1123 . [DOI] [PubMed] [Google Scholar]
- 29. Jana A, Pahan K. Fibrillar amyloid-beta peptides kill human primary neurons via NADPH oxidase-mediated activation of neutral sphingomyelinase: Implications for Alzheimer's disease. J Biol Chem 2004; 279: 51451– 51459 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lee JT, Xu J, Lee JM, et al. Amyloid-beta peptide induces oligodendrocyte death by activating the neutral sphingomyelinase-ceramide pathway. J Cell Biol 2004; 164: 123– 131 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mattson MP, Cutler RG, Jo DG. Alzheimer peptides perturb lipid-regulating enzymes. Nat Cell Biol 2005; 7: 1045– 1047 . [DOI] [PubMed] [Google Scholar]
- 32. Cordy JM, Hussain I, Dingwall C, Hooper NM, Turner AJ. Exclusively targeting beta-secretase to lipid rafts by GPI-anchor addition up-regulates beta-site processing of the amyloid precursor protein. Proc Natl Acad Sci USA 2003; 100: 11735– 11740 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kalvodova L, Kahya N, Schwille P, et al. Lipids as modulators of proteolytic activity of BACE: involvement of cho-lesterol, glycosphingolipids, and anionic phospholipids in vitro. J Biol Chem 2005; 280: 36815– 36823 . [DOI] [PubMed] [Google Scholar]
- 34. Vetrivel KS, Cheng H, Lin W, et al. Association of gamma-secretase with lipid rafts in post-Golgi and endosome membranes. J Biol Chem 2004; 279: 44945– 44954 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Puglielli L, Ellis BC, Saunders AJ, Kovacs DM. Ceramide stabilizes beta-site amyloid precursor protein-cleaving enzyme 1 and promotes amyloid beta-peptide biogenesis. J Biol Chem 2003; 278: 19777– 19783 . [DOI] [PubMed] [Google Scholar]
- 36. Bandaru VV, Mielke MM, Chu M, et al. CSF sphingolipids are biomarkers for neurocognitive status in HIV-infected patients. In: 10th International Symposium on Neurovirology, Milan, Italy, October 12–16, 2010. .. [Google Scholar]
- 37. Ichi I, Nakahara K, Miyashita Y, et al. Association of ceramides in human plasma with risk factors of atherosclerosis. Lipids 2006; 41: 859– 863 . [DOI] [PubMed] [Google Scholar]
- 38. Nelson JC, Jiang XC, Tabas I, Tall A, Shea S. Plasma sphingomyelin and subclinical atherosclerosis: findings from the multi-ethnic study of atherosclerosis. Am J Epidemiol 2006; 163: 903– 912 . [DOI] [PubMed] [Google Scholar]
- 39. Summers SA. Sphingolipids and insulin resistance: the five Ws. Curr Opin Lipidol 2010; 21: 128– 135 . [DOI] [PubMed] [Google Scholar]